Does Root Tensile Strength Exhibit Seasonal Variation? Evidence from Two Herbaceous Species
Abstract
1. Introduction
2. Results
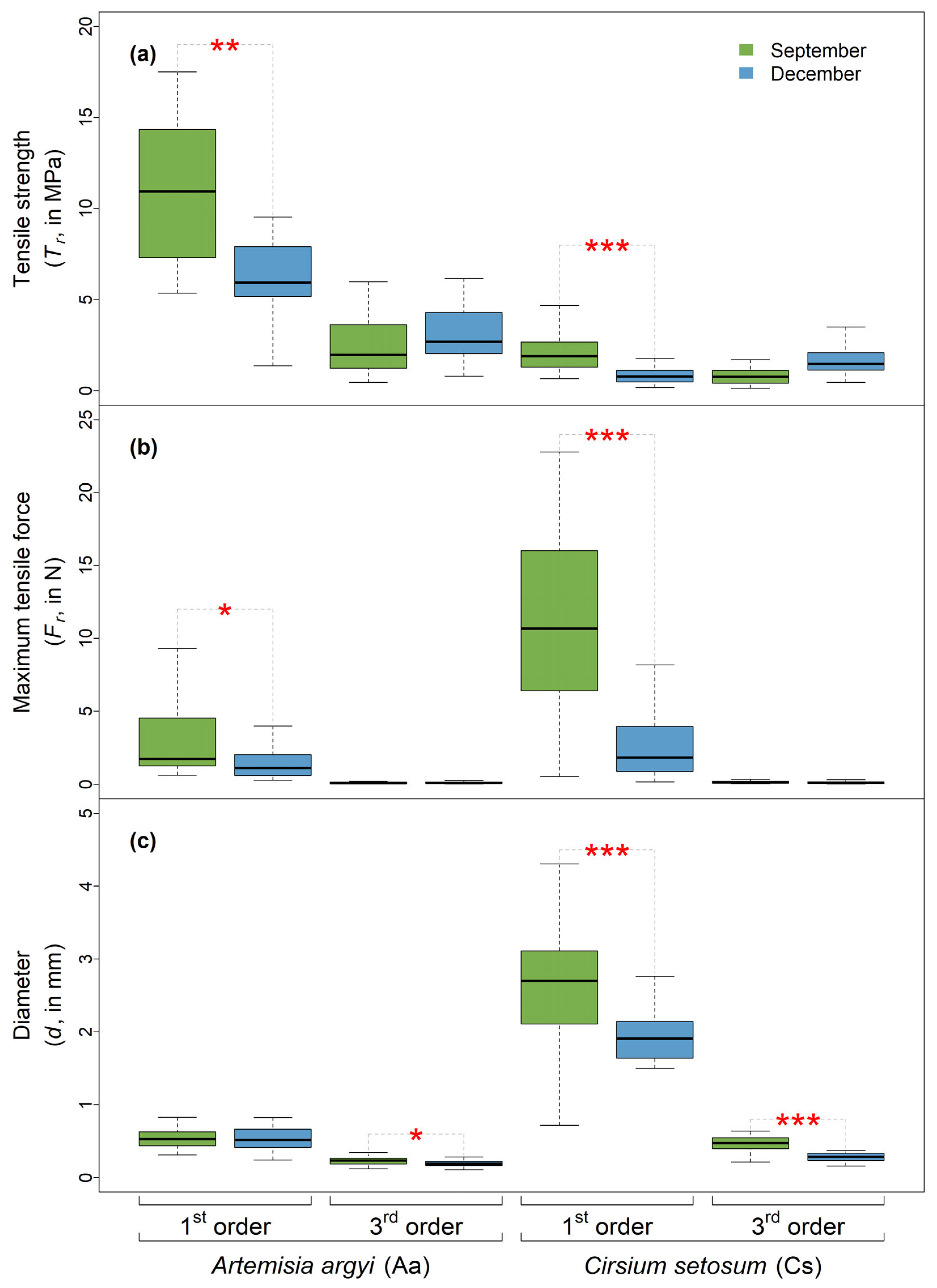
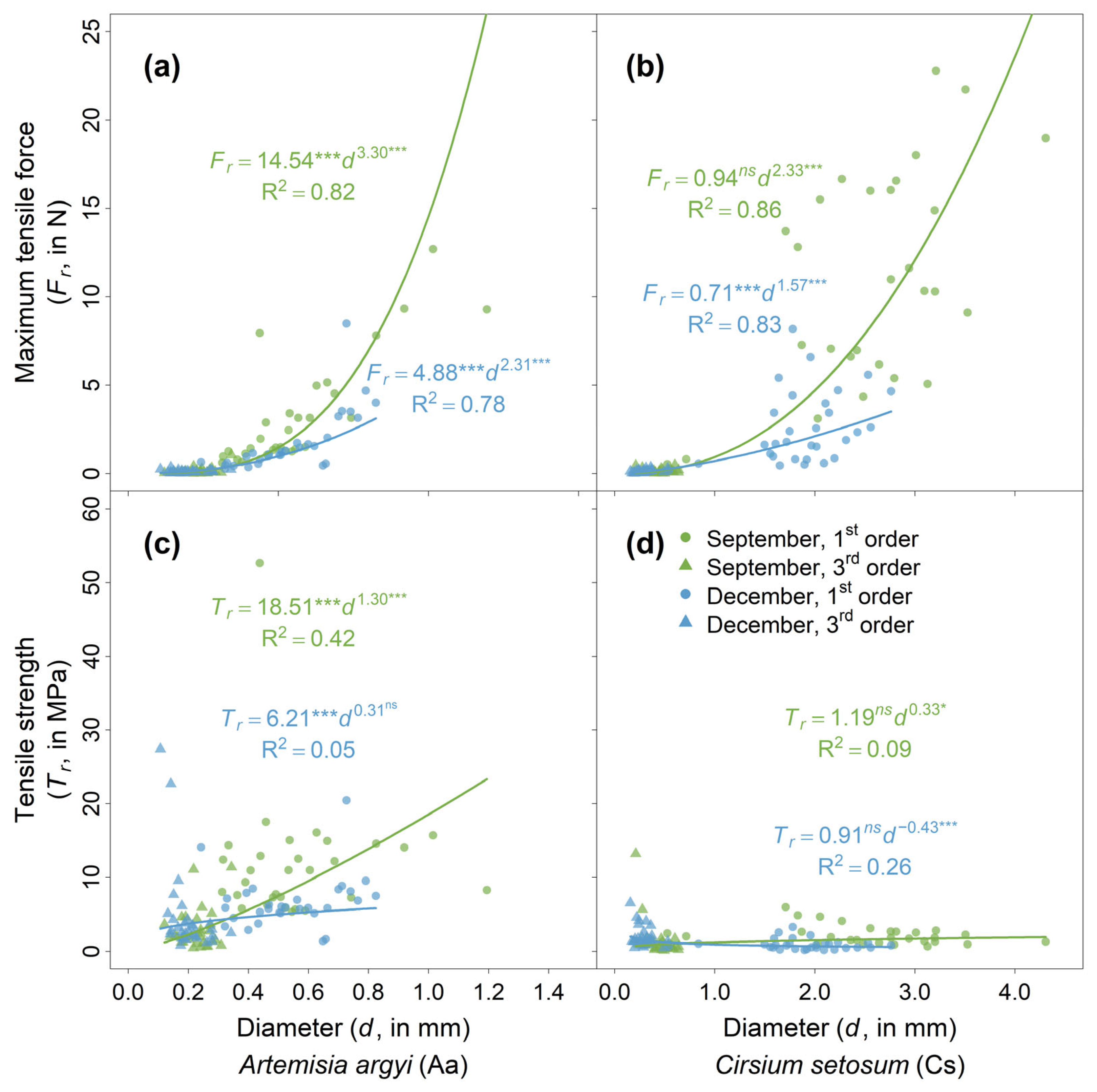
2.1. Seasonal Variation in Soil and Root Characteristics
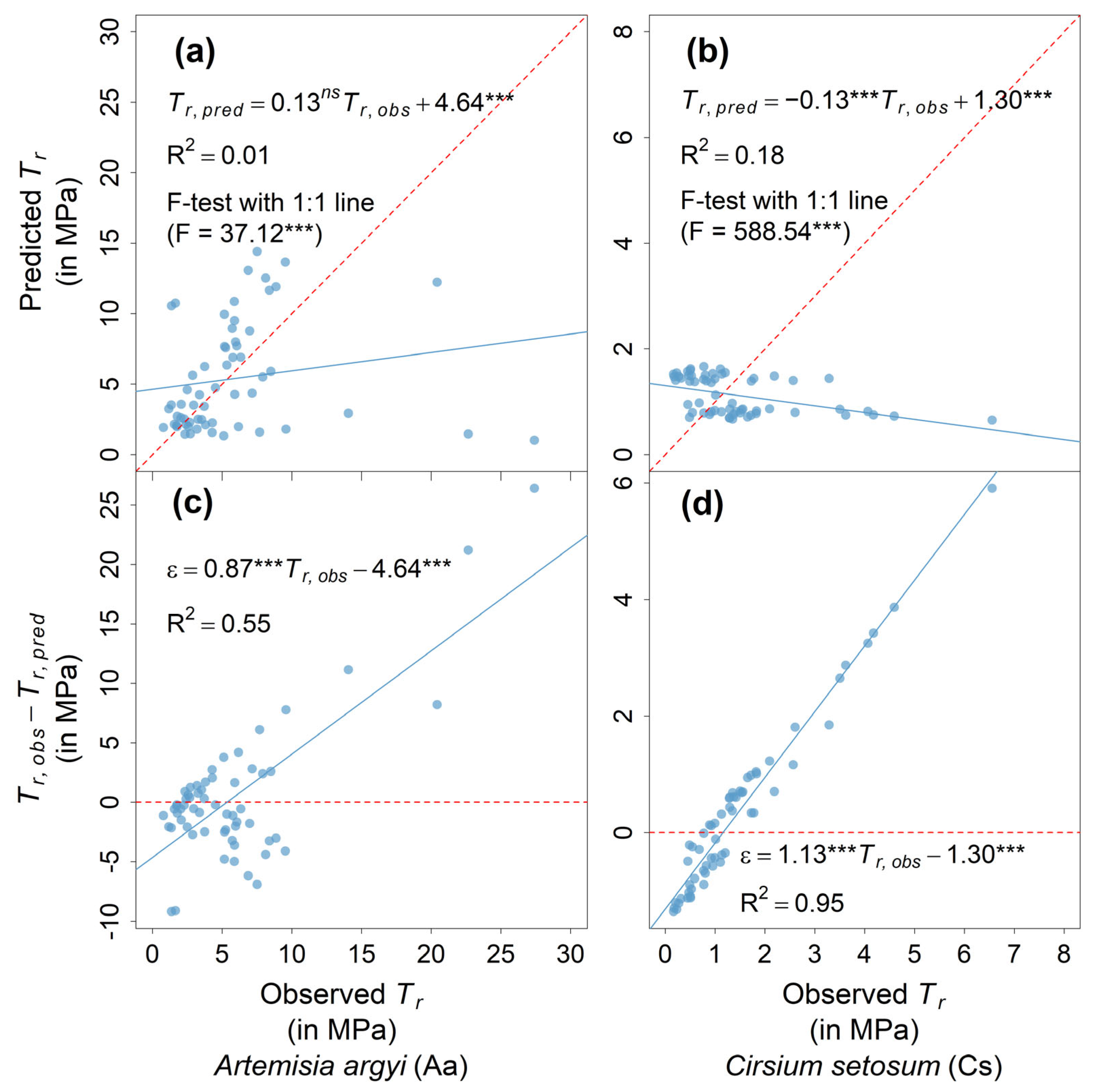
2.2. Comparison of Predicted and Observed Root Tensile Strength in Dormant Season
3. Discussion
4. Materials and Methods
4.1. Study Site and Model Species
4.2. Soil and Root Sampling
4.3. Soil Moisture and Bulk Density Measurements
4.4. Measurement of Root Tensile Strength
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, Z.; Roumet, C.; Rossi, L.M.W.; Merino-Martín, L.; Nespoulous, J.; Taugourdeau, O.; Boukcim, H.; Fourtier, S.; Del Rey-Granado, M.; Ramel, M.; et al. Intra- and Inter-specific Variation in Root Mechanical Traits for Twelve Herbaceous Plants and Their Link with the Root Economics Space. Oikos 2023, 2023, e0903. [Google Scholar] [CrossRef]
- Freschet, G.T. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable Plant Root Traits for Protecting Natural and Engineered Slopes against Landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Cislaghi, A.; Vergani, C.; Hubble, T.; Phillips, C.; Stokes, A. Methods to Measure the Mechanical Behaviour of Tree Roots: A Review. Ecol. Eng. 2017, 109, 256–271. [Google Scholar] [CrossRef]
- Tosi, M. Root Tensile Strength Relationships and Their Slope Stability Implications of Three Shrub Species in the Northern Apennines (Italy). Geomorphology 2007, 87, 268–283. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root Tensile Strength and Root Distribution of Typical Mediterranean Plant Species and Their Contribution to Soil Shear Strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Ghestem, M.; Veylon, G.; Bernard, A.; Vanel, Q.; Stokes, A. Influence of Plant Root System Morphology and Architectural Traits on Soil Shear Resistance. Plant Soil 2014, 377, 43–61. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Knapen, A.; Barberá, G.G.; Navarro, J.A. Root Characteristics of Representative Mediterranean Plant Species and Their Erosion-Reducing Potential during Concentrated Runoff. Plant Soil 2007, 294, 169–183. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, Y.; McCormack, M.; Rowe, N.; Deng, X.; Yang, X.; Xia, S.; Nespoulous, J.; Sidle, R.; Guo, D.; et al. Mechanical Traits of Fine Roots as a Function of Topology and Anatomy. Ann. Bot. 2018, 122, 1103–1116. [Google Scholar] [CrossRef]
- Loades, K.; Bengough, A.; Bransby, M.; Hallett, P. Reinforcement of Soil by Fibrous Roots. In Enhancing Understanding and Quantification of Soil-Root Growth Interactions; Timlin, D., Ahuja, L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 4, pp. 197–228. ISBN 2163-2774. [Google Scholar]
- Loades, K.; Bengough, A.; Bransby, M.; Hallett, P. Effect of Root Age on the Biomechanics of Seminal and Nodal Roots of Barley (Hordeum vulgare L.) in Contrasting Soil Environments. Plant Soil 2015, 395, 253–261. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.F.; Van Beek, R. The Influence of Cellulose Content on Tensile Strength in Tree Roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Mao, Z.; Saint-André, L.; Genet, M.; Mine, F.; Jourdan, C.; Rey, H.; Courbaud, B.; Stokes, A. Engineering Ecological Protection against Landslides in Diverse Mountain Forests: Choosing Cohesion Models. Ecol. Eng. 2012, 45, 55–69. [Google Scholar] [CrossRef]
- Nilaweera, N.S.; Nutalaya, P. Role of Tree Roots in Slope Stabilisation. Bull. Eng. Geol. Environ. 1999, 57, 337–342. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; Simonato, T.; Speziali, B.; Vitali, B.; Vullo, P.; Zocco, A. Root Strength and Root Area Ratio of Forest Species in Lombardy (Northern Italy). Plant Soil 2005, 278, 11–22. [Google Scholar] [CrossRef]
- Burylo, M.; Hudek, C.; Rey, F. Soil Reinforcement by the Roots of Six Dominant Species on Eroded Mountainous Marly Slopes (Southern Alps, France). Catena 2011, 84, 70–78. [Google Scholar] [CrossRef]
- Boldrin, D.; Leung, A.; Bengough, A. Effects of Root Dehydration on Biomechanical Properties of Woody Roots of Ulex europaeus. Plant Soil 2018, 431, 347–369. [Google Scholar] [CrossRef]
- Dumlao, M.R.; Ramananarivo, S.; Goyal, V.; DeJong, J.T.; Waller, J.; Silk, W.K. The Role of Root Development of Avena Fatua in Conferring Soil Strength. Am. J. Bot. 2015, 102, 1050–1060. [Google Scholar] [CrossRef]
- Hales, T.; Miniat, C. Soil Moisture Causes Dynamic Adjustments to Root Reinforcement That Reduce Slope Stability. Earth Surf. Process. Landf. 2017, 42, 803–813. [Google Scholar] [CrossRef]
- Zhu, J.; Mao, Z.; Wang, Y.; Wang, Y.; Tong, L.; Wang, K.; Langendoen, E.; Zheng, B. Soil Moisture and Hysteresis Affect Both Magnitude and Efficiency of Root Reinforcement. Catena 2022, 219, 106574. [Google Scholar] [CrossRef]
- Rossi, R.; Picuno, P.; Fagnano, M.; Amato, M. Soil Reinforcement Potential of Cultivated Cardoon (Cynara cardunculus L.): First Data of Root Tensile Strength and Density. Catena 2022, 21, 106016. [Google Scholar] [CrossRef]
- Hu, W.; Shao, M.A.; Si, B.C. Seasonal Changes in Surface Bulk Density and Saturated Hydraulic Conductivity of Natural Landscapes. Eur. J. Soil Sci. 2012, 63, 820–830. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, N.; Wei, T.; Liu, B.; Shen, L.; Liu, Y.; Liu, D. Adaptation Strategies of Leaf Traits and Leaf Economic Spectrum of Two Urban Garden Plants in China. BMC Plant Biol. 2023, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- Loades, K.W.; Bengough, A.G.; Bransby, M.F.; Hallett, P.D. Planting Density Influence on Fibrous Root Reinforcement of Soils. Ecol. Eng. 2010, 36, 276–284. [Google Scholar] [CrossRef]
- Cleland, E.; Chuine, I.; Menzel, A.; Mooney, H.; Schwartz, M. Shifting Plant Phenology in Response to Global Change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J. The Relationship between Landslide Activity and Weather: Examples from Hungary. Nat. Hazards Earth Syst. Sci. 2003, 3, 43–52. [Google Scholar] [CrossRef]
- Genet, M.; Li, M.; Luo, T.; Fourcaud, T.; Clément-Vidal, A.; Stokes, A. Linking Carbon Supply to Root Cell-Wall Chemistry and Mechanics at High Altitudes in Abies Georgei. Ann. Bot. 2011, 107, 311–320. [Google Scholar] [CrossRef]
- Hales, T.C.; Cole-Hawthorne, C.; Lovell, L.; Evans, S.L. Assessing the Accuracy of Simple Field Based Root Strength Measurements. Plant Soil 2013, 372, 553–565. [Google Scholar] [CrossRef]
- Boldrin, D.; Bengough, A.; Lin, Z.; Loades, K. Root Age Influences Failure Location in Grass Species during Mechanical Testing. Plant Soil 2021, 461, 457–469. [Google Scholar] [CrossRef]
- Zhai, D.; Wang, Y.; Liao, C.; Men, X.; Wang, C.; Cheng, X. Soil Carbon Accumulation Under Afforestation Is Driven by Contrasting Responses of Particulate and Mineral-Associated Organic Carbon. Glob. Biogeochem. Cycles 2024, 38, e2024GB008116. [Google Scholar] [CrossRef]
- Li, L.; Chen, P.; Wang, K.; Zhang, R.; Yuan, X.; Ge, L.; Li, Q.; Liu, Y.; Zhang, X.; Li, Z. Gramineae-Legumes Mixed Planting Effectively Reduces Soil and Nutrient Loss in Orchards. Agric. Water Manag. 2023, 289, 108513. [Google Scholar] [CrossRef]
- Berntson, G.M. Topological Scaling and Plant Root System Architecture: Developmental and Functional Hierarchies. New Phytol. 1997, 135, 621–634. [Google Scholar] [CrossRef]
- Cohen, D.; Schwarz, M.; Or, D. An Analytical Fiber Bundle Model for Pullout Mechanics of Root Bundles. J. Geophys. Res. Earth Surf. 2011, 116, F03010. [Google Scholar] [CrossRef]
- Meijer, G.J. A Generic Form of Fibre Bundle Models for Root Reinforcement of Soil. Plant Soil 2021, 468, 45–65. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2024. Available online: https://www.R-project.org/ (accessed on 8 June 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019; ISBN 978-1-5443-3647-3. [Google Scholar]

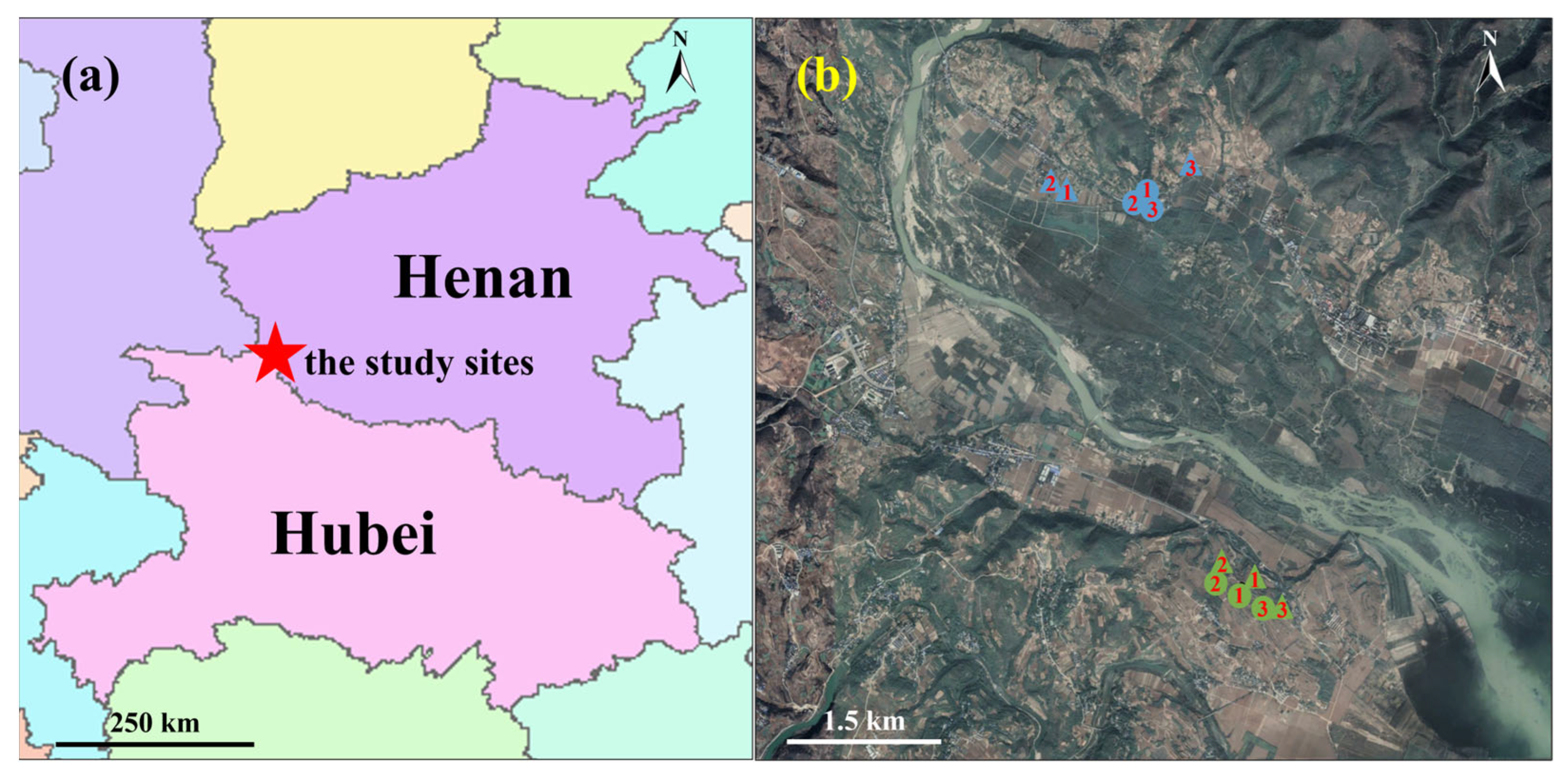
| Month | Species | Abbreviation | Sampling Site | Latitude | Longitude | Altitude (m) |
|---|---|---|---|---|---|---|
| September | Artemisia argyi | Aa | 1 | 33.0349° N | 111.2498° E | 224.6 |
| 2 | 33.0353° N | 111.2478° E | 213.1 | |||
| 3 | 33.0336° N | 111.2523° E | 215.7 | |||
| Cirsium setosum | Cs | 1 | 33.0350° N | 111.2498° E | 223.9 | |
| 2 | 33.0354° N | 111.2478° E | 215.7 | |||
| 3 | 33.0336° N | 111.2522° E | 210.5 | |||
| December | Artemisia argyi | Aa | 1 | 33.0688° N | 111.2391° E | 180.0 |
| 2 | 33.0687° N | 111.2390° E | 182.6 | |||
| 3 | 33.0687° N | 111.2389° E | 182.9 | |||
| Cirsium setosum | Cs | 1 | 33.0697° N | 111.2315° E | 162.3 | |
| 2 | 33.0722° N | 111.2443° E | 192.6 | |||
| 3 | 33.0707° N | 111.2299° E | 169.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, K.; Deng, C.; Ye, L.; Liu, Y.; Liu, F.; Mao, Z.; Zuo, J. Does Root Tensile Strength Exhibit Seasonal Variation? Evidence from Two Herbaceous Species. Plants 2025, 14, 2957. https://doi.org/10.3390/plants14192957
Ji K, Deng C, Ye L, Liu Y, Liu F, Mao Z, Zuo J. Does Root Tensile Strength Exhibit Seasonal Variation? Evidence from Two Herbaceous Species. Plants. 2025; 14(19):2957. https://doi.org/10.3390/plants14192957
Chicago/Turabian StyleJi, Kang, Chaochao Deng, Luping Ye, Yi Liu, Feng Liu, Zhun Mao, and Juan Zuo. 2025. "Does Root Tensile Strength Exhibit Seasonal Variation? Evidence from Two Herbaceous Species" Plants 14, no. 19: 2957. https://doi.org/10.3390/plants14192957
APA StyleJi, K., Deng, C., Ye, L., Liu, Y., Liu, F., Mao, Z., & Zuo, J. (2025). Does Root Tensile Strength Exhibit Seasonal Variation? Evidence from Two Herbaceous Species. Plants, 14(19), 2957. https://doi.org/10.3390/plants14192957







