Physiological, Biochemical, and Transcriptomic Responses to Iron Deficiency in Two Potato Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of Morphological Indicators
2.3. Measurement of Physiological Indicators
2.4. Determination of Metal Element Content
2.5. Illumina Sequencing, RNA Extraction, Reverse Transcription, qRT-PCR Analysis
2.6. Identification of Differentially Expressed Genes
2.7. Analysis of the Functions of Differentially Expressed Genes
2.8. Statistical Analysis
3. Result
3.1. The Effect of Iron Deficiency Stress on the Growth of Potato Tissue Culture Seedlings
3.2. The Influence of Iron Deficiency Stress on the Morphological Indicators of Potato Tissue Culture Seedlings
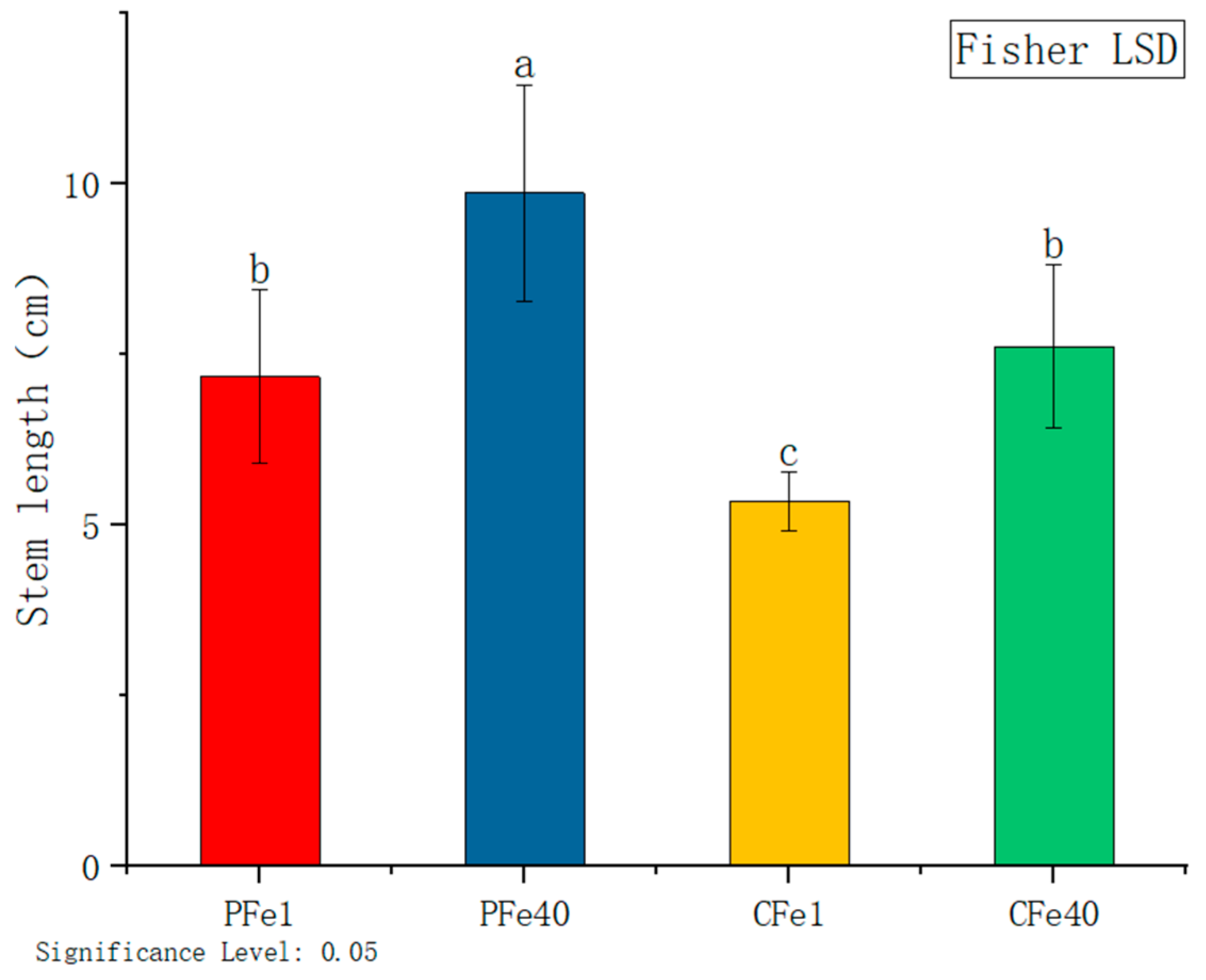
3.2.1. The Effect of Iron Deficiency Stress on the Height of Potato Tissue Culture Seedlings
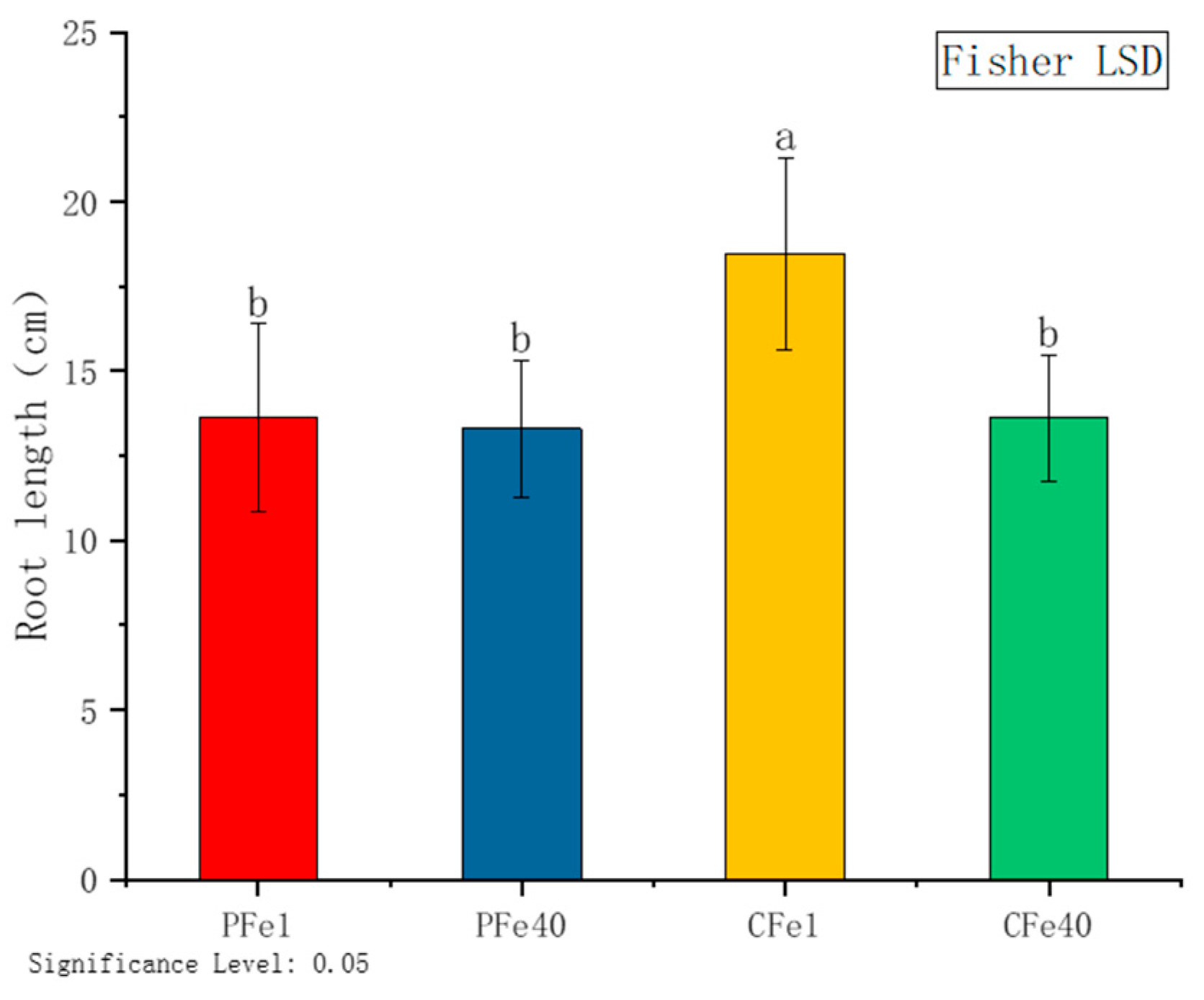
3.2.2. The Effect of Iron Deficiency Stress on the Root Length of Potato Tissue Culture Seedlings
3.2.3. The Effect of Iron Stress on the Fresh Shoot Weight of Potato Tissue Culture Seedlings
3.3. The Influence of Iron Deficiency Stress on the Physiological Indicators of Potato Tissue Culture Seedlings
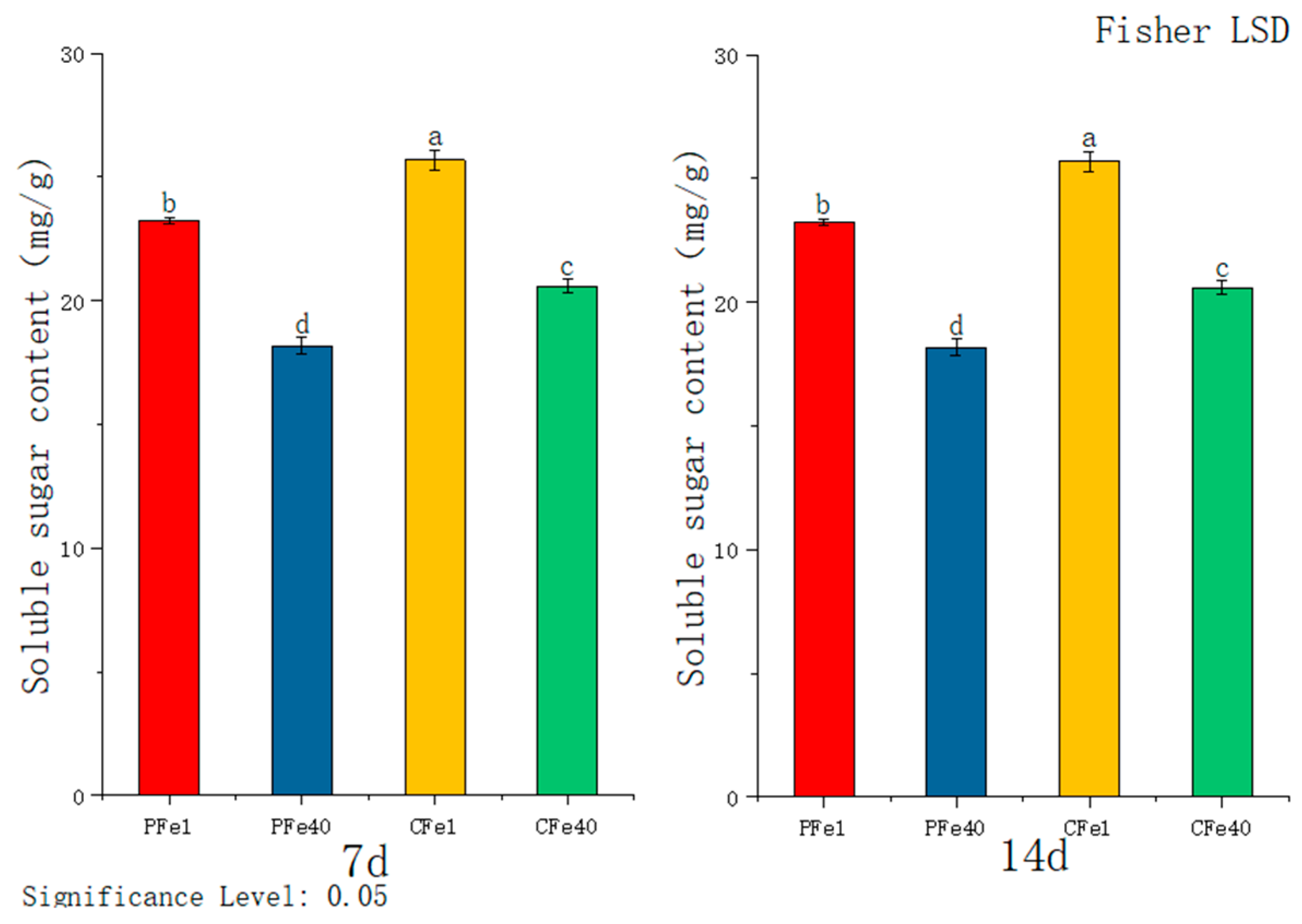
3.3.1. The Effect of Iron Deficiency Stress on the Soluble Sugar Content of Potato Tissue Culture Seedlings
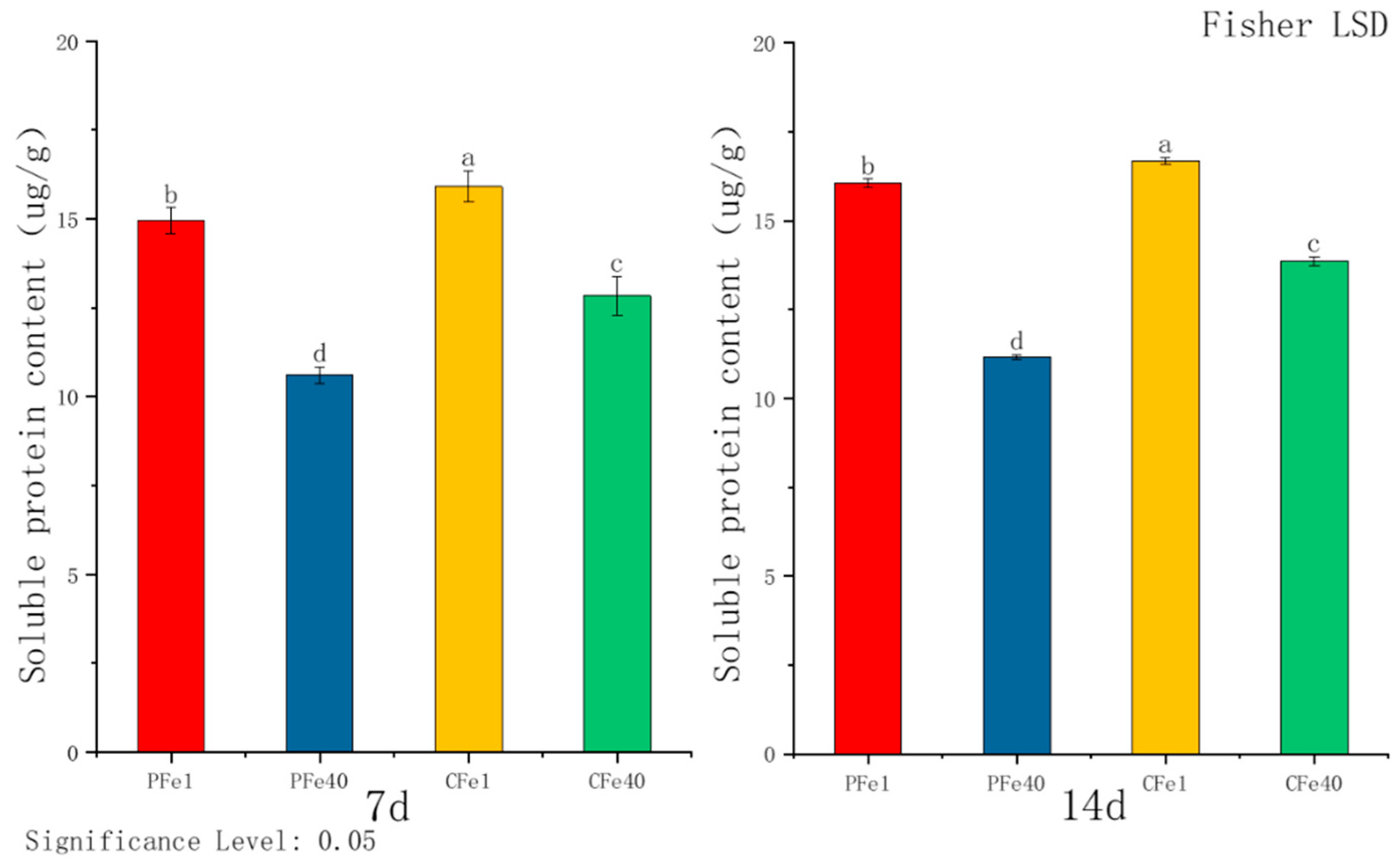
3.3.2. The Effect of Iron Deficiency Stress on the Soluble Protein Content of Potato Tissue Culture Seedlings
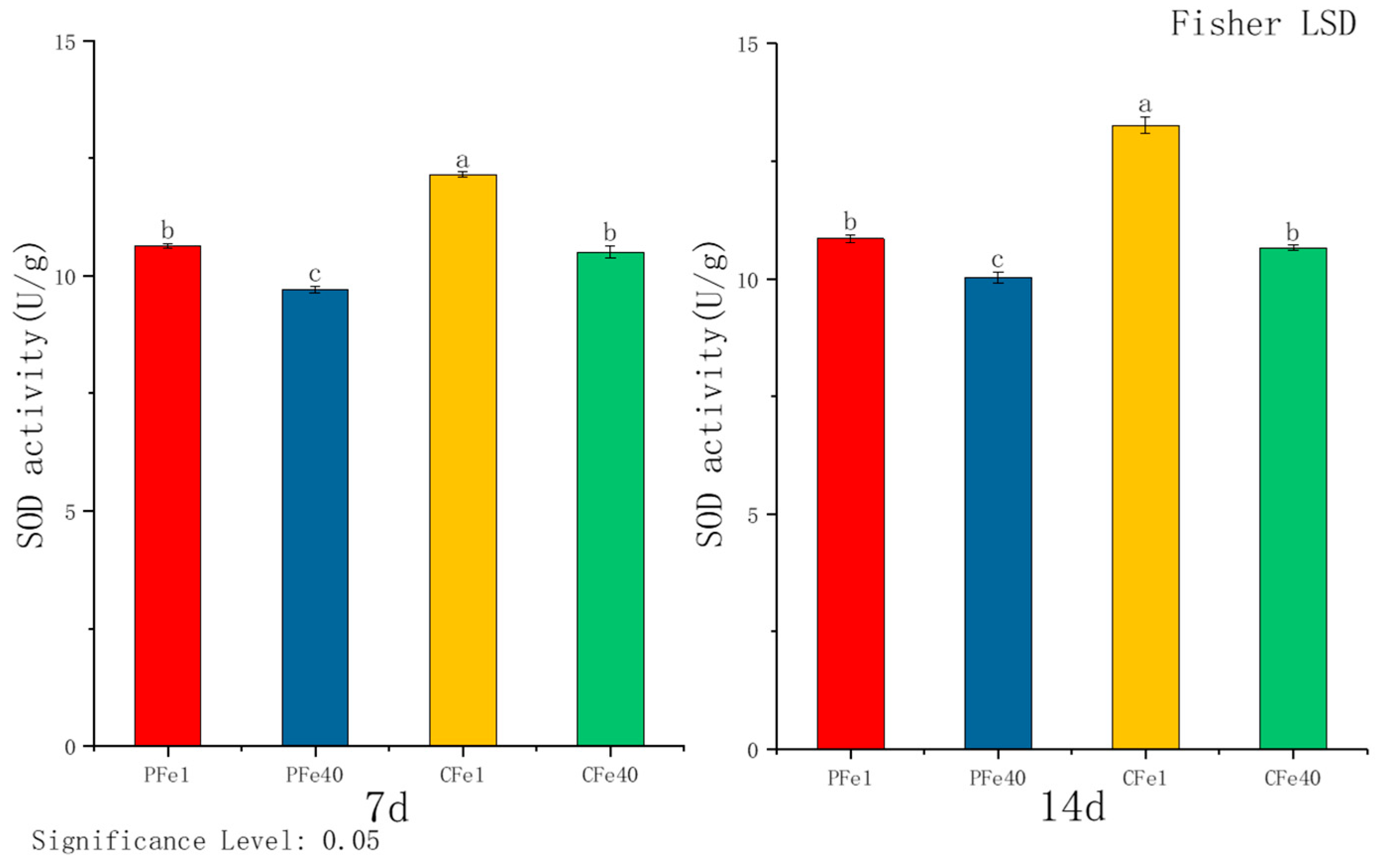
3.3.3. The Effect of Iron Deficiency Stress on the Superoxide Dismutase (SOD) Activity of Potato Tissue Culture Seedlings
3.3.4. The Effect of Iron Deficiency Stress on the Peroxidase (POD) Activity of Potato Tissue Culture Seedlings
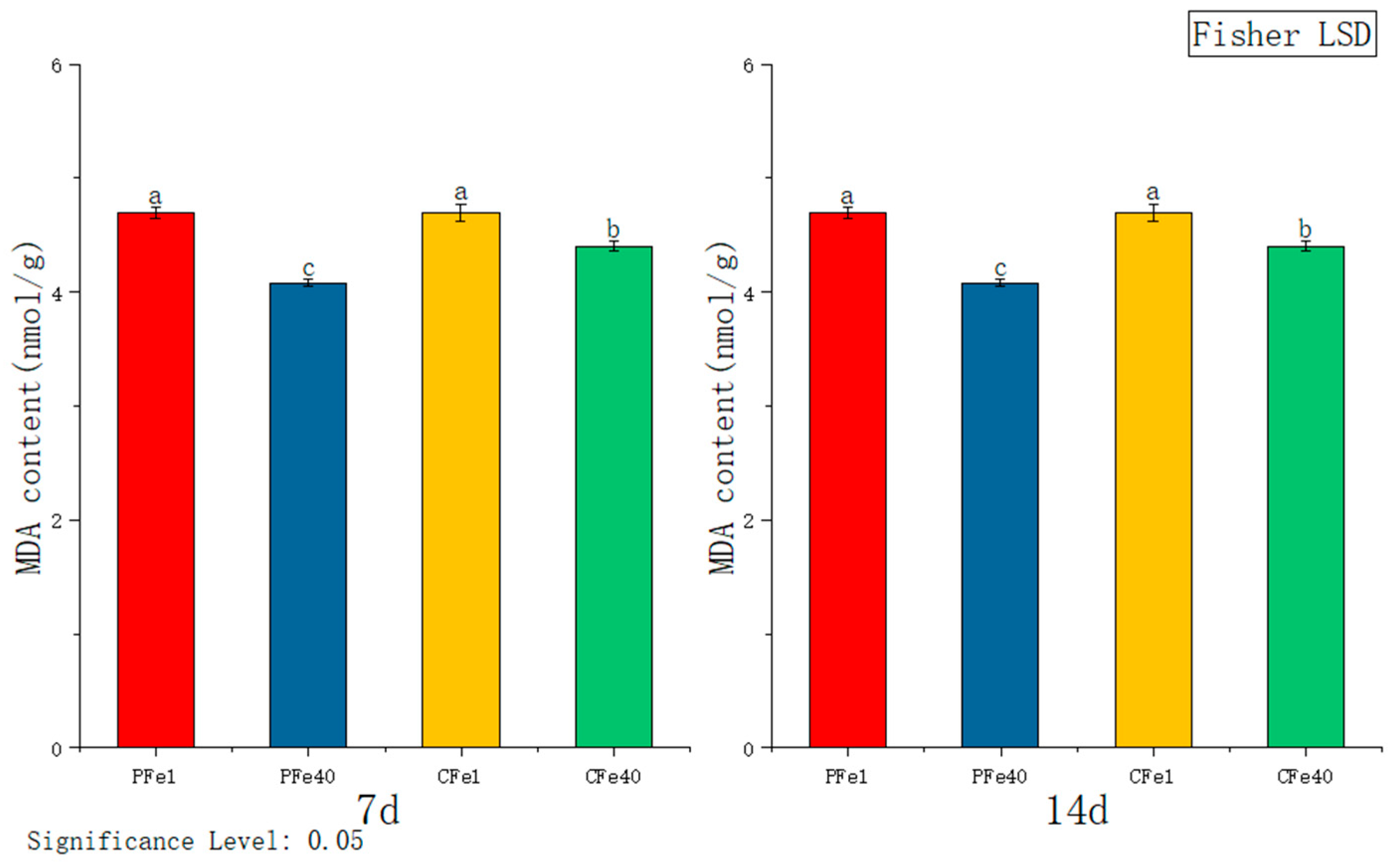
3.3.5. The Effect of Iron Deficiency Stress on the Malondialdehyde (MDA) Activity of Potato Tissue Culture Seedlings
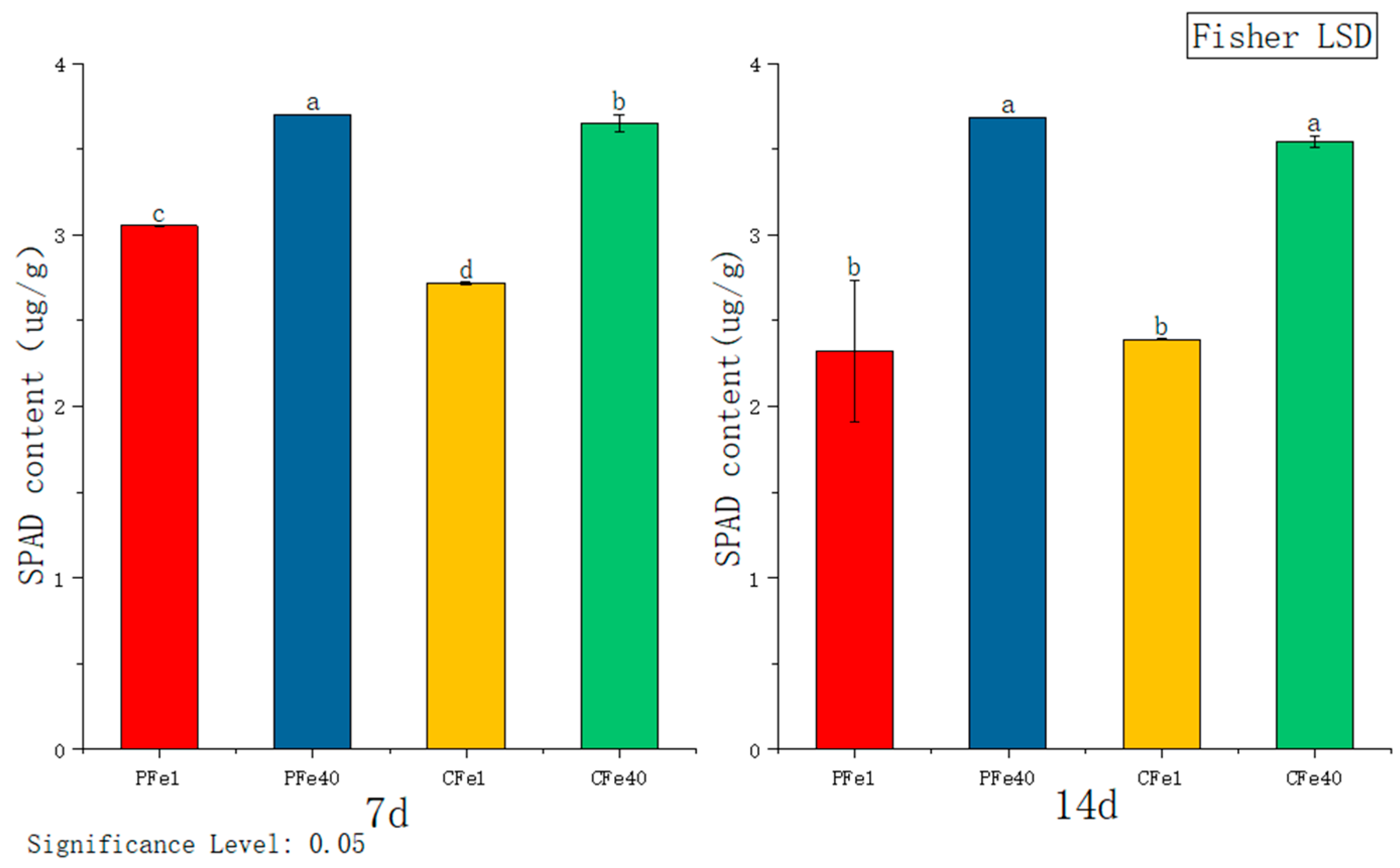
3.3.6. The Effect of Iron Deficiency Stress on the SPAD Content of Chlorophyll in Potato Tissue Culture Seedlings
3.4. The Effect of Iron Deficiency Stress on Metal Elements in Different Tissues of Potato Tissue Culture Seedlings
3.5. Identification of Differentially Expressed Genes in Different Tissues of 05P and CI5 Under Iron Deficiency Stress
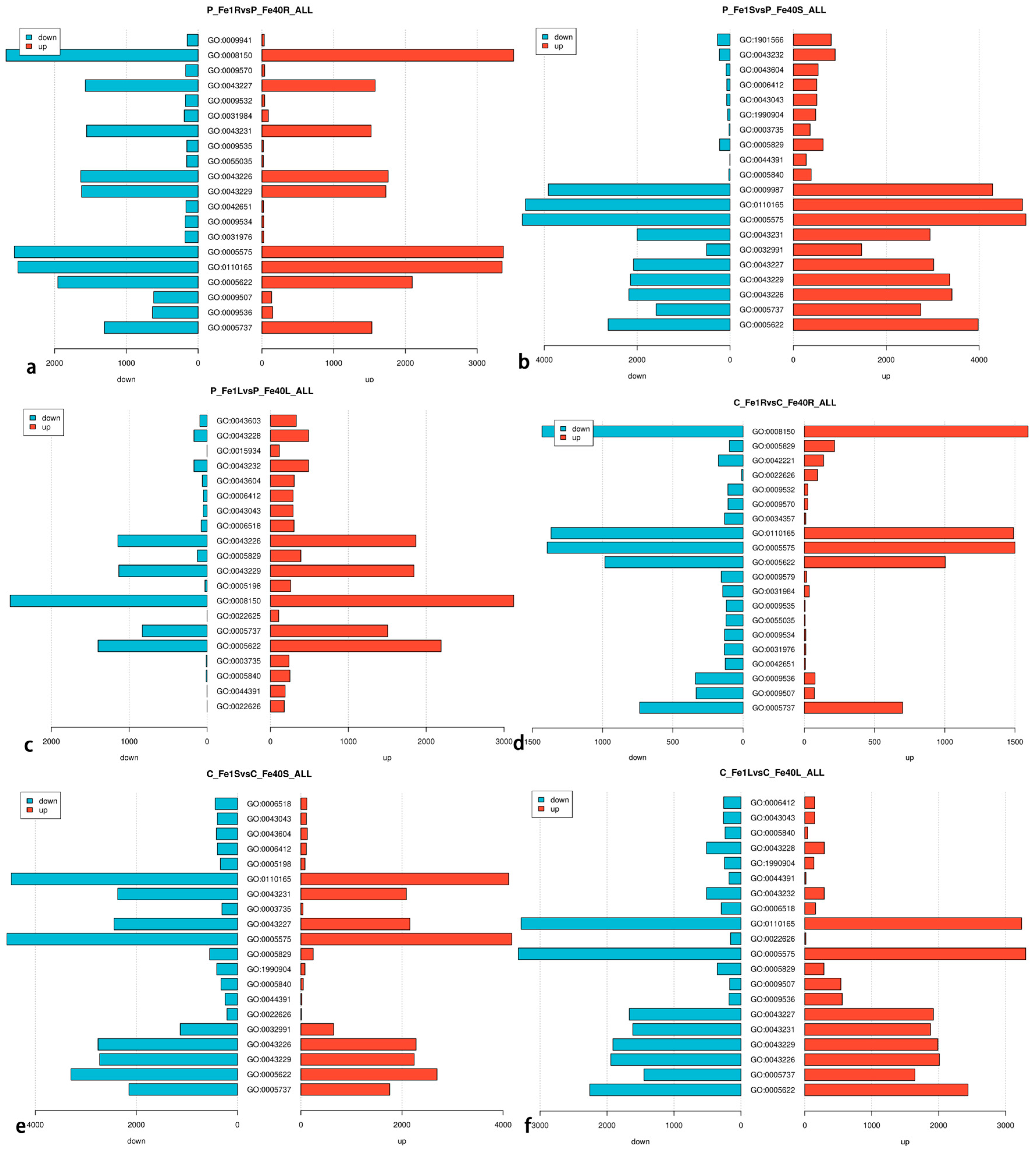
3.6. Differential Expression Gene GO Enrichment Analysis
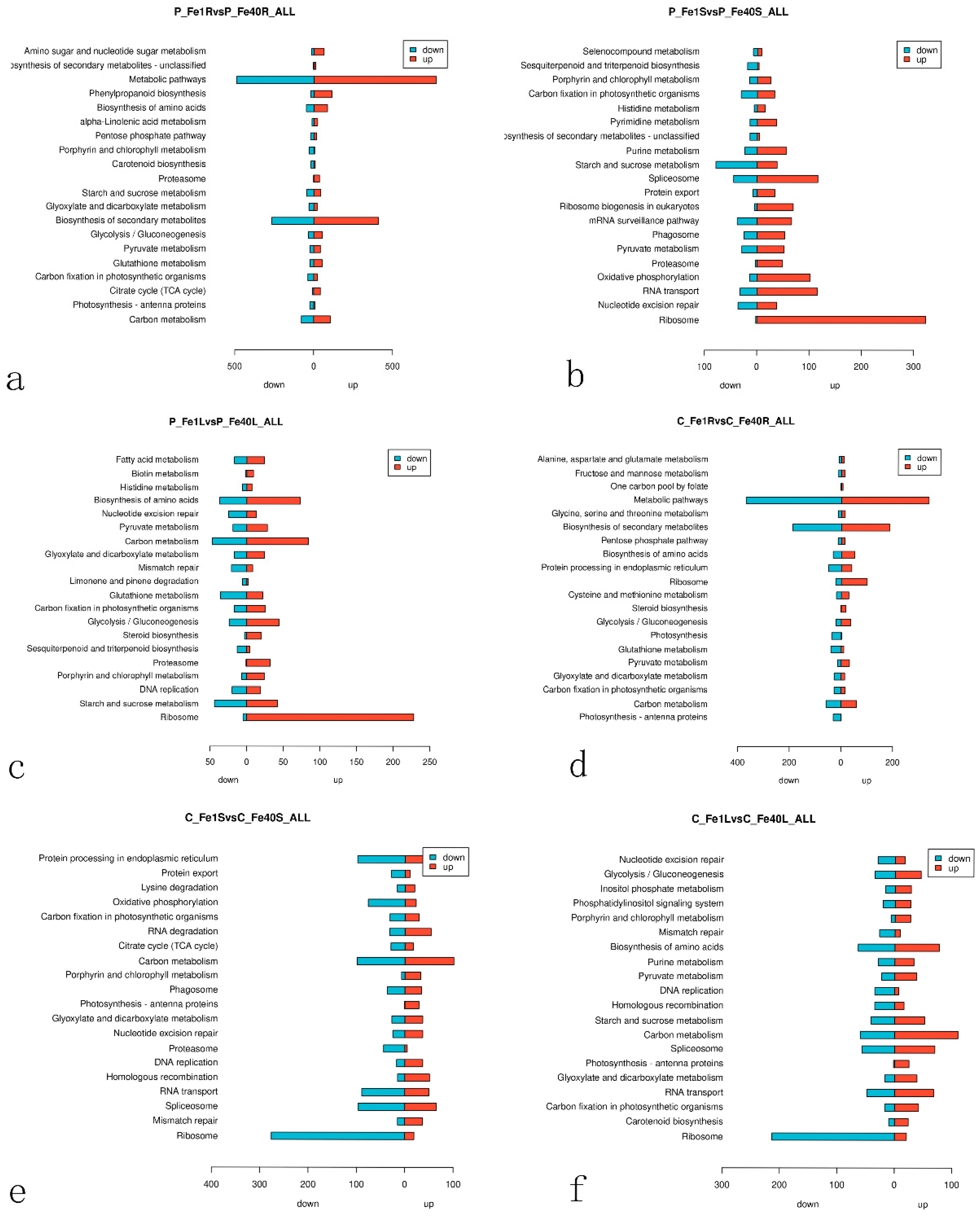
3.7. KEGG Enrichment Analysis of Differentially Expressed Genes in Potatoes Under Iron Deficiency Stress
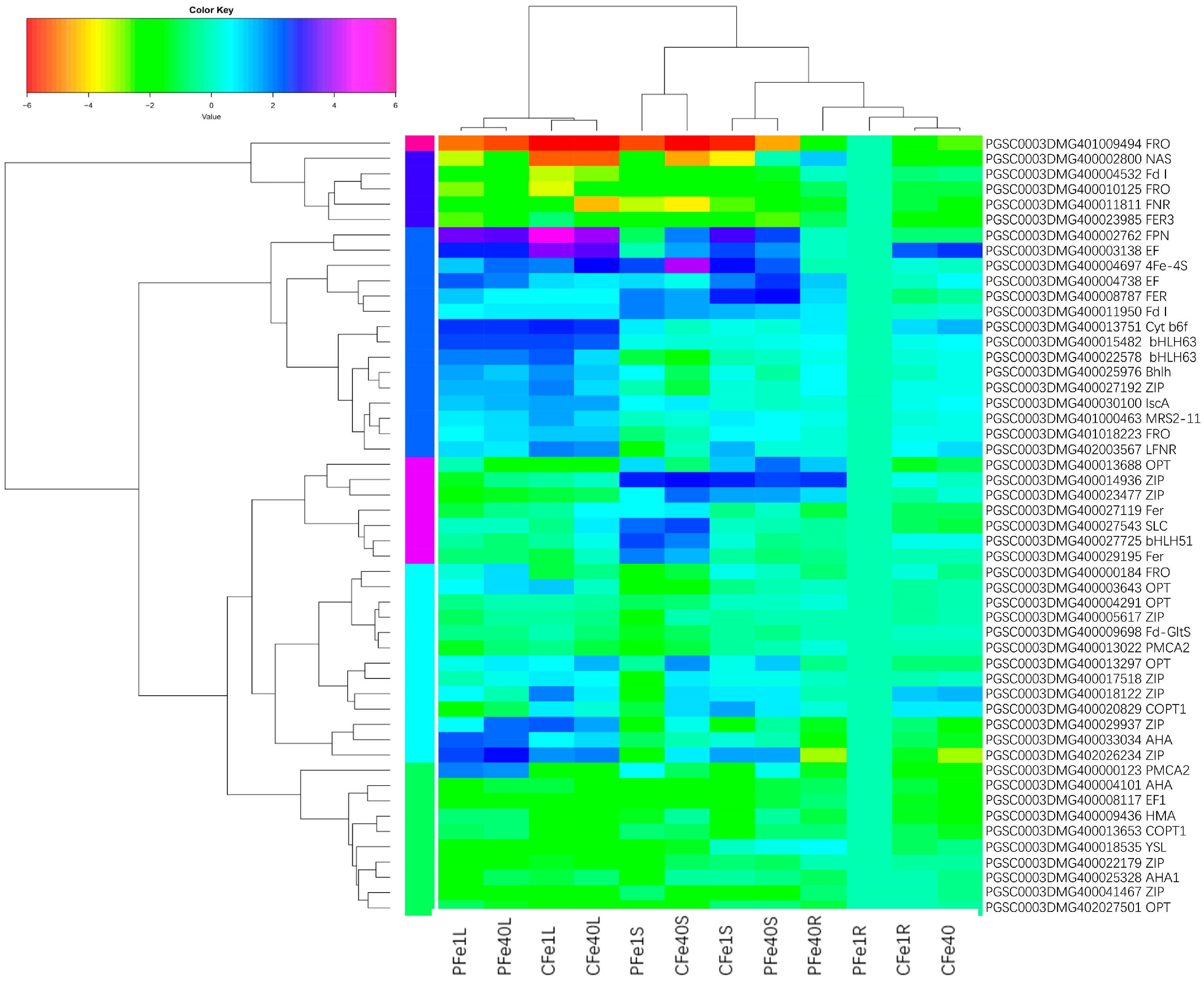
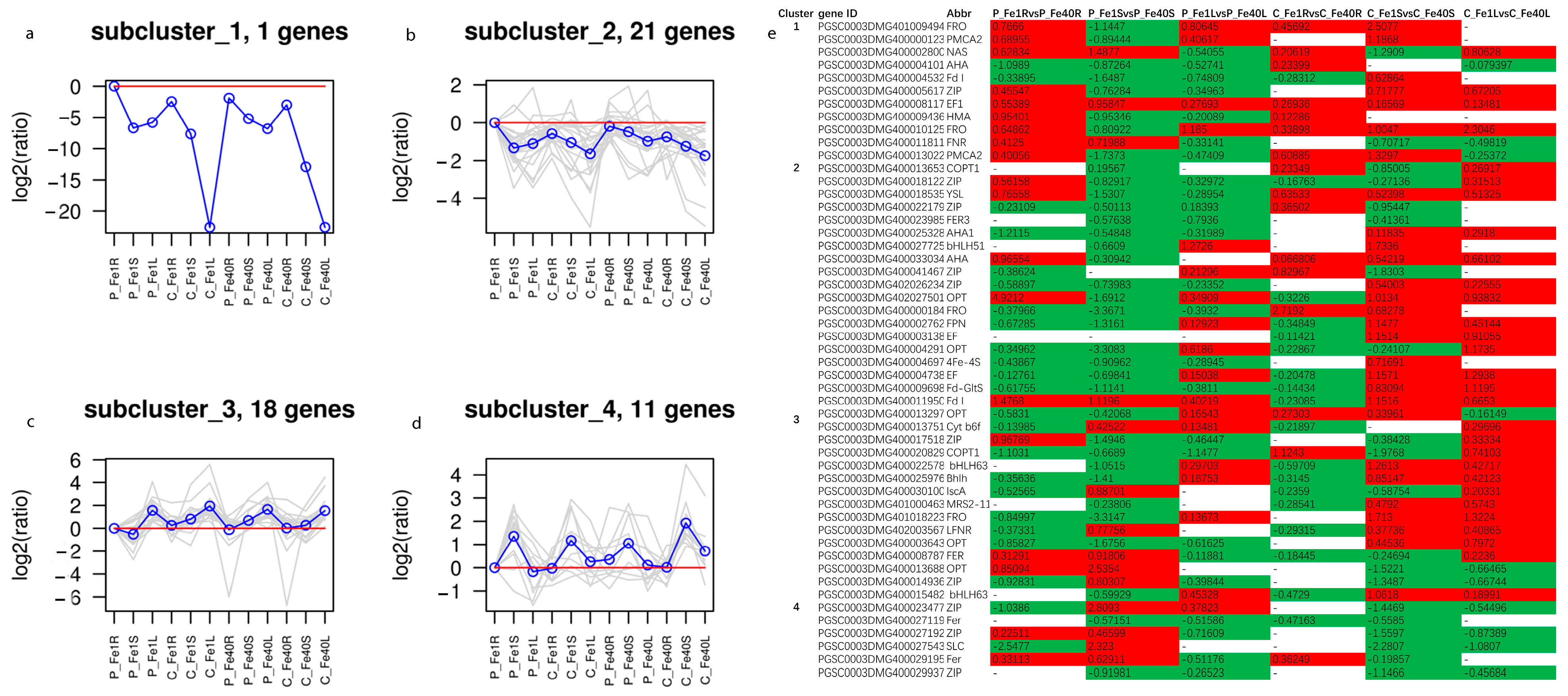
3.8. Potato Root, Stem and Leaf DEGs Related to Fe
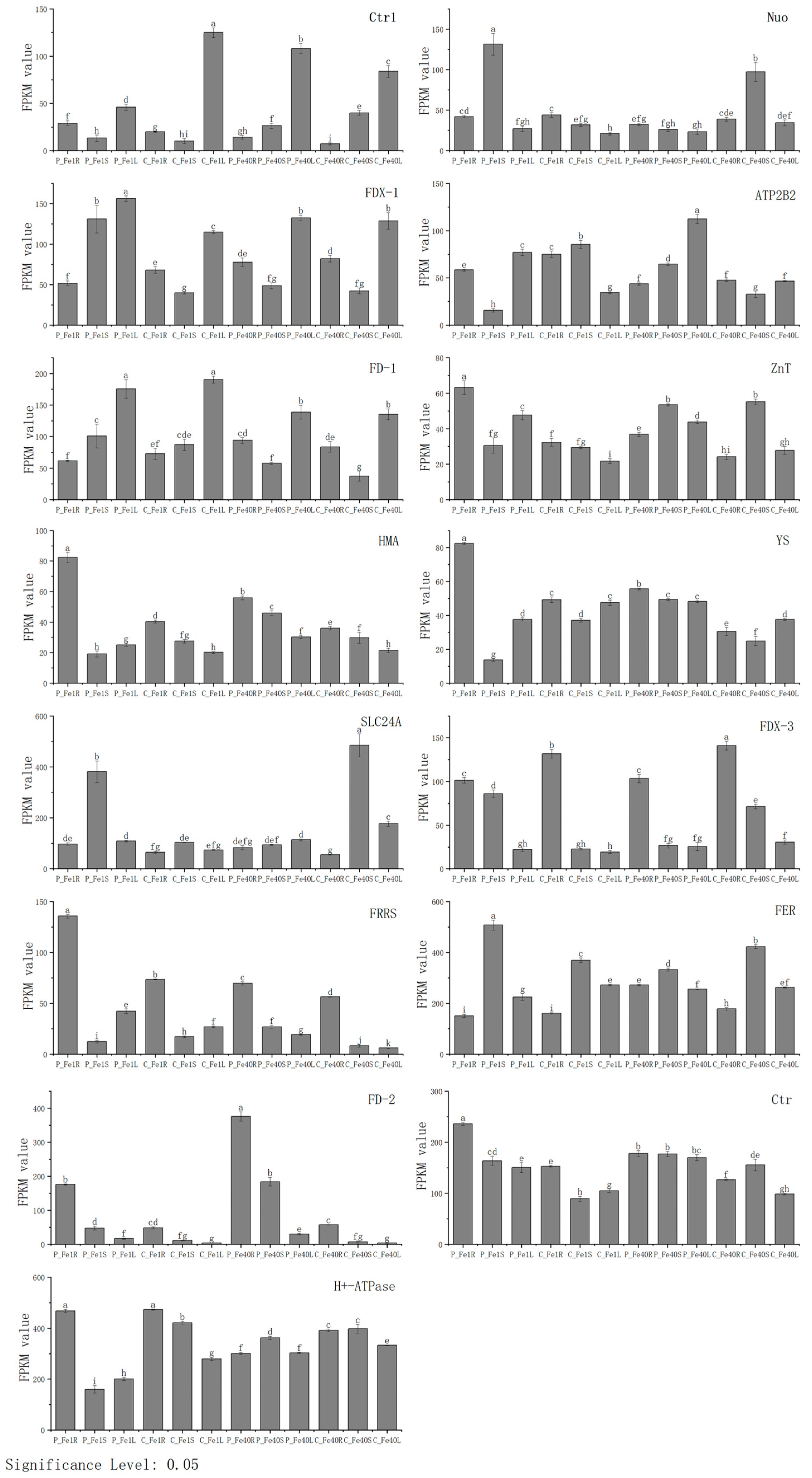
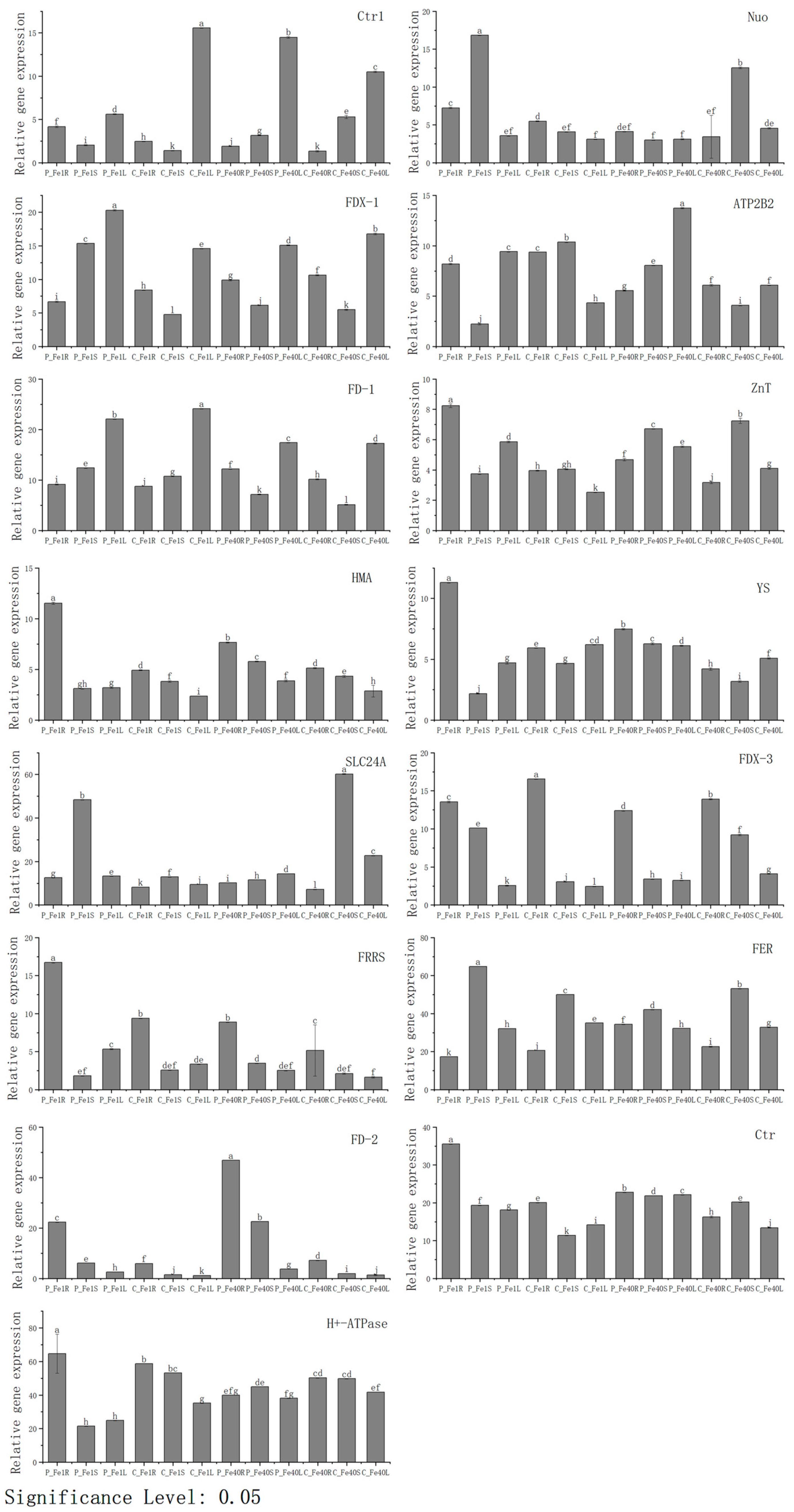
3.9. Real-Time Fluorescence Quantitative PCR Verification of DEGs
4. Discussion
- Fe2+ transport: Two IRT homologs (*Iron-regulated transporter 1/2*, PGSC0003DMG400010343/369) were specifically induced in roots, analogous to AtIRT1 [66].
5. Conclusions
- (1)
- First elucidation of the physiological compensation mechanism via ionomic remodeling under iron deficiencyThe study reveals that iron deficiency not only induces typical chlorosis symptoms (SPAD value reduced by 42.3%) and growth inhibition (biomass decreased by 35.7%) but also triggers a unique ionomic reprogramming, characterized by the specific accumulation of divalent metals such as Zn, Mg, Ca, Mn, and Cu in various tissues (increased by 20–150%). This multi-element synergistic regulation suggests that potato may activate a metal compensation mechanism through ionomic remodeling, providing new insights into plant micronutrient homeostasis.
- (2)
- Deciphering the molecular switch network of iron uptake and transport in potatoThrough whole-genome transcriptome analysis, we constructed, for the first time, a three-tiered iron absorption regulatory module (“H+-ATPase–FRO–IRT”) in potato roots. Under iron deficiency, root-specific induction of H+-ATPase genes (e.g., PGSC0003DMG400004101, up-regulated 5.2-fold) promotes rhizosphere acidification, followed by activation of the iron reduction system mediated by FRO family genes (PGSC0003DMG400000184 et al., up-regulated 3.8–7.1-fold), and finally facilitates Fe2+ transmembrane transport via IRT transporters. This regulatory cascade provides novel targets for genetic improvement of iron nutrition in crops.
- (3)
- Uncovering the synergistic defense strategy of oxidative stress and carbon metabolic reprogrammingThis study provides the first evidence that iron deficiency triggers a dual defense response in potato: (i) activation of the SOD/POD antioxidant system (activity increased by 2.3–4.5-fold) to alleviate membrane lipid peroxidation (MDA accumulation elevated by 89%), and (ii) remodeling of carbon metabolism, manifested by a significant increase in soluble sugars and proteins (by 60–80%). This metabolic reprogramming may supply carbon skeletons for iron chelate synthesis, offering new perspectives for understanding plant adaptation mechanisms under iron stress.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aung, M.S.; Masuda, H. Corrigendum: How Does Rice Defend against Excess Iron?: Physiological and Molecular Mechanisms. Front. Plant Sci. 2020, 11, 601527. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Touraine, B.; Vignols, F.; Przybyla-Toscano, J.; Ischebeck, T.; Dhalleine, T.; Wu, H.-C.; Magno, C.; Berger, N.; Couturand, D.; Dubos, C.; et al. Iron-Sulfur Protein NFU2 Is Required for Branched-Chain Amino Acid Synthesis in Arabidopsis Roots. J. Exp. Bot. 2019, 70, 1875–1889. [Google Scholar] [CrossRef]
- Murgia, I.; Marzorati, F.; Vigani, G.; Morandini, P. Plant Iron Nutrition: The Long Road from Soil to Seeds. J. Exp. Bot. 2022, 73, 1809–1824. [Google Scholar] [CrossRef]
- Li, W.F.; Lan, P. The Understanding of the Plant Iron Deficiency Responses in Strategy I Plants and the Role of Ethylene in This Process by Omic Approaches. Front. Plant Sci. 2017, 8, 40. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef]
- Eroglu, S.; Meier, B.; von Wirén, N.; Peiter, E. The Vacuolar Manganese Transporter MTP8 Determines Tolerance to Iron Deficiency-Induced Chlorosis in Arabidopsis. Plant Physiol. 2016, 170, 1030–1045. [Google Scholar] [CrossRef]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.-F. Towards a Knowledge-Based Correction of Iron Chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef]
- van Leeuwe, M.A.; Stefels, J. Photosynthetic Responses in Phaeocystis antarctica towards Varying Light and Iron Conditions. Biogeochemistry 2007, 83, 61–70. [Google Scholar] [CrossRef]
- Laganowsky, A.; Gómez, S.M.; Whitelegge, J.P.; Nishio, J.N. Hydroponics on a Chip: Analysis of the Fe Deficient Arabidopsis Thylakoid Membrane Proteome. J. Proteom. 2009, 72, 397–415. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V.; Kissel, M. Different Strategies in Higher Plants in Mobilization and Uptake of Iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Ivanov, R.; Brumbarova, T.; Bauer, P. Fitting into the Harsh Reality: Regulation of Iron-Deficiency Responses in Dicotyledonous Plants. Mol. Plant 2012, 5, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Legay, S.; Guignard, C.; Ziebel, J.; Evers, D. Iron Uptake and Homeostasis Related Genes in Potato Cultivated in Vitro under Iron Deficiency and Overload. Plant Physiol. Biochem. 2012, 60, 180–189. [Google Scholar] [CrossRef]
- Chan-Rodriguez, D.; Walker, E.L. Analysis of Yellow Striped Mutants of Zea mays Reveals Novel Loci Contributing to Iron Deficiency Chlorosis. Front. Plant Sci. 2018, 9, 157. [Google Scholar] [CrossRef]
- Expert, D.; O’Brian, M.R. (Eds.) Molecular Aspects of Iron Metabolism in Pathogenic and Symbiotic Plant-Microbe Associations; SpringerBriefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Varotto, C.; Maiwald, D.; Pesaresi, P.; Jahns, P.; Salamini, F.; Leister, D. The Metal Ion Transporter IRT1 Is Necessary for Iron Homeostasis and Efficient Photosynthesis in Arabidopsis thaliana. Plant J. 2002, 31, 589–599. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef]
- Mai, H.J.; Pateyron, S.; Bauer, P. Iron Homeostasis in Arabidopsis thaliana: Transcriptomic Analyses Reveal Novel FIT-Regulated Genes, Iron Deficiency Marker Genes and Functional Gene Networks. BMC Plant Biol. 2016, 16, 211. [Google Scholar] [CrossRef]
- Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The Transcriptional Control of Iron Homeostasis in Plants: A Tale of bHLH Transcription Factors? Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef]
- Brumbarova, T.; Bauer, P. Iron-Mediated Control of the Basic Helix-Loop-Helix Protein FER, a Regulator of Iron Uptake in Tomato. Plant Physiol. 2005, 137, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, E.P.; Guerinot, M.L. The Essential Basic Helix-Loop-Helix Protein FIT1 Is Required for the Iron Deficiency Response. Plant Cell 2004, 16, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.X.; Zhang, J.; Wang, D.W.; Ling, H.Q. AtbHLH29 of Arabidopsis thaliana Is a Functional Ortholog of Tomato FER Involved in Controlling Iron Acquisition in Strategy I Plants. Cell Res. 2005, 15, 613–621. [Google Scholar] [CrossRef]
- Palmer, C.M.; Hindt, M.N.; Schmidt, H.; Clemens, S.; Guerinot, M.L. MYB10 and MYB72 Are Required for Growth under Iron-Limiting Conditions. PLoS Genet. 2013, 9, e1003953. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron Nutrition, Biomass Production, and Plant Product Quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its By-Products: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Kotíková, Z. Carotenoids in Potatoes—A Short Overview. Plant Soil Environ. 2016, 62, 474–481. [Google Scholar] [CrossRef]
- Vreugdenhil, D. (Ed.) Potato Biology and Biotechnology: Advances and Perspectives; Elsevier B.V.: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of Iron Stress on Biomass, Yield, Metabolism and Quality of Potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Bacaicoa, E.; Zamarreño, Á.M.; Leménager, D.; Baigorri, R.; García-Mina, J.M. Relationship between the Hormonal Balance and the Regulation of Iron Deficiency Stress Responses in Cucumber. J. Am. Soc. Hortic. Sci. 2009, 134, 589–601. [Google Scholar] [CrossRef]
- Andre, C.M.; Ghislain, M.; Bertin, P.; Oufir, M.; Herrera, M.d.R.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Andean Potato Cultivars (Solanum tuberosum L.) as a Source of Antioxidant and Mineral Micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Morote, M.; Stangoulis, J.; Bonierbale, M. Iron and Zinc Concentration of Native Andean Potato Cultivars from a Human Nutrition Perspective. J. Sci. Food Agric. 2007, 87, 668–675. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for Linking Genomes to Life and the Environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.M.; Zhang, F.S. Effect of Peanut Mixed Cropping with Gramineous Species on Micronutrient Concentrations and Iron Chlorosis of Peanut Plants Grown in a Calcareous Soil. Plant Soil 2008, 306, 23–36. [Google Scholar] [CrossRef]
- Han, Z.H.; Zhu, J.T.; Wang, Q.; Shi, D.G.; Yang, G.H. Effect of Iron Deficiency Stress on Iron-Reductase Activity at Plasmalemma of Apple Root Cells. J. Plant Nutr. 2002, 25, 2849–2855. [Google Scholar] [CrossRef]
- Dobbels, A.A.; Lorenz, A.J. Soybean Iron Deficiency Chlorosis High Throughput Phenotyping Using an Unmanned Aircraft System. Plant Methods 2019, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of Iron, Zinc and Manganese Shortage-Induced Change on Photosynthetic Pigments, Some Osmoregulators and Chlorophyll Fluorescence Parameters in Lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Abadia, J.; Morales, F.; Abadia, A. Photosystem II Efficiency in Low Chlorophyll, Iron-Deficient Leaves. Plant Soil 1999, 215, 183–192. [Google Scholar] [CrossRef]
- Roháček, K.; Barták, M. Technique of the Modulated Chlorophyll Fluorescence: Basic Concepts, Useful Parameters, and Some Applications. Photosynthetica 1999, 37, 339–363. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Loboda, T. Photosystem II of Barley Seedlings under Cadmium and Lead Stress. Plant Soil Environ. 2007, 53, 511–516. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI Content Limits the Photoprotection of PSI and PSII in Early Growth Stages of Chlorophyll b-Deficient Wheat Mutant Lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V.; Pilbeam, D.J. (Eds.) Handbook of Plant Nutrition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, A.; Abadía, J. Chlorophyll Fluorescence and Photon Yield of Oxygen Evolution in Iron-Deficient Sugar Beet (Beta vulgaris L.) Leaves. Plant Physiol. 1991, 97, 886–893. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, Y.; Zhong, M.; Zhang, L.; Liu, L.; Zhang, Y.; Lin, X.; Huang, H. Effects of Iron Deficiency Stress on Plant Growth and Quality in Flowering Chinese Cabbage and Its Adaptive Response. Agronomy 2022, 12, 875. [Google Scholar] [CrossRef]
- Merry, R.; Espina, M.J.; Lorenz, A.J.; Stupar, R.M. Development of a Controlled-Environment Assay to Induce Iron Deficiency Chlorosis in Soybean by Adjusting Calcium Carbonates, pH, and Nodulation. Plant Methods 2022, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative Damage in Pea Plants Exposed to Water Deficit or Paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant Defense Mechanism under Salt Stress in Wheat Seedlings. Biol. Plant. 2006, 50, 227–231. [Google Scholar] [CrossRef]
- Alhoshan, M.; Zahedi, M.; Ramin, A.A.; Sabzalian, M.R. Effect of Soil Drought on Biomass Production, Physiological Attributes and Antioxidant Enzymes Activities of Potato Cultivars. Russ. J. Plant Physiol. 2019, 66, 265–277. [Google Scholar] [CrossRef]
- Wang, X.J.; Meng, M.L.; Cao, C.M.; Xu, F.; Chen, Y.J. Effects of Water Stress on Physiological Characteristics and Endogenous Hormone IAA and ABA Contents in Seedling Root of Potato. J. Northeast Norm. Univ. Nat. Sci. Ed. 2018, 50, 103–109. [Google Scholar] [CrossRef]
- Singh, R.; Pandey, N.; Kumar, A.; Shirke, P.A. Physiological Performance and Differential Expression Profiling of Genes Associated with Drought Tolerance in Root Tissue of Four Contrasting Varieties of Two Gossypium Species. Protoplasma 2016, 253, 163–174. [Google Scholar] [CrossRef]
- Bi, Z.; Dekomah, S.D.; Wang, Y.; Wang, L.; Wang, Z.; Wang, Y.; Wang, H.; Wang, X.; Qin, Y.; Xu, J. Overexpression of StCDPK13 in Potato Enhances Tolerance to Drought Stress. Int. J. Mol. Sci. 2024, 25, 12620. [Google Scholar] [CrossRef]
- Cao, Q.; Xv, C.; Jiang, Q.; Wu, X.; Wang, X.; Wang, X.; Zhang, Z.; Wang, Y.; Wang, Z. Comparative Transcriptome Analysis Reveals Key Genes Responsible for the Homeostasis of Iron and Other Divalent Metals in Peanut Roots under Iron Deficiency. Plant Soil 2019, 445, 513–531. [Google Scholar] [CrossRef]
- Cohen, C.K.; Fox, T.C.; Garvin, D.F.; Kochian, L.V. The Role of Iron-Deficiency Stress Responses in Stimulating Heavy-Metal Transport in Plants. Plant Physiol. 1998, 116, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cuenca, M.-R.; Quiñones, A.; Iglesias, D.J.; Forner-Giner, M.Á.; Primo-Millo, E.; Legaz, F. Effects of High Levels of Zinc and Manganese Ions on Strategy I Responses to Iron Deficiency in Citrus. Plant Soil 2013, 373, 943–953. [Google Scholar] [CrossRef]
- Kobraee, S.; Shamsi, K. Some of Micronutrients Homeostasis in Soybean. J. Basic Appl. Sci. Res. 2011, 1, 1659–1665. [Google Scholar]
- Shanmugam, V.; Lo, J.C.; Wu, C.L.; Wang, S.L.; Lai, C.C.; Connolly, E.L.; Huang, J.L.; Yeh, K.C. Differential Expression and Regulation of Iron-Regulated Metal Transporters in Arabidopsis halleri and Arabidopsis thaliana—The Role in Zinc Tolerance. New Phytol. 2011, 190, 125–137. [Google Scholar] [CrossRef]
- Nishida, S.; Tsuzuki, C.; Kato, A.; Aisu, A.; Yoshida, J.; Mizuno, T. AtIRT1, the Primary Iron Uptake Transporter in the Root, Mediates Excess Nickel Accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1433–1442. [Google Scholar] [CrossRef]
- Nishida, S.; Aisu, A.; Mizuno, T. Induction of IRT1 by the Nickel-Induced Iron-Deficient Response in Arabidopsis. Plant Signal. Behav. 2012, 7, 329–331. [Google Scholar] [CrossRef]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M.L. A Ferric-Chelate Reductase for Iron Uptake from Soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Zha, Q.; Zhang, Q.; Zhang, X.; Zhang, L.; Zhang, W.; Xi, Z. Cloning and Characterization of MxHA7, a Plasma Membrane H+-ATPase Gene Related to High Tolerance of Malus xiaojinensis to Iron Deficiency. Acta Physiol. Plant. 2014, 36, 955–962. [Google Scholar] [CrossRef]
- Ning, X.; Lin, M.; Huang, G.; Mao, J.; Gao, Z.; Wang, X. Research Progress on Iron Absorption, Transport, and Molecular Regulation Strategy in Plants. Front. Plant Sci. 2023, 14, 1190768. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.; Broderius, M.; Fett, J.; Guerinot, M.L. A Novel Iron-Regulated Metal Transporter from Plants Identified by Functional Expression in Yeast. Proc. Natl. Acad. Sci. USA 1996, 93, 5624–5628. [Google Scholar] [CrossRef] [PubMed]






| Variety | Sampling Tissue | Iron Deficiency Stress (FeNaEDTA: 1 mg/L) | Control (FeNaEDTA: 40.4 mg/L) |
|---|---|---|---|
| Sample Naming | Sample Naming | ||
| 05P | root | PFe1R | PFe40R |
| stem | PFe1S | PFe40S | |
| leaf | PFe1L | PFe40L | |
| CI5 | root | CFe1R | CFe40R |
| stem | CFe1S | CFe40S | |
| leaf | CFe1L | CFe40L |
| Gene ID | Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| PGSC0003DMG400020829 | Ctr1 | GCATGGAATGGCTATGGGGC | ATCATCATCCCGCTACCCGC |
| PGSC0003DMG400028681 | Nuo | TACAGGGAACAAAGGGCGAT | ATTCGGTTCCTACGTTGATACTCT |
| PGSC0003DMG400035711 | FDX-1 | TGCAAGCTAGGAGTGTGCAT | CAATCATCGGCAGTTGCGAG |
| PGSC0003DMG400013022 | ATP2B2 | AGGAGCCTTTGTGGTGTTGT | CACTTGGTTGCATGCCTTGTAT |
| PGSC0003DMG400011950 | FD-1 | CCGAGGAAACCGGGATAGAT | AACGCAAGTGAGCACAAATCC |
| PGSC0003DMG400022179 | ZnT | CAAGGGCAGTCACAGGTCT | TGGCTATTCCCTCAAAGACGG |
| PGSC0003DMG400009436 | HMA | TGCAGTTGCTAAGGAGGTTGG | AACGGACGACCTCAGCTTTC |
| PGSC0003DMG400018535 | YS | AGTATTGCTGTAGGAGGTGGC | TGCCACTTGTATCAACCCCAG |
| PGSC0003DMG400027543 | SLC24A | TCTGCTCCTTATTTCCTCCGTT | CAGTGGCACACCATCCTGAG |
| PGSC0003DMG400026360 | FDX-3 | TGCTGCCAACTTTGCCTAAC | ACGGTCGACATGTTTTACCAAT |
| PGSC0003DMG400010125 | FRRS | GAAAGGGAGCTGGAAAGCCT | TGGTAGAAGCAAGCAGAGAGC |
| PGSC0003DMG400008787 | FER | AGTGCAGAGGAAAGAGAGCAC | CGCAAGCTCCATTGCATAC |
| PGSC0003DMG400004532 | FD-2 | TGCGGACACGTGGAAGATTA | TGTGCTTGGCTGGATCTCAC |
| PGSC0003DMG400013653 | Ctr | TACGGCGATCGTTTTTGGGA | TGACATCATCCTCATCGCCG |
| PGSC0003DMG400004101 | H+-ATPase | GCTGGAGTATTTCCTGAGCAC | AAGCAGGAGCATCGTTCACA |
| Reference Genes | StActin | AGATGCTTACGCTGGATGGAATGC | TTCCGGTGTGGTTGGATTCTGTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, Y.; Yang, S.; He, M.; Zhou, Y.; Ye, G.; Wang, J. Physiological, Biochemical, and Transcriptomic Responses to Iron Deficiency in Two Potato Varieties. Plants 2025, 14, 2934. https://doi.org/10.3390/plants14182934
Ma X, Zhang Y, Yang S, He M, Zhou Y, Ye G, Wang J. Physiological, Biochemical, and Transcriptomic Responses to Iron Deficiency in Two Potato Varieties. Plants. 2025; 14(18):2934. https://doi.org/10.3390/plants14182934
Chicago/Turabian StyleMa, Xiangying, Yanping Zhang, Shenglong Yang, Miaomiao He, Yun Zhou, Guangji Ye, and Jian Wang. 2025. "Physiological, Biochemical, and Transcriptomic Responses to Iron Deficiency in Two Potato Varieties" Plants 14, no. 18: 2934. https://doi.org/10.3390/plants14182934
APA StyleMa, X., Zhang, Y., Yang, S., He, M., Zhou, Y., Ye, G., & Wang, J. (2025). Physiological, Biochemical, and Transcriptomic Responses to Iron Deficiency in Two Potato Varieties. Plants, 14(18), 2934. https://doi.org/10.3390/plants14182934








