Morphological and Transcriptomic Analyses Provide New Insights into Linseed (Linum usitatissimum L.) Seedling Roots Response to Nitrogen Stress
Abstract
1. Introduction
2. Results
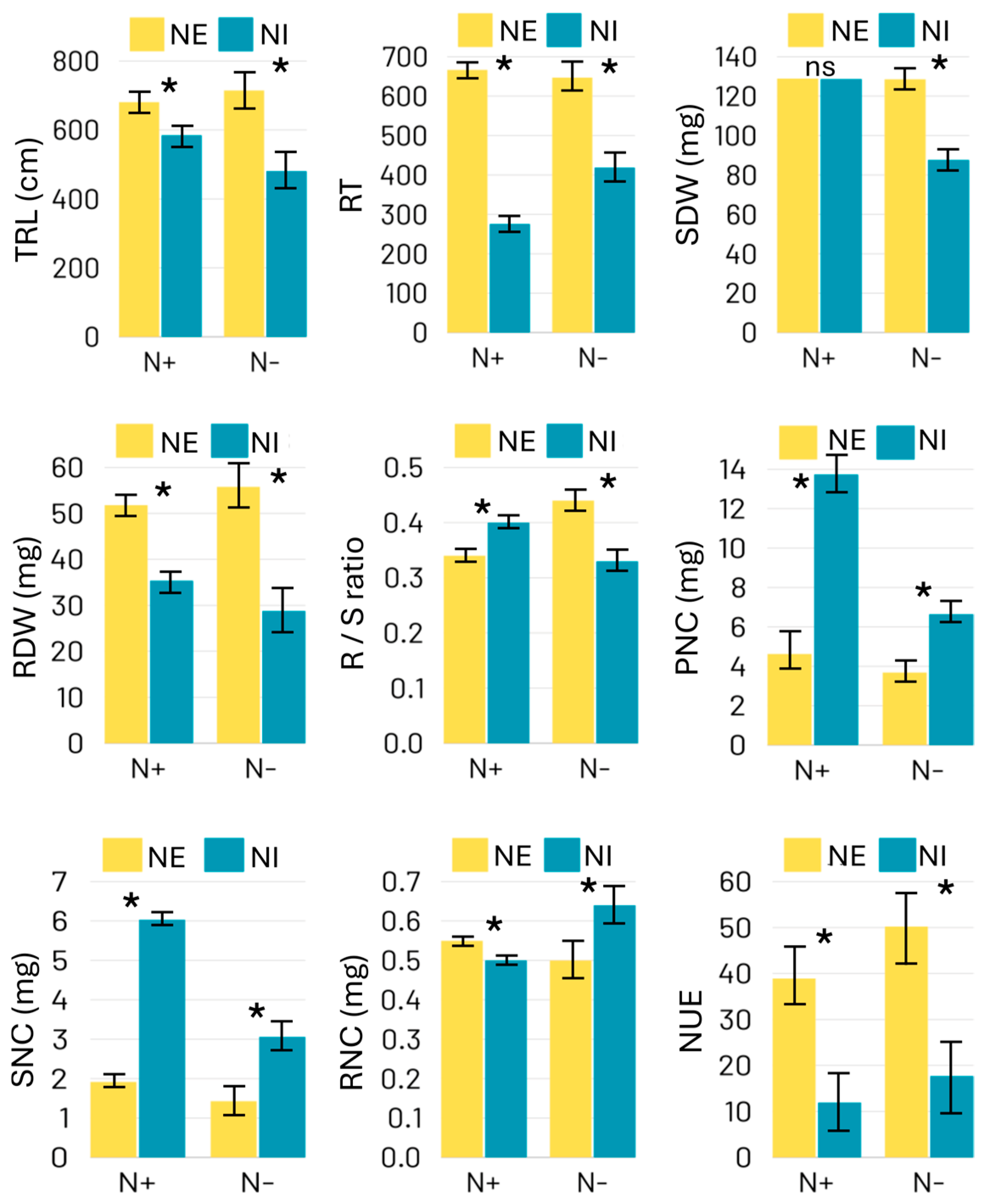
2.1. Phenotypic Performance of Linseed Genotypes
2.2. Transcriptome Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Phenotyping
4.2. Statistical Analysis of Phenotypic Data
4.3. Isolation of Total RNA, Illumina Sequencing and Mapping of Reads
4.4. Functional Annotation and Identification of Transcription Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRL | Total Root Length (cm) |
| RV | Root Volume (cm3) |
| RD | Root Diameter (mm) |
| RT | Root Tips |
| PDW | Plant Dry Weight (mg) |
| SDW | Shoot Dry Weight (mg) |
| RDW | Root Dry Weight (mg) |
| R/S | Root-to-Shoot Ratio (%) |
| PNC | Plant Nitrogen Content (mg) |
| SNC | Shoot Nitrogen Content (mg) |
| RNC | Root Nitrogen Content (mg) |
| NUE | Nitrogen Use Efficiency |
| NE | Nitrogen-Efficient |
| NI | Nitrogen-Inefficient |
| DEG | Differentially Expressed Gene |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| TF | Transcription Factor |
| RSA | Root System Architecture |
References
- Liu, Q.; Wu, K.; Song, W.; Zhong, N.; Wu, Y.; Fu, X. Improving Crop Nitrogen Use Efficiency Toward Sustainable Green Revolution. Annu. Rev. Plant Biol. 2022, 73, 523–551. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Trends and Outlook to 2022; FAO: Rome, Italy, 2022.
- Zhang, Y.; Wang, N.; He, C.; Gao, Z.; Chen, G. Comparative Transcriptome Analysis Reveals Major Genes, Transcription Factors and Biosynthetic Pathways Associated with Leaf Senescence in Rice under Different Nitrogen Application. BMC Plant Biol. 2024, 24, 419. [Google Scholar] [CrossRef]
- Bouchet, A.S.; Laperche, A.; Bissuel-Belaygue, C.; Snowdon, R.; Nesi, N.; Stahl, A. Nitrogen Use Efficiency in Rapeseed. A Review. Agron. Sustain. Dev. 2016, 36, 38. [Google Scholar] [CrossRef]
- Billen, G.; Garnier, J.; Lassaletta, L. The Nitrogen Cascade from Agricultural Soils to the Sea: Modelling Nitrogen Transfers at Regional Watershed and Global Scales. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130123. [Google Scholar] [CrossRef]
- Ahmad, N.; Su, B.; Ibrahim, S.; Kuang, L.; Tian, Z.; Wang, X.; Wang, H.; Dun, X. Deciphering the Genetic Basis of Root and Biomass Traits in Rapeseed (Brassica napus L.) through the Integration of GWAS and RNA-Seq under Nitrogen Stress. Int. J. Mol. Sci. 2022, 23, 7958. [Google Scholar] [CrossRef]
- Du, Q.; Yang, J.; Shah, S.M.S.; Yang, R.; Yu, J.; Li, W. Comparative Transcriptome Analysis of Different Nitrogen Responses in Low-Nitrogen Sensitive and Tolerant Maize Genotypes. J. Integr. Agric. 2021, 20, 2043–2055. [Google Scholar] [CrossRef]
- Cormier, F.; Le Gouis, J.; Dubreuil, P.; Lafarge, S.; Praud, S. A Genome-Wide Identification of Chromosomal Regions Determining Nitrogen Use Efficiency Components in Wheat (Triticum aestivum L.). Theor. Appl. Genet. 2014, 127, 2679–2693. [Google Scholar] [CrossRef]
- Kotur, Z.; Glass, A.D.M. A 150kDa Plasma Membrane Complex of AtNRT2.5 and AtNAR2.1 Is the Major Contributor to Constitutive High-Affinity Nitrate Influx in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 1490–1502. [Google Scholar] [CrossRef]
- Monostori, I.; Szira, F.; Tondelli, A.; Árendás, T.; Gierczik, K.; Cattivelli, L.; Galiba, G.; VÁgújfalvi, A. Genome-Wide Association Study and Genetic Diversity Analysis on Nitrogen Use Efficiency in a Central European Winter Wheat (Triticum aestivum L.) Collection. PLoS ONE 2017, 12, e0189265. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How Does Nitrogen Shape Plant Architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Chu, C. Nitrogen Use Efficiency in Crops: Lessons from Arabidopsis and Rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Wang, J.; Song, K.; Sun, L.; Qin, Q.; Sun, Y.; Pan, J.; Xue, Y. Morphological and Transcriptome Analysis of Wheat Seedlings Response to Low Nitrogen Stress. Plants 2019, 8, 98. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed Proteins: Food Uses and Health Benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Gui, B.; Shim, Y.Y.; Reaney, M.J.T. Distribution of Cyclolinopeptides in Flaxseed Fractions and Products. J. Agric. Food Chem. 2012, 60, 8580–8589. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Cloutier, S.; Quian, R.; Gajardo, H.A.; Olivos, M.; You, F.M. Genome-Wide Association Analysis of Mucilage and Hull Content in Flax (Linum usitatissimum L.) Seeds. Int. J. Mol. Sci. 2018, 19, 2870. [Google Scholar] [CrossRef]
- Bassett, C.M.C.; Rodriguez-Leyva, D.; Pierce, G.N. Experimental and Clinical Research Findings on the Cardiovascular Benefits of Consuming Flaxseed. Appl. Physiol. Nutr. Metab. 2009, 34, 965–974. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Hosseiniana, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant Capacity of Flaxseed Lignans in Two Model Systems. J. Am. Oil Chem. Soc. 2006, 83, 835–840. [Google Scholar] [CrossRef]
- Stepień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Oxidant and Anti-Cancer Properties of Flaxseed. Int. J. Mol. Sci. 2025, 26, 1226. [Google Scholar] [CrossRef]
- Dash, P.K.; Cao, Y.; Jailani, A.K.; Gupta, P.; Venglat, P.; Xiang, D.; Rai, R.; Sharma, R.; Thirunavukkarasu, N.; Abdin, M.Z.; et al. Genome-Wide Analysis of Drought Induced Gene Expression Changes in Flax (Linum usitatissimum). GM Crops Food 2014, 5, 106–119. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Cloutier, S.; Gajardo, H.A.; Aravena, G.; Quian, R. Identifying Drought-Resilient Flax Genotypes and Related-Candidate Genes Based on Stress Indices, Root Traits and Selective Sweep. Euphytica 2019, 215, 41. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Wang, L.; Tan, M.; Ogutu, C.O.; Yin, Z.; Zhou, J.; Wang, J.; Wang, L.; Yan, X. Transcriptome Analysis and Molecular Mechanism of Linseed (Linum usitatissimum L.) Drought Tolerance under Repeated Drought Using Single-Molecule Long-Read Sequencing. BMC Genom. 2021, 22, 109. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, H.; Yu, H.; Ma, Y.; Hu, H.; Han, Z.; Zhang, Y.; Zhen, Z.; Yi, L.; Hou, J. Combined GWAS and Transcriptome Analyses Provide New Insights into the Response Mechanisms of Sunflower Against Drought Stress. Front. Plant Sci. 2022, 13, 847435. [Google Scholar] [CrossRef]
- Subudhi, P.K.; Garcia, R.S.; Coronejo, S.; Tapia, R. Comparative Transcriptomics of Rice Genotypes with Contrasting Responses to Nitrogen Stress Reveals Genes Influencing Nitrogen Uptake through the Regulation of Root Architecture. Int. J. Mol. Sci. 2020, 21, 5759. [Google Scholar] [CrossRef]
- Kaur, S.; Shamshad, M.; Jindal, S.; Kaur, A.; Singh, S.; Sharma, A.; Kaur, S. RNA-Seq-Based Transcriptomics Study to Investigate the Genes Governing Nitrogen Use Efficiency in Indian Wheat Cultivars. Front. Genet. 2022, 13, 853910. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, K.; Jha, A.K.; Yadava, P.; Pal, M.; Rakshit, S.; Singh, I. Global Gene Expression Profiling under Nitrogen Stress Identifies Key Genes Involved in Nitrogen Stress Adaptation in Maize (Zea mays L.). Sci. Rep. 2022, 12, 4211. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Li, Z.; Chen, Z.; Li, J.; Du, H. An Integrated Nitrogen Utilization Gene Network and Transcriptome Analysis Reveal Candidate Genes in Response to Nitrogen Deficiency in Brassica napus. Front. Plant Sci. 2023, 14, 1187552. [Google Scholar] [CrossRef]
- You, F.M.; Xiao, J.; Li, P.; Yao, Z.; Jia, G.; He, L.; Zhu, T.; Luo, M.C.; Wang, X.; Deyholos, M.K.; et al. Chromosome-Scale Pseudomolecules Refined by Optical, Physical and Genetic Maps in Flax. Plant J. 2018, 95, 371–384. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Pushkova, E.N.; Novakovskiy, R.O.; Beniaminov, A.D.; Rozhmina, T.A.; Zhuchenko, A.A.; Bolsheva, N.L.; Muravenko, O.V.; Povkhova, L.V.; Dvorianinova, E.M.; et al. Genome Sequencing of Fiber Flax Cultivar Atlant Using Oxford Nanopore and Illumina Platforms. Front. Genet. 2021, 11, 590282. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Wang, L.; Wang, L.; Yan, X.; Dang, Z.; Li, W.; Zhao, W.; Pei, X.; Li, X.; et al. Genomic Comparison and Population Diversity Analysis Provide Insights into the Domestication and Improvement of Flax. iScience 2020, 23, 100967. [Google Scholar] [CrossRef]
- Sa, R.; Yi, L.; Siqin, B.; An, M.; Bao, H.; Song, X.; Wang, S.; Li, Z.; Zhang, Z.; Hazaisi, H.; et al. Chromosome-Level Genome Assembly and Annotation of the Fiber Flax (Linum usitatissimum) Genome. Front. Genet. 2021, 12, 735690. [Google Scholar] [CrossRef]
- Dvorianinova, E.M.; Bolsheva, N.L.; Pushkova, E.N.; Rozhmina, T.A.; Zhuchenko, A.A.; Novakovskiy, R.O.; Povkhova, L.V.; Sigova, E.A.; Zhernova, D.A.; Borkhert, E.V.; et al. Isolating Linum usitatissimum L. Nuclear DNA Enabled Assembling High-Quality Genome. Int. J. Mol. Sci. 2022, 23, 3244. [Google Scholar] [CrossRef]
- Dvorianinova, E.M.; Pushkova, E.N.; Bolsheva, N.L.; Borkhert, E.V.; Rozhmina, T.A.; Zhernova, D.A.; Novakovskiy, R.O.; Turba, A.A.; Sigova, E.A.; Melnikova, N.V.; et al. Genome of Linum usitatissimum Convar. Crepitans Expands the View on the Section Linum. Front. Genet. 2023, 14, 1269837. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, L.; Zuo, Y.; Gao, F.; Cheng, Y.; Zhang, H.; Zhou, Y.; Jia, X.; Su, S.; Zhang, D.; et al. High-Quality Genome Assembly and Genome-Wide Association Study of Male Sterility Provide Resources for Flax Improvement. Plants 2023, 12, 2773. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Krasnov, G.S.; Rozhmina, T.A.; Novakovskiy, R.O.; Snezhkina, A.V.; Fedorova, M.S.; Yurkevich, O.Y.; Muravenko, O.V.; Bolsheva, N.L.; Kudryavtseva, A.V.; et al. Differential Gene Expression in Response to Fusarium Oxysporum Infection in Resistant and Susceptible Genotypes of Flax (Linum usitatissimum L.). BMC Plant Biol. 2017, 17, 29–40. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kozak, B.; Zalewski, I.; Szopa, J.; Kulma, A. Transcriptomic Profiling of Susceptible and Resistant Flax Seedlings after Fusarium oxysporum Lini Infection. PLoS ONE 2021, 16, e0246052. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Krasnov, G.S.; Rozhmina, T.A.; Kishlyan, N.V.; Zyablitsin, A.V.; Sadritdinova, A.F.; Snezhkina, A.V.; Fedorova, M.S.; Yurkevich, O.Y.; Muravenko, O.V.; et al. Glutathione S-Transferases and UDP-Glycosyltransferases Are Involved in Response to Aluminum Stress in Flax. Front. Plant Sci. 2016, 7, 1920. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Q.; Sun, D.; Wu, G.; Zhang, L.; Yuan, H.; Yu, Y.; Zhang, S.; Yang, X.; Li, Z.; et al. Transcriptome Analysis of Flax (Linum usitatissimum L.) Undergoing Osmotic Stress. Ind. Crops Prod. 2018, 116, 215–223. [Google Scholar] [CrossRef]
- Miart, F.; Fontaine, J.X.; Mongelard, G.; Wattier, C.; Lequart, M.; Bouton, S.; Molinié, R.; Dubrulle, N.; Fournet, F.; Demailly, H.; et al. Integument-Specific Transcriptional Regulation in the Mid-Stage of Flax Seed Development Influences the Release of Mucilage and the Seed Oil Content. Cells 2021, 10, 2677. [Google Scholar] [CrossRef]
- Xie, D.; Dai, Z.; Yang, Z.; Tang, Q.; Deng, C.; Xu, Y.; Wang, J.; Chen, J.; Zhao, D.; Zhang, S.; et al. Combined Genome-Wide Association Analysis and Transcriptome Sequencing to Identify Candidate Genes for Flax Seed Fatty Acid Metabolism. Plant Sci. 2019, 286, 98–107. [Google Scholar] [CrossRef]
- Gorshkova, T.; Chernova, T.; Mokshina, N.; Gorshkov, V.; Kozlova, L.; Gorshkov, O. Transcriptome Analysis of Intrusively Growing Flax Fibers Isolated by Laser Microdissection. Sci. Rep. 2018, 8, 14570. [Google Scholar] [CrossRef]
- Guo, D.; Jiang, H.; Ye, J.; Zhang, A.; Wang, Y.; Gao, Y.; Yan, Q.; Chen, J.; Duan, L.; Liu, H.; et al. Transcriptome Combined with Population Level Validation Reveals Genomic Loci Controlling Plant Height in Flax (Linum usitatissimum L.). Ind. Crops Prod. 2021, 172, 113998. [Google Scholar] [CrossRef]
- Povkhova, L.V.; Melnikova, N.V.; Rozhmina, T.A.; Novakovskiy, R.O.; Pushkova, E.N.; Dvorianinova, E.M.; Zhuchenko, A.A.; Kamionskaya, A.M.; Krasnov, G.S.; Dmitriev, A.A. Genes Associated with the Flax Plant Type (Oil or Fiber) Identified Based on Genome and Transcriptome Sequencing Data. Plants 2021, 10, 2616. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012; ISBN 9780191810046. [Google Scholar]
- Xie, Y.; Gan, Y.; Li, Y.; Niu, J.; Gao, Y.; An, H.; Li, A. Effect of Nitrogen Fertilizer on Nitrogen Accumulation, Translocation, and Use Efficiency in Dryland Oilseed Flax. Agron. J. 2015, 107, 1931–1939. [Google Scholar] [CrossRef]
- Sharma, N.; Sinha, V.B.; Gupta, N.; Rajpal, S.; Kuchi, S.; Sitaramam, V.; Parsad, R.; Raghuram, N. Phenotyping for Nitrogen Use Efficiency: Rice Genotypes Differ in N-Responsive Germination, Oxygen Consumption, Seed Urease Activities, Root Growth, Crop Duration, and Yield at Low N. Front. Plant Sci. 2018, 9, 1452. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Wang, F.; Lu, J.; Tan, G.; Wang, N.; Qi, F.; Zhang, C.; Deyholos, M.K.; Zang, Z.; et al. Transcriptome Analysis and Physiological Response to Heat and Cold Stress in Flax (Linum usitatissimum L) at the Seedling Stage. Environ. Exp. Bot. 2025, 229, 106076. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Li, X.; Jiang, H.; Guo, D.; Xie, F.; Zhang, Z.; Xie, L. Adaptive Response and Transcriptomic Analysis of Flax (Linum usitatissimum L.) Seedlings to Salt Stress. Genes 2022, 13, 1904. [Google Scholar] [CrossRef]
- Bao, Y.; Zou, Y.; Tian, R.; Huang, X.; Liu, L.; Wang, B.; Peng, D. Transcriptome Analysis of Fiber Development under High-Temperature Stress in Flax (Linum usitatissimum L.). Ind. Crops Prod. 2023, 195, 116019. [Google Scholar] [CrossRef]
- Shao, A.; Ma, W.; Zhao, X.; Hu, M.; He, X.; Teng, W.; Li, H.; Tong, Y. The Auxin Biosynthetic TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2.1-3A Increases Grain Yield of Wheat. Plant Physiol. 2017, 174, 2274–2288. [Google Scholar] [CrossRef]
- Liu, C.; Gu, W.; Liu, C.; Shi, X.; Li, B.; Chen, B.; Zhou, Y. Tryptophan Regulates Sorghum Root Growth and Enhances Low Nitrogen Tolerance. Plant Physiol. Biochem. 2024, 212, 108737. [Google Scholar] [CrossRef]
- Yang, J.; Yin, Y.; Yu, D.; He, L.; Shen, S. Activation of MAPK Signaling in Response to Nitrogen Deficiency in Ulva prolifera (Chlorophyta). Algal Res. 2021, 53, 102153. [Google Scholar] [CrossRef]
- Mira, H.; Martínez, N.; Peñarrubia, L. Expression of a Vegetative-Storage-Protein Gene from Arabidopsis Is Regulated by Copper, Senescence and Ozone. Planta 2002, 214, 939–946. [Google Scholar] [CrossRef]
- Abbaraju, H.K.R.; Gupta, R.; Appenzeller, L.M.; Fallis, L.P.; Hazebroek, J.; Zhu, G.; Bourett, T.M.; Howard, R.J.; Weers, B.; Lafitte, R.H.; et al. A Vegetative Storage Protein Improves Drought Tolerance in Maize. Plant Biotechnol. J. 2022, 20, 374–389. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, D.G.; Avice, J.C.; Kim, T.H. Characterization of Vegetative Storage Protein (VSP) and Low Molecular Proteins Induced by Water Deficit in Stolon of White Clover. Biochem. Biophys. Res. Commun. 2014, 443, 229–233. [Google Scholar] [CrossRef]
- Dexter, R.; Qualley, A.; Kish, C.M.; Ma, C.J.; Koeduka, T.; Nagegowda, D.A.; Dudareva, N.; Pichersky, E.; Clark, D. Characterization of a Petunia Acetyltransferase Involved in the Biosynthesis of the Floral Volatile Isoeugenol. Plant J. 2007, 49, 265–275. [Google Scholar] [CrossRef]
- Berdeja, M.; Nicolas, P.; Kappel, C.; Dai, Z.W.; Hilbert, G.; Peccoux, A.; Lafontaine, M.; Ollat, N.; Gomès, E.; Delrot, S. Water Limitation and Rootstock Genotype Interact to Alter Grape Berry Metabolism through Transcriptome Reprogramming. Hortic. Res. 2015, 2, 15012. [Google Scholar] [CrossRef]
- Sultana, N.; Islam, S.; Juhasz, A.; Yang, R.; She, M.; Alhabbar, Z.; Zhang, J.; Ma, W. Transcriptomic Study for Identification of Major Nitrogen Stress Responsive Genes in Australian Bread Wheat Cultivars. Front. Genet. 2020, 11, 583785. [Google Scholar] [CrossRef]
- Vega, A.; Canessa, P.; Hoppe, G.; Retamal, I.; Moyano, T.C.; Canales, J.; Gutiérrez, R.A.; Rubilar, J. Transcriptome Analysis Reveals Regulatory Networks Underlying Differential Susceptibility to Botrytis cinerea in Response to Nitrogen Availability in Solanum lycopersicum. Front. Plant Sci. 2015, 6, 911. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The Central Role of Transcription Factors in Bridging Biotic and Abiotic Stress Responses for Plants’ Resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Ma, J.; Su, S.; Chen, L.; Cheng, Y.; Buter, S.; Zhao, X.; Yi, L.; Lu, Z. Analyzing the Diversity of MYB Family Response Strategies to Drought Stress in Different Flax Varieties Based on Transcriptome Data. Plants 2024, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Huang, W.; He, C.; Wu, B.; Duan, H.; Ruan, J.; Zhao, Q.; Fang, Z. Transcription Factor OsMYB2 Triggers Amino Acid Transporter OsANT1 Expression to Regulate Rice Growth and Salt Tolerance. Plant Physiol. 2025, 197, kiae559. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Chen, P.; Liang, T.; Li, X.; Liu, H. UV-B Photoreceptor UVR8 Interacts with MYB73/MYB77 to Regulate Auxin Responses and Lateral Root Development. EMBO J. 2020, 39, e101928. [Google Scholar] [CrossRef]
- Thilakarathne, A.S.; Liu, F.; Zou, Z. Plant Signaling Hormones and Transcription Factors: Key Regulators of Plant Responses to Growth, Development, and Stress. Plants 2025, 14, 1070. [Google Scholar] [CrossRef]
- Lv, B.; Wei, K.; Hu, K.; Tian, T.; Zhang, F.; Yu, Z.; Zhang, D.; Su, Y.; Sang, Y.; Zhang, X.; et al. MPK14-Mediated Auxin Signaling Controls Lateral Root Development via ERF13-Regulated Very-Long-Chain Fatty Acid Biosynthesis. Mol. Plant 2021, 14, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, B.N.; Karthikeyan, A.S.; Raghothama, K.G. WRKY75 Transcription Factor Is a Modulator of Phosphate Acquisition and Root Development in Arabidopsis. Plant Physiol. 2007, 143, 1789–1801. [Google Scholar] [CrossRef]
- Heerah, S.; Katari, M.; Penjor, R.; Coruzzi, G.; Marshall-Colon, A. WRKY1 Mediates Transcriptional Regulation of Light and Nitrogen Signaling Pathways. Plant Physiol. 2019, 181, 1371–1388. [Google Scholar] [CrossRef]
- Aghaee, P.; Rahmani, F. Biochemical and Molecular Responses of Flax to 24-Epibrassinosteroide Seed Priming under Drought Stress. J. Plant Interact. 2019, 14, 242–253. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Kudryavtseva, A.V.; Krasnov, G.S.; Koroban, N.V.; Speranskaya, A.S.; Krinitsina, A.A.; Belenikin, M.S.; Snezhkina, A.V.; Sadritdinova, A.F.; Kishlyan, N.V.; et al. Gene Expression Profiling of Flax (Linum usitatissimum L.) under Edaphic Stress. BMC Plant Biol. 2016, 16, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ma, W.; Hu, J.; Cai, W. The Nitrate-Inducible NAC Transcription Factor NAC056 Controls Nitrate Assimilation and Promotes Lateral Root Growth in Arabidopsis thaliana. PLoS Genet. 2022, 18, e1010090. [Google Scholar] [CrossRef]
- Xie, Q.; Frugis, G.; Colgan, D.; Chua, N.H. Arabidopsis NAC1 Transduces Auxin Signal Downstream of TIR1 to Promote Lateral Root Development. Genes. Dev. 2000, 14, 3024–3036. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Teng, K.; Dong, D.; Liu, Z.; Zhang, T.; Han, L. Transcriptome Profiling Revealed Candidate Genes, Pathways and Transcription Factors Related to Nitrogen Utilization and Excessive Nitrogen Stress in Perennial Ryegrass. Sci. Rep. 2022, 12, 3353. [Google Scholar] [CrossRef] [PubMed]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis Nitrate Transporter NRT2.5 Plays a Role in Nitrate Acquisition and Remobilization in Nitrogen-Starved Plants. Plant J. 2014, 80, 230–241. [Google Scholar] [CrossRef]
- Yang, X.; Nong, B.; Chen, C.; Wang, J.; Xia, X.; Zhang, Z.; Wei, Y.; Zeng, Y.; Feng, R.; Wu, Y.; et al. OsNPF3.1, a Member of the NRT1/PTR Family, Increases Nitrogen Use Efficiency and Biomass Production in Rice. Crop J. 2023, 11, 108–118. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; Von Wirén, N. Three Functional Transporters for Constitutive, Diurnally Regulated, and Starvation-Induced Uptake of Ammonium into Arabidopsis Roots. Plant Cell 1999, 11, 937–947. [Google Scholar] [CrossRef]
- Liu, L.; An, M.-M.; Li, X.-J.; Han, Z.; Li, S.-X.; Li, B. Molybdenum-Induced Effects on Nitrogen Absorption and Utilization under Different Nitrogen Sources in Vitis vinifera. J. Plant Interact. 2022, 17, 756–765. [Google Scholar] [CrossRef]
- Fischer, W.N.; Kwart, M.; Hummel, S.; Frommer, W.B. Substrate Specificity and Expression Profile of Amino Acid Transporters (AAPs) in Arabidopsis. J. Biol. Chem. 1995, 270, 16315–16320. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Frommer, W.B.; Ludewig, U. Molecular and Functional Characterization of a Family of Amino Acid Transporters from Arabidopsis. Plant Physiol. 2004, 136, 3104–3113. [Google Scholar] [CrossRef]
- Goel, P.; Sharma, N.K.; Bhuria, M.; Sharma, V.; Chauhan, R.; Pathania, S.; Swarnkar, M.K.; Chawla, V.; Acharya, V.; Shankar, R.; et al. Transcriptome and Co-Expression Network Analyses Identify Key Genes Regulating Nitrogen Use Efficiency in Brassica juncea L. Sci. Rep. 2018, 8, 7451. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Buckseth, T.; Zinta, R.; Saraswati, A.; Singh, R.K.; Rawat, S.; Dua, V.K.; Chakrabarti, S.K. Transcriptome Analysis of Potato Shoots, Roots and Stolons under Nitrogen Stress. Sci. Rep. 2020, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Nezamivand-Chegini, M.; Metzger, S.; Moghadam, A.; Tahmasebi, A.; Koprivova, A.; Eshghi, S.; Mohammadi-Dehchesmeh, M.; Kopriva, S.; Niazi, A.; Ebrahimie, E. Integration of Transcriptomic and Metabolomic Analyses Provides Insights into Response Mechanisms to Nitrogen and Phosphorus Deficiencies in Soybean. Plant Sci. 2023, 326, 111498. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A.; Kusters, P.M.; Kessler, D.; Bainas, Z.; Gugel, R.K. Assembling a Core Collection from the Flax World Collection Maintained by Plant Gene Resources of Canada. Genet. Resour. Crop Evol. 2013, 60, 1479–1485. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- VSN International GenStat for Windows 2015; VSN International Ltd.: Hemel Hempstead, UK, 2015.
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 11 June 2025).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog Assignment Based on Profile HMM and Adaptive Score Threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef] [PubMed]



| Trait | Treatment | Genotype | Treatment × Genotype |
|---|---|---|---|
| TRL | 0.024 | <0.001 | 0.013 |
| RV | 0.315 | <0.001 | 0.084 |
| RD | 0.605 | <0.001 | 0.713 |
| RT | 0.017 | <0.001 | <0.001 |
| PDW | 0.060 | 0.004 | 0.035 |
| SDW | 0.018 | 0.018 | 0.019 |
| RDW | 0.012 | <0.001 | 0.161 |
| R/S | 0.048 | <0.001 | 0.572 |
| PNC | <0.001 | <0.001 | <0.001 |
| SNC | <0.001 | <0.001 | <0.001 |
| RNC | <0.001 | <0.001 | 0.002 |
| NUE | 0.012 | <0.001 | 0.009 |
| Genotype | Treatment | TRL | RT | R/S | SDW | RDW | PNC | SNC | RNC | NUE |
|---|---|---|---|---|---|---|---|---|---|---|
| NE | N+ | 680.8 | 667 | 0.40 | 128.9 | 51.8 | 4.6 | 1.9 | 0.55 | 38.9 |
| N- | 749.0 | 697 | 0.44 | 129.6 | 55.8 | 3.7 | 1.4 | 0.50 | 50.2 | |
| NI | N+ | 585.2 | 276 | 0.28 | 128.7 | 35.4 | 13.7 | 6.1 | 0.50 | 12.0 |
| N- | 481.0 | 319 | 0.30 | 87.7 | 28.8 | 6.7 | 3.1 | 0.64 | 17.7 |
| Sample ID | Raw Reads | HQ Reads | %HQ | Number of Mapped Reads | % Mapped (from HQ Reads) |
|---|---|---|---|---|---|
| NE-N-(R1) | 26,186,612 | 25,634,568 | 98.07 | 24,026,638 | 91.75 |
| NE-N-(R2) | 31,440,357 | 30,754,943 | 97.98 | 29,123,044 | 92.63 |
| NE-N-(R3) | 31,591,373 | 30,902,313 | 97.98 | 28,951,668 | 91.64 |
| NE-N-(R4) | 23,958,564 | 23,444,602 | 97.97 | 22,092,449 | 92.21 |
| NE-N+(R1) | 30,771,046 | 30,125,083 | 98.03 | 28,168,431 | 91.54 |
| NE-N+(R2) | 33,547,421 | 32,808,879 | 97.96 | 30,490,536 | 90.89 |
| NE-N+(R3) | 33,866,998 | 33,158,610 | 98.06 | 30,784,145 | 90.90 |
| NE-N+(R4) | 21,871,133 | 21,448,333 | 98.23 | 19,980,893 | 91.36 |
| NI-N-(R1) | 22,384,872 | 21,953,587 | 98.22 | 20,731,804 | 92.62 |
| NI-N-(R2) | 25,810,407 | 25,196,537 | 97.79 | 23,513,530 | 91.10 |
| NI-N-(R3) | 21,399,235 | 20,955,647 | 98.11 | 19,551,670 | 91.37 |
| NI-N-(R4) | 22,296,996 | 21,870,222 | 98.24 | 20,458,320 | 91.75 |
| NI-N+(R1) | 21,309,014 | 20,826,784 | 97.88 | 19,387,170 | 90.98 |
| NI-N+(R2) | 21,864,092 | 21,470,869 | 98.36 | 19,844,818 | 90.76 |
| NI-N+(R3) | 22,994,955 | 22,583,208 | 98.35 | 21,266,395 | 92.48 |
| NI-N+(R4) | 20,003,344 | 19,599,262 | 98.11 | 18,052,452 | 90.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© His Majesty the King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food Canada [2025] and by other authors.
Share and Cite
Soto-Cerda, B.J.; Larama, G.; Fofana, B.; Soto, I. Morphological and Transcriptomic Analyses Provide New Insights into Linseed (Linum usitatissimum L.) Seedling Roots Response to Nitrogen Stress. Plants 2025, 14, 2920. https://doi.org/10.3390/plants14182920
Soto-Cerda BJ, Larama G, Fofana B, Soto I. Morphological and Transcriptomic Analyses Provide New Insights into Linseed (Linum usitatissimum L.) Seedling Roots Response to Nitrogen Stress. Plants. 2025; 14(18):2920. https://doi.org/10.3390/plants14182920
Chicago/Turabian StyleSoto-Cerda, Braulio J., Giovanni Larama, Bourlaye Fofana, and Izsavo Soto. 2025. "Morphological and Transcriptomic Analyses Provide New Insights into Linseed (Linum usitatissimum L.) Seedling Roots Response to Nitrogen Stress" Plants 14, no. 18: 2920. https://doi.org/10.3390/plants14182920
APA StyleSoto-Cerda, B. J., Larama, G., Fofana, B., & Soto, I. (2025). Morphological and Transcriptomic Analyses Provide New Insights into Linseed (Linum usitatissimum L.) Seedling Roots Response to Nitrogen Stress. Plants, 14(18), 2920. https://doi.org/10.3390/plants14182920








