Halotolerant Endophytic Fungi: Diversity, Host Plants, and Mechanisms in Plant Salt–Alkali Stress Alleviation
Abstract
1. Introduction
2. Diversity and Host Specificity
| Crops | Floristics | Geographical Distribution | Soil pH | Electrical Conductivity | Sodium Adsorption Ratio | The Main Fungal Genera Isolated | References |
|---|---|---|---|---|---|---|---|
| Wilding | Robinia pseudoacacia L. | 100° E, 35° N | 6.3 | 5.1 | 8.9 | Fusarium | [20] |
| Wilding | Elaeagnus angustifolia Linn. | 124° E, 43° N | 8.4 | 9.3 | 15.2 | Fusarium | [21] |
| Wilding | Puccinellia tenuiflora | 110° E, 38° N | 9.0 | 11.2 | 18.7 | Fusarium | [18,22] |
| Wilding | Arundo donax L. | 70° E, 40° N | 8.3 | 5.4 | 7.9 | Fusarium | [20] |
| Wilding | Setaria viridis | 124° E, 43° N | 8.2 | 9.4 | 14.3 | Fusarium | [20] |
| Wilding | Saussurea japonica (Thunb.) DC. | 102° E, 35° N | 7.5 | 3.2 | 8.8 | Penicillium | [23] |
| Wilding | Anthemis nobilis | 90° E, 55° N | 7.2 | 4.1 | 8.1 | Penicillium | [24] |
| Wilding | Suaeda salsa | 126° E, 35° N | 9.2 | 10.3 | 13.7 | Penicillium | [25] |
| Wilding | Arabidopsis thaliana (L.) Heynh. | Europe, western Asia | 6.8–7.5 | 3.9 | 7.3 | Penicillium | [26] |
| Wilding | Spartina anglica Hubb | 100° E, 44° N | 6.5–8.5 | 6.8 | 7.2 | Fusarium | [26] |
| Wilding | Lolium perenne L. | 106° E, 26° N | 6.5 | 4.1 | 6.5 | Aspergillus | [27] |
| Wilding | Festuca elata Keng ex E. B. Alexeev | 102° E, 40° N | 8.5 | 6.2 | 8.5 | Neotyphodium | [26] |
| Wilding | Populus L. | 90° E, 45° N | 6.5–8.0 | 6.7 | 5.1 | Penicillium | [25] |
| Wilding | Trifolium repens L. | Europe and West Asia | 6.0–7.0 | 4.5 | 12.5 | Alternaria arborescens | [16] |
| Wilding | Glycyrrhiza uralensis Fisch. | 123° E, 44° N | 7.8 | 8.2 | 5.6 | Fusarium | [28] |
| Wilding | Medicago sativa L. | 105° E, 45° N | 6.5–7.5 | 5.0 | 6.3 | Fusarium | [25] |
| Cultivated | Solanum lycopersicum | 125° E, 43° N | 6.0–7.0 | 5.1 | 7.1 | Fusarium oxysporum | [20] |
| Cultivated | Zea mays L. | 125° E, 43° N | 6.0–7.5 | 3.9 | 5.8 | Fusarium oxysporum | [21] |
| Cultivated | Cucumis | 125° E, 43° N | 5.5–7.0 | 7.2 | 7.6 | Fusarium oxysporum | [26] |
| Cultivated | Triticum aestivum L. | Subtropical | 6.0–7.5 | 4.9 | 9.8 | Fusarium oxysporum | [29] |
| Cultivated | Gossypium hirsutum | 102° E, 47° N | 6.0–8.0 | 8.1 | 5.3 | Fusarium oxysporum | [30] |
| Cultivated | Arachis hypogaea | 120° E, 40° N | 5.6–6.0 | 7.5 | 11.2 | Fusarium | [26] |
| Cultivated | Oryza sativa | 122° E, 45° N | 8.2 | 5.2 | 13.8 | Fusarium | [27] |
| Cultivated | Beta vulgaris L. | 122° E, 45° N | 6.0–6.7 | 5.9 | 7.9 | Fusarium oxysporum | [30] |
| Cultivated | Cucumis melo L. | 122° E, 45° N | 6.0–7.5 | 5.4 | 6.8 | Fusarium oxysporum | [30] |
3. Isolation and Identification Techniques
3.1. Separation Method
3.1.1. Traditional Tissue Culture Method
3.1.2. Alkali Adaptability Separation Technology
3.1.3. Hydroscopic Method for Separation of Mycelium
3.1.4. High-Throughput Metagenomic Technology
3.2. Identification Techniques: From Morphological Characteristics to Molecular Phylogenetic Analysis
3.2.1. Morphological Identification
3.2.2. Molecular Identification
4. Functional Validation: From Lab to Field
4.1. Experimental Designs
4.2. Field Trials
5. Mechanisms of Fungal-Mediated Alleviation of Saline–Alkali Stress
5.1. Physiological Adaptations: Osmolyte Production, Ion Exclusion, Antioxidant Defense, and Phytohormone Modulation
5.1.1. Osmolyte Production: Fungal vs. Host-Derived Compounds
5.1.2. Ion Exclusion Mechanisms: Na+/K+ Balance Regulation
5.1.3. Antioxidant Enzyme Enhancement
5.1.4. Phytohormone Modulation
5.2. Molecular Mechanisms: Signaling Networks and Metabolic Reprogramming
5.2.1. SOS Pathway Regulation
5.2.2. MAPK-Transcription Factor Networks
5.3. Halotolerant Endophytic Fungi: Definition and Mechanistic Distinctions
6. Bioactive Metabolites and Their Applications
7. Summary
8. Prospects and Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEFs | Halotolerant endophytic fungi |
| PDA | Potato Dextrose Agar |
| MEA | Malt extract agar |
| ITS | Internal Transcribed Spacer |
| PCR | Polymerase Chain Reaction |
| OTU | Operational Taxonomic Unit |
| IAA | Indole-3-Acetic Acid |
| ABA | Abscisic acid |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| SPAD | Soil Plant Analysis Development Index |
| MAPK | Mitogen-Activated Protein Kinase |
| NRPS | Non-Ribosomal Peptide Synthetases |
| PGPR | Plant Growth-Promoting Rhizobacteria |
| AMF | Arbuscular Mycorrhizal Fungi |
| EPS | Exopolysaccharides |
| GSH | Glutathione |
References
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Endophytic halotolerant Bacillus velezensis FMH2 alleviates salt stress on tomato plants by improving plant growth and altering physiological and antioxidant responses. Plant Physiol. Biochem. 2021, 165, 217–227. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Anik, A.R.; Ranjan, R.; Ranganathan, T. Estimating the Impact of Salinity Stress on Livelihood Choices and Incomes in Rural Bangladesh. J. Int. Dev. 2018, 30, 1414–1438. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal Fungi (AMF) on Salt Stress Tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 601004. [Google Scholar] [CrossRef]
- Soares, D.A.; Rosa, L.H.; Da Silva, J.F.M.; Pimenta, R.S. A review of bioactive compounds produced by endophytic fungi associated with medicinal plants. Bol. Mus. Para. Emílio Goeldi-Ciências Nat. 2017, 12, 331–352. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, Z.; Zhang, Y.; Wang, Y.; Ding, Y.; Wang, C.; Cao, S. Sulfur-Containing Compounds from Endophytic Fungi: Sources, Structures and Bioactivities. J. Fungi 2022, 8, 628. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Piernik, A.; Hrynkiewicz, K.; Wojciechowska, A.; Szymańska, S.; Lis, M.I.; Muscolo, A. Effect of halotolerant endophytic bacteria isolated from Salicornia europaea L. on the growth of fodder beet (Beta vulgaris L.) under salt stress. Arch. Agron. Soil Sci. 2017, 63, 1404–1418. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kim, Y.-H.; Kang, S.-M.; Lee, I.-J. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol. Biochem. 2011, 49, 852–861. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Subramanian, S.; Smith, D.L. Phytomicrobiome Coordination Signals Hold Potential for Climate Change-Resilient Agriculture. Front. Plant Sci. 2020, 11, 634. [Google Scholar] [CrossRef]
- Antar, M.; Gopal, P.; Msimbira, L.; Naamala, J. Inter-Organismal Signaling in the Rhizosphere. In Microbes and Signaling Biomolecules Against Plant Stress; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Lyu, D.; Zajonc, J.; Pagé, A.; Tanney, C.A.S.; Shah, A.; Monjezi, N.; Msimbira, L.A.; Antar, M.; Nazari, M.; Backer, R.; et al. Plant Holobiont Theory: The Phytomicrobiome Plays a Central Role in Evolution and Success. Microorganisms 2021, 9, 675. [Google Scholar] [CrossRef]
- Lyu, D.; Msimbira, L.A.; Nazari, M.; Antar, M.; Pagé, A.; Shah, A.; Monjezi, N.; Zajonc, J.; Tanney, C.A.S.; Backer, R.; et al. The Coevolution of Plants and Microbes Underpins Sustainable Agriculture. Microorganisms 2021, 9, 1036. [Google Scholar] [CrossRef]
- Nicoletti, R.; Ferranti, P.; Caira, S.; Misso, G.; Castellano, M.; Di Lorenzo, G.; Caraglia, M. Myrtucommulone production by a strain of Neofusicoccum australe endophytic in myrtle (Myrtus communis). World J. Microbiol. Biotechnol. 2014, 30, 1047–1052. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Tong, C.-L.; Wang, Q.-S.; Bie, S. Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress. Plants 2024, 13, 805. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Wang, G.; Liu, X.; Amombo, E.; Xie, Y.; Fu, J. The Fungus Aspergillus aculeatus Enhances Salt-Stress Tolerance, Metabolite Accumulation, and Improves Forage Quality in Perennial Ryegrass. Front. Microbiol. 2017, 8, 1664. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, W.; Wang, Y.; Zhang, L.; Huang, S.; Lin, J. Metabolomics Analysis Reveals the Alkali Tolerance Mechanism in Puccinellia tenuiflora Plants Inoculated with Arbuscular Mycorrhizal Fungi. Microorganisms 2020, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Shi, Y.; Mu, C.; Wang, J. Differences in Organic Solute and Metabolites of Leymus chinensis in Response to Different Intensities of Salt and Alkali Stress. Plants 2023, 12, 1916. [Google Scholar] [CrossRef]
- Liu, H.; Tang, H.; Ni, X.; Zhang, Y.; Wang, Y. Impact of an arbuscular mycorrhizal fungal inoculum and exogenous methyl jasmonate on the performance of tall fescue under saline-alkali condition. Front. Microbiol. 2022, 13, 902667. [Google Scholar] [CrossRef]
- Zai, X.-M.; Fan, J.-J.; Hao, Z.-P.; Liu, X.-M.; Zhang, W.-X. Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci. Rep. 2021, 11, 5761. [Google Scholar] [CrossRef]
- Xia, F.; Hao, H.; Qi, Y.; Bai, H.; Li, H.; Shi, Z.; Shi, L. Effect of Salt Stress on Microbiome Structure and Diversity in Chamomile (Matricaria chamomilla L.) Rhizosphere Soil. Agronomy 2023, 13, 1444. [Google Scholar] [CrossRef]
- Fang, L.; Xu, J.; Yang, C. Arbuscular Mycorrhizal Fungi Alleviates Salt-Alkali Stress Demage on Syneilesis aconitifolia. Phyton 2023, 92, 3195–3209. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Guo, X.; Peng, W.; Xu, X.; Xie, K.; Yang, X. The Potential of Endophytes in Improving Salt–Alkali Tolerance and Salinity Resistance in Plants. Int. J. Mol. Sci. 2023, 24, 16917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mallik, A.; Zhang, J.; Huang, Y.; Zhou, L. Effects of arbuscular mycorrhizal fungi on inoculated seedling growth and rhizosphere soil aggregates. Soil Tillage Res. 2019, 194, 104340. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, C.; Li, J.; Zhao, Z.; Liu, Y. Metabolomics Revealed the Tolerance and Growth Dynamics of Arbuscular Mycorrhizal Fungi (AMF) to Soil Salinity in Licorice. Plants 2024, 13, 2652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; White, J.F.; Li, C. Epichloë bromicola from wild barley improves salt-tolerance of cultivated barley by altering physiological responses to salt stress. Front. Microbiol. 2022, 13, 1044735. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M. Insights into Microbially Induced Salt Tolerance and Endurance Mechanisms (STEM) in Plants. Front. Microbiol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Han, S.; Cheng, Y.; Wu, G.; He, X.; Zhao, G. Enhancing Salt Tolerance in Poplar Seedlings through Arbuscular Mycorrhizal Fungi Symbiosis. Plants 2024, 13, 233. [Google Scholar] [CrossRef]
- Li, B.; Mamuti, R.; Xiao, L.; Qian, B.; Wang, Y.; Wei, X. The adaptation of lichen symbiosis to desert saline-alkali stress depends more on their symbiotic algae. Physiol. Plant. 2024, 176, e14510. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, T.; Qi, D.; Chang, W.; Li, K.; Fan, X.; Zhang, M.; Ping, Y.; Song, F. Synergistic effect of Rhizophagus irregularis and biochar improves saline-alkaline tolerance physiology of Panicum virgatum. Physiol. Plant. 2024, 176, e14367. [Google Scholar] [CrossRef]
- Wang, P.; Su, C.; Wu, J.; Xie, Y.; Fan, J.; Wang, J.; Hui, W.; Yang, H.; Gong, W. Response of Photosynthetic Characteristics to Different Salicylic Acid Concentrations in Relation to Waterlogging Resistance in Zanthoxylum armatum. Hortic. Sci. Technol. 2023, 41, 349–360. [Google Scholar] [CrossRef]
- Kumar Yadav, V.; Krishna Jha, R.; Kaushik, P.; Altalayan, F.H.; Al Balawi, T.; Alam, P. Traversing arbuscular mycorrhizal fungi and Pseudomonas fluorescens for carrot production under salinity. Saudi J. Biol. Sci. 2021, 28, 4217–4223. [Google Scholar] [CrossRef] [PubMed]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Rahman, M.H.U.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Zare, N. Silicon Pretreatment at the Transplanting Stage, a Tool to Improve the Drought Tolerance and Subsequent Growth of Melons in the Field. Silicon 2023, 15, 4921–4929. [Google Scholar] [CrossRef]
- Ndiate, N.I.; Zaman, Q.U.; Francis, I.N.; Dada, O.A.; Rehman, A.; Asif, M.; Goffner, D.; Kane, A.; Liqun, C.; Haider, F.U. Soil Amendment with Arbuscular Mycorrhizal Fungi and Biochar Improves Salinity Tolerance, Growth, and Lipid Metabolism of Common Wheat (Triticum aestivum L.). Sustainability 2022, 14, 3210. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Rehman, M.Z.U.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Ren, C.-G.; Kong, C.-C.; Yan, K.; Xie, Z.-H. Transcriptome analysis reveals the impact of arbuscular mycorrhizal symbiosis on Sesbania cannabina expose to high salinity. Sci. Rep. 2019, 9, 278. [Google Scholar] [CrossRef]
- Satir, N.Y.; Ortas, I.; Satir, O. The Influence of Mycorrhizal Species on Sour Orange (Citrusaurantium L.) Growth Under Saline Soil Conditions. Pak. J. Agri. Sci. 2016, 53, 399–406. [Google Scholar]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- García-Gaytán, V.; Bojórquez-Quintal, E.; Hernández-Mendoza, F.; Tiwari, D.K.; Corona-Morales, N.; Moradi-Shakoorian, Z. Polymerized Silicon (SiO2·nH2O) in Equisetum Arvense: Potential Nanoparticle in Crops. J. Chil. Chem. Soc. 2019, 64, 4298–4302. [Google Scholar] [CrossRef]
- D’iMperio, M.; Montesano, F.F.; Renna, M.; Leoni, B.; Buttaro, D.; Parente, A.; Serio, F. NaCl stress enhances silicon tissue enrichment of hydroponic “baby leaf” chicory under biofortification process. Sci. Hortic. 2018, 235, 258–263. [Google Scholar] [CrossRef]
- Saleem, A.; Raza, M.A.S.; Tahir, M.A.; Iqbal, R.; Aslam, M.U.; Toleikiene, M.; Khan, M.S.; Alwahibi, M.S.; Elshikh, M.S.; Ditta, A. Impact of Biogas Slurry on Physiological and Antioxidant Mechanisms of Wheat Under Drought Stress. Pol. J. Environ. Stud. 2025, 34, 1721–1731. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Kang, S.; Zhen, S. Sulfur Supplementation Enhanced the Growth and Photosynthesis of Lettuce in Hydroponic Production Using One-bag Complete Fertilizer. Hort. Sci. 2024, 59, 412–420. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, N.-L.; Wang, M.-Q.; He, X.-B.; Lv, Z.-Q.; Wei, J.; Su, X.; Wu, A.-P.; Li, Y. Sex-Specific Differences in the Physiological and Biochemical Performance of Arbuscular Mycorrhizal Fungi-Inoculated Mulberry Clones Under Salinity Stress. Front. Plant Sci. 2021, 12, 614162. [Google Scholar] [CrossRef] [PubMed]
- Feghhenabi, F.; Hadi, H.; Khodaverdiloo, H.; van Genuchten, M.T.; Lake, L.; Acuna, T. Quantitative evaluation of silicon applications on wheat response to salinity: Changes in photosynthetic pigments, chlorophyll fluorescence parameters, yield and yield components. Crop. Pasture Sci. 2022, 73, 1118–1130. [Google Scholar] [CrossRef]
- El-Nashar, Y.I. Response of snapdragon (Antirrhinum majus L.) to blended water irrigation and arbuscular mycorrhizal fungi inoculation: Uptake of minerals and leaf water relations. Photosynthetica 2017, 55, 201–209. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. Sci. 2015, 22, 773–779. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.-B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Song, Z.; Su, Q.-W.; Wei, Z.-H.; Li, W.-C.; Jiang, Z.-X.; Tian, P.; Wang, Z.-H.; Yang, X.; Yang, M.-Y.; et al. Transcriptomic and metabolomic reveals silicon enhances adaptation of rice under dry cultivation by improving flavonoid biosynthesis, osmoregulation, and photosynthesis. Front. Plant Sci. 2022, 13, 967537. [Google Scholar] [CrossRef]
- Peng, L.; Shan, X.; Yang, Y.; Wang, Y.; Druzhinina, I.S.; Pan, X.; Jin, W.; He, X.; Wang, X.; Zhang, X.; et al. Facultative symbiosis with a saprotrophic soil fungus promotes potassium uptake in American sweetgum trees. Plant Cell Environ. 2021, 44, 2793–2809. [Google Scholar] [CrossRef]
- Wen, Y.; Shi, F.; Zhang, B.; Li, K.; Chang, W.; Fan, X.; Dai, C.L.; Song, F. Rhizophagus irregularis and biochar can synergistically improve the physiological characteristics of saline-alkali resistance of switchgrass. Physiol. Plant. 2024, 176, e14367. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-O. Effects of Salt Stress on the Growthand Physiological Characteristics of Two Desert Plants Mediated by AMF. Acta Agrestia Sin. 2023, 31, 2712–2772. [Google Scholar]
- Xie, K.; Wang, M.; Wang, X.; Li, F.; Xu, C.; Feng, J.; Fang, F. Effect of rice cultivar on greenhouse-gas emissions from rice–fish co-culture. Crop J. 2024, 12, 888–896. [Google Scholar] [CrossRef]
- Thapa, A.; Hasan, R.; Kabir, A.H. Trichoderma afroharzianum T22 Induces Rhizobia and Flavonoid-Driven Symbiosis to Promote Tolerance to Alkaline Stress in Garden Pea. Plant Cell Environ. 2025; early view. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.d.C.; Afridi, M.S.; Mitra, D.; Valencia-Cantero, E.; Macías-Rodríguez, L. Trichoderma and Bacillus multifunctional allies for plant growth and health in saline soils: Recent advances and future challenges. Front. Microbiol. 2024, 15, 1423980. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Yasin, N.A.; Wahid, A.; Sardar, R. Exogenous application of glutathione enhanced growth, nutritional orchestration and physiochemical characteristics of Brassica oleracea L. under lead stress. Physiol. Mol. Biol. Plants 2023, 29, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.; Zhou, T.; Zhang, C.; Zhang, J.; Zhao, B. Salinity-driven differentiation of bacterial and fungal communities in coastal wetlands: Contrasting assembly processes and spatial dynamics. Environ. Res. 2025, 279, 121895. [Google Scholar] [CrossRef]
- Wen, B.; Huang, H.; Lu, L.; Lui, T.; Lui, R. Overexpression of Geranylgeranyl Diphosphate Synthase and Cyclase Enhances Pleuromutilin Production in Clitopilus Passeckerianus T6. Biotechnol. J. 2025, 20, e202500004. [Google Scholar] [CrossRef]
- Hmissi, M.; Chaieb, M.; Krouma, A. Exogenous IAA Application Enhances Durum Wheat Tolerance to Salinity by Regulating Osmotic Adjustment and Ionic Homeostasis. Russ. J. Plant Physiol. 2025, 72, 60. [Google Scholar] [CrossRef]
- Kraševec, N. Pore-forming aegerolysin and MACPF proteins in extremotolerant or extremophilic fungi. IUBMB Life 2024, 76, 922–936. [Google Scholar] [CrossRef]
- Saeedi, R.; Seyedi, A.; Esmaeilizadeh, M.; Seyedi, N.; Zahedi, S.M. Efficiency of Nanostructures Containing Chitosan-Selenium in Grafted Citrus Seedlings Under Salinity Stress: Element Uptake, Biochemical and Morphological Changes. J. Soil Sci. Plant Nutr. 2025, 25, 1813–1829. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, S.; Wang, M.; Zeb, S.; Khan, M.N.; Chen, Y.; Zhu, G.; Zhu, Z. Enhancement of sweetpotato tolerance to chromium stress through melatonin and glutathione: Insights into photosynthetic efficiency, oxidative defense, and growth parameters. Plant Physiol. Biochem. 2024, 208, 108509. [Google Scholar] [CrossRef]
- Li, Z.; Jin, N.; Jin, L.; Wang, S.; Li, Y.; Sun, M.; Huang, S.; Xie, Y.; Meng, X.; Xu, Z.; et al. Use of silicon to protect tomato (Solanum lycopersicum L.) seedlings from low-calcium stress-derived oxidative damage. Sci. Hortic. 2025, 349, 114231. [Google Scholar] [CrossRef]
- Akhzari, D.; Mahdavi, S.; Pessarakli, M.; Ebrahimi, M. Effects of Arbuscular Mycorrhizal Fungi on Seedling Growth and Physiological Traits of Melilotus officinalis L. Grown Under Salinity Stress Conditions. Commun. Soil Sci. Plant Anal. 2016, 47, 822–831. [Google Scholar] [CrossRef]
- Zeng, W.; Hou, Y.; Ao, C.; Huang, J. Effects of PGPR and γ-PGA on maize growth and rhizosphere microbial community in saline soil. Agric. Water Manag. 2024, 295, 108736. [Google Scholar] [CrossRef]
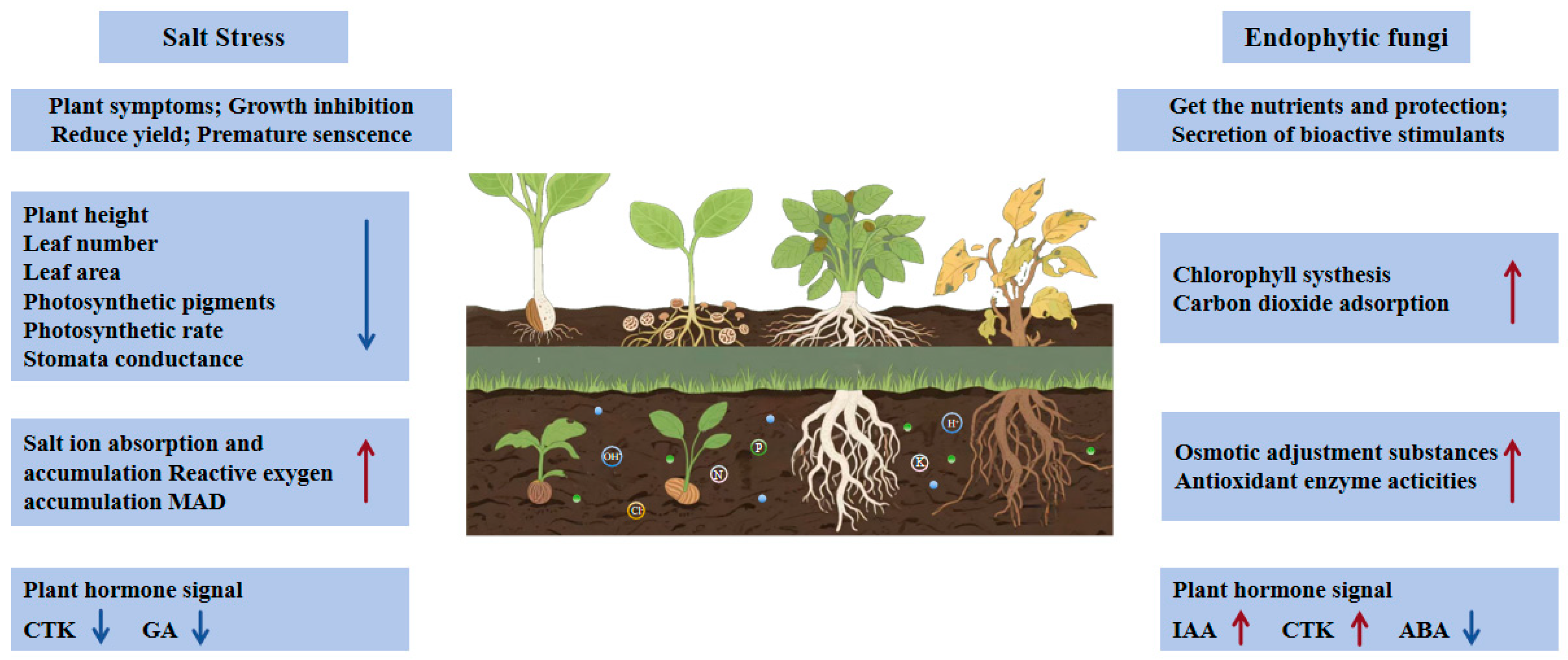

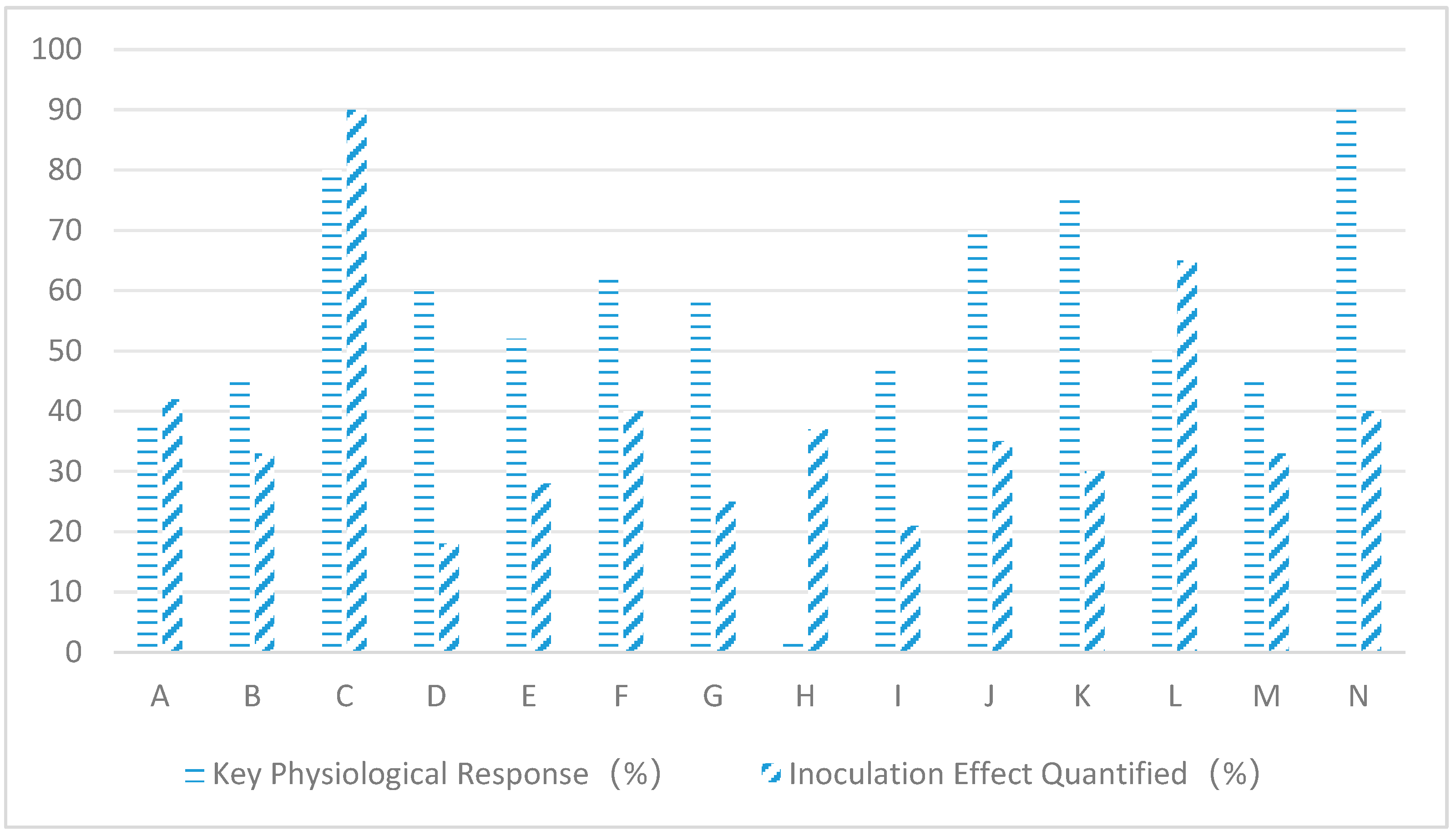
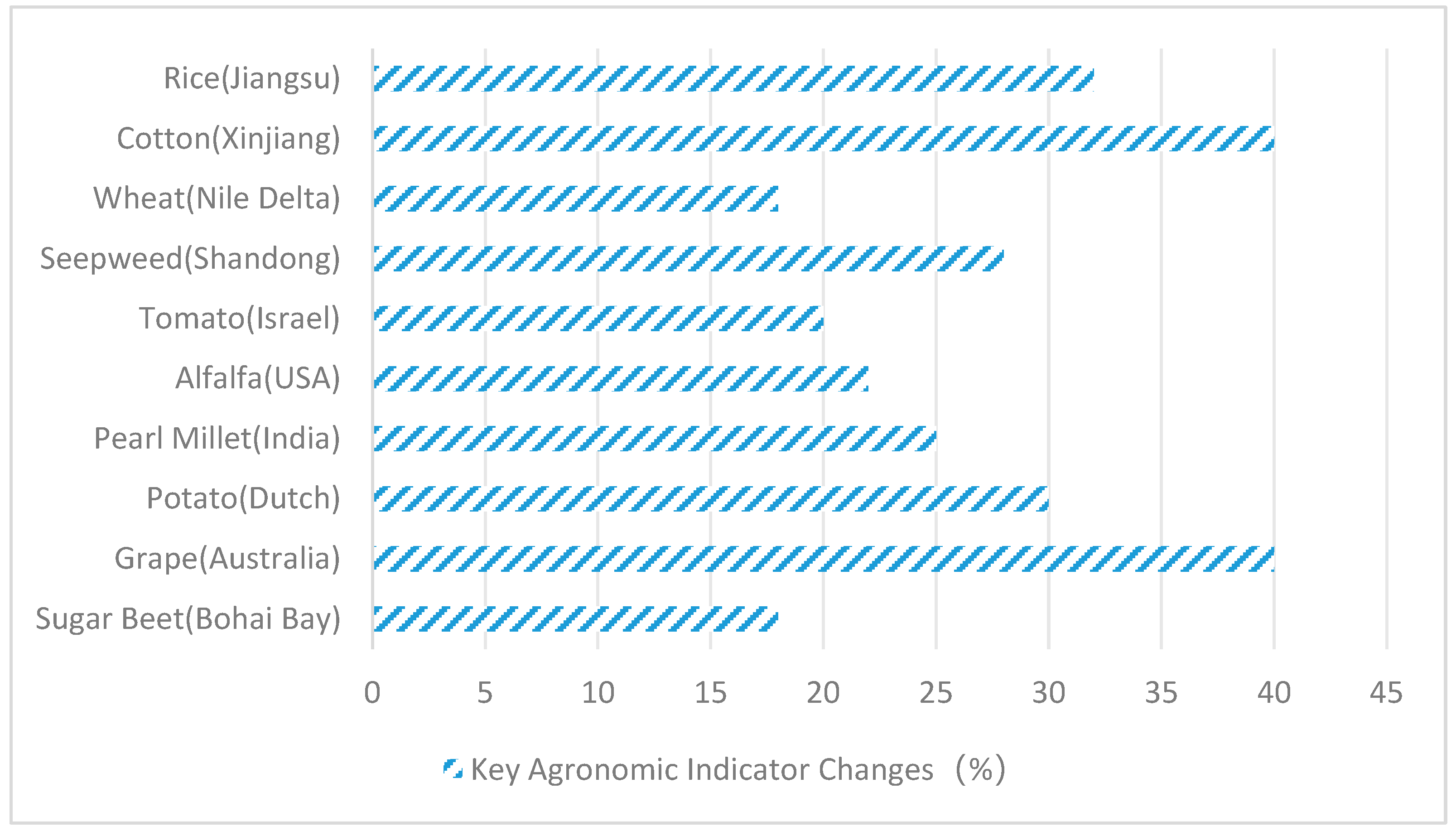


| Host Plant | Endophytic Fungus | Salt Stress Conditions | Stress Damage Markers | Adaptive Responses | Inoculation Effect Quantified | References |
|---|---|---|---|---|---|---|
| Wheat | Penicillium simplicissimum | 150 mM NaCl | Na+ ↓38%, K+/Na+ ↑2.1×, Proline ↑67% | Biomass ↑42% | [34] | |
| Rice | Aspergillus terreus | 200 mM NaCl | Photosynthetic rate ↑45%, SOS1 gene ↑3.2× | Tillering number ↑33% | [35] | |
| Seepweed | Alternaria alternata | 300 mM NaCl | Superoxide dismutase (SOD) ↑80% | Survival rate ↑90% | [38] | |
| Cotton | Fusarium oxysporum | 100 mM NaCl | Root IAA ↑55%, Biofilm stability ↑60% | Fiber length ↑18% | [39] | |
| Maize | Trichoderma harzianum | 250 mM NaCl | MDA ↓52% | Chlorophyll ↑39% | Grain yield ↑28% | [36] |
| Tomato | Epicoccum nigrum | 150 mM NaCl | H2O2 ↓62% | APX activity ↑75% | Fruit setting rate ↑40% | [40] |
| Soybean | Chaetomium globosum | 200 mM NaCl | Proline ↑58%, Nodule nitrogenase ↑3.5× | Protein content ↑25% | [41] | |
| Alfalfa | Phoma glomerata | 100 mM NaCl | Na+/K+ ↓1.8×, Aquaporin gene ↑2.4× | Dry matter accumulation ↑37% | [42] | |
| Sunflower | Cladosporium cladosporioides | 200 mM NaCl | Stomatal conductance ↑48%, ABA ↓43% | Seed oil content ↑21% | [43] | |
| Goji Berry | Talaromyces wortmannii | 300 mM NaCl | Betaine ↑120%, Ionic compartmentalization efficiency ↑70% | Carotenoids ↑35% | [44] | |
| Sugar Beet | Fusarium redolens | 150 mM NaCl | Sucrose synthase ↑2.1×, Soluble sugars ↑75% | Root sugar content ↑30% | [45] | |
| Cucumber | Trichoderma asperellum | 100 mM NaCl | Lignin deposition ↑50%, Vessel density ↑30% | Fusarium wilt incidence ↓65% | [46] | |
| Sorghum | Curvularia lunata | 250 mM NaCl | Rhizosphere pH ↓0.8 units, Na+ adsorption ↓45% | Water use efficiency ↑33% | [47] | |
| Arabidopsis | Acremonium strictum | 150 mM NaCl | SOS2 gene ↑4.5×, Ion efflux ↑60% | Flowering advanced by 7 days | [48] | |
| Alkali Grass | Sarocladium strictum | 300 mM NaCl | Organic acid secretion ↑90%, Na+ chelation ↑80% | Salt gland density ↑40% | [49] |
| Location/Soil Type | Crop | Endophytic Fungus | Treatment Method | Key Agronomic Indicator Changes | Economic Benefit | References |
|---|---|---|---|---|---|---|
| Jiangsu Coastal Saline Soil (pH 8.7) | Rice | Aspergillus flavus | Seed coating | Yield ↑32%, Empty grains rate ↓40% | Net profit ↑USD 220/ha | [35] |
| Xinjiang Saline–Alkali Soil (pH 9.2) | Cotton | P. simplicissimum + T. harzianum | Root drenching | Fiber yield ↑40%, Lint percentage ↑15% | Water saving ↑25% | [50] |
| Nile Delta, Egypt (pH 8.9) | Wheat | Fusarium verticillioides | Foliar spray | 1000-grain weight ↑18%, Protein ↑12% | Nitrogen fertilizer ↓30% | [51] |
| Yellow River Estuary Wetland, Shandong (pH 8.5) | Seepweed | Alternaria alternata | Rhizosphere inoculation | Biodiesel output ↑28%, Na+ enrichment ↑50% | Marginal cost ↓USD 15/ton | [38] |
| Negev Desert, Israel (pH 9.0) | Tomato | Epicoccum nigrum | Drip irrigation | Soluble solids ↑20%, Fruit cracking rate ↓60% | Market price ↑15% | [40] |
| Central Valley, CA, USA (pH 8.6) | Alfalfa | Phoma glomerata | Seed pelleting | Crude protein ↑22%, Overwintering survival rate ↑35% | Harvest cycle shortened by 10 days | [42] |
| Saline Soil, Gujarat, India (pH 9.1) | Pearl Millet | Curvularia lunata | Furrow application | Grain yield ↑25%, Water requirement ↓20% | Marginal return ↑USD 45/ha | [47] |
| Dutch Polder Area (pH 8.4) | Potato | Sarocladium strictum | Seed tuber soaking | Tuber starch ↑30%, Scab ↓70% | Storage loss ↓25% | [49] |
| Murray-Darling Basin, Australia (pH 8.8) | Grape | Talaromyces wortmannii | Drip + Inoculant | Sugar-acid ratio optimized ↑1.8×, Resveratrol ↑40% | Premium wine price ↑30% | [44] |
| Bohai Bay Coastal Saline Soil (pH 8.7) | Sugar Beet | Fusarium redolens | Root dipping at transplanting | Sugar content ↑18%, Brown spot ↓55% | Processing efficiency ↑20% | [45] |
| Metabolite | Function | Example |
|---|---|---|
| Siderophores | Fe3+ chelation (logK = 32) | Harzianic acid increases leaf Fe by 36%, reducing ROS accumulation [57]. |
| Cyclopeptides | SOS1 autoinhibition relief (KD = 1.2 μM) | cyclo(L-Phe-L-Pro) enhances Na+ efflux 2.3-fold [60]. |
| Organic acids | Metal chelation/compartmentalization | A. niger citrate (12 mM) sequesters Zn2+/Pb2+ [61]. |
| Trehalose | Protein stabilization via H-bonding | Maintains RuBisCO activity at 85% of control under K+ limitation [53]. |
| Compound Category/Structure | Source/Origin | Functional Mechanism | Potential Patent Application Direction | References |
|---|---|---|---|---|
| Fungal-Derived Metabolites | ||||
| Siderophores (e.g., Ferrichrome) | Fungal secretion | Chelate Fe3+ (logK = 32) to alleviate iron deficiency; inhibit pathogen growth. | Biocontrol agent (CA2887654) | [62] |
| Exopolysaccharides (EPS) | Fungal secretion | Form rhizosphere biofilm, reducing Na+ permeation; improve soil aggregate stability. | Microbial encapsulation material (DE102019117890) | [62] |
| Cyclopeptides (e.g., cyclo(L-Phe-L-Pro)) | Fungal secretion | Act as signaling molecules; relieve SOS1 autoinhibition (KD = 1.2 μM), enhancing Na+ efflux. | Bioeffector compound | [58] |
| Fungal terpenoids (e.g., Trichoderins) | Fungal secretion | Induce plant stress response genes (e.g., OsNHX1); enhance vacuolar Na+ compartmentalization. | Genetic engineering promoter element (WO202209876) | [63] |
| Chitinase | Fungal secretion | Degrade pathogen cell walls; induce plant systemic resistance (ISR). | Biopesticide (US10709123) | [62] |
| Fungal-Induced Plant Metabolites | ||||
| Proline | Fungal-induced plant synthesis | Osmotic adjustment, maintaining cell water potential; protects protein structure and function. | Biostimulant composition (CN107384123A) | [64,65] |
| Betaine (Glycine betaine) | Fungal-induced plant synthesis | Osmoprotection, stabilizes photosynthetic complexes; enhances Rubisco activity. | Foliar spray for saline–alkali soil crops (US20200154621) | [52,66] |
| Flavonoid derivatives (e.g., Quercetin) | Fungal-induced plant synthesis | ROS scavenging, membrane stabilization; upregulate plant SOD, CAT gene expression. | Crop stress resistance enhancer (WO202112345) | [58,59] |
| Polyamines (Putrescine, Spermidine) | Fungal-induced plant synthesis | Inhibit ethylene synthesis; maintain DNA stability, delay senescence. | Seed coating agent (AU2018300567) | [67,68] |
| Metabolites of Dual or Uncertain Origin | ||||
| γ-Polyglutamic acid (γ-PGA) | Mainly from associated Bacillus spp. | Promotes rhizosphere probiotics colonization; reduces leaf Na+. | Soil amendment (JP2020156789) | [69] |
| Silicic acid polymer | Fungal-enhanced plant uptake/assimilation | Enhances cell wall silicification, reduces Na+ influx; increases plant SOD activity. | Silicon fertilizer technology (RU2017145678) | [43,47] |
| Melatonin | Putative: Fungal-induced or co-synthesis | Protects chloroplast ultrastructure; upregulates photosynthetic genes PsbA, PsbD. | Stress mitigation formulation (EP3456218) | [52,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Liu, Y.; Liu, Z.; Xu, Y.; Yin, S.; Bai, H.; Wang, J. Halotolerant Endophytic Fungi: Diversity, Host Plants, and Mechanisms in Plant Salt–Alkali Stress Alleviation. Plants 2025, 14, 2907. https://doi.org/10.3390/plants14182907
Ma Q, Liu Y, Liu Z, Xu Y, Yin S, Bai H, Wang J. Halotolerant Endophytic Fungi: Diversity, Host Plants, and Mechanisms in Plant Salt–Alkali Stress Alleviation. Plants. 2025; 14(18):2907. https://doi.org/10.3390/plants14182907
Chicago/Turabian StyleMa, Qiurui, Yangyuxin Liu, Zi Liu, Yang Xu, Shuren Yin, Helong Bai, and Jing Wang. 2025. "Halotolerant Endophytic Fungi: Diversity, Host Plants, and Mechanisms in Plant Salt–Alkali Stress Alleviation" Plants 14, no. 18: 2907. https://doi.org/10.3390/plants14182907
APA StyleMa, Q., Liu, Y., Liu, Z., Xu, Y., Yin, S., Bai, H., & Wang, J. (2025). Halotolerant Endophytic Fungi: Diversity, Host Plants, and Mechanisms in Plant Salt–Alkali Stress Alleviation. Plants, 14(18), 2907. https://doi.org/10.3390/plants14182907






