Cascade Oxygen Control Enhances Growth of Nicotiana benthamiana Cell Cultures in Stirred-Tank Bioreactors
Abstract
1. Introduction
2. Results
2.1. Effect of DO Cascade Control by Agitation and Aeration
2.2. Process Scale-Up Using Reynolds Number and kLa Parameters
2.3. Effect of DO Cascade Control at Larger Scale
3. Discussion
3.1. Effect of DO Control on Culture Performance
3.2. Scaling Up the Process with NRE, kLa and Geometry
4. Materials and Methods
4.1. Plant Material
4.2. Callus Culture
4.3. Shake Flask Cultures
4.4. Bioreactor Configuration and Scale-Up Strategy
4.5. Specific Growth Rate Calculation
4.6. Determination of kLa via Sodium Sulphite Method
- OTR is the oxygen transfer rate (mol·L−1·h−1);
- kLa is the volumetric mass transfer coefficient (h−1);
- C* is the saturation concentration of oxygen in the medium (mol·L−1);
- CL is the actual dissolved oxygen concentration in the medium (mol·L−1).
4.7. Reynolds Number Calculation
- ρ is the fluid density (assumed 1000 kg/m3);
- N is the impeller speed (rev/s);
- D is the impeller diameter (m);
- μ is the dynamic viscosity (Pa·s).
4.8. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHO | Chinese Hamster Ovary |
| CSTR | Continuous Stirring Tank Bioreactor |
| DO | Dissolved Oxygen |
| H/D | Height/Diameter |
| hESCs | Human Embryonic Stem Cells |
| hiPSCs | Human Pluripotent Stem Cells |
| kLa | Volumetric Mass Transfer Coefficient |
| NRE | Reynolds Number |
| OTR | Oxygen Transfer Rate |
| P/V | Power Input Per Unit Volume |
| PCV | Packed Cell Volume |
| slpm | Standard Liter Per Minute |
| tm | Mixing Time |
| vvm | Volume of Gas per Volume of Medium per Minute |
| WV | Working Volume |
References
- Lehr, F.; Posten, C. Closed Photo-Bioreactors as Tools for Biofuel Production. Curr. Opin. Biotechnol. 2009, 20, 280–285. [Google Scholar] [CrossRef]
- Chidambarampadmavathy, K.; Karthikeyan, O.P.; Heimann, K. Biopolymers Made from Methane in Bioreactors. Eng. Life Sci. 2015, 15, 689–699. [Google Scholar] [CrossRef]
- Acedos, M.G.; Moreno-Cid, J.; Verdú, F.; González, J.A.; Tena, S.; López, J.C. Exploring the Potential of Slaughterhouse Waste Valorization: Development and Scale-up of a New Bioprocess for Medium-Chain Length Polyhydroxyalkanoates Production. Chemosphere 2022, 287, 132401. [Google Scholar] [CrossRef]
- Fang, Z.; Lyu, J.; Li, J.; Li, C.; Zhang, Y.; Guo, Y.; Wang, Y.; Zhang, Y.; Chen, K. Application of Bioreactor Technology for Cell Culture-Based Viral Vaccine Production: Present Status and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 921755. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Selvaganapathy, P.R.; Geng, F. Advancing Our Understanding of Bioreactors for Industrial-Sized Cell Culture: Health Care and Cellular Agriculture Implications. Am. J. Physiol.-Cell Physiol. 2023, 325, C580–C591. [Google Scholar] [CrossRef] [PubMed]
- Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The Advent of Plant Cells in Bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef]
- Karki, U.; Fang, H.; Guo, W.; Unnold-Cofre, C.; Xu, J. Cellular Engineering of Plant Cells for Improved Therapeutic Protein Production. Plant Cell Rep. 2021, 40, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Abdulhafiz, F.; Mohammed, A.; Reduan, M.F.H.; Kari, Z.A.; Wei, L.S.; Goh, K.W. Plant Cell Culture Technologies: A Promising Alternatives to Produce High-Value Secondary Metabolites. Arab. J. Chem. 2022, 15, 104161. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.-K.; Park, J.-S.; Lee, K.-R. Plant-Made Pharmaceuticals: Exploring Studies for the Production of Recombinant Protein in Plants and Assessing Challenges Ahead. Plant Biotechnol. Rep. 2023, 17, 53–65. [Google Scholar] [CrossRef]
- Jia, J.; Wilson, W.; Karmaker, A.; Nishimura, A.; Otsuka, H.; Ohara, K.; Okawa, H.; McDonald, K.; Nandi, S.; Albeck, J.G.; et al. Applications of Plant-Made Fibroblast Growth Factor for Human Pluripotent Stem Cells. Stem Cells Dev. 2024, 33, 57–66. [Google Scholar] [CrossRef]
- Bero, J.; Frédérich, M.; Quetin-Leclercq, J. Antimalarial Compounds Isolated from Plants Used in Traditional Medicine. J. Pharm. Pharmacol. 2009, 61, 1401–1433. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Moreira, V.M.; Gonçalves, B.M.F.; Leal, A.S.; Jing, Y. Ursane-Type Pentacyclic Triterpenoids as Useful Platforms to Discover Anticancer Drugs. Nat. Prod. Rep. 2012, 29, 1463–1479. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of Plant (Tri)Terpenoids: From Metabolic Engineering of Plants to Synthetic Biology in Vivo and in Vitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef]
- Xu, X.; Cocco, E.; Guerriero, G.; Sergeant, K.; Jourdan, S.; Renaut, J.; Hausman, J.-F.; Legay, S. Harnessing Apple Cell Suspension Cultures in Bioreactors for Triterpene Production: Transcriptomic Insights into Biomass and Triterpene Biosynthesis. Int. J. Mol. Sci. 2025, 26, 3188. [Google Scholar] [CrossRef]
- Venkat, K. Paclitaxel Production through Plant Cell Culture: An Exciting Approach to Harnessing Biodiversity. Pure Appl. Chem. 1998, 70, 2127–2128. [Google Scholar]
- Choi, H.-K.; Son, J.-S.; Na, G.-H.; Hong, S.-S.; Park, Y.-S.; Song, J.-Y. Mass Production of Paclitaxel by Plant Cell Culture. J. Plant Biotechnol. 2002, 29, 59–62. [Google Scholar] [CrossRef]
- Almagro, L.; Belchí-Navarro, S.; Martínez-Márquez, A.; Bru, R.; Pedreño, M.A. Enhanced Extracellular Production of Trans-Resveratrol in Vitis Vinifera Suspension Cultured Cells by Using Cyclodextrins and Coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced Extracellular Production of Trans-Resveratrol in Vitis Vinifera Suspension Cultured Cells by Using Cyclodextrins and Methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, F. Effect of Yeast Elicitor on the Secondary Metabolism of Ti-Transformed Salvia Miltiorrhiza Cell Suspension Cultures. Plant Cell Rep. 2000, 19, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under in Vitro Salinity Stress. Plant Cell Tissue Organ. Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Xing, Z.; Kenty, B.M.; Li, Z.J.; Lee, S.S. Scale-up Analysis for a CHO Cell Culture Process in Large-Scale Bioreactors. Biotechnol. Bioeng. 2009, 103, 733–746. [Google Scholar] [CrossRef]
- Mete, T.; Ozkan, G.; Hapoglu, H.; Alpbaz, M. Control of Dissolved Oxygen Concentration Using Neural Network in a Batch Bioreactor. Comput. Appl. Eng. Educ. 2012, 20, 619–628. [Google Scholar] [CrossRef]
- Cárcamo, M.; Saa, P.; Torres, J.; Torres, S.; Mandujano, P.; Ricardo Perez Correa, J.; Agosin, E. Effective Dissolved Oxygen Control Strategy for High-Cell-Density Cultures. IEEE Lat. Am. Trans. 2014, 12, 389–394. [Google Scholar] [CrossRef]
- Jay, V.; Genestier, S.; Courduroux, J.C. Bioreactor Studies on the Effect of Dissolved Oxygen Concentrations on Growth and Differentiation of Carrot (Daucus carota L.) Cell Cultures. Plant Cell Rep. 1992, 11, 605–608. [Google Scholar] [CrossRef]
- Corbin, J.M.; Hashimoto, B.I.; Karuppanan, K.; Kyser, Z.R.; Wu, L.; Roberts, B.A.; Noe, A.R.; Rodriguez, R.L.; McDonald, K.A.; Nandi, S. Semicontinuous Bioreactor Production of Recombinant Butyrylcholinesterase in Transgenic Rice Cell Suspension Cultures. Front. Plant Sci. 2016, 7, 412. [Google Scholar] [CrossRef]
- Arias, J.P.; Mendoza, D.; Arias, M. Agitation Effect on Growth and Metabolic Behavior of Plant Cell Suspension Cultures of Thevetia Peruviana at Bench Scale Reactor. Plant Cell Tissue Organ. Cult. 2021, 145, 307–319. [Google Scholar] [CrossRef]
- Cheung, C.K.-L.; Leksawasdi, N.; Doran, P.M. Bioreactor Scale-down Studies of Suspended Plant Cell Cultures. AIChE J. 2018, 64, 4281–4288. [Google Scholar] [CrossRef]
- Hogue, R.S.; Lee, J.M.; An, G. Production of a Foreign Protein Product with Genetically Modified Plant Cells. Enzym. Microb. Technol. 1990, 12, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lee, J.M. Effect of Oxygen Supply on the Suspension Culture of Genetically Modified Tobacco Cells. Biotechnol. Prog. 1992, 8, 285–290. [Google Scholar] [CrossRef]
- Vazquez-Marquez, A.M.; Zepeda-Gómez, C.; Burrola-Aguilar, C.; Bernabé-Antonio, A.; Nieto-Trujillo, A.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Estrada-Zúñiga, M.E. Effect of Stirring Speed on the Production of Phenolic Secondary Metabolites and Growth of Buddleja Cordata Cells Cultured in Mechanically Agitated Bioreactor. Plant Cell Tissue Organ Cult. 2019, 139, 155–166. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and Engineering Aspects on the Scale-up of Bioreactors for Different Bioprocesses. Syst. Microbiol. Biomanuf. 2024, 4, 365–385. [Google Scholar] [CrossRef]
- Shin, W.-S.; Lee, D.; Kim, S.; Jeong, Y.-S.; Chun, G.-T. Application of Scale-Up Criterion of Constant Oxygen Mass Transfer Coefficient (kLa) for Production of Itaconic Acid in a 50 L Pilot-Scale Fermentor by Fungal Cells of Aspergillus Terreus. J. Microbiol. Biotechnol. 2013, 23, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hoshan, L.; Jiang, R.; Gupta, B.; Brodean, E.; O’Neill, K.; Seamans, T.C.; Bowers, J.; Chen, H. A Practical Approach in Bioreactor Scale-up and Process Transfer Using a Combination of Constant P/V and Vvm as the Criterion. Biotechnol. Prog. 2017, 33, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, V.M.; Anupindi, K.; Bhatt, N.; Srivastava, S. Characterizing the Effect of Impeller Design in Plant Cell Fermentations Using CFD Modeling. Sci. Rep. 2025, 15, 9322. [Google Scholar] [CrossRef]
- Ding, H.; Cheng, H.; Wu, J.; Zhang, F.; Cao, C.; Mualif, S.A.; Xie, Z. A New Strategy in Bioreactor Scale-up and Process Transfer Using a Dynamic Initial Vvm According to Different Aeration Pore Size. Front. Bioeng. Biotechnol. 2024, 12, 1461253. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.; Salzig, D. Impact of Bioreactor Geometry on Mesenchymal Stem Cell Production in Stirred-Tank Bioreactors. Chem. Ing. Tech. 2021, 93, 1537–1554. [Google Scholar] [CrossRef]
- Han, J.-E.; Lee, H.; Ho, T.-T.; Park, S.-Y. Brazzein Protein Production in Transgenic Carrot Cells Using Air-Lift Bioreactor Culture. Plant Biotechnol. Rep. 2022, 16, 161–171. [Google Scholar] [CrossRef]
- Macharoen, K.; Du, M.; Jung, S.; McDonald, K.A.; Nandi, S. Production of Recombinant Butyrylcholinesterase from Transgenic Rice Cell Suspension Cultures in a Pilot-Scale Bioreactor. Biotechnol. Bioeng. 2021, 118, 1431–1443. [Google Scholar] [CrossRef]
- Breuling, M.; Alfermann, A.W.; Reinhard, E. Cultivation of Cell Cultures of Berberis Wilsonae in 20-l Airlift Bioreactors. Plant Cell Rep. 1985, 4, 220–223. [Google Scholar] [CrossRef]
- Thanh, N.T.; Murthy, H.N.; Paek, K.Y. Optimization of Ginseng Cell Culture in Airlift Bioreactors and Developing the Large-Scale Production System. Ind. Crops Prod. 2014, 60, 343–348. [Google Scholar] [CrossRef]
- Tekoah, Y.; Shulman, A.; Kizhner, T.; Ruderfer, I.; Fux, L.; Nataf, Y.; Bartfeld, D.; Ariel, T.; Gingis–Velitski, S.; Hanania, U.; et al. Large-Scale Production of Pharmaceutical Proteins in Plant Cell Culture—The Protalix Experience. Plant Biotechnol. J. 2015, 13, 1199–1208. [Google Scholar] [CrossRef]
- Navia-Osorio, A.; Garden, H.; Cusidó, R.M.; Palazón, J.; Alfermann, A.W.; Piñol, M.T. Taxol® and Baccatin III Production in Suspension Cultures of Taxus baccata and Taxus wallichiana in an Airlift Bioreactor. J. Plant Physiol. 2002, 159, 97–102. [Google Scholar] [CrossRef]
- Eibl, R.; Eibl, D. Design and Use of the Wave Bioreactor for Plant Cell Culture. Plant Tissue Cult. Eng. 2008, 203–227. [Google Scholar] [CrossRef]
- Gubser, G.; Vollenweider, S.; Eibl, D.; Eibl, R. Food Ingredients and Food Made with Plant Cell and Tissue Cultures: State-of-the Art and Future Trends. Eng. Life Sci. 2021, 21, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, K.; Pilarek, M. Mass Transfer Characteristics in Disposable Rocking Bioreactors: A Critical Review and Quantitative Data Catalogue. Chem. Eng. J. 2024, 499, 155966. [Google Scholar] [CrossRef]
- Lobato-Gómez, M.; Laurel, M.; Vázquez-Vilar, M.; Rambla, J.L.; Orzáez, D.; Rischer, H.; Granell, A. Novel Plant Cell Suspension Platforms for Saffron Apocarotenoid Production and Its Impact on Carotenoid and Volatile Profiles. Plant Biotechnol. J. 2025, 23, 3903–3918. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, S.C.; Karuppanan, K.; Li, Q.; Lebrilla, C.B.; Nandi, S.; McDonald, K.A. Transient Recombinant Protein Production in Glycoengineered Nicotiana Benthamiana Cell Suspension Culture. Int. J. Mol. Sci. 2018, 19, 1205. [Google Scholar] [CrossRef]
- Hanittinan, O.; Oo, Y.; Chaotham, C.; Rattanapisit, K.; Shanmugaraj, B.; Phoolcharoen, W. Expression Optimization, Purification and in Vitro Characterization of Human Epidermal Growth Factor Produced in Nicotiana benthamiana. Biotechnol. Rep. 2020, 28, e00524. [Google Scholar] [CrossRef]
- Selma, S.; Sanmartín, N.; Espinosa-Ruiz, A.; Gianoglio, S.; Lopez-Gresa, M.P.; Vázquez-Vilar, M.; Flors, V.; Granell, A.; Orzaez, D. Custom-Made Design of Metabolite Composition in N. Benthamiana Leaves Using CRISPR Activators. Plant Biotechnol. J. 2022, 20, 1578–1590. [Google Scholar] [CrossRef]
- Calvache, C.; Vazquez-Vilar, M.; Moreno-Giménez, E.; Orzaez, D. A Quantitative Autonomous Bioluminescence Reporter System with a Wide Dynamic Range for Plant Synthetic Biology. Plant Biotechnol. J. 2024, 22, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.F.; Levine, A.; Meijer, P.-J.; Tapon, N.A.; Pennell, R.I. A Programmed Cell Death Pathway Activated in Carrot Cells Cultured at Low Cell Density. Plant J. 1997, 12, 267–280. [Google Scholar] [CrossRef]
- McCabe, P.F.; Leaver, C.J. Programmed Cell Death in Cell Cultures. Plant Mol. Biol. 2000, 44, 359–368. [Google Scholar] [CrossRef]
- Jay, V.; Genestier, S.; Courduroux, J.-C. Bioreactor Studies of the Effect of Medium pH on Carrot (Daucus carota L.) Somatic Embryogenesis. Plant Cell Tissue Organ. Cult. 1994, 36, 205–209. [Google Scholar] [CrossRef]
- Shigeta, J.; Sato, K.; Mii, M. Effects of Initial Cell Density, pH and Dissolved Oxygen on Bioreactor Production of Carrot Somatic Embryos. Plant Sci. 1996, 115, 109–114. [Google Scholar] [CrossRef]
- Amini, S.; Ziaratnia, S.M.; Hemmati, K. Optimization of Conditions for Increasing of Saffron Cell Biomass and Crocin Production in Stirred Bioreactor. Plant Cell Tissue Organ. Cult. 2022, 149, 243–255. [Google Scholar] [CrossRef]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering Considerations to Produce Bioactive Compounds from Plant Cell Suspension Culture in Bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef]
- Snape, J.B.; Thomas, N.H.; Callow, J.A. How Suspension Cultures of Catharanthus Roseus Respond to Oxygen Limitation: Small-Scale Tests with Applications to Large-Scale Cultures. Biotechnol. Bioeng. 1989, 34, 1058–1062. [Google Scholar] [CrossRef]
- Tate, J.L.; Payne, G.F. Plant Cell Growth under Different Levels of Oxygen and Carbon Dioxide. Plant Cell Rep. 1991, 10, 22–25. [Google Scholar] [CrossRef]
- Pavlov, A.I.; Georgiev, M.I.; Ilieva, M.P. Production of Rosmarinic Acid byLavandula Vera MM Cell Suspension in Bioreactor: Effect of Dissolved Oxygen Concentration and Agitation. World J. Microbiol. Biotechnol. 2005, 21, 389–392. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, H.; Zhong, J. Scale-up Study on Suspension Cultures of Taxus Chinensis Cells for Production of Taxane Diterpene. Enzyme Microb. Technol. 2000, 27, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, M.; Wierzchowski, K.; Pilarek, M. Mass Transfer in a Liter-Scale Wave Mixed Single-Use Bioreactor: Influence of Viscosity and Antifoaming Agent. Ind. Eng. Chem. Res. 2023, 62, 10893–10902. [Google Scholar] [CrossRef]
- Eibl, R.; Werner, S.; Eibl, D. Bag Bioreactor Based on Wave-Induced Motion: Characteristics and Applications. Adv. Biochem. Eng. Biotechnol. 2009, 115, 55–87. [Google Scholar] [CrossRef]
- Huang, T.-K.; Plesha, M.A.; Falk, B.W.; Dandekar, A.M.; McDonald, K.A. Bioreactor Strategies for Improving Production Yield and Functionality of a Recombinant Human Protein in Transgenic Tobacco Cell Cultures. Biotechnol. Bioeng. 2009, 102, 508–520. [Google Scholar] [CrossRef]
- Pérez-Hernández, J.; Nicasio-Torres, M.d.P.; Sarmiento-López, L.G.; Rodríguez-Monroy, M. Production of Anti-Inflammatory Compounds in Sphaeralcea Angustifolia Cell Suspension Cultivated in Stirred Tank Bioreactor. Eng. Life Sci. 2019, 19, 196–205. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Sahu, B.B.; Singh, V.R.; Patra, N. Enhanced Production of Bacopa Saponins by Repeated Batch Strategy in Bioreactor. Bioprocess Biosyst. Eng. 2022, 45, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Kargupta, R.; Rivera, S.; Kochert, B.; Devenney, K.; Donelly, D.; Atieh, T.; Li, F.; Pan, J.; Patel, D.; Tayi, V.; et al. Elucidation of Cell Culture Impacts on Hydroxylysine Levels in Monoclonal Antibodies Using High-Throughput Analytical Quantification and Media Components. Biotechnol. Prog. 2025, e70068. [Google Scholar] [CrossRef]
- Andaluz, A.; Monteverde, B.; Vera, K.; Tse, B.; Gajic, I.; Forelich, C.; Motevalian, S.P. Accelerated Adeno Associated Virus Upstream Process Development from High-Throughput Systems to Clinical Scale. Biotechnol. Bioeng. 2025. [Google Scholar] [CrossRef]
- Zhang, X.; Moroney, J.; Hoshan, L.; Jiang, R.; Xu, S. Systematic Evaluation of High-Throughput Scale-down Models for Single-Use Bioreactors (SUB) Using Volumetric Gas Flow Rate as the Criterion. Biochem. Eng. J. 2019, 151, 107307. [Google Scholar] [CrossRef]
- Gelves, R.; Dietrich, A.; Takors, R. Modeling of Gas–Liquid Mass Transfer in a Stirred Tank Bioreactor Agitated by a Rushton Turbine or a New Pitched Blade Impeller. Bioprocess Biosyst. Eng. 2014, 37, 365–375. [Google Scholar] [CrossRef]
- Leckie, F.; Scragg, A.H.; Cliffe, K.C. An Investigation into the Role of Initial KLa on the Growth and Alkaloid Accumulation by Cultures of Catharanthus Roseus. Biotechnol. Bioeng. 1991, 37, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Henderson, K.A.; Rorrer, G.L. Cell Damage and Oxygen Mass Transfer during Cultivation of Nicotiana Tabacum in a Stirred-Tank Bioreactor. Biotechnol. Prog. 1995, 11, 140–145. [Google Scholar] [CrossRef]
- Doran, P.M. Design of Mixing Systems for Plant Cell Suspensions in Stirred Reactors. Biotechnol. Prog. 1999, 15, 319–335. [Google Scholar] [CrossRef]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The Rise and Rise of Nicotiana Benthamiana: A Plant for All Reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Huang, T.-K.; Plesha, M.A.; McDonald, K.A. Semicontinuous Bioreactor Production of a Recombinant Human Therapeutic Protein Using a Chemically Inducible Viral Amplicon Expression System in Transgenic Plant Cell Suspension Cultures. Biotechnol. Bioeng. 2010, 106, 408–421. [Google Scholar] [CrossRef]
- Imai, Y.; Takei, H.; Matsumura, M. A Simple Na2SO3 Feeding Method for KLa Measurement in Large-Scale Fermentors. Biotechnol. Bioeng. 1987, 29, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, C.; Almeida, G.; Cuvelier, G.; Menut, P. Modelling Shear Viscosity of Soft Plant Cell Suspensions. Food Hydrocoll. 2021, 118, 106776. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]

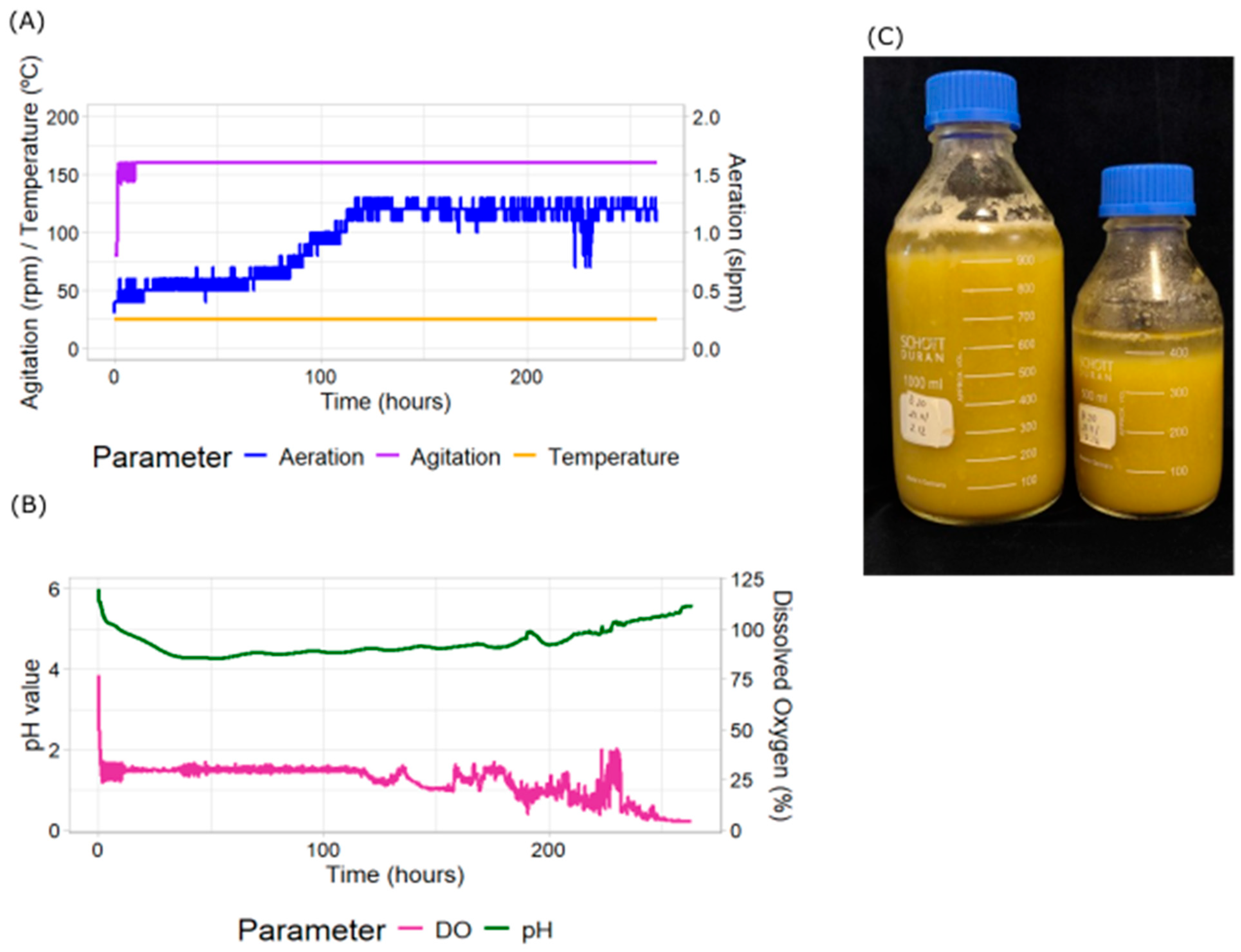
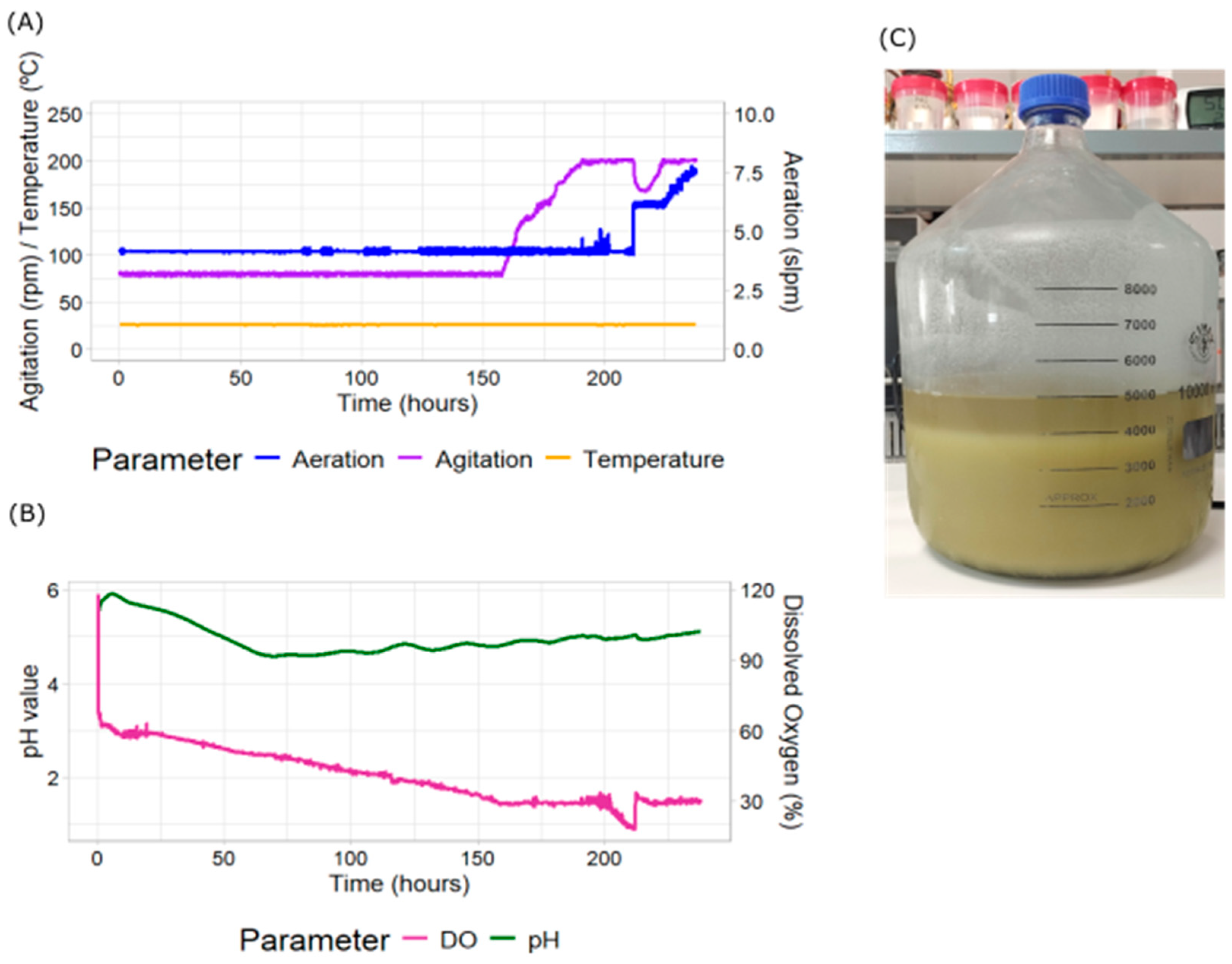

| Treatment | Replicate Number | Batch Duration (Days) | Final Packed Cell Volume (%) | Specific Growth Rate (d−1) |
|---|---|---|---|---|
| Uncontrolled Dissolved Oxygen | 1 | 19 | 85 | 0.089 |
| 2 | 21 | 79 | 0.076 | |
| 3 | 19 | 70 | 0.078 | |
| 4 | 19 | 80 | 0.085 | |
| Controlled Dissolved Oxygen | 1 | 9 | 70 | 0.164 |
| 2 | 12 | 75 | 0.129 | |
| 3 | 10 | 90 | 0.173 | |
| 4 | 14 | 75 | 0.110 | |
| Statistical differences between treatments | p = 0.002 | p = 0.85 | p = 0.02 | |
| Equipment | Working Volume (L) | Agitator Speed (rpm) | Aeration Rate (slpm) | Volume of CuSO4 320 mg/L Solution (mL) | Total Mass Na2SO3 (g) | Net Mass Na2SO3 (g) | Test Duration (min) | Calculated Oxygen Transfer Rate (mmol/L-h) | Calculated Volumetric Mass Transfer Coefficient, kLa T (h−1) |
|---|---|---|---|---|---|---|---|---|---|
| F0 | 2 | 80 | 0.4 | 4 | 10 | 9.87 | 854.8 | 1.4 | 5.6 |
| F0 | 2 | 80 | 0.4 | 4 | 10 | 9.87 | 729.7 | 1.6 | 6.6 |
| F0 | 2 | 80 | 0.4 | 4 | 10 | 9.87 | 778.4 | 1.5 | 6.2 |
| F0 | 2 | 160 | 1.2 | 4 | 10 | 9.87 | 238.5 | 4.9 | 20.2 |
| F0 | 2 | 160 | 1.2 | 4 | 10 | 9.87 | 243.0 | 4.8 | 19.8 |
| F0 | 2 | 160 | 1.2 | 4 | 10 | 9.87 | 239.3 | 4.9 | 20.1 |
| F2 | 7 | 80 | 4 | 14 | 35 | 34.55 | 346.4 | 3.4 | 13.9 |
| F2 | 7 | 80 | 4 | 14 | 35 | 34.55 | 403.2 | 2.9 | 11.9 |
| F2 | 7 | 80 | 4 | 14 | 35 | 34.55 | 333.8 | 3.5 | 14.4 |
| F2 | 7 | 200 | 8 | 14 | 35 | 34.55 | 115.0 | 10.2 | 41.9 |
| F2 | 7 | 200 | 8 | 14 | 35 | 34.55 | 118.0 | 10.0 | 40.8 |
| F2 | 7 | 200 | 8 | 14 | 35 | 34.55 | 114.3 | 10.3 | 42.1 |
| Parameter | F0 (No Cascade) | F0 (Cascade Dissolved Oxygen Control) | F2 (Pilot Scale, Cascade Dissolved Oxygen Control) | Control |
|---|---|---|---|---|
| Working volume (L) | 2.0 | 2.0 | 7.0 | Maintained geometric similarity (Height/Diameter ratio) |
| Impeller diameter (mm) | 54 | 54 | 70.5 | Proportional |
| Agitation range (rpm) | Constant, 80 | 80–160 | 80–200 | Adjusted via cascade control |
| Tip speed (m/s) | 0.23 | 0.23–0.45 | 0.3–0.82 | Adjusted to control kLa |
| Aeration rate (vvm) | 0.2 | 0.2–0.6 | 0.6–1.1 | Adjusted to control kLa |
| Dissolved oxygen setpoint (%) | None (natural decline) | 30 | 30 | Constant (cascade-based) |
| Average volumetric oxygen transfer coefficient, kLa (h−1) | ~6.1 | 13.4–20.0 | 13.4–41.6 | Targeted and matched |
| Reynolds number (NRE) | ~373 | 580–910 | 580–910 | Maintained hydrodynamic regime |
| Culture duration (days) | 21 | 10 | 10 | Significantly reduced |
| Final Packed Cell Volume (%) | 80–90 | ~90 | ~80 | Equivalent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. Cascade Oxygen Control Enhances Growth of Nicotiana benthamiana Cell Cultures in Stirred-Tank Bioreactors. Plants 2025, 14, 2879. https://doi.org/10.3390/plants14182879
Verdú-Navarro F, Moreno-Cid JA, Weiss J, Egea-Cortines M. Cascade Oxygen Control Enhances Growth of Nicotiana benthamiana Cell Cultures in Stirred-Tank Bioreactors. Plants. 2025; 14(18):2879. https://doi.org/10.3390/plants14182879
Chicago/Turabian StyleVerdú-Navarro, Fuensanta, Juan Antonio Moreno-Cid, Julia Weiss, and Marcos Egea-Cortines. 2025. "Cascade Oxygen Control Enhances Growth of Nicotiana benthamiana Cell Cultures in Stirred-Tank Bioreactors" Plants 14, no. 18: 2879. https://doi.org/10.3390/plants14182879
APA StyleVerdú-Navarro, F., Moreno-Cid, J. A., Weiss, J., & Egea-Cortines, M. (2025). Cascade Oxygen Control Enhances Growth of Nicotiana benthamiana Cell Cultures in Stirred-Tank Bioreactors. Plants, 14(18), 2879. https://doi.org/10.3390/plants14182879










