Decoding Lodging Resistance in Elite Chinese Conventional Rice Varieties: A Phenotypic and Biomechanical Perspective
Abstract
1. Introduction
2. Results
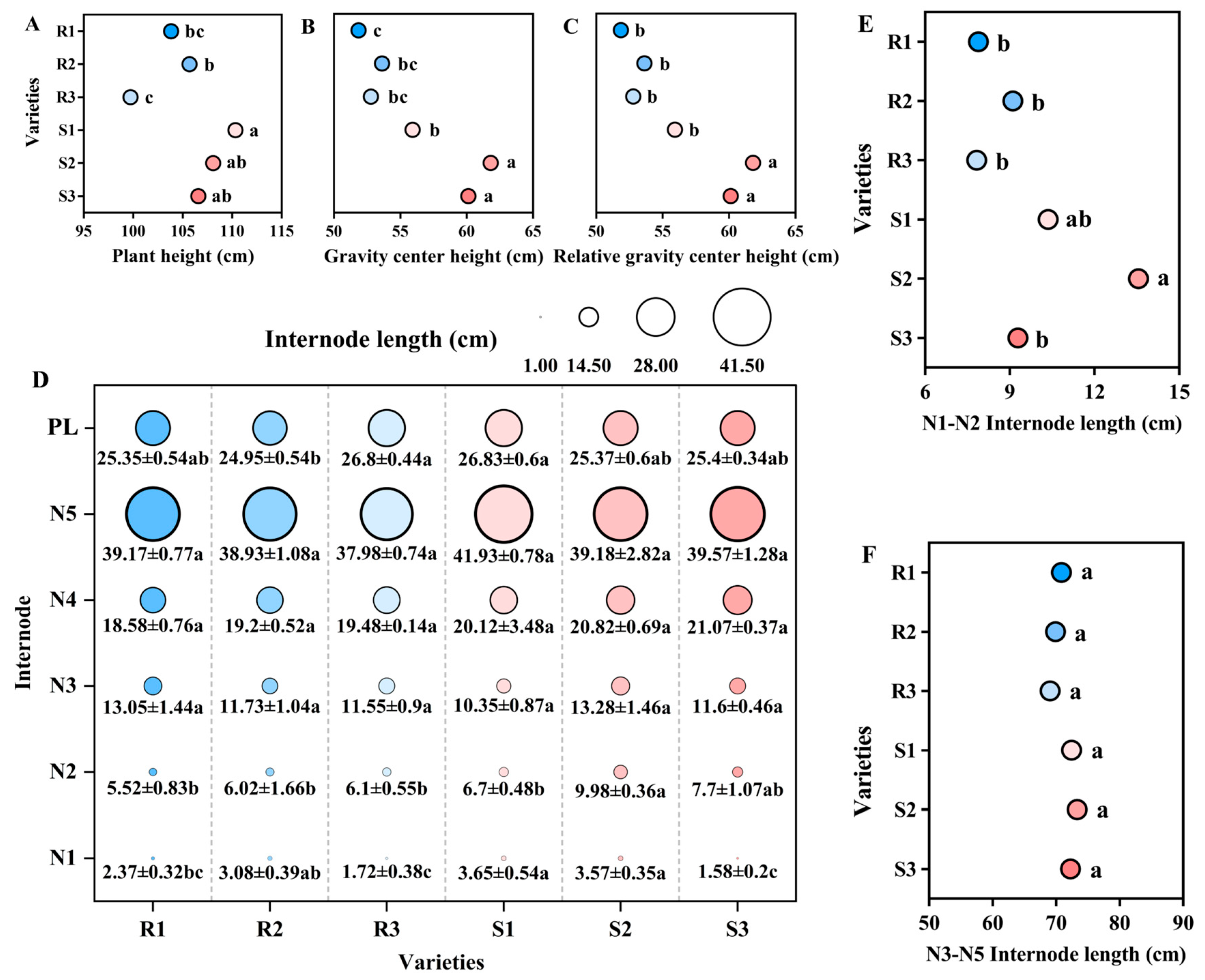
2.1. Comparison of Main Stem Internode Physical Traits of Different High-Quality Conventional Rice
2.1.1. Plant Height and Internode Length
2.1.2. Internode Physical Traits
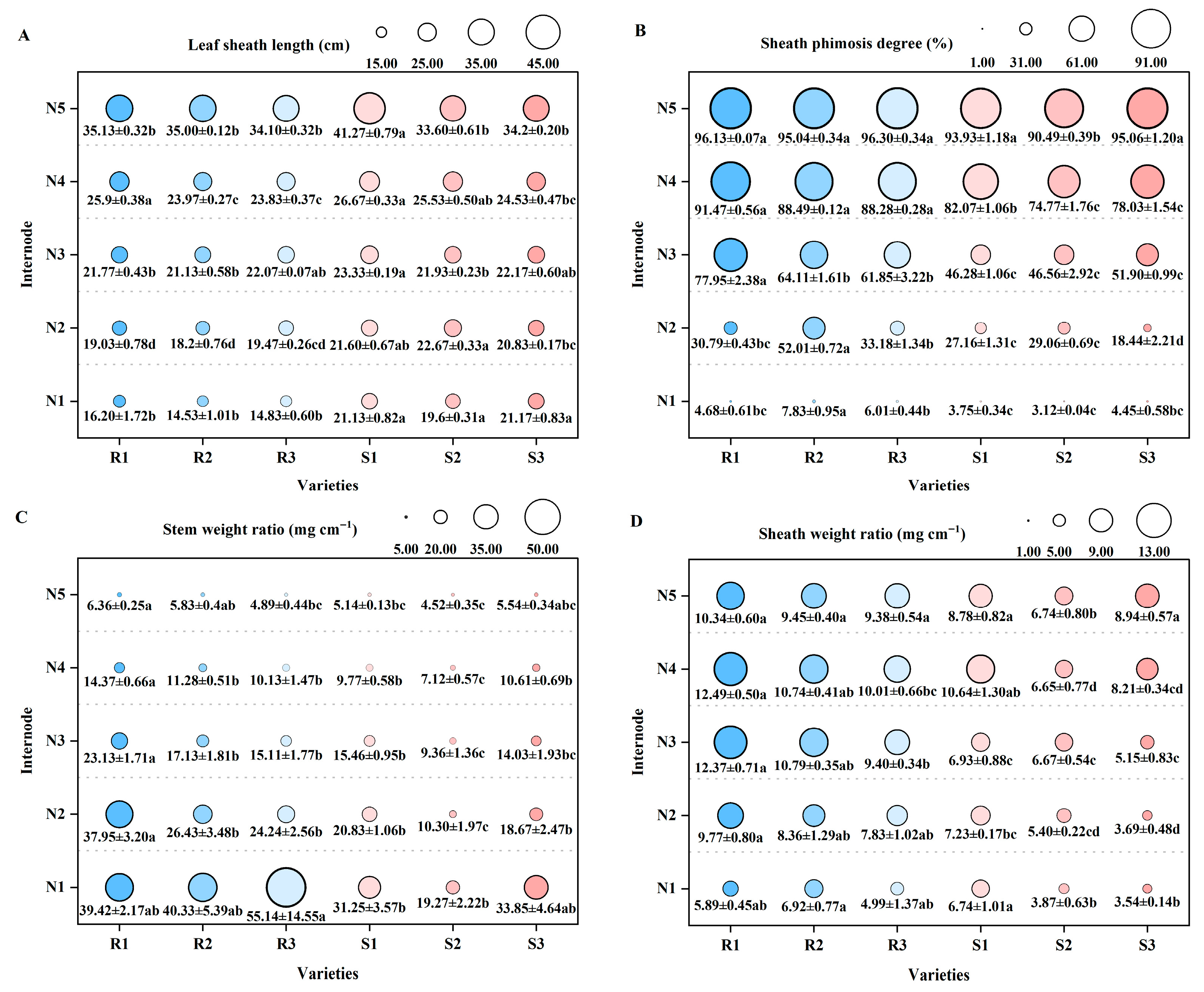
2.2. Comparison of Stem and Sheath Plumpness Traits of Different High-Quality Conventional Rice
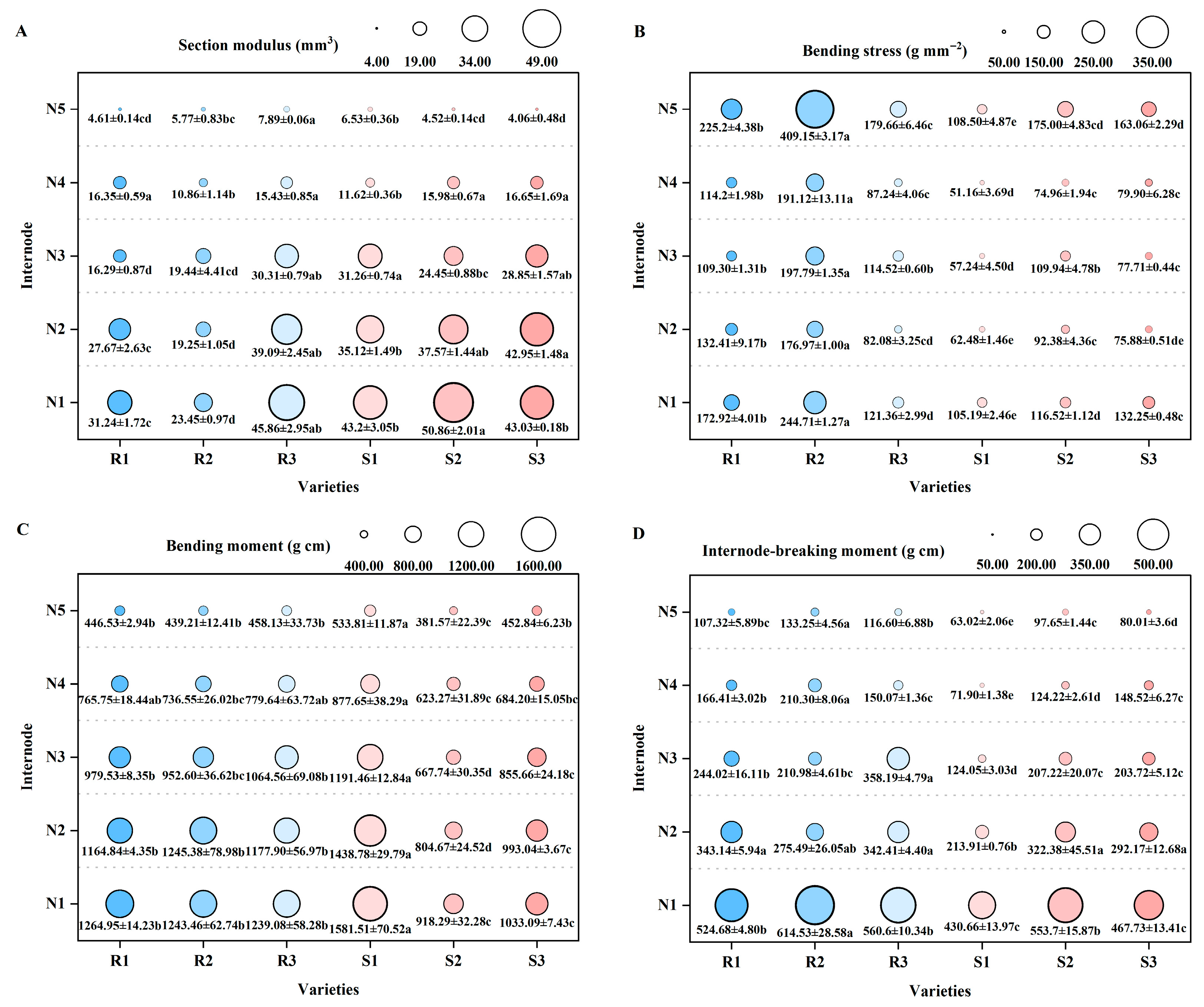
2.3. Comparison of Main Stem Mechanical Properties and Lodging Resistance of Different High-Quality Conventional Rice
2.3.1. Main Stem Mechanical Properties
2.3.2. Lodging Resistance
2.4. Comparison of Main Stem Yield-Related Traits and Panicle Characteristics of Different High-Quality Conventional Rice
2.4.1. Main Stem Yield-Related Traits
2.4.2. Main Stem Primary Branch Traits
2.4.3. Main Stem Secondary Branch Traits
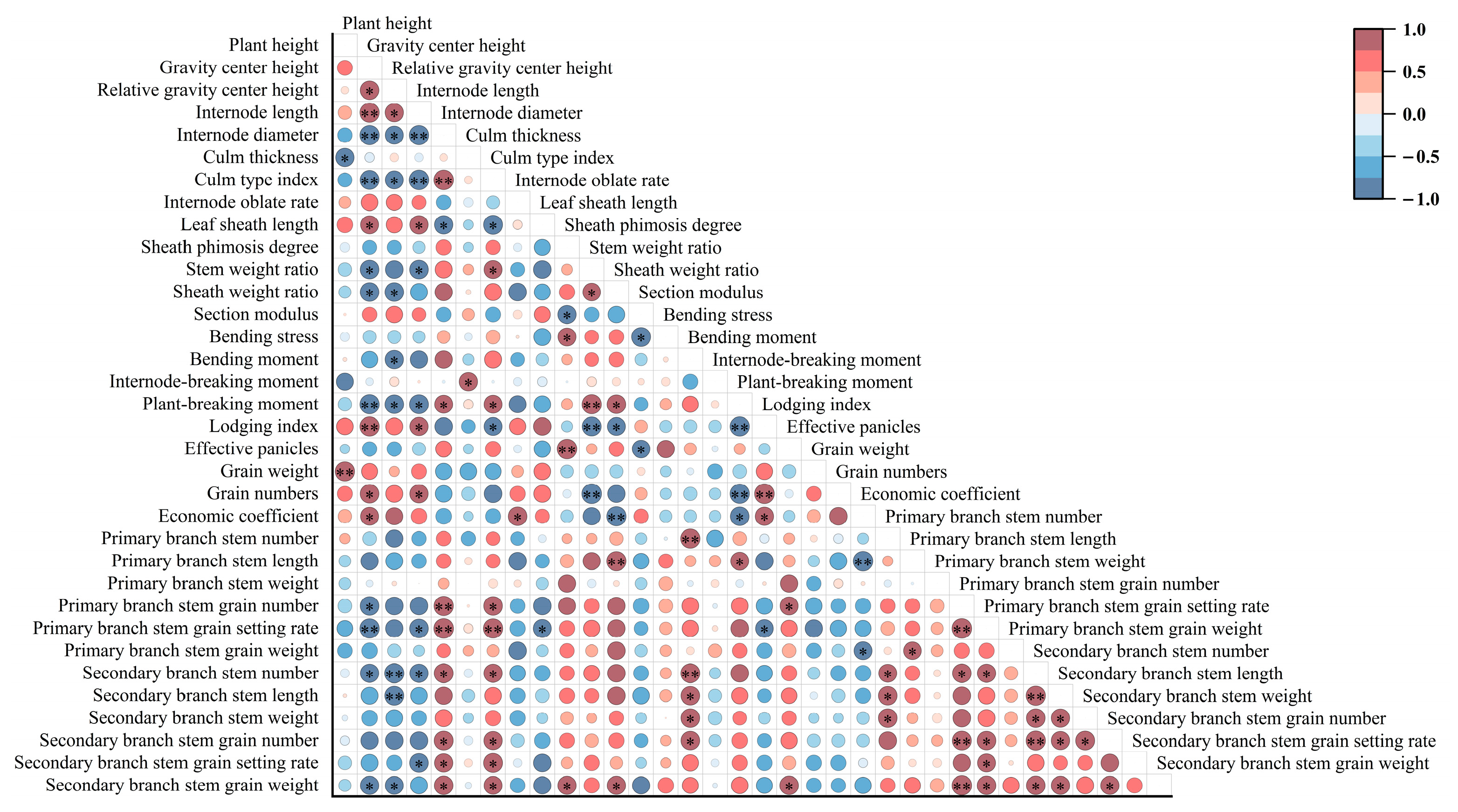
2.5. Correlation Analysis
2.6. Principal Component Analysis
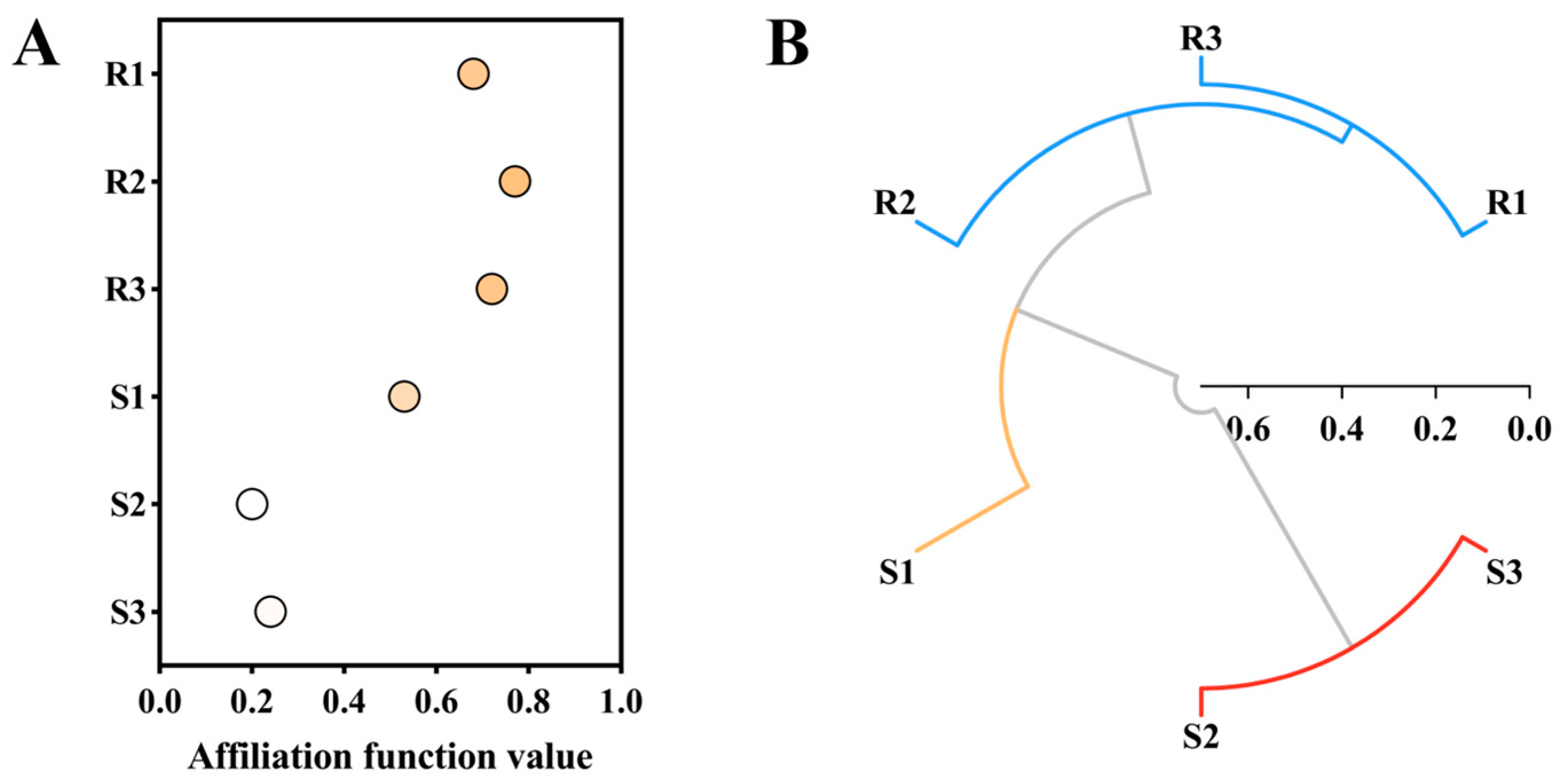
2.7. Membership Function Analysis and Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Material
4.2. Experimental Design
4.3. Determination Indicators and Methods
4.3.1. Main Stem Internode Physical Traits
minor axes, cm)/Culm length (cm)] × 100
Major axis of internode (mm))] × 100
4.3.2. Stem and Sheath Plumpness Traits
to leaf sheath opening (cm)/Leaf sheath length (cm) × 100
Internode length (cm)] × 1000
Leaf sheath length (cm)] × 1000
4.3.3. Main Stem Mechanical Properties
Section modulus (mm3)
of the tested internode to the panicle top (g) × Length
from the base of the internode to the panicle top (cm)
Plant-breaking moment (g cm) × 100.
4.3.4. Yield-Related Traits and Panicle Characteristics
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, B.; Ranawana, V.; Henry, J. The Glycemic Index of Rice and Rice Products: A Review, and Table of GI Values. Crit. Rev. Food Sci. Nutr. 2015, 56, 215–236. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; He, Y.Q. Rice grain quality—Traditional traits for high quality rice and health-plus substances. Mol. Breed. 2019, 40, 201–204. [Google Scholar] [CrossRef]
- Peng, S.B.; Khush, G.S.; Virk, P.; Tang, Q.Y.; Zou, Y.B. Progress in Ideotype Breeding to Increase Rice Yield Potential. Field Crops Res. 2008, 108, 32–38. [Google Scholar] [CrossRef]
- Chen, S.B.; Chen, S.J.; Jiang, Y.H.; Lu, Q.; Liu, Z.Y.; Liu, W.Y.; Wang, X.H.; Shi, W.H.; Xu, Q.; Sun, J.; et al. Dissecting of the Deterioration in Eating Quality for Erect Panicle (Ep) Type High Yield Japonica Super Rice in Northest China. Rice 2022, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.W.; Yu, H.; Wang, B.; Li, J.Y. Retrospective and Perspective of Rice Breeding in China. J. Genet. Genom. 2018, 45, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Wang, C.Y.; Linderholm, H.W.; Fu, Y.; Cai, W.Y.; Xu, J.X.; Zhuang, L.W.; Wu, M.X.; Shi, Y.X.; Wang, G.F.; et al. The Negative Impact of Increasing Temperatures on Rice Yields in Southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar] [CrossRef]
- Zhao, W.X.; Chou, J.M.; Li, J.N.; Xu, Y.; Li, Y.M.; Hao, Y.D. Impacts of Extreme Climate Events on Future Rice Yields in Global Major Rice-Producing Regions. Int. J. Environ. Res. Public Health 2022, 19, 4437. [Google Scholar] [CrossRef]
- Takai, T. Potential of Rice Tillering for Sustainable Food Production. J. Exp. Bot. 2024, 75, 708–720. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yu, C.S.; Lin, J.Z.; Liu, J.; Liu, B.; Wang, J.; Huang, A.; Li, H.Y.; Zhao, T. OsMPH1 regulates plant height and improves grain yield in rice. PLoS ONE 2017, 12, e0180825. [Google Scholar] [CrossRef]
- Xu, C.M.; Zhou, C.N.; Zheng, G.S.; Wang, D.Y.; Zhao, F.; Zhu, X.D.; Zhang, X.F. Effects of Nitrogen Fertilizer Application and Densities on Non-structural Carbohydrates Accumulation, Distribution and Quality of Super Late Rice Tianyouhuazhan. J. Agric. Sci. Technol. 2010, 12, 86–91. [Google Scholar]
- Li, Q.; Fu, C.F.; Liang, C.L.; Ni, X.J.; Zhao, X.H.; Chen, M.; Ou, L.J. Crop Lodging and The Roles of Lignin, Cellulose, and Hemicellulose in Lodging Resistance. Agronomy 2022, 12, 1795. [Google Scholar] [CrossRef]
- He, Y.; Mouthier, T.M.; Kabel, M.A.; Dijkstra, J.; Hendriks, W.H.; Struik, P.C.; Cone, J.W. Lignin composition is more important than content for maize stem cell wall degradation. J. Sci. Food Agric. 2018, 98, 384–390. [Google Scholar] [CrossRef]
- Fan, C.L.; Hu, Y.G.; Yang, G.T.; Fan, Y.Y.; Tang, G.J.; Zhou, F.; Li, Q.M.; Tang, H. Correlation Between Stem Physical Characters Lodging Resistance of Hybrid Rice Mianhui 725 with Massive Spikes. Guizhou Agric. Sci. 2015, 43, 1–4. [Google Scholar] [CrossRef]
- Lai, S.K.; Chen, C.; Lai, S.K.; Wang, L.; Chen, W.J. Genotypic Differences and Correlations Between Rice Main Agronomic Traits and Lodging-resistance. J. Nucl. Agric. Sci. 2018, 32, 1256–1266. [Google Scholar] [CrossRef]
- Li, H.J.; Zhang, X.J.; Li, W.J.; Xu, Z.J.; Xu, H. Lodging Resistance in japonica Rice Varieties with Different Panicle Types. Chin. J. Rice Sci. 2009, 23, 191–196. [Google Scholar] [CrossRef]
- Shrestha, S.; Laza, M.R.C.; Mendez, K.V.; Bhosale, S.; Dingkuhn, M. The Blaster: A Methodology to Induce Rice Lodging at Plot Scale to Study Lodging Resistance. Field Crops Res. 2020, 245, 107663. [Google Scholar] [CrossRef]
- Ouyang, H.; Yang, X.L.; Wang, L.Z.; Zhang, T.C.; Chi, L.Y.; Zhao, Q.; Zhang, X.J.; Li, M.X.; Li, Z.J.; Li, R.; et al. Research Advances and Prospects of Evaluation Methods and Mechanisms of Rice Lodging Resistance. China Rice 2023, 29, 12–17. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, L.; Li, X.X.; Yang, G.D.; Wang, F.; Peng, S.B. Grain yield and lodging-related traits of ultrashort-duration varieties for direct-seeded and double-season rice in Central China. J. Integr. Agric. 2022, 21, 2888–2899. [Google Scholar] [CrossRef]
- Luo, X.Y.; Wu, Z.F.; Fu, L.; Dan, Z.W.; Yuan, Z.Q.; Liang, T.; Zhu, R.S. Evaluation of lodging resistance in rice based on an optimized parameter from lodging index. Crop Sci. 2022, 62, 1318–1332. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ma, Z.B.; He, N.; Tang, Z.Q.; Wang, C.H.; Zheng, W.J.; Wang, H.; Sui, G.S.; Gao, H.; Wang, L.L. Lodging Resistance of Japonica Hybrid Rice Plants Studied in Relation to Mechanical and Physicochemical Characteristics. Agronomy 2025, 15, 699. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, N.; Lai, S.; Frei, M.; Wang, Y.; Yang, L. Elevated CO2 Improves Lodging Resistance of Rice by Changing Physicochemical Properties of the Basal Internodes. Sci. Total Environ. 2019, 647, 223–231. [Google Scholar] [CrossRef]
- Gu, H.Z.; Wang, C.; Zhang, Y.; Wu, H.; Xiao, Z.L.; Jing, W.J.; Zhang, H. Research progress on lodging resistance evaluation of rice stem and its physiological mechanism. Jiangsu Agric. Sci. 2023, 51, 1–7. [Google Scholar] [CrossRef]
- Feng, S.W.; Kong, D.C.; Ding, W.H.; Ru, Z.G.; Li, G.; Niu, L.Y. A novel wheat lodging resistance evaluation method and device based on the thrust force of the stalks. PLoS ONE 2019, 14, e0224732. [Google Scholar] [CrossRef]
- Luo, X.Y.; Zheng, X.F.; Peng, X.G.; Yu, Q.Z.; Dong, H.L.; Yin, D.S.; Wang, H.B.; Hu, J.L.; Xue, L.; Hu, P.; et al. Research on rice lodging resistance: Current status, challenges, and future directions. Chin. J. Rice Sci. 2025, 1–16. [Google Scholar]
- Chen, G.H.; Deng, H.B.; Zhang, G.L.; Tang, W.B.; Huang, H. The Correlation of Stem Characters and Lodging Resistance and Combining Ability Analysis in Rice. Sci. Agric. Sin. 2016, 49, 407–417. [Google Scholar]
- Wu, D.H.; Chen, C.T.; Yang, M.D.; Wu, Y.C.; Lin, C.Y.; Lai, M.H.; Yang, C.Y. Controlling the Lodging Risk of Rice Based on a Plant Height Dynamic Model. Bot. Stud. 2022, 63, 25. [Google Scholar] [CrossRef]
- Sun, Q.; Gu, X.H.; Chen, L.P.; Xu, X.B.; Pan, Y.C.; Hu, X.Q.; Xu, B. Monitoring Rice Lodging Grade via Sentinel-2A Images Based on Change Vector Analysis. Int. J. Remote Sens. 2022, 43, 1549–1576. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Zhong, X.C.; Jiang, M.; Jin, X.L.; Zhou, P.; Liu, S.P.; Sun, C.M.; Guo, W.S. Estimates of Rice Lodging Using Indices Derived from UAV Visible and Thermal Infrared Images. Agric. For. Meteorol. 2018, 252, 144–154. [Google Scholar] [CrossRef]
- Ban, H.Y.; Baek, J.K.; Sang, W.G.; Kim, J.H.; Seo, M.C. Estimation of the Lodging Area in Rice Using Deep Learning. Korean J. Crop Sci. 2021, 66, 105–111. [Google Scholar] [CrossRef]
- Liu, B.L.; Wang, W.H.; Wei, S.F.; Feng, R.; Shi, Y.M. Research on the Physiological Changes of Preharvest Sprouting in Rice. Seed 2013, 32, 89–91. [Google Scholar] [CrossRef]
- Lang, Y.Z.; Yang, X.D.; Wang, M.E.; Zhu, Q.S. Effects of Lodging at Different Filling Stages on Rice Yield and Grain Quality. Rice Sci. 2012, 19, 315–319. [Google Scholar] [CrossRef]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Wang, L.; Riaz, M.W.; Rehman, S.; Wu, W.X.; Khan, R.M.; et al. Improving Lodging Resistance: Using Wheat and Rice as Classical Examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Chen, H.L.; Hu, C.D.; Guo, P. Effects of Lodging at the Late Growth Stage on Dry Matter Distribution and Yield of Winter Wheat. Chin. J. Agrometeorol. 2017, 38, 321–329. [Google Scholar] [CrossRef]
- Yuan, X.J.; Liu, X.; Chen, G.X. Stem lodging resistance of rice core germplasm. J. Huazhong Agric. Univ. 2021, 40, 147–153. [Google Scholar] [CrossRef]
- Zhong, X.H.; Liang, K.M.; Peng, B.L.; Tian, K.; Li, X.J.; Huang, N.R.; Liu, Y.Z.; Pan, J.F. Basal Internode Elongation of Rice as Affected by Light Intensity and Leaf Area. Crop J. 2020, 8, 62–70. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Xu, Y.B.; Wang, Y.L.; Li, Q.; Hu, J.B. Establishment of a Comprehensive Evaluation System for Chilling Tolerance in Melon Seedlings Based on Principal Component Analysis and Cluster Analysis. Chin. Bull. Bot. 2017, 52, 520–529. [Google Scholar] [CrossRef]
- Satoru, T.; Hiroyuki, S.; Yukitaka, I.; Kazuya, I. Thickness-stiffness trade-off improves lodging resistance in rice. Sci. Rep. 2023, 13, 10828. [Google Scholar] [CrossRef]
- Tomohiro, N.; Satoshi, O.; Nagano, A.J.; Fahim, S.A.; Shunsuke, A.; Taiichiro, O. Physiological and morphological factors affecting leaf sheath reinforcement and their contribution to lodging resistance in rice. Plant Prod. Sci. 2023, 26, 48–64. [Google Scholar] [CrossRef]
- Cornwall, J.; Stubbs, C.J.; McMahan, C.S.; Robertson, D.J. The Overlooked Biomechanical Role of the Clasping Leaf Sheath in Wheat Stalk Lodging. Front. Plant Sci. 2021, 12, 617880. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.H.; Song, Y.P.; Liu, Z.H.; Yang, C.D.; Tang, S.; Zheng, C.Y.; Wang, S.H.; Ding, Y.F. Lodging Resistance Characteristics of High-Yielding Rice Populations. Field Crops Res. 2014, 161, 64–74. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhang, P.; Wang, X.Z.; Chen, R.F.; Wang, W.Y.; Wan, S.Y.; Song, W.M.; Zhang, G.L.; Li, Y.; Qian, Y.D. Effects of Combined Application of Nitrogen and Potassium on Lodging Resistance of Rice in Cold Region under Condition of Returning Straw to Field. J. Henan Agric. Sci. 2021, 50, 17–24. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Wang, Y.B.; Ye, D.L.; Wang, W.; Qiu, X.M.; Duan, L.S.; Li, Z.H.; Zhang, M.C. Ethephon Improved Stalk Strength of Maize (Zea mays L.) Mainly through Altering Internode Morphological Traits to Modulate Mechanical Properties under Field Conditions. Agronomy 2019, 9, 186. [Google Scholar] [CrossRef]
- Robertson, D.J.; Brenton, Z.W.; Kresovich, S.; Cook, D.D. Maize lodging resistance: Stalk architecture is a stronger predictor of stalk bending strength than chemical composition. Biosyst. Eng. 2022, 219, 124–134. [Google Scholar] [CrossRef]
- Santosa, E.; Dulbari, D.; Agusta, H.; Zaman, S. Phenomenon of lodging and its implication on improvement of rice adaptive to extreme weather in Indonesia. In Proceedings of the Seminar Nasional Perhimpunan Ilmu Pemuliaan Indonesia (PERIPI) “Strategi Pemuliaan dalam Mengantisipasi Perubahan Iklim Global”, Riau, Indonesia, 2015–2017; Available online: https://www.researchgate.net/publication/322202139 (accessed on 20 May 2025).
- Liao, P.; Bell, S.M.; Chen, L.; Huang, S.; Wang, H.Y.; Miao, J.H.; Qi, Y.M.; Sun, Y.N.; Liao, B.; Zeng, Y.J.; et al. Improving rice grain yield and reducing lodging risk simultaneously: A meta-analysis. Eur. J. Agron. 2023, 143, 126709. [Google Scholar] [CrossRef]
- Huang, M.; Tao, Z.; Lei, T.; Cao, F.B.; Chen, J.N.; Yin, X.H.; Zou, Y.B.; Liang, T.F. Improving Lodging Resistance While Maintaining High Grain Yield by Promoting Pre-Heading Growth in Rice. Field Crops Res. 2021, 270, 108212. [Google Scholar] [CrossRef]
- Wang, Z.C.; Guo, X.P.; Yang, J.H.; Chen, S.; Huang, S.S.; Wang, F.; Qiu, R.J.; Liu, C.W.; Cao, X.C.; Zhu, J.B.; et al. Effect of alternate flooding and drought stress on biomass production, distribution and lodging characteristic of rice. Trans. Chin. Soc. Agric. Eng. 2016, 32, 114–123. [Google Scholar] [CrossRef]
- Deng, W.; Xian, Q.G.; Ma, G.H.; Ai, Z.Y. Progress of Research on Lodging Resistance in Rice. Hybrid Rice 2006, 6, 6–10. [Google Scholar] [CrossRef]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.G. Evaluation of 14 Morphological, Yield-Related and Physiological Traits as Indicators of Drought Tolerance in Chinese Winter Bread Wheat Revealed by Analysis of the Membership Function Value of Drought Tolerance (MFVD). Field Crops Res. 2012, 137, 195–201. [Google Scholar] [CrossRef]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Zeng, Y.; Xiang, L.; Cheng, Q. A Salt Tolerance Evaluation Method for Sunflower (Helianthus annuus L.) at the Seed Germination Stage. Sci. Rep. 2020, 10, 10626. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, P.; Tafreshi, H.A.S.; Ebrahimi, A.M.; Haerinasab, M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci. Hortic. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Zhang, S.X.; Jia, Z.X.; Fang, T.; Liu, Y.F.; Zhao, W.; Wang, R.; Chang, H.C.; Luo, F.L.; Zhu, Y.J.; Yu, F.H. Methods to evaluate plant tolerance to environmental stresses. Biodivers. Sci. 2025, 33, 106–118. [Google Scholar] [CrossRef]
- Gu, H.Z.; Xiao, Z.L.; Meng, Q.H.; Fa, X.T.; Jing, W.J.; Wang, W.L.; Zhu, K.Y.; Zhang, W.Y.; Gu, J.F.; Liu, L.J.; et al. The synergistic effect of variety improvement and alternate wetting and drying irrigation on yield, water use efficiency and lodging resistance in rice. Eur. J. Agron. 2025, 164, 127507. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Cui, J.H.; Bao, S.Y.; Liu, W.Y.; Geng, Y.Q.; Liang, X.H.; Li, S.Z.; Guo, L.Y.; Shao, X.W. Effects of nitrogen fertilizer application rate on lodging resistance for rice (Oryza sativa L.) stem. Sci. Rep. 2025, 15, 2149. [Google Scholar] [CrossRef]
- Li, Z.Z.; Deng, F.; Zhu, L.; Tang, L.; Zhou, T.; Lu, H.; Xia, H.X.; Zhong, X.Y.; Wang, L.; Tao, Y.F.; et al. Fewer hills with more seedlings improved lodging resistance of whole hill and yield stability of machine-transplanted rice. Agron. J. 2023, 115, 620–634. [Google Scholar] [CrossRef]
- Dorairaj, D.; Ismail, R.M.; Ismail, R.M. Distribution of Silicified Microstructures, Regulation of Cinnamyl Alcohol Dehydrogenase and Lodging Resistance in Silicon and Paclobutrazol Mediated Oryza sativa. Front. Physiol. 2017, 8, 491. [Google Scholar] [CrossRef]
- Xiao, C.L.; Xie, B.S.; Wan, A.D.; Wang, S.Q.; Li, C.G.; Na, Y.G. Effects of Nitrogen and Uniconazole Regulation on Lodging Resistance and Yield of Rice in Cold Region. Crops 2017, 6, 96–103. [Google Scholar] [CrossRef]
- Lei, X.L.; Liu, L.; Gou, W.; Ma, R.C.; Ren, W.J. Effects of Planting Methods on Culm Lodging Resistance of Indica Hybrid Rice (Oryza sativa L.). Acta Agron. Sin. 2013, 39, 1814–1825. [Google Scholar] [CrossRef]
- Ookawa, T.; Ishihra, K. Varietal difference of physical characteristic of the culm related to lodging resistance in paddy rice. Jpn. J. Crop Sci. 1992, 61, 419–425. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, L.; Zhu, F.; Tang, Y.; Ni, Q.; Ren, J.; Huang, B.; Zhang, Z.; Wang, Y.; Peng, Y. Decoding Lodging Resistance in Elite Chinese Conventional Rice Varieties: A Phenotypic and Biomechanical Perspective. Plants 2025, 14, 2878. https://doi.org/10.3390/plants14182878
Li Y, Zhou L, Zhu F, Tang Y, Ni Q, Ren J, Huang B, Zhang Z, Wang Y, Peng Y. Decoding Lodging Resistance in Elite Chinese Conventional Rice Varieties: A Phenotypic and Biomechanical Perspective. Plants. 2025; 14(18):2878. https://doi.org/10.3390/plants14182878
Chicago/Turabian StyleLi, Yufei, Lu Zhou, Fan Zhu, Yinmei Tang, Qun Ni, Jing Ren, Biyu Huang, Zhenqian Zhang, Yue Wang, and Yulin Peng. 2025. "Decoding Lodging Resistance in Elite Chinese Conventional Rice Varieties: A Phenotypic and Biomechanical Perspective" Plants 14, no. 18: 2878. https://doi.org/10.3390/plants14182878
APA StyleLi, Y., Zhou, L., Zhu, F., Tang, Y., Ni, Q., Ren, J., Huang, B., Zhang, Z., Wang, Y., & Peng, Y. (2025). Decoding Lodging Resistance in Elite Chinese Conventional Rice Varieties: A Phenotypic and Biomechanical Perspective. Plants, 14(18), 2878. https://doi.org/10.3390/plants14182878




