Nanopesticides in Brazilian Crops: Classes, Mechanisms, Efficacy, Risks, and Photocatalytic Remediation
Abstract
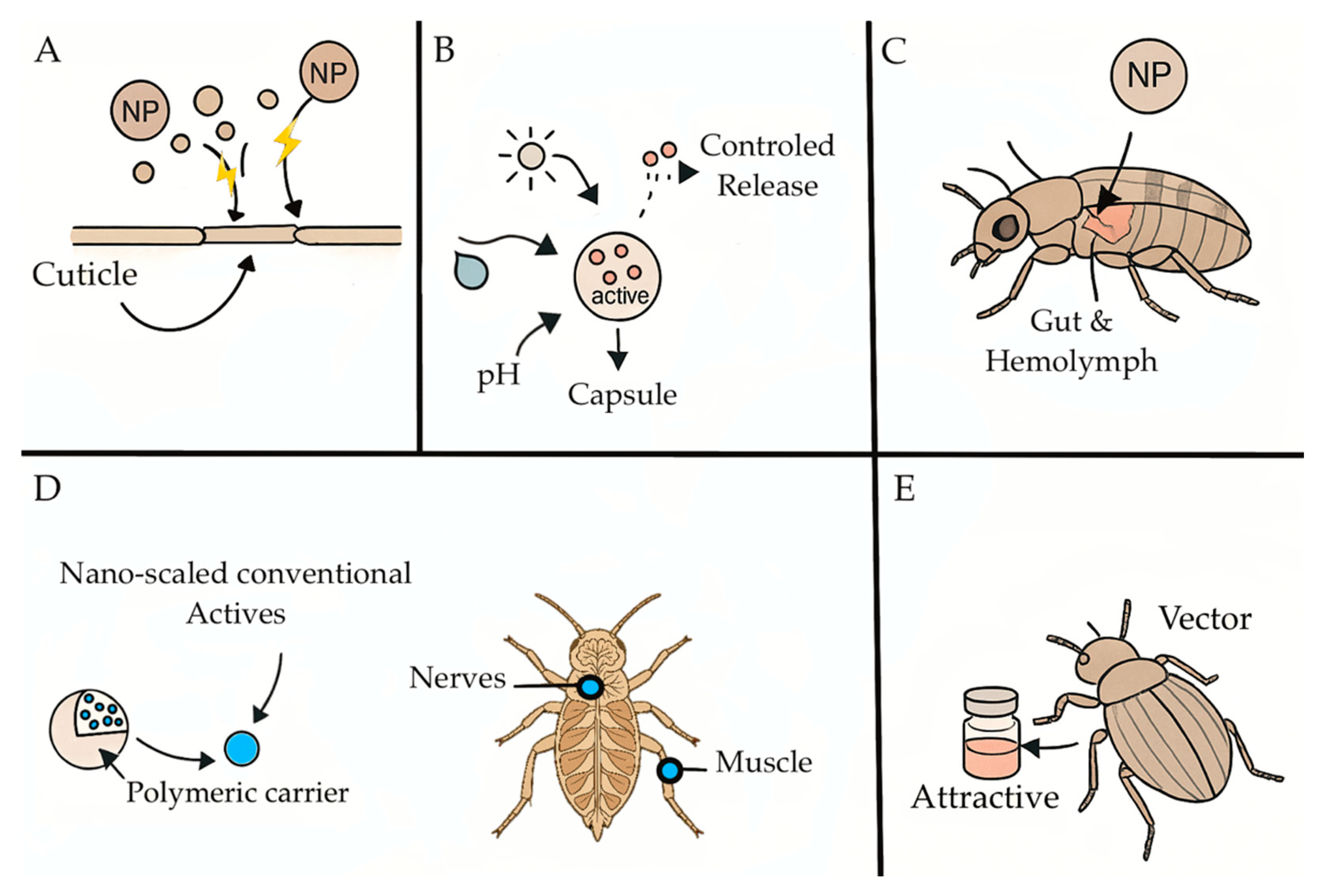
1. Introduction
2. Nanopesticide Concepts, Formulations, Efficacy, and Modes of Action
3. Nanotechnology in Coffee Crops
4. Nanotechnology in Sugarcane Crops
5. Nanotechnology in Orange Crops
6. Nanotechnology in Soybean Crops
7. Environmental Risk and Human Health Considerations
8. Photocatalytic Remediation and Environmental Fate and Cleanup of Pesticide Residues
9. Future Perspectives and Challenges for Nanopesticides in Crops
10. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arruda, D.; Sills, J.; Candido, H.G.; Fonseca, R. Amazon fires threaten Brazil’s agribusiness. Science 2019, 365, 1387. [Google Scholar] [CrossRef] [PubMed]
- MAPA. Exportações do Agronegócio Fecham 2022 Com US$ 159 Bilhões em Vendas. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/exportacoes-do-agronegocio-fecham-2022-com-us-159-bilhoes-em-vendas (accessed on 20 April 2025).
- Ibge. Levantamento Sistemático da Produção Agropecuária–LSPA. Available online: https://www.gov.br/fazenda/pt-br/central-de-conteudo/publicacoes/conjuntura-economica/agricola/2023/2023-3-10_lspa-ibge.pdf (accessed on 20 April 2025).
- Chaves, M.E.D.; Mataveli, G.; zu Ermgassen, E.; de A. Aragão, R.B.; Adami, M.; Sanches, I.D. Reverse the Cerrado’s neglect. Nat. Sustain. 2023, 6, 1028–1029. [Google Scholar] [CrossRef]
- Bustos, P.; Caprettini, B.; Ponticelli, J. Agricultural Productivity and Structural Transformation: Evidence from Brazil. Am. Econ. Rev. 2016, 106, 1320–1365. [Google Scholar] [CrossRef]
- Feng, X.; Merow, C.; Liu, Z.; Park, D.S.; Roehrdanz, P.R.; Maitner, B.; Newman, E.A.; Boyle, B.L.; Lien, A.; Burger, J.R.; et al. How deregulation, drought and increasing fire impact Amazonian biodiversity. Nature 2021, 597, 516–521. [Google Scholar] [CrossRef]
- Metzger, J.P.; Bustamante, M.M.C.; Ferreira, J.; Fernandes, G.W.; Librán-Embid, F.; Pillar, V.D.; Prist, P.R.; Rodrigues, R.R.; Vieira, I.C.G.; Overbeck, G.E. Why Brazil needs its Legal Reserves. Perspect. Ecol. Conserv. 2019, 17, 91–103. [Google Scholar] [CrossRef]
- Rajão, R.; Soares-Filho, B.; Nunes, F.; Börner, J.; Machado, L.; Assis, D.; Oliveira, A.; Pinto, L.; Ribeiro, V.; Rausch, L.; et al. The rotten apples of Brazil’s agribusiness. Science 2020, 369, 246–248. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Protect. 2014, 56, 50–54. [Google Scholar] [CrossRef]
- Wilby, A.; Dalin, P.; Kindvall, O.; Björkman, C. Reduced Population Control of an Insect Pest in Managed Willow Monocultures. PLoS ONE 2009, 4, e5487. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Mota, T.F.M.; Oliveira, W.L.; Gonçalves, S.; Vasconcelos, M.W.; Miglioranza, K.S.B.; Ghisi, N.C. Are the issues involving acephate already resolved? A scientometric review. Environ. Res. 2023, 237, 117034. [Google Scholar] [CrossRef]
- Lucio, F.T.; Almeida, I.V.; Buzo, M.G.; Vicentini, V.E.P. Genetic instability in farmers using pesticides: A study in Brazil with analysis combining alkaline comet and micronucleus assays. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2023, 886, 503587. [Google Scholar] [CrossRef]
- Valentim, J.M.B.d.M.; Fagundes, T.R.; Okamoto Ferreira, M.; Lonardoni Micheletti, P.; Broto Oliveira, G.E.; Cremer Souza, M.; Geovana Leite Vacario, B.; Silva, J.C.d.; Scandolara, T.B.; Gaboardi, S.C.; et al. Monitoring residues of pesticides in food in Brazil: A multiscale analysis of the main contaminants, dietary cancer risk estimative and mechanisms associated. Front. Public Health 2023, 11, 893. [Google Scholar] [CrossRef]
- Braga, A.R.C.; de Rosso, V.V.; Harayashiki, C.A.Y.; Jimenez, P.C.; Castro, Í.B. Global health risks from pesticide use in Brazil. Nat. Food 2020, 1, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, G.A.; de Syllos Colus, I.M. Chromosomal aberrations analysis in a Brazilian population exposed to pesticides. Teratog. Carcinog. Mutagen. 2000, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.F. Significance of Pesticide Residues to Human Health. J. Dairy Sci. 1971, 54, 701–706. [Google Scholar] [CrossRef]
- González, R.H. Plant Protection in Latin America. Pans 2009, 22, 26–34. [Google Scholar] [CrossRef]
- Hay, A. A recent assessment of cocoa and pesticides in Brazil: An unhealthy blend for plantation workers. Sci. Total Environ. 1991, 106, 97–109. [Google Scholar] [CrossRef]
- Matuo, Y.K.; Lopes, J.N.C.; Casanova, I.C.; Matuo, T. Organochlorine pesticide residues in human milk in the Ribeirão Preto region, state of São Paulo, Brazil. Arch. Environ. Contam. Toxicol. 1992, 22, 167–175. [Google Scholar] [CrossRef]
- Moraes, R.; Elfvendahl, S.; Kylin, H.; Molander, S. Pesticide Residues in Rivers of a Brazilian Rain Forest Reserve: Assessing Potential Concern for Effects on Aquatic Life and Human Health. AMBIO J. Hum. Environ. 2003, 32, 258–263. [Google Scholar] [CrossRef]
- Richardson, L.A.; Foter, M.J. Pesticides and the Food Supply. J. Milk Food Technol. 1966, 29, 148–155. [Google Scholar] [CrossRef]
- Recena, M.C.P.; Caldas, E.D.; Pires, D.X.; Pontes, E.R.J.C. Pesticides exposure in Culturama, Brazil—Knowledge, attitudes, and practices. Environ. Res. 2006, 102, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Waichman, A.V.; Eve, E.; Nina, N.C.d.S. Do farmers understand the information displayed on pesticide product labels? A key question to reduce pesticides exposure and risk of poisoning in the Brazilian Amazon. Crop Protect. 2007, 26, 576–583. [Google Scholar] [CrossRef]
- Guida, Y.d.S.; Meire, R.O.; Torres, J.P.M.; Malm, O. Air contamination by legacy and current-use pesticides in Brazilian mountains: An overview of national regulations by monitoring pollutant presence in pristine areas. Environ. Pollut. 2018, 242, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Pasiani, J.O.; Torres, P.; Roniery Silva, J.; Diniz, B.Z.; Caldas, E. Knowledge, Attitudes, Practices and Biomonitoring of Farmers and Residents Exposed to Pesticides in Brazil. Int. J. Environ. Res. Public Health 2012, 9, 3051–3068. [Google Scholar] [CrossRef]
- Sousa, A.S.; Duaví, W.C.; Cavalcante, R.M.; Milhome, M.A.L.; do Nascimento, R.F. Estimated Levels of Environmental Contamination and Health Risk Assessment for Herbicides and Insecticides in Surface Water of Ceará, Brazil. Bull. Environ. Contam. Toxicol. 2015, 96, 90–95. [Google Scholar] [CrossRef]
- Rocha, G.M.; Grisolia, C.K. Why pesticides with mutagenic, carcinogenic and reproductive risks are registered in Brazil. Dev. World Bioeth. 2018, 19, 148–154. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef]
- Fernandes, C.L.F.; Volcão, L.M.; Ramires, P.F.; Moura, R.R.D.; Da Silva Júnior, F.M.R. Distribution of pesticides in agricultural and urban soils of Brazil: A critical review. Environ. Sci. Process. Impacts 2020, 22, 256–270. [Google Scholar] [CrossRef]
- Williams, C.M. Third-Generation Pesticides. Sci. Am. 1967, 217, 13–17. [Google Scholar] [CrossRef]
- Yorinori, J.T. Pesticides in Brazilian Agriculture; EMBRAPA-CNPSO: Londrina, Brazil, 1982. [Google Scholar]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Brodt, S.; Six, J.; Feenstra, G.; Ingels, C.; Campbell, D. Sustainable agriculture. Nat. Educ. Knowl. 2011, 3. [Google Scholar]
- Li, N.; Sun, C.; Jiang, J.; Wang, A.; Wang, C.; Shen, Y.; Huang, B.; An, C.; Cui, B.; Zhao, X.; et al. Advances in Controlled-Release Pesticide Formulations with Improved Efficacy and Targetability. J. Agric. Food Chem. 2021, 69, 12579–12597. [Google Scholar] [CrossRef] [PubMed]
- Abdollahdokht, D.; Gao, Y.; Faramarz, S.; Poustforoosh, A.; Abbasi, M.; Asadikaram, G.; Nematollahi, M.H. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022, 9, 13. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2016, 15, 15–22. [Google Scholar] [CrossRef]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. Nano-pesticides: A great challenge for biodiversity? The need for a broader perspective. Nano Today 2020, 30, 100808. [Google Scholar] [CrossRef]
- Kah, M. Nanopesticides and Nanofertilizers: Emerging Contaminants or Opportunities for Risk Mitigation? Front. Chem. 2015, 3, 64. [Google Scholar] [CrossRef]
- Kookana, R.S.; Boxall, A.B.A.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding Principles for Regulatory Evaluation of Environmental Risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Kah, M.; Walch, H.; Hofmann, T. Environmental fate of nanopesticides: Durability, sorption and photodegradation of nanoformulated clothianidin. Environ. Sci. Nano 2018, 5, 882–889. [Google Scholar] [CrossRef]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Yin, M.; Shen, J.; Du, X.; Yan, S.; Dong, M. Star Polymer-Based Nanodelivery System for Pesticides: Enhanced Broad-Spectrum Toxicity and Selective Toxicity. ACS Omega 2023, 8, 41595–41602. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 7589. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Sun, C.; Wang, A.; An, C.; Li, N.; Shen, Y.; Hu, J.; Liu, H.; Xie, J.; et al. A unimolecule nanopesticide delivery system applied in field scale for enhanced pest control. Nat. Commun. 2025, 16, 6809. [Google Scholar] [CrossRef]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional Nanoparticles and Nanopesticides in Agricultural Application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef]
- Ali, H.M.; Abdel-Aty, B.; El-Sayed, W.; Mariy, F.M.; Hegazy, G.M. Comparison between imidacloprid effects on AChE and nAChRα1 in target Aphis craccivora and non-target Apis mellifera: Experimental and theoretical approaches. Chem. Biol. Technol. Agric. 2024, 11, 125. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green synthesis of silver nanoparticles: A comprehensive review of methods, influencing factors, and applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Eskin, A.; Nurullahoğlu, Z.U. Effects of zinc oxide nanoparticles (ZnO NPs) on the biology of Galleria mellonella L. (Lepidoptera: Pyralidae). J. Basic Appl. Zool. 2022, 83, 54. [Google Scholar] [CrossRef]
- Abd-Elnabi, A.D.; El-sawy, E.A.F.; Badawy, M.E.I. Plant Oil Nano-Emulsions as a Potential Solution for Pest Control in Sustainable Agriculture. Neotrop. Entomol. 2025, 54, 35. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A Light-Triggered pH-Responsive Metal–Organic Framework for Smart Delivery of Fungicide to Control Sclerotinia Diseases of Oilseed Rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, S.; Jiang, C.; Zhang, T.; Chen, W. Recent advances in stimuli-response mechanisms of nano-enabled controlled-release fertilizers and pesticides. Eco-Environ. Health 2023, 2, 161–175. [Google Scholar] [CrossRef]
- Shangguan, W.J.; Mei, X.D.; Chen, H.P.; Hu, S.; Xu, C.L.; Wang, L.; Lv, K.F.; Huang, Q.L.; Xu, H.L.; Cao, L.D. Biodegradable electrospun fibers as sustained-release carriers of insect pheromones for field trapping of Spodoptera litura (Lepidoptera: Noctuidae). Pest Manag. Sci. 2023, 79, 4774–4783. [Google Scholar] [CrossRef]
- Xiang, H.M.; Wei, X.H.; Li, Y.R.; Zhang, B.J.; Li, M.; Ma, R.Y. Electrospun nanofibers as controlled release systems for the combined pheromones of Grapholita molesta and Cydia pomonella. Pest Manag. Sci. 2025, 81, 2257–2265. [Google Scholar] [CrossRef]
- Giannuzzi, V.A.; Rossi, V.; Moujahed, R.; Poccia, A.; D’Archivio, F.; Rossi Magi, T.; Chierici, E.; Casoli, L.; Rondoni, G.; Conti, E. Evaluation of Lure and Dispenser Combinations for Halyomorpha halys (Hemiptera: Pentatomidae) Trapping. Insects 2025, 16, 341. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, W.Y.; Han, Z.H.; Wang, D.; Yin, M.Z.; Du, X.G.; Shen, J. Nanometerization of thiamethoxam by a cationic star polymer nanocarrier efficiently enhances the contact and plant-uptake dependent stomach toxicity against green peach aphids. Pest Manag. Sci. 2021, 77, 1954–1962. [Google Scholar] [CrossRef]
- Ito, E.; Cui, B.; Feng, L.; Wang, C.; Yang, D.; Yu, M.; Zeng, Z.; Wang, Y.; Sun, C.; Zhao, X.; et al. Stability and Biological Activity Evaluation of Chlorantraniliprole Solid Nanodispersions Prepared by High Pressure Homogenization. PLoS ONE 2016, 11, e0160877. [Google Scholar] [CrossRef]
- Assalin, M.R.; Souza, D.R.C.d.; Rosa, M.A.; Duarte, R.R.M.; Castanha, R.F.; Vilela, E.S.D.; Tasic, L.; Durán, N. Thiamethoxam used as nanopesticide for the effective management of Diaphorina citri psyllid: An environmental-friendly formulation. Int. J. Pest Manag. 2022, 70, 801–809. [Google Scholar] [CrossRef]
- Bonser, C.A.R.; Astete, C.E.; Sabliov, C.M.; Davis, J.A.; Stark, J. Elucidating the insecticidal mechanisms of zein nanoparticles on Anticarsia gemmatalis (Lepidoptera: Erebidae). J. Econ. Entomol. 2023, 116, 1196–1204. [Google Scholar] [CrossRef]
- Pittarate, S.; Rajula, J.; Rahman, A.; Vivekanandhan, P.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Insecticidal Effect of Zinc Oxide Nanoparticles against Spodoptera frugiperda under Laboratory Conditions. Insects 2021, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Pittarate, S.; Perumal, V.; Rajula, J.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response. Insects 2022, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Villca, W.L.L. Efeito de Nanopartículas de Óxido de Cério (CeO2), Zinco (ZnO) e Cobre (CuO) Sobre Características Biológicas da Broca-do-Café Hypothenemus hampei (Ferrari, 1867) (Coleoptera: Curculionidae: Scolytinae); Federal University of Lavras: Lavras, MG, Brazil, 2021. [Google Scholar]
- Santás-Miguel, V.; Arias-Estévez, M.; Rodríguez-Seijo, A.; Arenas-Lago, D. Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 2023, 334, 122222. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Jing, M.; Liu, S.; Feng, J.; Wu, H.; Zhou, Y.; Zhang, X.; Ma, Z. Self-assembled mixed micelle loaded with natural pyrethrins as an intelligent nano-insecticide with a novel temperature-responsive release mode. Chem. Eng. J. 2019, 361, 1381–1391. [Google Scholar] [CrossRef]
- Shan, P.; Lu, Y.; Lu, W.; Yin, X.; Liu, H.; Li, D.; Lian, X.; Wang, W.; Li, Z.; Li, Z. Biodegradable and Light-Responsive Polymeric Nanoparticles for Environmentally Safe Herbicide Delivery. ACS Appl. Mater. Interfaces 2022, 14, 43759–43770. [Google Scholar] [CrossRef]
- Meyer, W.L.; Gurman, P.; Stelinski, L.L.; Elman, N.M. Functional nano-dispensers (FNDs) for delivery of insecticides against phytopathogen vectors. Green Chem. 2015, 17, 4173–4177. [Google Scholar] [CrossRef]
- Bisotto-de-Oliveira, R.; De Jorge, B.C.; Roggia, I.; Sant’Ana, J.; Pereira, C.N. Nanofibers as a Vehicle for the Synthetic Attactant TRIMEDLURE to be Used for Ceratitis capitata Wied: (Diptera, Tethritidae) Capture. J. Res. Updates Polym. Sci. 2014, 3, 40–47. [Google Scholar] [CrossRef]
- Kah, M.; Weniger, A.-K.; Hofmann, T. Impacts of (Nano)formulations on the Fate of an Insecticide in Soil and Consequences for Environmental Exposure Assessment. Environ. Sci. Technol. 2016, 50, 10960–10967. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Meredith, A.N.; Harper, B.; Harper, S.L. The influence of size on the toxicity of an encapsulated pesticide: A comparison of micron- and nano-sized capsules. Environ. Int. 2016, 86, 68–74. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Hooven, L.A.; Chakrabarti, P.; Harper, B.J.; Sagili, R.R.; Harper, S.L. Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules 2019, 24, 4458. [Google Scholar] [CrossRef]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Assalin, M.R.; dos Santos, L.D.L.; de Souza, D.R.C.; Rosa, M.A.; Duarte, R.R.M.; Castanha, R.F.; Donaire, P.P.R.; Durán, N. Nanoformulation as a tool for improvement of thiamethoxam encapsulation and evaluation of ecotoxicological impacts. Energy Ecol. Environ. 2019, 4, 310–317. [Google Scholar] [CrossRef]
- Basso Abraham, A.L.; Rockenbach de Avila, M.; Torres, R.; Diz, V. Magnetite nanoparticles as a promising non contaminant method to control populations of fruit flies (DIPTERA: Tephritidae). J. Appl. Biotechnol. Bioeng. 2021, 8, 112–117. [Google Scholar] [CrossRef]
- Elek, N.; Hoffman, R.; Raviv, U.; Resh, R.; Ishaaya, I.; Magdassi, S. Novaluron nanoparticles: Formation and potential use in controlling agricultural insect pests. Colloids Surf. Physicochem. Eng. Asp. 2010, 372, 66–72. [Google Scholar] [CrossRef]
- Pittarate, S.; Perumal, V.; Kannan, S.; Mekchay, S.; Thungrabeab, M.; Suttiprapan, P.; Sengottayan, S.-N.; Krutmuang, P. Insecticidal efficacy of nanoparticles against Spodoptera frugiperda (J.E. Smith) larvae and their impact in the soil. Heliyon 2023, 9, e16133. [Google Scholar] [CrossRef] [PubMed]
- Magda, S.; Hussein, M.M. Determinations of the effect of using silca gel and nano-silica gel against Tuta absoluta (Lepidoptera: Gelechiidae) in tomato fields. J. Chem. Pharm. Res. 2016, 8, 506–512. [Google Scholar]
- Moussa, S.H.; Tayel, A.A.; Alsohim, A.S.; Abdallah, R.R. Botryticidal Activity of Nanosized Silver-Chitosan Composite and Its Application for the Control of Gray Mold in Strawberry. J. Food Sci. 2013, 78, M1589–M1594. [Google Scholar] [CrossRef] [PubMed]
- MAPA. Brasil é o Maior Produtor Mundial e o Segundo Maior Consumidor de Café. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/brasil-e-o-maior-produtor-mundial-e-o-segundo-maior-consumidor-de-cafe (accessed on 20 April 2025).
- Costa, J.N.M.; Teixeira, C.A.D.; Trevisan, O. Pragas do cafeeiro. In Café na Amazônia; Marcolan, A.L., Espindula, M.C., Eds.; Embrapa: Brasília, DF, Brazil, 2015; pp. 255–278. [Google Scholar]
- Mesquita, C.M.d.; Melo, E.M.d.; Rezende, J.E.d.; Carvalho, J.S.; Júnior, M.A.F.; Moraes, N.C.; Dias, P.T.; Carvalho, R.M.d.; Araújo, W.G.d. Manual do Café: Implantação de Cafezais Coffea arábica L.; Emater: Balo Horizonte, MG, Brazil, 2016. [Google Scholar]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Economic impact of exotic insect pests in Brazilian agriculture. J. Appl. Entomol. 2012, 137, 1–15. [Google Scholar] [CrossRef]
- Carvalho, J.P.; Souza, J.C. Manual de Prevenção e Combate a Broca-do-Café; Região do Cerrado Mineiro: Patrocínio, MG, Brazil, 2016. [Google Scholar]
- Johnson, M.A.; Ruiz-Diaz, C.P.; Manoukis, N.C.; Verle Rodrigues, J.C. Coffee Berry Borer (Hypothenemus hampei), a Global Pest of Coffee: Perspectives from Historical and Recent Invasions, and Future Priorities. Insects 2020, 11, 882. [Google Scholar] [CrossRef]
- Cardoso, F.H.; Moraes, M.V.P.d.; Serra, J.; Filho, J.S. Decreto Nº 4074, de 04 de Janeiro de 2002. 2002. Available online: https://www.planalto.gov.br/ccivil_03/decreto/2002/D4074.htm (accessed on 10 September 2025).
- Menezes, R.G.; Qadir, T.F.; Moin, A.; Fatima, H.; Hussain, S.A.; Madadin, M.; Pasha, S.B.; Al Rubaish, F.A.; Senthilkumaran, S. Endosulfan poisoning: An overview. J. Forensic Leg. Med. 2017, 51, 27–33. [Google Scholar] [CrossRef]
- Goel, P.; Arora, M. Photocatalytic degradation efficiency of Cu/Cu2O core–shell structured nanoparticles for endosulfan mineralization. J. Nanoparticle Res. 2022, 24, 56. [Google Scholar] [CrossRef]
- Almeida, J.D.d.; Motta, I.d.O.; Vidal, L.d.A.; Bílio, J.F.; Pupe, J.M.; Veiga, A.D.; Carvalho, C.H.S.d.; Lopes, R.B.; Rocha, T.L.; Silva, L.P.d.; et al. Bicho mineiro (Leucoptera coffeella): Uma revisão sobre o inseto e perspectivas para o manejo da praga. Embrapa Recursos Genéticos e Biotecnologia: Brasília, DF, Brazil, 2020; Volume 372. [Google Scholar]
- Dantas, J.; Motta, I.O.; Vidal, L.A.; Nascimento, E.F.M.B.; Bilio, J.; Pupe, J.M.; Veiga, A.; Carvalho, C.; Lopes, R.B.; Rocha, T.L.; et al. A Comprehensive Review of the Coffee Leaf Miner Leucoptera coffeella (Lepidoptera: Lyonetiidae)—A Major Pest for the Coffee Crop in Brazil and Others Neotropical Countries. Insects 2021, 12, 1130. [Google Scholar] [CrossRef]
- Ibama. Relatórios de Comercialização de Agrotóxicos. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos (accessed on 10 September 2025).
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Saunders, M.; Magnanti, B.L.; Correia Carreira, S.; Yang, A.; Alamo-Hernández, U.; Riojas-Rodriguez, H.; Calamandrei, G.; Koppe, J.G.; Krayer von Krauss, M.; Keune, H.; et al. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health 2012, 11, S5. [Google Scholar] [CrossRef]
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.K.; Alstrup, A.K.O.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006. [Google Scholar] [CrossRef]
- Goel, P.; Arora, M. Photocatalytically driven mineralization of chlorpyrifos pesticide by copper nanoparticles. MRS Adv. 2021, 6, 774–779. [Google Scholar] [CrossRef]
- Nascimento, A.F.O. Uso de nanotecnologia e controle biológico conservativo para o manejo de Leucoptera coffeella. Review. Master’s Thesis, Federal University of Uberlandia, Uberlândia, MG, Brazil, 2022. [Google Scholar] [CrossRef]
- Conab. Informações Agropecuárias: Séries Históricas. 2024. Available online: https://www.gov.br/conab/pt-br/atuacao/informacoes-agropecuarias/safras/series-historicas (accessed on 10 September 2025).
- White, W.H.; Hensley, S.D. Techniques to quantify the effect of Diatraea saccharalis (Lepidoptera: Pyralidae) on sugarcane quality. Field Crops Res. 1987, 15, 341–348. [Google Scholar] [CrossRef]
- Casteliani, A.; de Fatima Martins, L.; Maringoli Cardoso, J.F.; Oliveira Silva, M.S.; Abe da Silva, R.S.; Chacon-Orozco, J.G.; Barbosa Casteliani, A.G.; Půža, V.; Harakava, R.; Leite, L.G. Behavioral aspects of Sphenophorus levis (Coleoptera: Curculionidae), damage to sugarcane and its natural infection by Steinernema carpocapsae (Nematoda: Rhabditidae). Crop Protect. 2020, 137, 105262. [Google Scholar] [CrossRef]
- Manoukis, N.C.; Francischini, F.J.B.; Cordeiro, E.M.G.; de Campos, J.B.; Alves-Pereira, A.; Viana, J.P.G.; Wu, X.; Wei, W.; Brown, P.; Joyce, A.; et al. Diatraea saccharalis history of colonization in the Americas. The case for human-mediated dispersal. PLoS ONE 2019, 14, e0220031. [Google Scholar] [CrossRef]
- Silva, M.F.d.; Funichello, M.; Souza, D.M.d. Performance of insecticides in control of Diatraea saccharalis (Lepidoptera: Crambidae) in sugarcane. Arq. Inst. Biológico 2020, 87, e0782018. [Google Scholar] [CrossRef]
- Friedrich, K.; Silveira, G.R.d.; Amazonas, J.C.; Gurgel, A.d.M.; Almeida, V.E.S.d.; Sarpa, M. Situação regulatória internacional de agrotóxicos com uso autorizado no Brasil: Potencial de danos sobre a saúde e impactos ambientais. Cad. Saúde Pública 2021, 37. [Google Scholar] [CrossRef]
- Hwang, J.-M.; Bae, J.-W.; Jung, E.-J.; Lee, W.-J.; Kwon, W.-S. Novaluron Has Detrimental Effects on Sperm Functions. Int. J. Environ. Res. Public Health 2021, 19, 61. [Google Scholar] [CrossRef]
- Zhu, N.; Li, R.; Zhang, J.; Yan, Q.; Jiao, J.; Liang, D.; Yue, H.; Sang, N.; Li, G. Photo-degradation behavior of seven benzoylurea pesticides with C3N4 nanofilm and its aquatic impacts on Scendesmus obliquus. Sci. Total Environ. 2021, 799, 149470. [Google Scholar] [CrossRef]
- Vaurie, P. Revision of the Genus Sphenophorus in South America (Coleoptera, Curculionidae, Rhynchophorinae); American Museum of Natural History: New York, NY, USA, 1978; Volume 2656. [Google Scholar]
- Pavlu, F.A.; Molin, J.P. A sampling plan and spatial distribution for site-specific control of Sphenophorus levis in sugarcane. Acta Scientiarum. Agron. 2016, 38, 279–287. [Google Scholar] [CrossRef]
- Paula Silva, D.d.; Jacomassi, L.M.; Oliveira, J.A.V.; Oliveira, M.P.; Momesso, L.; de Siqueira, G.F.; Foltran, R.; Soratto, R.P.; Dinardo-Miranda, L.L.; Crusciol, C.A.C. Growth-Promoting Effects of Thiamethoxam on Sugarcane Ripened with Sulfometuron-Methyl. Sugar Tech 2022, 25, 339–351. [Google Scholar] [CrossRef]
- Martins, R.G.; Martins, M.B.G.; Silva, J.M.; Pereira, M.A.; Appezzato-da-Glória, B.; Castro, P.R.d.C.e. Thiamethoxam on the histological characteristics of sugarcane young roots. Cienc. Rural 2012, 42, 1936–1940. [Google Scholar] [CrossRef][Green Version]
- Paula Silva, D.d.; Oliveira, M.P.; Oliveira, J.A.V.; Jacomassi, L.M.; Momesso, L.; Garcia, A.; Ferraz de Siqueira, G.; Foltran, R.; Soratto, R.P.; Dinardo-Miranda, L.L.; et al. Phytotonic effects of thiamethoxam on sugarcane managed with glyphosate as a ripener. Pest Manag. Sci. 2022, 78, 4006–4017. [Google Scholar] [CrossRef]
- Ramanathan, S.; Kumar, M.S.; Sanjeevi, G.; Narayanan, B.; Kurien, A.A. Thiamethoxam, a Neonicotinoid Poisoning Causing Acute Kidney Injury via a Novel Mechanism. Kidney Int. Rep. 2020, 5, 1111–1113. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef]
- Yan, S.; Li, N.; Guo, Y.; Chen, Y.; Ji, C.; Yin, M.; Shen, J.; Zhang, J. Chronic exposure to the star polycation (SPc) nanocarrier in the larval stage adversely impairs life history traits in Drosophila melanogaster. J. Nanobiotechnol. 2022, 20, 515. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Daniel, A.B. Synthesizes of graphene oxide grafted with chitosan and copper oxide nanoparticles for the removal of lambda-cyhalothrin and thiamethoxam insecticides from wastewater. Z. Für Phys. Chem. 2023, 237, 479–501. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Removal of chlorpyrifos, thiamethoxam, and tebuconazole from water using green synthesized metal hexacyanoferrate nanoparticles. Environ. Sci. Pollut. Res. Int. 2018, 25, 10878–10893. [Google Scholar] [CrossRef]
- Freitas, D.A.d.; Barbosa, J.A.; Labuto, G.; Nocelli, R.C.F.; Carrilho, E.N.V.M. Removal of the pesticide thiamethoxam from sugarcane juice by magnetic nanomodified activated carbon. Environ. Sci. Pollut. Res. 2022, 29, 79855–79865. [Google Scholar] [CrossRef]
- Ibge. Produção de Laranja. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/laranja/br (accessed on 20 April 2025).
- Bassanezi, R.B.; Behlau, F.; Lopes, S.A.; Wulff, N.A.; Miranda, M.P.d.; Barbosa, J.C.; Ayres, A.J.; Sala, I.; Trombin, V.G.; Reina, R.; et al. Levantamento da Incidência das Doenças dos Citros: Greening, CVC e Cancro Cítrico; Fundo de Defesa da Citricultura: Araraquara, SP, Brazil, 2024; p. 75. [Google Scholar]
- Zapata, S.D.; Peguero, F.; Setamou, M.; Alabi, O. Economic Implications of Citrus Greening Disease Management Strategies. J. Agric. Resour. Econ. 2021, 47, 300–323. [Google Scholar] [CrossRef]
- Paiva, P.E.B.; Parra, J.R.P. Hidrogenionic potential (pH) of the attractant, trap density and control threshold for Ceratitis capitata (DIPTERA: TEPHRITIDAE) on Hamlin oranges in São Paulo central region, Brazil. Rev. Bras. Frutic. 2013, 35, 464–470. [Google Scholar] [CrossRef][Green Version]
- Fernandes, N.G. Combate ao Greening em Citros Necessita de Legislação Específica; Visão Agrícola: 2004. Available online: https://www.esalq.usp.br/visaoagricola/edicoes/citrus (accessed on 10 September 2025).[Green Version]
- Lima, A.d.C. Insetos do Brasil. Tomo 2, capítulo 22–Hemípteros; BR Escola Nacional de Agronomia: Goiânia, Brazil, 1940. [Google Scholar][Green Version]
- Sabry, A.-K.H.; Hussein, M.A. Relative Toxicity of Some Nanopesticides and Their Conventional Formulations against the Alossy Clover Snails, Monacha cartusiana. J. Biol. Nat. 2021, 13, 54–60. [Google Scholar][Green Version]
- David, D.; George, I.A.; Peter, J.V. Toxicology of the newer neonicotinoid insecticides: Imidacloprid poisoning in a human. Clin. Toxicol. 2008, 45, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Proença, P.; Teixeira, H.; Castanheira, F.; Pinheiro, J.; Monsanto, P.V.; Marques, E.P.; Vieira, D.N. Two fatal intoxication cases with imidacloprid: LC/MS analysis. Forensic Sci. Int. 2005, 153, 75–80. [Google Scholar] [CrossRef]
- Shadnia, S.; Moghaddam, H.H. Fatal intoxication with imidacloprid insecticide. Am. J. Emerg. Med. 2008, 26, 634.e1–634.e4. [Google Scholar] [CrossRef]
- Stokstad, E. European Union expands ban of three neonicotinoid pesticides. Science 2018. Available online: https://www.science.org/content/article/european-union-expands-ban-three-neonicotinoid-pesticides (accessed on 10 September 2025). [CrossRef]
- Kathuria, D.; Bhattu, M.; Bankar, A.; Puri, P. A Review on Methods for the Elimination of Imidacloprid: 2018–2022. ChemistrySelect 2023, 8, e202302293. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Identification of Active Species in Photodegradation of Aqueous Imidacloprid over g-C3N4/TiO2 Nanocomposites. Catalysts 2022, 12, 120. [Google Scholar] [CrossRef]
- Thomas, M.C.; Heppner, J.B.; Woodruff, R.E.; Weems, J.H.V.; Steck, G.J.; Fasulo, T.R. Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann) (Insecta: Diptera: Tephritidae): EENY-214/IN371, rev. 9/2001. EDIS 2004, 2004. [Google Scholar] [CrossRef]
- Hallman, G.J. Ionizing radiation quarantine treatments against tephritid fruit flies. Postharvest Biol. Technol. 1999, 16, 93–106. [Google Scholar] [CrossRef]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest categorisation of non-EU Tephritidae. EFSA J. 2020, 18, e05931. [Google Scholar] [CrossRef]
- Tabanca, N.; Masi, M.; Epsky, N.D.; Nocera, P.; Cimmino, A.; Kendra, P.E.; Niogret, J.; Evidente, A. Laboratory Evaluation of Natural and Synthetic Aromatic Compounds as Potential Attractants for Male Mediterranean fruit Fly, Ceratitis capitata. Molecules 2019, 24, 2409. [Google Scholar] [CrossRef]
- Bruzek, J.; Malmstrom, C. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. 2009, 50, 32009R1107. Available online: https://www.legislation.gov.uk/eur/2009/1107 (accessed on 10 September 2025).
- Smith, J.B. A Catalogue, Bibliographical and Synonymical of the Species of Moths of the Lepidopterous Superfamily Noctuidae Found in Boreal America: With Critical Notes. 1893. Available online: https://www.biodiversitylibrary.org/bibliography/38378 (accessed on 10 September 2025).
- Anvisa. T51–Trimedlure. 2020. Available online: https://www.gov.br/anvisa/pt-br/setorregulado/regularizacao/agrotoxicos/monografias/monografias-autorizadas/t/4554json-file-1/view (accessed on 10 September 2025).
- Embrapa. Soja em Números (Safra 2024/25). Available online: https://www.embrapa.br/soja/cultivos/soja1/dados-economicos (accessed on 20 April 2025).
- Silva, M.T.B.d.; Costa, E.C.; Boss, A. Controle de Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) com reguladores de crescimento de insetos. Cienc. Rural. 2003, 33, 601–605. [Google Scholar] [CrossRef][Green Version]
- Peruca, R.D. Consumo Alimentar e Biologia de Spodoptera frugiperda (J. E. Smith, 1797) (Noctuidae) Alimentada Com Folhas de soja, Submetidas à Herbivoria Previa; Dom Bosco Catholical University: Campo Grande, MS, Brazil, 2015. [Google Scholar][Green Version]
- Leiderman, L.; Sauer, H.F.G. L. frugiperda in Maize Fields. Open Biol. 1953, 6, 105–113. [Google Scholar][Green Version]
- Bernardi, O.; Bernardi, D.; Horikoshi, R.; Omoto, C. Manejo da Resistência de Insetos a Plantas Bt; Promip: Engenheiro Coelho, SP, Brazil, 2016; Volume 1. [Google Scholar][Green Version]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Farias, J.R.; Bernardi, D. Estratégias de manejo da resistência e importância das áreas de refúgio para tecnologia Bt. In Diversidade e Inovações na Cadeia Produtiva de Milho e Sorgo na Era dos Transgênicos; Paterniani, M.E.A.G.Z., Duarte, A.P., Tsunechiro, A., Sorgo, A.B.d.M.e., Eds.; Instituto Agronômico de Campinas (IAC): Campinas, SP, Brazil, 2012; pp. 303–314. [Google Scholar][Green Version]
- Kadala, A.; Charreton, M.; Charnet, P.; Collet, C. Honey bees long-lasting locomotor deficits after exposure to the diamide chlorantraniliprole are accompanied by brain and muscular calcium channels alterations. Sci. Rep. 2019, 9, 2153. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef]
- Botelho, P.S.M.; Silveira Neto, S.; Magrini, E.A. Fator chave para Anticarsia gemmatalis Hübner, 1818 (Lepidoptera: Noctuidae) em cultura de soja, para o estado de São Paulo. Sci. Agric. 1999, 56, 867–873. [Google Scholar] [CrossRef]
- Moscardi, F.; de Freitas Bueno, A.; Sosa-Gómez, D.; Roggia, S.; Hoffmann-Campo, C.; Pomari-Fernandes, A.; Corso, I.C.; Yano, S.A.C. Artrópodes que Atacam as Folhas da Soja; Embrapa Soja: Londrina, Brazil, 2012; pp. 213–334. [Google Scholar]
- Guedes, J.V.C.; Fiorin, R.A.; Stürmer, G.R.; Dal Prá, E.; Perini, C.R.; Bigolin, M. Sistemas de aplicação e inseticidas no controle de Anticarsia gemmatalis na soja. Rev. Bras. Eng. Agríc. Ambient. 2012, 16, 910–914. [Google Scholar] [CrossRef][Green Version]
- Silva, A.C.J.; Sosa-Gomez, D.R. Suscetibilidade relativa de espécies de noctuoídeos ao inseticida clorfenapir. In Proceedings of the Jornada Acadêmica da Embrapa Soja, Londrina, PR, Brazil, 12 July 2017; pp. 9–13. [Google Scholar][Green Version]
- Pereira, A.G.; Davico, C.E.; Oliveira, B.R.F.; Izídio, G.S. Critical Review: Regulatory Differences Among Countries Expose the Permissiveness in the Use of Hazardous Pesticides in Brazil. Ecotoxicol. Environ. Contam. 2022, 17, 2–19. [Google Scholar] [CrossRef]
- Pignati, W.A.; Lima, F.A.N.d.S.e.; Lara, S.S.d.; Correa, M.L.M.; Barbosa, J.R.; Leão, L.H.d.C.; Pignatti, M.G. Distribuição espacial do uso de agrotóxicos no Brasil: Uma ferramenta para a Vigilância em Saúde. Ciência Saúde Coletiva 2017, 22, 3281–3293. [Google Scholar] [CrossRef]
- Cruces, L.; de la Peña, E.; De Clercq, P. Field Evaluation of Cypermethrin, Imidacloprid, Teflubenzuron and Emamectin Benzoate against Pests of Quinoa (Chenopodium quinoa Willd.) and Their Side Effects on Non-Target Species. Plants 2021, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.S.; Souza, J.P.; Winkaler, E.U.; Carraschi, S.P.; Cruz, C.; Souza-Júnior, S.C.; Machado-Neto, J.G. Acute toxicity and environmental risk of teflubenzuron to Daphnia magna, Poecilia reticulata and Lemna minorin the absence and presence of sediment. J. Environ. Sci. Health Part B 2013, 48, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.E.; Samuelsen, O.B.; Johnsen, I.A.; Hannisdal, R.; Tjensvoll, T.; Husa, V. Distribution and Persistence of Diflubenzuron and Teflubenzuron in the Marine Environment Around Salmonid Aquaculture Facilities. Front. Mar. Sci. 2021, 8, 694577. [Google Scholar] [CrossRef]
- Samuelsen, O.B.; Lunestad, B.T.; Farestveit, E.; Grefsrud, E.S.; Hannisdal, R.; Holmelid, B.; Tjensvoll, T.; Agnalt, A.-L. Mortality and deformities in European lobster (Homarus gammarus) juveniles exposed to the anti-parasitic drug teflubenzuron. Aquat. Toxicol. 2014, 149, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M.; Nassar, M.M.; Farrag, T.E.; Mahmoud, M.S. Effective photodegradation of methomyl pesticide in concentrated solutions by novel enhancement of the photocatalytic activity of TiO2 using CdSO4 nanoparticles. Environ. Sci. Pollut. Res. 2013, 21, 1425–1435. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abd El-Aty, A.M.; Kim, S.-W.; Lee, Y.-J.; Na, T.-W.; Park, J.-S.; Shin, H.-C.; Shim, J.-H. Simultaneous determination and identity confirmation of thiodicarb and its degradation product methomyl in animal-derived foodstuffs using high-performance liquid chromatography with fluorescence detection and tandem mass spectrometry. J. Chromatogr. B 2017, 1040, 97–104. [Google Scholar] [CrossRef]
- Aliste, M.; Garrido, I.; Flores, P.; Hellín, P.; Pérez-Lucas, G.; Navarro, S.; Fenoll, J. Photocatalytic degradation of four insecticides and their main generated transformation products in soil and pepper crop irrigated with reclaimed agro-wastewater under natural sunlight. J. Hazard. Mater. 2021, 414, 125603. [Google Scholar] [CrossRef]
- Romeh, A.A.; Ibrahim Saber, R.A. Green nano-phytoremediation and solubility improving agents for the remediation of chlorfenapyr contaminated soil and water. J. Environ. Manag. 2020, 260, 110104. [Google Scholar] [CrossRef]
- Laabs, V.; Amelung, W.; Pinto, A.; Zech, W. Fate of Pesticides in Tropical Soils of Brazil under Field Conditions. J. Environ. Qual. 2002, 31, 256–268. [Google Scholar] [CrossRef]
- Laabs, V.; Amelung, W.; Pinto, A.; Altstaedt, A.; Zech, W. Leaching and degradation of corn and soybean pesticides in an Oxisol of the Brazilian Cerrados. Chemosphere 2000, 41, 1441–1449. [Google Scholar] [CrossRef]
- Correia, F.V.; Macrae, A.; Guilherme, L.R.G.; Langenbach, T. Atrazine sorption and fate in a Ultisol from humid tropical Brazil. Chemosphere 2007, 67, 847–854. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef] [PubMed]
- Gravenor, M.B.; Mohamed, F.; Gawarammana, I.; Robertson, T.A.; Roberts, M.S.; Palangasinghe, C.; Zawahir, S.; Jayamanne, S.; Kandasamy, J.; Eddleston, M.; et al. Acute Human Self-Poisoning with Imidacloprid Compound: A Neonicotinoid Insecticide. PLoS ONE 2009, 4, e5127. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2019, 27, 2522–2565. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Zhu, Y.; Long, C.; Su, L.; Liu, S.; Tang, Z. Applications of Nanomaterials in Asymmetric Photocatalysis: Recent Progress, Challenges, and Opportunities. Adv. Mater. 2020, 33, 2001731. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2011, 112, 1555–1614. [Google Scholar] [CrossRef]
- Thakur, S.; Ojha, A.; Kansal, S.K.; Gupta, N.K.; Swart, H.C.; Cho, J.; Kuznetsov, A.; Sun, S.; Prakash, J. Advances in powder nano-photocatalysts as pollutant removal and as emerging contaminants in water: Analysis of pros and cons on health and environment. Adv. Powder Mater. 2024, 3, 100233. [Google Scholar] [CrossRef]
- Intisar, A.; Ramzan, A.; Sawaira, T.; Kareem, A.T.; Hussain, N.; Din, M.I.; Bilal, M.; Iqbal, H.M.N. Occurrence, toxic effects, and mitigation of pesticides as emerging environmental pollutants using robust nanomaterials—A review. Chemosphere 2022, 293, 133538. [Google Scholar] [CrossRef] [PubMed]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Shanker, U. Degradation of traditional and new emerging pesticides in water by nanomaterials: Recent trends and future recommendations. Int. J. Environ. Sci. Technol. 2017, 15, 1347–1380. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2020, 19, 355–374. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- MAPA. Exportações do Agronegócio Atingem Novo Recorde no Mês de Maio e no Acumulado do ano. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/exportacoes-do-agronegocio-atingem-novo-recorde-no-mes-de-maio-e-no-acumulado-do-ano (accessed on 20 April 2025).
- Viana, G. Brasil Caminha Para Liderar Exportações de Milho em 2023, Preveem Especialistas. Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/78910453/brasil-caminha-para-liderar-exportacoes-de-milho-em-2023-preveem-especialistas (accessed on 20 April 2025).
- Gaboardi, S.C.; Candiotto, L.Z.P.; Panis, C. Agribusiness in Brazil and its dependence on the use of pesticides. Hyg. Environ. Health Adv. 2023, 8, 100080. [Google Scholar] [CrossRef]
- Brovini, E.M.; de Deus, B.C.T.; Vilas-Boas, J.A.; Quadra, G.R.; Carvalho, L.; Mendonça, R.F.; Pereira, R.d.O.; Cardoso, S.J. Three-bestseller pesticides in Brazil: Freshwater concentrations and potential environmental risks. Sci. Total Environ. 2021, 771, 144754. [Google Scholar] [CrossRef]
- Paumgartten, F.J.R. Pesticides and public health in Brazil. Curr. Opin. Toxicol. 2020, 22, 7–11. [Google Scholar] [CrossRef]
- Dias, T.C.C.d.C.; Lopes, M.A.C.Q.; Leite, J.A.P. Decreto Nº 10833, de 07 de Outubro de 2021. 2021. Available online: https://www.planalto.gov.br/ccivil_03/_ato2019-2022/2021/decreto/d10833.htm (accessed on 20 April 2025).
- Hazarika, A.; Yadav, M.; Yadav, D.K.; Yadav, H.S. An overview of the role of nanoparticles in sustainable agriculture. Biocataly. Agric. Biotechnol. 2022, 43, 102399. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929. [Google Scholar] [CrossRef] [PubMed]
- Hajji-Hedfi, L.; Chhipa, H. Nano-based pesticides: Challenges for pest and disease management. Euro-Mediterr. J. Environ. Integr. 2021, 6, 69. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Garcia, T.D.; Franco-Belussi, L.; Salla, R.F.; Souza, B.F.S.; de Melo, N.F.S.; Irazusta, S.P.; Jones-Costa, M.; Silva-Zacarin, E.C.M.; Fraceto, L.F. Pyrethrum extract encapsulated in nanoparticles: Toxicity studies based on genotoxic and hematological effects in bullfrog tadpoles. Environ. Pollut. 2019, 253, 1009–1020. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.-m. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- de Oliveira, R.; da Silva Martini, W.; Carlos Sant’Ana, A. Combined effect involving semiconductors and plasmonic nanoparticles in photocatalytic degradation of pesticides. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100657. [Google Scholar] [CrossRef]

| Crop | Target Pest or Objective | Nano System/Formulation | Intended Function | Reported Effect | Proposed Mechanism * | Refs. |
|---|---|---|---|---|---|---|
| Coffee | Coffee berry borer Hypothenemus hampei | ZnO nanoparticles | Direct bioactivity | Sublethal and adverse biological effects in lab assays | Membrane disruption and ROS generation typical of metal oxide NPs | [47,48,69] |
| Coffee | Coffee berry borer H. hampei | CuO nanoparticles | Direct bioactivity | As above, dose-dependent effects on biological traits | Membrane damage, oxidative stress, and ionic release | [47,48,69] |
| Coffee | Coffee berry borer H. hampei | CeO2 nanoparticles | Direct bioactivity | Adverse effects on biological characteristics | Redox imbalance and enzyme perturbation at nano–bio interface | [69] |
| Citrus | Vector Diaphorina citri | Thiamethoxam nanoformulation (encapsulation and release control) | Delivery optimization | Effective psyllid management with improved encapsulation and profiling | Improved leaf wetting and retention, controlled release, and enhanced uptake | [65,82] |
| Citrus | Vector D. citri | Functional nano-dispenser for imidacloprid | Spatial targeting | Vector suppression with point-source release | Sustained, localized release from nano-dispenser matrices | [74] |
| Citrus | Medfly Ceratitis capitata | Electrospun nanofibers loaded with trimedlure | Attract-and-kill, monitoring | Longer-lasting lure release, improved trap performance | Diffusion-controlled semiochemical release from nanofibers | [75] |
| Citrus | Medfly (alternative) | Magnetite nanoparticles (trimedlure-free concept) | Attract-and-kill or toxic contact | Dose-dependent larval lethality in the lab | ROS-mediated stress and membrane interactions of Fe oxide NPs | [83] |
| Sugarcane | Sugarcane borer Diatraea saccharalis | Novaluron nanoparticles | Delivery optimization of IGR | Comparable bioactivity to commercial formulations with potential dose economy | Better dispersion and controlled release from nano-capsules | [84] |
| Soybean | Fall armyworm Spodoptera frugiperda | ZnO nanoparticles | Direct bioactivity | Larval mortality, deformities, reduced fecundity, and hatch | Membrane disruption, ROS generation, ionic release | [48,67] |
| Soybean | Fall armyworm S. frugiperda | Copper-based nanoparticles | Direct bioactivity | Strong larvicidal and antifeedant activity; immune effects | Oxidative stress, membrane interactions | [68] |
| Soybean | Fall armyworm S. frugiperda | Mixed NPs (Cu, KI, Ag, and Bd) | Direct bioactivity | Significant insecticidal effects in the lab; soil impact assessed | Multi-modal surface reactivity and redox stress | [85] |
| Soybean | Velvetbean caterpillar Anticarsia gemmatalis | Zein protein nanoparticles | Carrier with intrinsic bioactivity | Direct insecticidal activity: mechanistic lesions documented | Contact toxicity and gut interaction of protein-based NPs | [66] |
| Various crops | Multiple targets | Chlorantraniliprole solid nanodispersions | Stabilize and improve the dispersion of poorly soluble AI | Retained potency after high-pressure homogenization; improved handling | Enhanced dissolution, better leaf coverage, and uptake | [64] |
| Field settings | Multiple pathosystems | Unimolecule nanopesticide delivery system | Field-scale delivery optimization | Improved field control across systems | Increased deposition and penetration from nano-architecture | [50] |
| Model: aphid | Green peach aphid Myzus persicae | Thiamethoxam complexed with star polycation (SPc) | Foliar adhesion and plant uptake boost | Higher contact and stomach toxicity at the same AI load | Improved wetting, retention, and plant uptake via cationic carrier | [45,63] |
| Botanicals | Soft-bodied pests, general | Plant oil nanoemulsions | Stabilize botanicals, enhance coverage | Improved control outcomes and dose economy vs. coarse emulsions | Better dispersion, smaller droplets, improved cuticular penetration | [55,71] |
| Tomato | The tomato leafminer, Tuta absoluta | Nano-silica gel | Nanodelivery systems for pesticides | Nano-silica gel significantly increased the weight of the harvested tomato crop (Kg/feddan) compared to silica gel and the control | Dissolution, biodegradation, diffusion, and osmotic pressure at a specific pH | [86] |
| Strawberry | Botrytis cinerea Pers | Nanosized silver-chitosan | Inhibit the growth of B. cinerea and prevent gray mold decay | Strawberry coated with nano Ag-IrCTS: Showed no signs of infection for 4 days. By the end of the 7-day storage period, fungal decay appeared in just 10% of strawberries | Membrane disruption, ROS generation, and ionic release | [87] |
| IPM dispensers | Spodoptera litura and tree-fruit moths | Electrospun pheromone nanofibers | Mating disruption or mass trapping | Sustained release with strong trapping performance | Diffusion-controlled release from nanofibers | [58,59] |
| Matrix or Context | Target Pesticide(s) | Nano Material or System | Reported Effect | Mechanism | Ref. |
|---|---|---|---|---|---|
| Water, soil | Chlorpyrifos | Cu nanoparticles under natural daylight | Photocatalytic mineralization of chlorpyrifos | Visible-light photocatalysis on Cu/Cu2O surfaces | [103] |
| Water | Chlorpyrifos | g-C3N4/TiO2 nanocomposite | Photodegradation with identified reactive species | Heterojunction-enabled charge separation and ROS production | [137] |
| Soil, aquatic tests | Novaluron; Teflubenzuron | C3N4 nanofilm (visible light) | 64 percent novaluron and 82 percent teflubenzuron degraded in 2 h | Photocatalytic oxidation via graphitic carbon nitride | [112] |
| Water | Methomyl | CdSO4-doped TiO2 nanoparticles | Fast removal with high capacity under sunlight | Doped TiO2 photocatalysis with enhanced charge separation | [163] |
| Field soil and irrigation water | Chlorantraniliprole, imidacloprid, pirimicarb, and thiamethoxam | TiO2/Na2S2O8, pilot-scale, sunlight | Removal of most parents and main transformation products | Photocatalysis with persulfate oxidation | [165] |
| Soil and water | Chlorfenapyr | Fe and Ag nanoparticles | Up to 93.7 percent degradation | Nano-catalyzed reduction and oxidative pathways | [166] |
| Sugarcane juice, water | Thiamethoxam | Magnetic nanomodified activated carbon | Efficient removal from juice and water | High-area adsorption with magnetic separation | [124] |
| Water | Thiamethoxam, chlorpyrifos, tebuconazole | Green-synthesized metal hexacyanoferrate NPs | 70 to 98 percent solar degradation of thiamethoxam; broad removal | Photocatalysis and adsorption on Prussian blue analogs | [123] |
| Water | Endosulfan | Cu/Cu2O core–shell nanoparticles | Mineralization under light | Plasmonic–semiconductor photocatalysis at the interface | [96] |
| Crop | Main Disease/Pest | Conventional Agrochemical Strategy | Nanoparticulate Treatment (s) |
|---|---|---|---|
| Coffee | Coffee berry borer | Endosulfan (banned since 2013) | Zinc oxide (ZnO), copper oxide (CuO), and cerium oxide (CeO2) nanoparticles |
| Coffee leaf miner | Chlorpyrifos (still prevalent) | Titanium dioxide (TiO2) nanoparticles (UV light), copper nanoparticles (natural daylight) for photodegradation | |
| Sugarcane | Sugarcane borer | Novaluron | Novaluron nanoparticles, carbon nitride nanofilm for photodegradation |
| Sugarcane weevil | Thiamethoxam | Nanometerization of thiamethoxam by polymers, nano-sized thiamethoxam/star polycation complexes | |
| Metal hexacyanoferrate nanoparticles, magnetic nanomodified activated carbon for removal | |||
| Orange | Citrus greening disease | Control of the vector: thiamethoxam, imidacloprid | Nanosized thiamethoxam, nano-dispenser strategy for imidacloprid, and imidacloprid nanoparticles |
| Mediterranean fruit fly (Medfly) | Trimedlure-baited traps | Nanofiber formulations containing trimedlure, magnetite nanoparticles (trimedlure-free) | |
| Soybean | Fall armyworm | Thiodicarb, methomyl, chlorantraniliprole, and flubendiamide (not all registered) | Cu, KI, Ag, and Bd nanoparticles, commercial zinc oxide nanoparticles, copper oxide nanoparticles, chlorantraniliprole nanoparticles, and functionalized thiodicarb nanoparticles |
| Velvetbean caterpillar | Benzoylphenylureas (novaluron, teflubenzuron), chlorfenapyr | Nanoparticle compositions of teflubenzuron, nanoparticles of chlorfenapyr, and zein nanoparticles | |
| Pesticide residues (general) | Various conventional applications | C3N4 nanofilm for the photodegradation of teflubenzuron and novaluron, CdSO4-doped TiO2 nanoparticles for methomyl (and potentially thiodicarb), TiO2/Na2S2O8 for chlorantraniliprole, imidacloprid, pirimicarb, and thiamethoxam, and iron and silver nanoparticles for chlorfenapyr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso e Bufalo, T.; Buttrós, V.H.; de Paiva, A.B.; de Oliveira, D.D.; Ribeiro, C.S.F.; Dória, J. Nanopesticides in Brazilian Crops: Classes, Mechanisms, Efficacy, Risks, and Photocatalytic Remediation. Plants 2025, 14, 2880. https://doi.org/10.3390/plants14182880
Cardoso e Bufalo T, Buttrós VH, de Paiva AB, de Oliveira DD, Ribeiro CSF, Dória J. Nanopesticides in Brazilian Crops: Classes, Mechanisms, Efficacy, Risks, and Photocatalytic Remediation. Plants. 2025; 14(18):2880. https://doi.org/10.3390/plants14182880
Chicago/Turabian StyleCardoso e Bufalo, Tatiana, Victor Hugo Buttrós, Aline Bastos de Paiva, Deyne Dehon de Oliveira, Caio Silas Ferreira Ribeiro, and Joyce Dória. 2025. "Nanopesticides in Brazilian Crops: Classes, Mechanisms, Efficacy, Risks, and Photocatalytic Remediation" Plants 14, no. 18: 2880. https://doi.org/10.3390/plants14182880
APA StyleCardoso e Bufalo, T., Buttrós, V. H., de Paiva, A. B., de Oliveira, D. D., Ribeiro, C. S. F., & Dória, J. (2025). Nanopesticides in Brazilian Crops: Classes, Mechanisms, Efficacy, Risks, and Photocatalytic Remediation. Plants, 14(18), 2880. https://doi.org/10.3390/plants14182880






