PtrIAA12-PtrARF8 Complex Regulates the Expression of PtrSAUR17 to Control the Growth of Roots in Poncirus trifoliata
Abstract
1. Introduction
2. Results
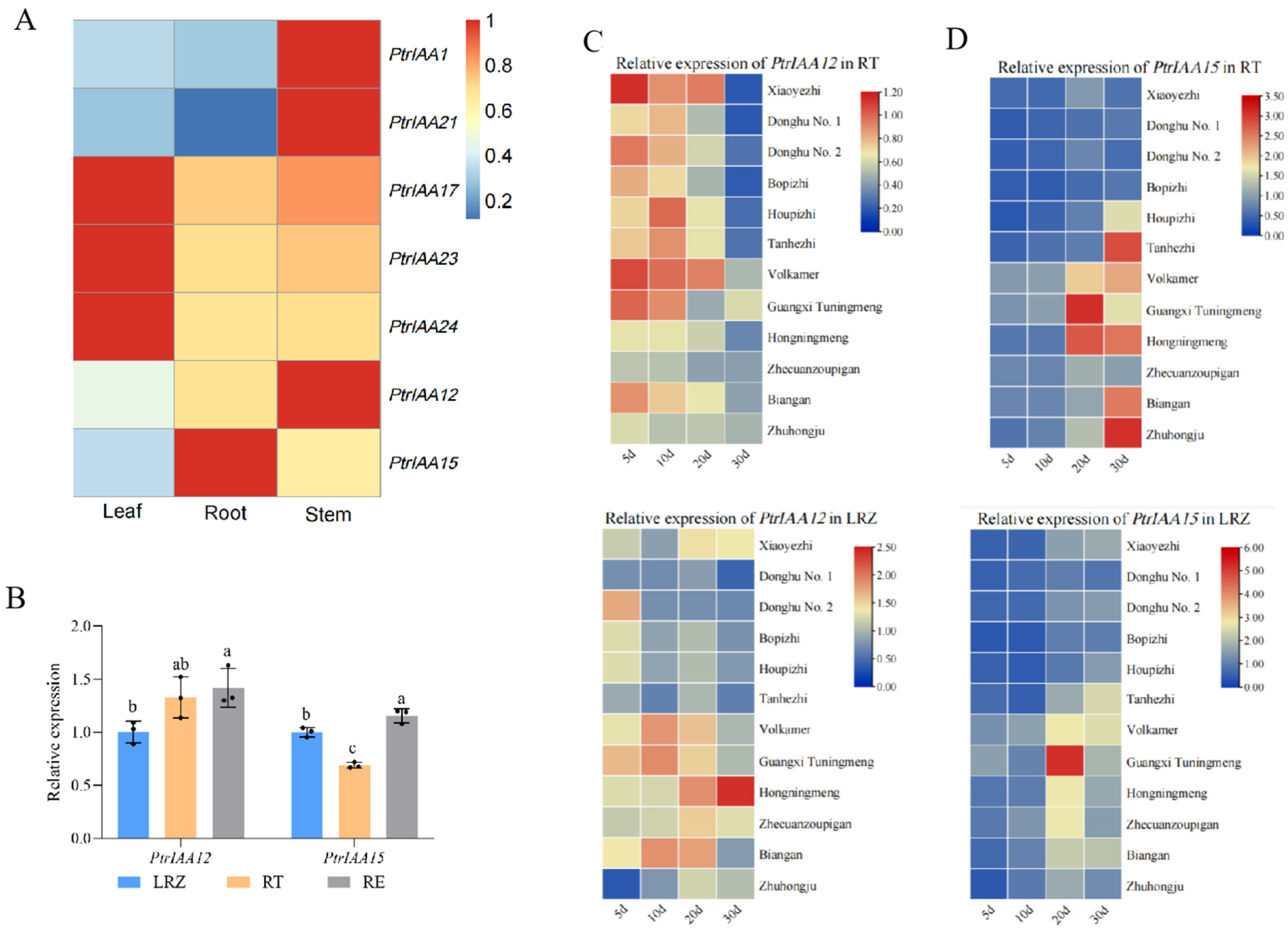
2.1. Identification of the Members of PtrAUX/IAAs Involved in Root Development in Citrus
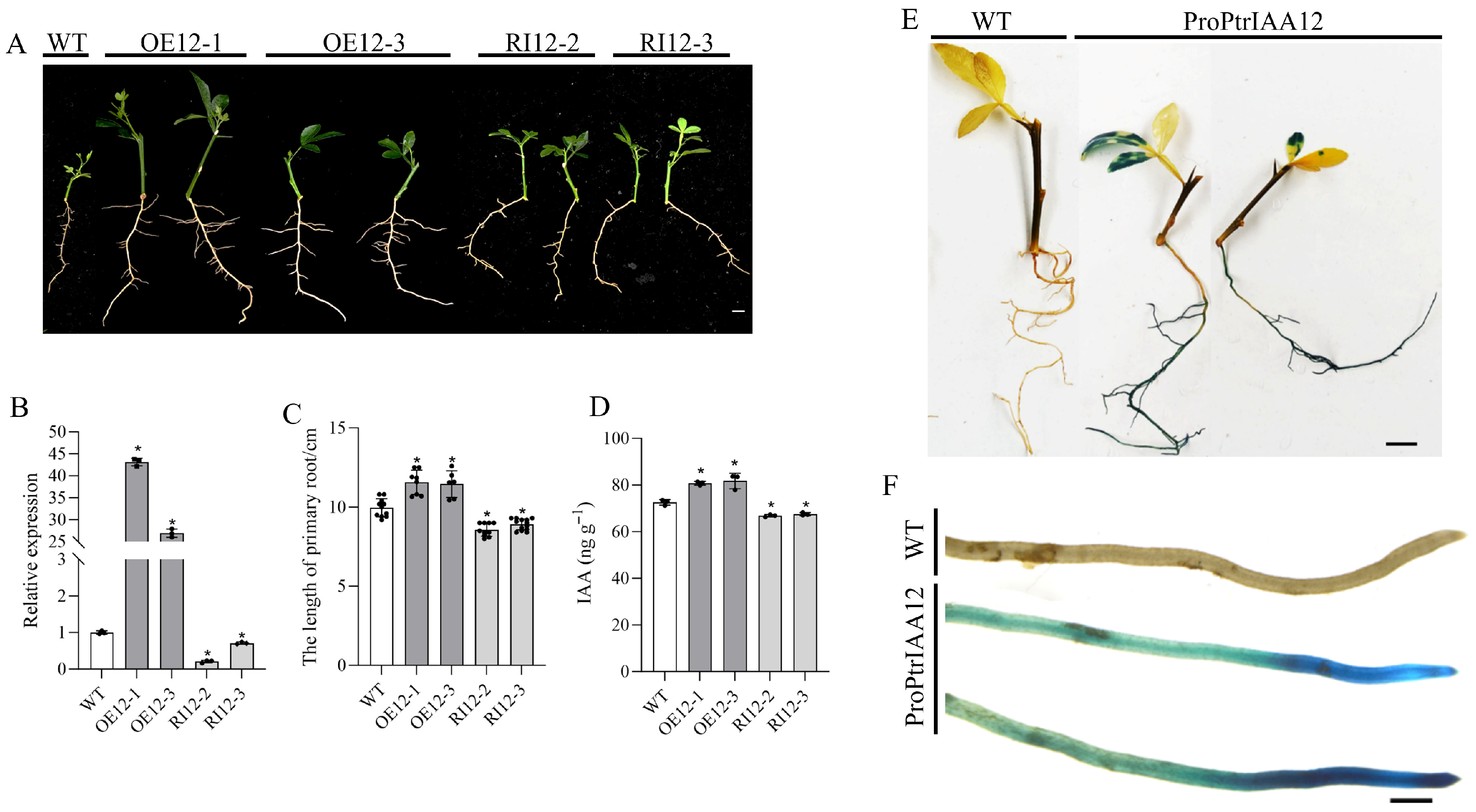
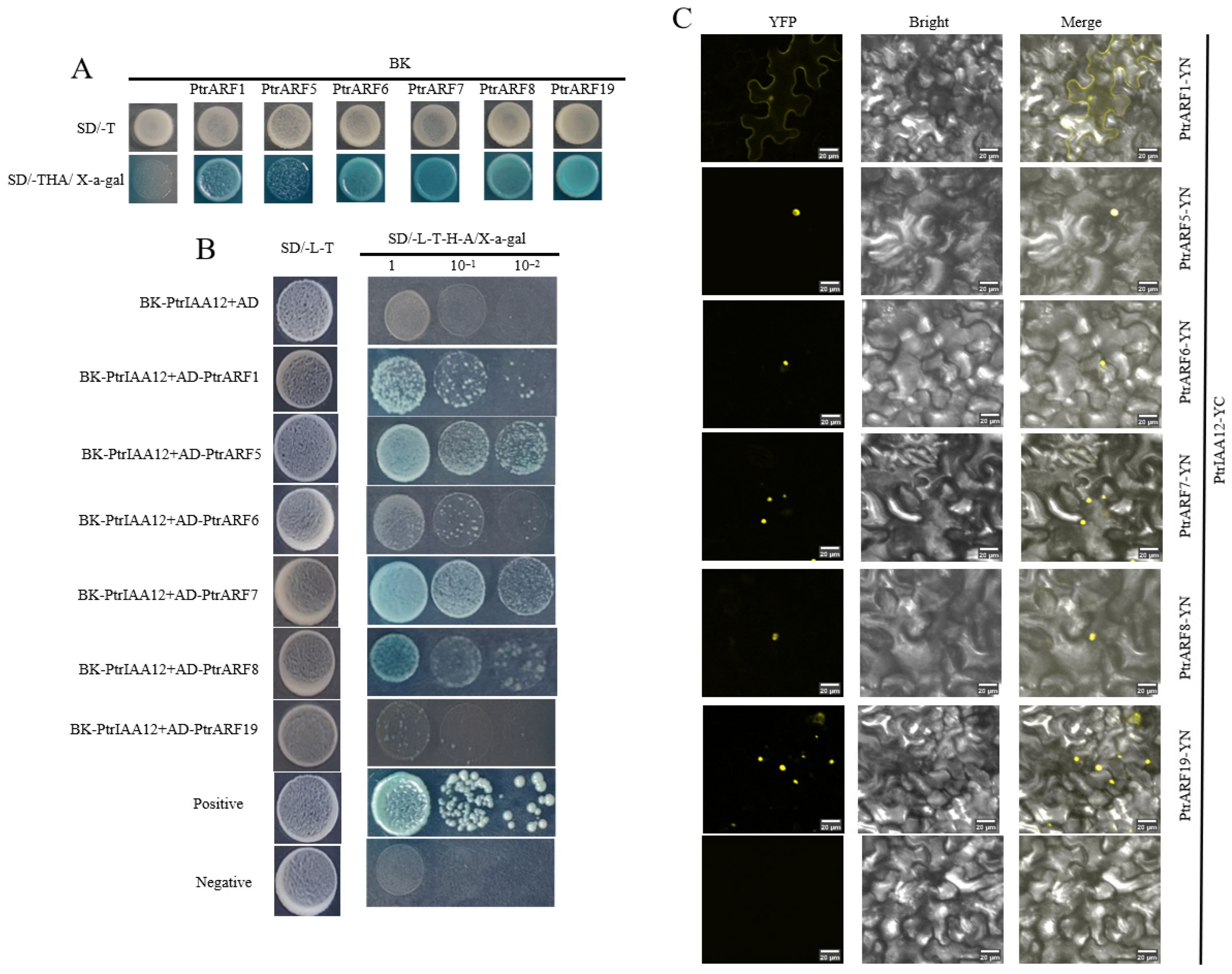
2.2. PtrIAA12 Participates in the Regulation of Root Growth in Citrus via Interacting with PtrARFs
2.3. PtrARF8 Regulates the Expression of PtrSAUR17
2.4. PtrSAUR17 Promotes Root Growth in Citrus
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Gene Expression Analysis
4.3. Phylogenetic Analysis and Multiple Sequence Alignments
4.4. Subcellular Localization Analysis
4.5. Stable Transformation in Citrus
4.6. GUS Staining
4.7. Auxin Analysis
4.8. Transactivation Analysis in Yeast
4.9. Y2H and BiFC Assay
4.10. Transient Overexpression of PtrARF Genes in Citrus Leaves
4.11. Y1H and Transient Transactivation Assay
4.12. Accession Numbers
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lavenus, J.; Goh, T.; Roberts, I.; Guyomarc’H, S.; Lucas, M.; De Smet, I.; Fukaki, H.; Beeckman, T.; Bennett, M.; Laplaze, L. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci. 2013, 18, 450–458. [Google Scholar] [CrossRef]
- Pacifici, E.; Polverari, L.; Sabatini, S. Plant hormone cross-talk: The pivot of root growth. J. Exp. Bot. 2015, 66, 1113–1121. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, J.; Qi, J.; Zhao, D.; Jin, M.; Wang, T.; Yang, Y.; Shi, H.; Guo, L.; Zhang, H. Crosstalk among plant hormone regulates the root development. Plant Signal. Behav. 2024, 19, 2404807. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. Cold Spring Harbor Perspect. Biol. 2022, 14, a039933. [Google Scholar] [CrossRef] [PubMed]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Jedlickova, V.; Ebrahimi, N.S.; Robert, H.S. On the trail of auxin: Reporters and sensors. Plant Cell 2022, 34, 3200–3213. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, F.; Friml, J.; Ding, Z. Auxin signaling: Research advances over the past 30 years. J. Integr. Plant Biol. 2022, 64, 371–392. [Google Scholar] [CrossRef]
- Hayashi, K.I. Chemical biology in auxin research. Cold Spring Harbor Perspect. Biol. 2021, 13, a040105. [Google Scholar] [CrossRef]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef]
- Fukaki, H.; Nakao, Y.; Okushima, Y.; Theologis, A.; Tasaka, M. Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 2005, 44, 382–395. [Google Scholar] [CrossRef]
- Goh, T.; Kasahara, H.; Mimura, T.; Kamiya, Y.; Fukaki, H. Multiple AUX/IAA-ARF modules regulate lateral root formation: The role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1461–1468. [Google Scholar] [CrossRef]
- Kim, S.H.; Bahk, S.; An, J.; Hussain, S.; Nguyen, N.T.; Do, H.L.; Kim, J.Y.; Hong, J.C.; Chung, W.S. A gain-of-function mutant of IAA15 inhibits lateral root development by transcriptional repression of LBD genes in Arabidopsis. Front. Plant Sci. 2020, 11, 1239. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Inahashi, H.; Nishizawa, N.K.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. Fine control of aerenchyma and lateral root development through AUX/IAA-and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 20770–20775. [Google Scholar] [CrossRef]
- Yang, F.; Shi, Y.; Zhao, M.; Cheng, B.; Li, X. ZmIAA5 regulates maize root growth and development by interacting with ZmARF5 under the specific binding of ZmTCP15/16/17. PeerJ 2022, 10, e13710. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, X.; Xu, X.; Han, Z.; Qiu, C. Transcription factor IAA27 positively regulates p uptake through promoted adventitious root development in apple plants. Int. J. Mol. Sci. 2022, 23, 14029. [Google Scholar] [CrossRef] [PubMed]
- Mcclure, B.A.; Guilfoyle, T. Characterization of a class of small auxin-inducible soybean polyadenylated RNAs. Plant Mol. Biol. 1987, 9, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wen, S.S.; Sun, T.T.; Wang, R.; Zuo, W.T.; Yang, T.; Wang, C.; Hu, J.J.; Lu, M.Z.; Wang, L.Q. PagWOX11/12a positively regulates the PagSAUR36 gene that enhances adventitious root development in poplar. J. Exp. Bot. 2022, 73, 7298–7311. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Pang, S.; Ma, Y.; Deng, L.; He, S.; Yi, S.; Lv, Q.; Zheng, Y. The ARF, AUX/IAA and GH3 gene families in citrus: Genome-wide identification and expression analysis during fruitlet drop from abscission zone A. Mol. Genet. Genom. 2015, 290, 2089–2105. [Google Scholar] [CrossRef]
- Wang, P.B.; Duan, Y.Y.; Quan, R.M.; Feng, M.Q.; Ren, J.; Tang, Y.Y.; Qing, M.; Xie, K.D.; Guo, W.W.; Wu, X.M. CsTCP14-CsIAA4 module-mediated repression of auxin signaling regulates citrus somatic embryogenesis. New Phytol. 2025, 246, 567–580. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zheng, S.; Wang, F.; Zhu, S.; Zhao, X. PtrSAUR32 interacts with PtrPP2C.Ds to regulate root growth in citrus. Plants 2025, 14, 1579. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Dello, I.R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Arase, F.; Nishitani, H.; Egusa, M.; Nishimoto, N.; Sakurai, S.; Sakamoto, N.; Kaminaka, H. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE 2012, 7, e43414. [Google Scholar] [CrossRef]
- Nam, H.; Han, S.; Lee, S.; Nam, H.; Lim, H.; Lee, G.; Cho, H.S.; Dang, T.; Choi, S.; Lee, M.M.; et al. CPR5-mediated nucleo-cytoplasmic localization of IAA12 and IAA19 controls lateral root development during abiotic stress. Proc. Natl. Acad. Sci. USA 2023, 120, e2085186176. [Google Scholar] [CrossRef]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002, 29, 153–168. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Lau, S.; Voss, U.; Vanneste, S.; Benjamins, R.; Rademacher, E.H.; Schlereth, A.; De Rybel, B.; Vassileva, V.; Grunewald, W.; et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Yamamoto, K.T. Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol. Plant. 2008, 133, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Yu, Q.; Liu, J.; Wen, X.; Yan, Z.; Hu, K.; Li, H.; Kong, X.; Li, C.; Tian, H.; et al. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J. 2020, 39, e101515. [Google Scholar] [CrossRef]
- Rogg, L.E.; Lasswell, J.; Bartel, B. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 2001, 13, 465–480. [Google Scholar] [CrossRef]
- Piya, S.; Shrestha, S.K.; Binder, B.; Stewart, C.N.; Hewezi, T. Protein-protein interaction and gene co-expression maps of ARFs and AUX/IAAs in Arabidopsis. Front. Plant Sci. 2014, 5, 744. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Lv, M.; Hepworth, S.R.; Li, D.; Ma, C.; Li, J.; Wang, S.M. SAUR15 promotes lateral and adventitious root development via activating H+-ATPases and auxin biosynthesis. Plant Physiol. 2020, 184, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Wang, X.J.; Hagen, G.; Guilfoyle, T.J. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef][Green Version]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. Dimerization and DNA binding of auxin response factors. Plant J. 1999, 19, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, C.; Chen, W.; Dai, H. The MdIAA29-MdARF4 complex plays an important role in balancing plant height with salt and drought stress responses. Plant Physiol. 2024, 196, 2795–2811. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.; Zhang, R.; Gao, A.; Oginga, Z.K.; Zheng, B.; Han, Y. ARF4 acting upstream of LBD16 promotes adventitious root formation in peach. Hortic. Plant J. 2023, 11, 145–161. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Di, P.; Wang, Y. Genome-wide identification and analysis of the AUX/IAA gene family in panax ginseng: Evidence for the role of PgIAA02 in lateral root development. Int. J. Mol. Sci. 2024, 25, 3470. [Google Scholar] [CrossRef]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/IAA14 regulates microRNA-mediated cold stress response in Arabidopsis roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Liang, Y.; Anwar, M.; Fatima, A.; Hassan, M.J.; Ali, A.; Tang, Q.; Peng, Y. Overexpression of auxin/indole-3-acetic acid gene TrIAA27 enhances biomass, drought, and salt tolerance in Arabidopsis thaliana. Plants 2024, 13, 2684. [Google Scholar] [CrossRef]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive AUX/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef]
- Yan, Z.; Li, K.; Li, Y.; Wang, W.; Leng, B.; Yao, G.; Zhang, F.; Mu, C.; Liu, X. The ZmbHLH32-ZmIAA9-ZmARF1 module regulates salt tolerance in maize. Int. J. Biol. Macromol. 2023, 253, 126978. [Google Scholar] [CrossRef] [PubMed]
- Cance, C.; Martin-Arevalillo, R.; Boubekeur, K.; Dumas, R. Auxin response factors are keys to the many auxin doors. New Phytol. 2022, 235, 18. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Vernoux, T.; Furutani, M.; Traas, J.; Tasaka, M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 2002, 129, 3965–3974. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and MicroRNA abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef]
- Hardtke, C.S.; Berleth, T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. Embo. J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Przemeck, G.; Mattsson, J.; Hardtke, C.S.; Sung, Z.R.; Berleth, T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 1996, 200, 229–237. [Google Scholar] [CrossRef]
- Dong, D.; Deng, Q.; Zhang, J.; Jia, C.; Gao, M.; Wang, Y.; Zhang, L.; Zhang, N.; Guo, Y.D. Transcription factor SlSTOP1 regulates small auxin-up RNA genes for tomato root elongation under aluminum stress. Plant Physiol. 2024, 196, 2654–2668. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Hong, Y.; Wen, Z.; Shang, C.; Li, Z.; Cai, X.; Qiao, G.; Wen, X. Molecular characterization of the SAUR gene family in sweet cherry and functional analysis of PavSAUR55 in the process of abscission. J. Integr. Agric. 2023, 22, 1720–1739. [Google Scholar] [CrossRef]
- Chae, K.; Isaacs, C.G.; Reeves, P.H.; Maloney, G.S.; Muday, G.K.; Nagpal, P.; Reed, J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant. J. 2012, 71, 684–697. [Google Scholar] [CrossRef]
- Nagpal, P.; Reeves, P.H.; Wong, J.H.; Armengot, L.; Chae, K.; Rieveschl, N.B.; Trinidad, B.; Davidsdottir, V.; Jain, P.; Gray, W.M.; et al. SAUR63 stimulates cell growth at the plasma membrane. PLoS Genet. 2022, 18, e1010375. [Google Scholar] [CrossRef]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, F.; Wang, X.; Feng, J.; Yi, Q.; Zhu, S.; Zhao, X. Mining key genes related to root morphogenesis through genome-wide identification and expression analysis of RR gene family in citrus. Front. Plant Sci. 2022, 13, 1068961. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J.; Muller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, C.Y. BiFC assay for detecting protein-protein interaction in tobacco leaves. Bio-Protocol 2018, 101, e1010133. (In Chinese) [Google Scholar]
- Zhang, M.; Wang, F.; Hu, Z.; Wang, X.; Yi, Q.; Feng, J.; Zhao, X.; Zhu, S. CcRR5 interacts with CcRR14 and CcSnRK2s to regulate the root development in citrus. Front. Plant Sci. 2023, 14, 1170825. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, M.; Li, X.; Zheng, S.; Wang, F.; Zhu, S.; Zhao, X. PtrIAA12-PtrARF8 Complex Regulates the Expression of PtrSAUR17 to Control the Growth of Roots in Poncirus trifoliata. Plants 2025, 14, 2875. https://doi.org/10.3390/plants14182875
Wang X, Zhang M, Li X, Zheng S, Wang F, Zhu S, Zhao X. PtrIAA12-PtrARF8 Complex Regulates the Expression of PtrSAUR17 to Control the Growth of Roots in Poncirus trifoliata. Plants. 2025; 14(18):2875. https://doi.org/10.3390/plants14182875
Chicago/Turabian StyleWang, Xiaoli, Manman Zhang, Xiaoya Li, Saihang Zheng, Fusheng Wang, Shiping Zhu, and Xiaochun Zhao. 2025. "PtrIAA12-PtrARF8 Complex Regulates the Expression of PtrSAUR17 to Control the Growth of Roots in Poncirus trifoliata" Plants 14, no. 18: 2875. https://doi.org/10.3390/plants14182875
APA StyleWang, X., Zhang, M., Li, X., Zheng, S., Wang, F., Zhu, S., & Zhao, X. (2025). PtrIAA12-PtrARF8 Complex Regulates the Expression of PtrSAUR17 to Control the Growth of Roots in Poncirus trifoliata. Plants, 14(18), 2875. https://doi.org/10.3390/plants14182875





