Bio-Ecological Indicators for Gentiana pneumonanthe L. Climatic Suitability in the Iberian Peninsula
Abstract
1. Introduction
2. Results
2.1. Bio-Ecological Indicators and Gentiana pneumonanthe Distribution
2.2. Accuracy Evaluation of SDMs
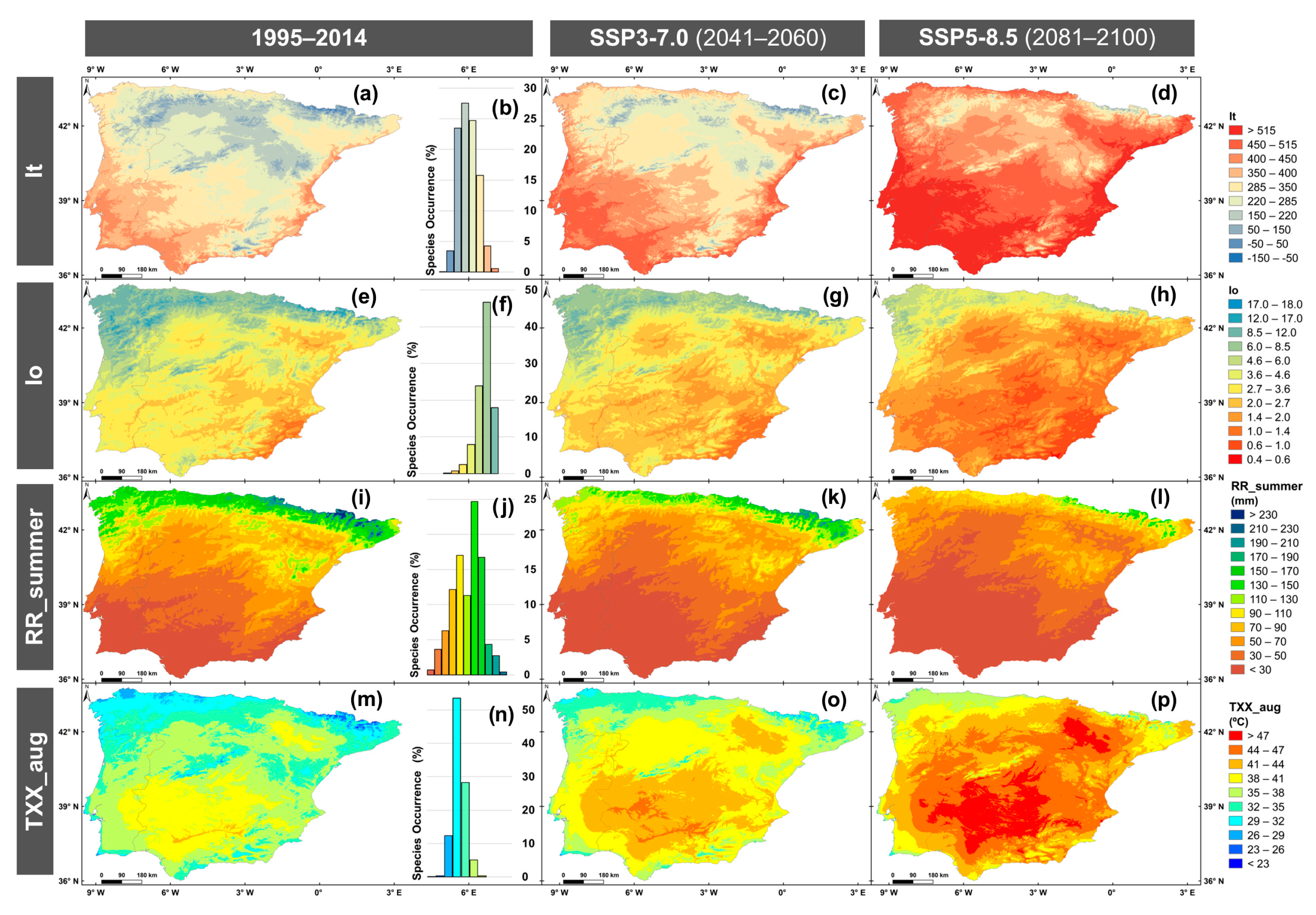
2.3. Climatic Suitability for Gentiana pneumonanthe
2.4. Dynamic Changes in Areas of Climatic Suitability
2.5. Impact of Conservation Areas on Species Viability
3. Discussion
4. Materials and Methods
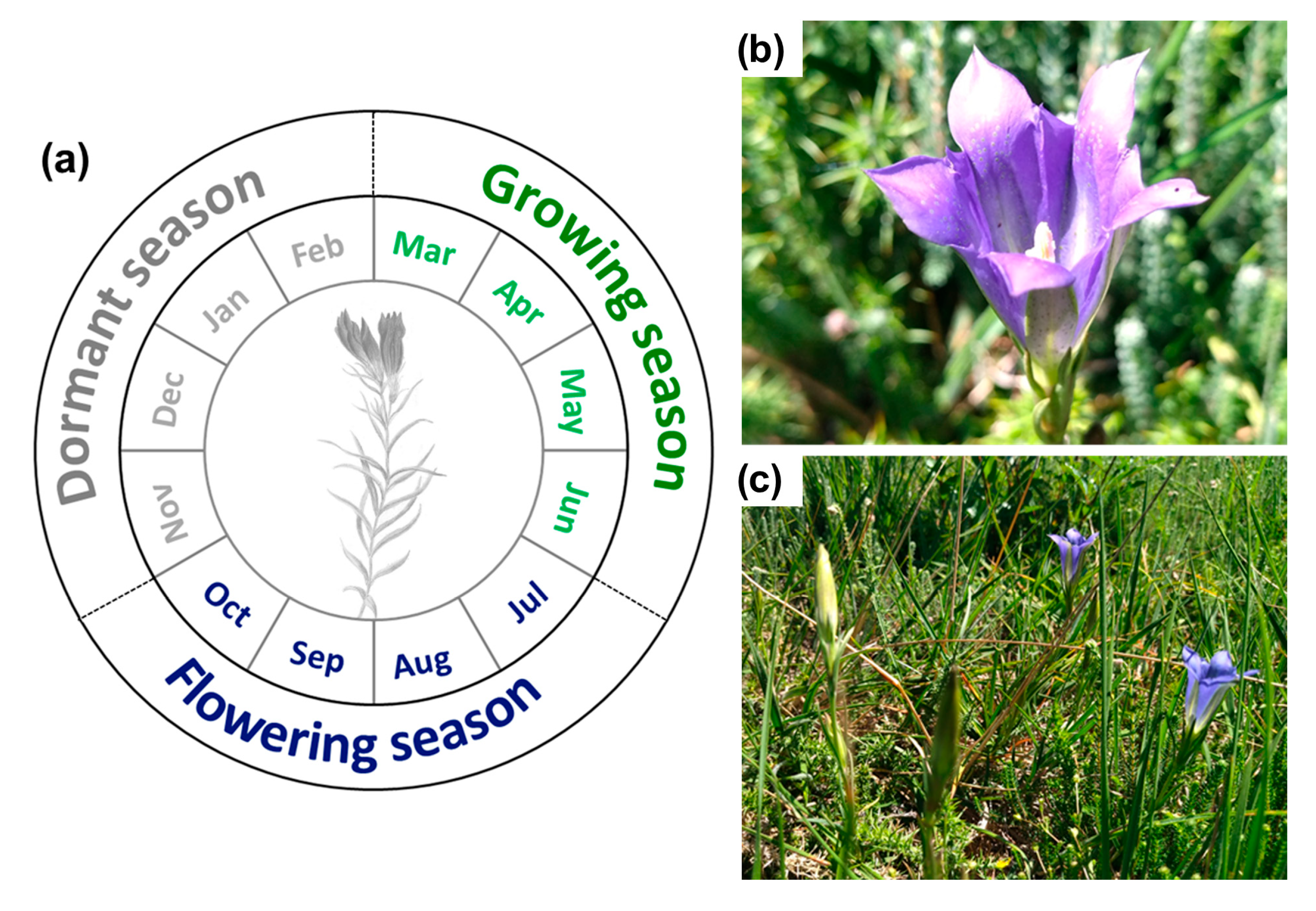
4.1. Description of Gentiana pneumonanthe L. Phenological Stages
4.2. Study Area Description and Gentiana pneumonanthe L. Occurrence Records
4.3. Climate Dataset
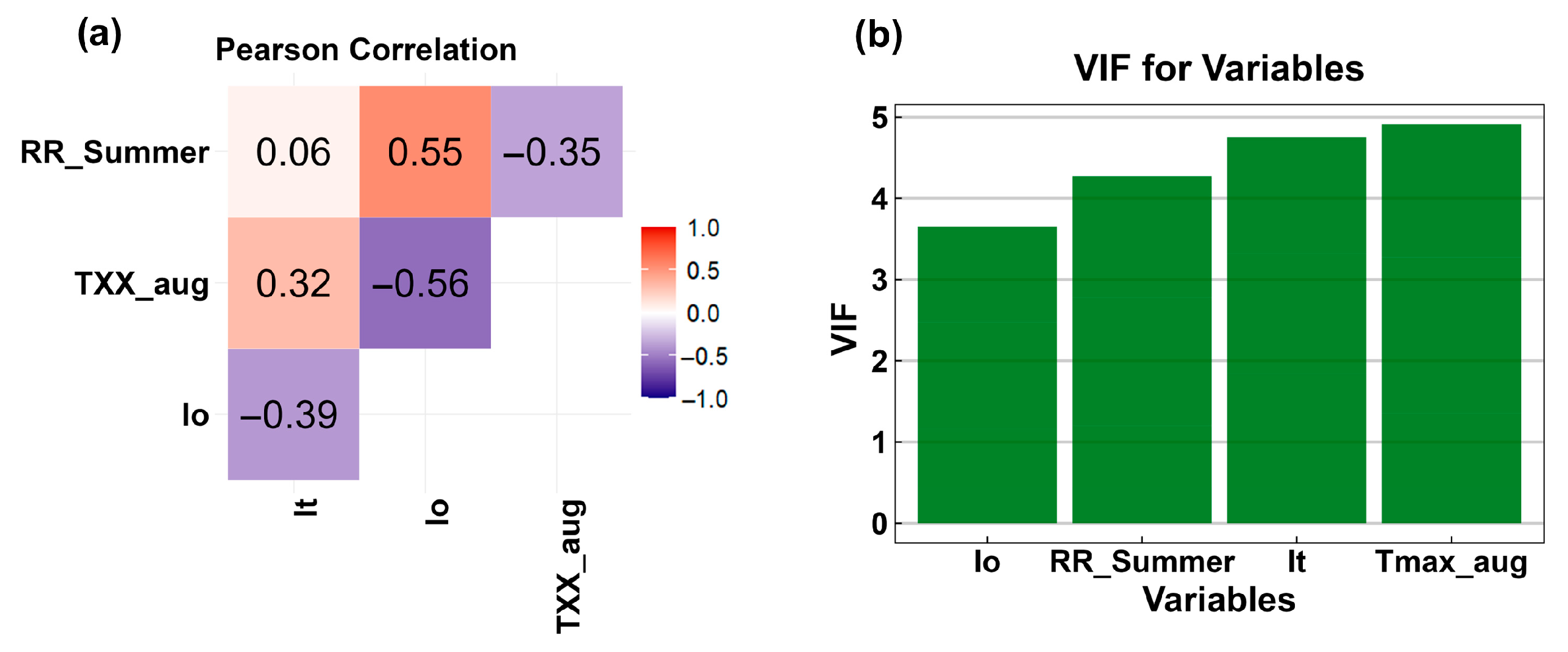
4.4. Bio-Ecological Indicators Related to Species Distribution
- Accumulated summer precipitation from June to August (RR_Summer; mm): This parameter represents the total precipitation from June to August. Since marsh gentian thrives in humid environments, low summer precipitation can be a critical limiting factor for its survival and reproduction [87];
- Maximum of the daily maximum temperature of August (TXX_aug; °C): The marsh gentian distribution is typically restricted at higher temperatures. Elevated maximum temperature, particularly in August, may negatively impact its physiological processes and limit its growth [88].
4.5. Species Distribution Models
4.6. Evaluation of Climatic Suitability in Ecological Conservation Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popović, Z.; Krstić-Milošević, D.; Stefanović, M.; Matić, R.; Vidaković, V.; Bojović, S. Chemical and Morphological Inter- and Intrapopulation Variability in Natural Populations of Gentiana pneumonanthe L. Chem. Biodivers. 2019, 16, e1800509. [Google Scholar] [CrossRef]
- Daoud-Bouattour, A.; Gammar-Ghrabi, Z.; Limam-Ben Saad, S.; Muller, S.D. The IUCN Red List of Threatened Species 2010—Gentiana pneumonanthe (Mediterranean Assessment). Available online: https://www.iucnredlist.org/species/164379/5849081 (accessed on 23 January 2025).
- Ben Saad, S.; Bilz, M.; Daoud-Bouattour, A.; Ghrabi, Z.; Muller, S. The IUCN Red List of Threatened Species 2012—Gentiana pneumonanthe (Europe Assessment). Available online: https://www.iucnredlist.org/species/164379/1047003#geographic-range (accessed on 23 January 2025).
- Oostermeijer, J.; Altenburg, R.M.; Den Nijs, H. Effects of Outcrossing Distance and Selling on Fitness 741 Components in the Rare Gentiana pneumonanthe (Gentianaceae). In Acta Botanica Neerlandica; Wiley: Hoboken, NJ, USA, 1995; pp. 257–268. [Google Scholar]
- Fonseca, A.; Santos, J.; Pádua, L.; Santos, M. Unveiling the Future of Relict Mediterranean Mountain Peatlands by Integrating the Potential Response of Ecological Indicators with Environmental Suitability Assessments. Ecol. Indic. 2023, 157, 111206. [Google Scholar] [CrossRef]
- Pierce, S.; Spada, A.; Caporali, E.; Puglisi, F.; Panzeri, A.; Luzzaro, A.; Cislaghi, S.; Mantegazza, L.; Cardarelli, E.; Labra, M.; et al. Identifying Population Thresholds for Flowering Plant Reproductive Success: The Marsh Gentian (Gentiana pneumonanthe) as a Flagship Species of Humid Meadows and Heathland. Biodivers. Conserv. 2018, 27, 891–905. [Google Scholar] [CrossRef]
- Ramsar Ramsar. Available online: https://www.ramsar.org/ (accessed on 21 May 2025).
- EEA Natura 2000. Available online: https://www.eea.europa.eu/en/datahub/datahubitem-view/6fc8ad2d-195d-40f4-bdec-576e7d1268e4 (accessed on 26 June 2025).
- Warson, J.; Baguette, M.; Stevens, V.M.; Honnay, O.; De Kort, H. The Impact of Habitat Loss on Molecular Signatures of Coevolution between an Iconic Butterfly (Alcon Blue) and Its Host Plant (Marsh Gentian). J. Hered. 2023, 114, 22–34. [Google Scholar] [CrossRef]
- Valdés, A.; Ehrlén, J. Microclimate Influences Plant Reproductive Performance via an Antagonistic Interaction. Basic Appl. Ecol. 2022, 64, 13–29. [Google Scholar] [CrossRef]
- Cormont, A.; Wieger Wamelink, G.W.; Jochem, R.; WallisDeVries, M.F.; Wegman, R.M.A. Host Plant-Mediated Effects of Climate Change on the Occurrence of the Alcon Blue Butterfly (Phengaris alcon). Ecol. Modell. 2013, 250, 329–337. [Google Scholar] [CrossRef]
- Rose, R.J.; Clarke, R.T.; Chapman, S.B. Individual Variation and the Effects of Weather, Age and Flowering History on Survival and Flowering of the Long-Lived Perennial Gentiana pneumonanthe. Ecography 1998, 21, 317–326. [Google Scholar] [CrossRef]
- De Kort, H.; Prunier, J.G.; Tessier, M.; Turlure, C.; Baguette, M.; Stevens, V.M. Interacting Grassland Species under Threat of Multiple Global Change Drivers. J. Biogeogr. 2018, 45, 2133–2145. [Google Scholar] [CrossRef]
- He, X.; Liang, J.; Zeng, G.; Yuan, Y.; Li, X. The Effects of Interaction between Climate Change and Land-Use/Cover Change on Biodiversity-Related Ecosystem Services. Glob. Chall. 2019, 3, 1800095. [Google Scholar] [CrossRef]
- Krzosek, K.; Nowicki, P. Quantification of Land Use Threats to a Flagship Species and Its Meadow Habitats within Urban Landscape. Eur. Zool. J. 2025, 92, 182–194. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Paredes, P.; Fraga, H. Future Aridity and Drought Risk for Traditional and Super-Intensive Olive Orchards in Portugal. Clim. Change 2024, 177, 155. [Google Scholar] [CrossRef]
- Claro, A.M.; Fonseca, A.; Fraga, H.; Santos, J.A. Susceptibility of Iberia to Extreme Precipitation and Aridity: A New High-Resolution Analysis over an Extended Historical Period. Water 2023, 15, 3840. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and Their Energy, Land Use, and Greenhouse Gas Emissions Implications: An Overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Adhikari, B.; Subedi, S.C.; Bhandari, S.; Baral, K.; Lamichhane, S.; Maraseni, T. Climate-Driven Decline in the Habitat of the Endemic Spiny Babbler (Turdoides nipalensis). Ecosphere 2023, 14, e4584. [Google Scholar] [CrossRef]
- Fernandes, A.; Kovač, N.; Fraga, H.; Fonseca, A.; Šućur Radonjić, S.; Simeunović, M.; Ratković, K.; Menz, C.; Costafreda-Aumedes, S.; Santos, J.A. Challenges to Viticulture in Montenegro under Climate Change. ISPRS Int. J. Geoinf. 2024, 13, 270. [Google Scholar] [CrossRef]
- Jia, T.; Qi, Y.; Zhao, H.; Xian, X.; Li, J.; Huang, H.; Yu, W.; Liu, W.X. Estimation of Climate-Induced Increased Risk of Centaurea solstitialis L. Invasion in China: An Integrated Study Based on Biomod2. Front. Ecol. Evol. 2023, 11, 1113474. [Google Scholar] [CrossRef]
- Bracho-Estévanez, C.A.; Arenas-Castro, S.; González-Varo, J.P.; González-Moreno, P. Spatially Explicit Metrics Improve the Evaluation of Species Distribution Models Facing Sampling Biases. Ecol. Inform. 2024, 84, 102916. [Google Scholar] [CrossRef]
- Guo, L.; Gao, Y.; He, P.; He, Y.; Meng, F. Modeling for Predicting the Potential Geographical Distribution of Three Ephedra Herbs in China. Plants 2023, 12, 787. [Google Scholar] [CrossRef] [PubMed]
- Sofaer, H.R.; Jarnevich, C.S.; Pearse, I.S.; Smyth, R.L.; Auer, S.; Cook, G.L.; Edwards, T.C.; Guala, G.F.; Howard, T.G.; Morisette, J.T.; et al. Development and Delivery of Species Distribution Models to Inform Decision-Making. Bioscience 2019, 69, 544–557. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming Limitations of Modelling Rare Species by Using Ensembles of Small Models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Adão, F.; Campos, J.C.; Santos, J.A.; Malheiro, A.C.; Fraga, H. Relocation of Bioclimatic Suitability of Portuguese Grapevine Varieties under Climate Change Scenarios. Front. Plant Sci. 2023, 14, 974020. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Martínez, S.; Rivas Sáenz, S.; Penas, A.; Alcaraz, F.; Amigo, J.; Asensi, A.; Barbour, M.; Biondi, E.; Cantó, P.; Capelo, J.; et al. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1–634. [Google Scholar]
- Costa, R.; Fraga, H.; Fernandes, P.M.; Santos, J.A. Implications of Future Bioclimatic Shifts on Portuguese Forests. Reg. Environ. Change 2017, 17, 117–127. [Google Scholar] [CrossRef]
- Andrade, C.; Fonseca, A.; Santos, J.A. Are Land Use Options in Viticulture and Oliviculture in Agreement with Bioclimatic Shifts in Portugal? Land 2021, 10, 869. [Google Scholar] [CrossRef]
- Araújo, P.V.; Portela-Pereira, E.; Lourenço, J.; Clemente, A.; Clamote, F.; Almeida, J.D.; Pereira, A.J.; Porto, M. Gentiana pneumonanthe L.—Mapa de Distribuição. Available online: http://www.flora-on.pt/#wGentiana+pneumonanthe (accessed on 2 January 2025).
- Cuena-Lombraña, A.; Porceddu, M.; Dettori, C.A.; Bacchetta, G. Predicting the Consequences of Global Warming on Gentiana lutea Germination at the Edge of Its Distributional and Ecological Range. PeerJ 2020, 8, e8894. [Google Scholar] [CrossRef]
- Quinta, L. A Borboleta Da Serra Do Alvão Que Parece Retirada de Uma Fábula. Available online: https://www.nationalgeographic.pt/meio-ambiente/a-borboleta-da-serra-do-alvao-que-parece-retirada-uma-fabula_1245 (accessed on 23 June 2025).
- Kanda, R.Z.; Da, S.S.; Maârouhi, I.M.; Issoufou, A.A.; Ouattara, D. Assessment of Climate Change Impact on Future Distribution of Palm Trees in Niger, West Africa. Discov. Sustain. 2024, 5, 195. [Google Scholar] [CrossRef]
- Karger, D.N.; Wilson, A.M.; Mahony, C.; Zimmermann, N.E.; Jetz, W. Global Daily 1 Km Land Surface Precipitation Based on Cloud Cover-Informed Downscaling. Sci. Data 2021, 8, 307. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuo, Z.; Liu, B.; Peng, Y.; Xu, D. Predicting the Future Geographic Distribution of the Traditional Chinese Medicinal Plant Epimedium acuminatum Franch. in China Using Ensemble Models Based on Biomod2. Plants 2025, 14, 1065. [Google Scholar] [CrossRef] [PubMed]
- Biancolini, D.; Pacifici, M.; Falaschi, M.; Bellard, C.; Blackburn, T.M.; Ficetola, G.F.; Rondinini, C. Global Distribution of Alien Mammals Under Climate Change. Glob. Change Biol. 2024, 30, e17560. [Google Scholar] [CrossRef]
- Campos, J.C.; Albuquerque, B.; Civantos, E.; Honrado, J.P.; Regos, A. Unveiling the Effects of Landscape–Fire Interactions on Functional Diversity in a Southern European Mountain. Ecol. Appl. 2025, 35, e3059. [Google Scholar] [CrossRef]
- Hu, H.; Wei, Y.; Wang, W.; Wang, C. The Influence of Climate Change on Three Dominant Alpine Species under Different Scenarios on the Qinghai–Tibetan Plateau. Diversity 2021, 13, 682. [Google Scholar] [CrossRef]
- Barbosa, W.L.; Alves-Souza, S.N. Data Quality Issues in Data Used in Species Distribution Models: A Systematic Literature Review. Ecol. Inform. 2025, 91, 103378. [Google Scholar] [CrossRef]
- Fiorentino, D.; Núñez-Riboni, I.; Pierce, M.E.; Oesterwind, D.; Akimova, A. Improving Species Distribution Models for Climate Change Studies: Ecological Plausibility and Performance Metrics. Ecol. Modell. 2025, 508, 111207. [Google Scholar] [CrossRef]
- Lienert, J.; Diemer, M.; Schmid, B. Effects of Habitat Fragmentation on Population Structure and Fitness Components of the Wetland Specialist Swertia perennis L. (Gentianaceae) Basic and Applied Ecology. Basic Appl. Ecol. 2002, 3, 101–114. [Google Scholar] [CrossRef]
- Petanidou, T.; Ellis-Adam, A.C.; Den, H.C.M.; Gerard, J.; Oostermeijer, B. Differential Pollination Success in the Course of Individual Flower Development and Flowering Time in Gentiana pneumonanthe L. (Gentianaceae). Bot. J. Linn. Soc. 2001, 135, 25–33. [Google Scholar] [CrossRef]
- Soares, P.O.; Crespi, A.L.; Rodrigues, M.C.; Arnaldo, P.S. The Habitat Vegetational Structure and the Success of the Blue Alcon, Maculinea alcon (Denis & Schiffermüller). Plant Biosyst. 2012, 146, 1–6. [Google Scholar] [CrossRef]
- Surrey Wildlife Trust Marsh Gentian. Available online: https://www.surreywildlifetrust.org/marsh-gentian (accessed on 23 June 2025).
- European Commission The Habitats Directive. Available online: https://environment.ec.europa.eu/topics/nature-and-biodiversity/habitats-directive_en (accessed on 26 June 2025).
- European Parliament of the European Union; Council of the European Union Regulation (EU) 2024/1991 of the European Parliament and of the Council of 24 June 2024 on Nature Restoration and Amending Regulation (EU) 2022/869. Available online: https://eur-lex.europa.eu/eli/reg/2024/1991/oj/eng (accessed on 4 September 2025).
- European Commission; Directorate-General for Environment EU Biodiversity Strategy for 2030. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52020DC0380 (accessed on 8 September 2025).
- Oostermeijer, J.G.B.; Den Nijs, J.C.M.; Raijmann, L.E.L.; Menken, S.B.J. Population Biology and Management of the Marsh Gentian (Gentiana pneumonanthe L.), a Rare Species in The Netherlands. Bot. J. Linn. Soc. 1992, 108, 117–130. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Malatesta, L.; Tardella, F.M. Effects of Grazing vs Mowing on the Functional Diversity of Sub-Mediterranean Productive Grasslands. Appl. Veg. Sci. 2014, 17, 658–669. [Google Scholar] [CrossRef]
- Maes, D.; Pardon, W.; Palmans, G.; Van Dyck, H. The Last of the Maculineans: Can We Save the Emblematic Alcon Blue Butterfly Phengaris alcon under Climate Change When Its Habitat Continues to Deteriorate? J. Insect. Conserv. 2024, 28, 1037–1049. [Google Scholar] [CrossRef]
- Moschetti, M.; Besnard, A.; Couturier, T.; Fonderflick, J. Grazing Intensity Negatively Affects the Maintenance of Gentiana pneumonanthe and the Survival of Phengaris alcon Egg-Laying. J. Insect. Conserv. 2020, 24, 343–351. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fonseca, A.; Fraga, H. Evaluation of Historical and Future Thermal Conditions for Almond Trees in North—Eastern Portugal. Clim. Change 2023, 176, 89. [Google Scholar] [CrossRef]
- Raijmann, L.E.L.; Van Leeuwen, N.C.; Kersten, R.; Oostermeijer, J.G.B.; Den Nijs, H.C.M.; Menken, S.B.J. Genetic Variation and Outcrossing Rate in Relation to Population Size in Gentiana pneumonanthe L. Conserv. Biol. 1994, 8, 1014–1026. [Google Scholar] [CrossRef]
- Anthos Listados de Gentiana Pneumonanthe (Fam. Gentianaceae) y Táxones Infraespecíficos. Available online: http://www.anthos.es/ (accessed on 15 May 2025).
- GBIF.org GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0051735-241126133413365 (accessed on 30 December 2024).
- iNaturalist.org INaturalist-Gentiana Pneumonanthe. Available online: https://www.inaturalist.org/observations/export?flow_task_id=516049 (accessed on 3 January 2025).
- GBIF.org GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0002669-250415084134356 (accessed on 16 April 2025).
- Huang, D.; An, Q.; Huang, S.; Tan, G.; Quan, H.; Chen, Y.; Zhou, J.; Liao, H. Biomod2 Modeling for Predicting the Potential Ecological Distribution of Three Fritillaria Species under Climate Change. Sci. Rep. 2023, 13, 18801. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Modell. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Swart, N.C.; Cole, J.N.S.; Kharin, V.V.; Lazare, M.; Scinocca, J.F.; Gillett, N.P.; Anstey, J.; Arora, V.; Christian, J.R.; Hanna, S.; et al. The Canadian Earth System Model Version 5 (CanESM5.0.3). Geosci. Model Dev. 2019, 12, 4823–4873. [Google Scholar] [CrossRef]
- Voldoire, A.; Saint-Martin, D.; Sénési, S.; Decharme, B.; Alias, A.; Chevallier, M.; Colin, J.; Guérémy, J.F.; Michou, M.; Moine, M.P.; et al. Evaluation of CMIP6 DECK Experiments With CNRM-CM6-1. J. Adv. Model. Earth Syst. 2019, 11, 2177–2213. [Google Scholar] [CrossRef]
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM Earth System Model, CNRM-ESM2-1: Role of Earth System Processes in Present-Day and Future Climate. J. Adv. Model. Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef]
- Döscher, R.; Acosta, M.; Alessandri, A.; Anthoni, P.; Arsouze, T.; Bergman, T.; Bernardello, R.; Boussetta, S.; Caron, L.P.; Carver, G.; et al. The EC-Earth3 Earth System Model for the Coupled Model Intercomparison Project 6. Geosci. Model Dev. 2022, 15, 2973–3020. [Google Scholar] [CrossRef]
- Lurton, T.; Balkanski, Y.; Bastrikov, V.; Bekki, S.; Bopp, L.; Braconnot, P.; Brockmann, P.; Cadule, P.; Contoux, C.; Cozic, A.; et al. Implementation of the CMIP6 Forcing Data in the IPSL-CM6A-LR Model. J. Adv. Model. Earth Syst. 2020, 12, e2019MS001940. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F.; et al. Description and Basic Evaluation of Simulated Mean State, Internal Variability, and Climate Sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Gutjahr, O.; Putrasahan, D.; Lohmann, K.; Jungclaus, J.H.; Von Storch, J.S.; Brüggemann, N.; Haak, H.; Stössel, A. Max Planck Institute Earth System Model (MPI-ESM1.2) for the High-Resolution Model Intercomparison Project (HighResMIP). Geosci. Model Dev. 2019, 12, 3241–3281. [Google Scholar] [CrossRef]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M.; et al. The Meteorological Research Institute Earth System Model Version 2.0, MRI-ESM2.0: Description and Basic Evaluation of the Physical Component. J. Meteorol. Soc. Jpn. 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Sellar, A.A.; Jones, C.G.; Mulcahy, J.P.; Tang, Y.; Yool, A.; Wiltshire, A.; O’Connor, F.M.; Stringer, M.; Hill, R.; Palmieri, J.; et al. UKESM1: Description and Evaluation of the U.K. Earth System Model. J. Adv. Model. Earth Syst. 2019, 11, 4513–4558. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Frieler, K.; Volkholz, J.; Lange, S.; Schewe, J.; Mengel, M.; Del Rocío Rivas López, M.; Otto, C.; Reyer, C.P.O.; Karger, D.N.; Malle, J.T.; et al. Scenario Setup and Forcing Data for Impact Model Evaluation and Impact Attribution within the Third Round of the Inter-Sectoral Impact Model Intercomparison Project (ISIMIP3a). Geosci. Model Dev. 2024, 17, 1–51. [Google Scholar] [CrossRef]
- Lange, S. ISIMIP3b Bias Adjustment Fact Sheet. 2021. Available online: https://www.isimip.org/documents/413/ISIMIP3b_bias_adjustment_fact_sheet_Gnsz7CO.pdf (accessed on 26 June 2025).
- Lange, S. ISIMIP3BASD (2.5.0). Zenodo 2021. Available online: https://zenodo.org/records/4686991 (accessed on 26 June 2025).
- Lange, S. Trend-Preserving Bias Adjustment and Statistical Downscaling with ISIMIP3BASD (v1.0). Geosci. Model Dev. 2019, 12, 3055–3070. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Brun, P.; Zimmermann, N.E.; Hari, C.; Pellissier, L.; Karger, D.N. Global Climate-Related Predictors at Kilometer Resolution for the Past and Future. Earth Syst. Sci. Data 2022, 14, 5573–5603. [Google Scholar] [CrossRef]
- Martinez, A.; Iglesias, G. Wind Resource Evolution in Europe under Different Scenarios of Climate Change Characterised by the Novel Shared Socioeconomic Pathways. Energy Convers. Manag. 2021, 234, 113961. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Fernandes, A.; Menz, C.; Paredes, P.; Fraga, H. Future Agroclimatic Suitability for Oliviculture in Portugal Based on a New High-Resolution Climate Dataset. Mitig. Adapt. Strateg. Glob. Change 2025, 30, 56. [Google Scholar] [CrossRef]
- Mesquita, S.; Sousa, A.J. Bioclimatic Mapping Using Geostatistical Approaches: Application to Mainland Portugal. Int. J. Climatol. 2009, 29, 2156–2170. [Google Scholar] [CrossRef]
- Loidi, J.; Navarro-Sánche, G.; Vynokurov, D. Climatic Definitions of the World’s Terrestrial Biomes. Veg. Classif. Surv. 2022, 3, 231–271. [Google Scholar] [CrossRef]
- Fonseca, A.R.; Santos, J.A. High-Resolution Temperature Datasets in Portugal from a Geostatistical Approach: Variability and Extremes. J. Appl. Meteorol. Climatol. 2018, 57, 627–644. [Google Scholar] [CrossRef]
- Andrade, C.; Contente, J.; Santos, J.A. Climate Change Projections of Aridity Conditions in the Iberian Peninsula. Water 2021, 13, 2035. [Google Scholar] [CrossRef]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance Inflation Factor: As a Condition for the Inclusion of Suppressor Variable(s) in Regression Analysis. Open J. Stat. 2015, 5, 754–767. [Google Scholar] [CrossRef]
- Gopar-Merino, L.F.; Velazquez, A.; De Azcarate, J.G. Bioclimatic Mapping as a New Method to Assess Effects of Climatic Change. Ecosphere 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the Worldwide Bioclimatic Classification. J. Maps 2014, 10, 538–553. [Google Scholar] [CrossRef]
- Monteiro-Henriques, T.; Martins, M.J.; Cerdeira, J.O.; Silva, P.; Arsénio, P.; Silva, A.; Bellu, A.; Costa, J.C. Bioclimatological Mapping Tackling Uncertainty Propagation: Application to Mainland Portugal. Int. J. Climatol. 2016, 36, 400–411. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Cahalan, C.; Ulak, S. Effects of Predicted Reduced Summer Rainfall on Growth and Development of Silver Birch (Betula Pendula Roth) and Downy Birch (Betula Pubescens Ehrh). For. J. Inst. For. 2018, 15, 28–44. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Santini, L.; Benítez-López, A.; Maiorano, L.; Čengić, M.; Huijbregts, M.A.J. Assessing the Reliability of Species Distribution Projections in Climate Change Research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive Performance of Presence-Only Species Distribution Models: A Benchmark Study with Reproducible Code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- McCullagh, P. Generalized Linear Models. Eur. J. Oper. Res. 1984, 16, 285–292. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models. Stat. Sci. 1986, 1, 297–310. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Ridgeway, G. The State of Boosting; University of Washington: Washington, DC, USA, 1999. [Google Scholar]
- Konstantinov, A.V.; Utkin, L.V. Interpretable Machine Learning with an Ensemble of Gradient Boosting Machines. Knowl. Based Syst. 2021, 222, 106993. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Routledge: Oxfordshire, UK, 2017; ISBN 9781315139470. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth: Belmont, CA, USA, 1984. [Google Scholar]
- Friedman, J.H. Multivariate Adaptive Regression Splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Boehmke, B.; Greenwell, B. Hands-On Machine Learning with R. Available online: https://bradleyboehmke.github.io/HOML/ (accessed on 23 May 2025).
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- CAWCR WWRP/WGNE Joint Working Group on Forecast Verification Research. Available online: https://community.wmo.int/en/activity-areas/wwrp/wwrp-working-groups/wwrp-forecast-verification-research (accessed on 9 September 2025).
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Modell. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Somodi, I.; Bede-Fazekas, Á.; Botta-Dukát, Z.; Molnár, Z. Confidence and Consistency in Discrimination: A New Family of Evaluation Metrics for Potential Distribution Models. Ecol. Modell. 2024, 491, 110667. [Google Scholar] [CrossRef]
- Guéguen, M.; Blancheteau, H.; Thuiller, W. Biomod2: Ensemble Platform for Species Distribution Modeling 2025. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 23 December 2024).
- Patiño, J.; Collart, F.; Vanderpoorten, A.; Martin-Esquivel, J.L.; Naranjo-Cigala, A.; Mirolo, S.; Karger, D.N. Spatial Resolution Impacts Projected Plant Responses to Climate Change on Topographically Complex Islands. Divers. Distrib. 2023, 29, 1245–1262. [Google Scholar] [CrossRef]
- Elith, J.; Ferrier, S.; Huettmann, F.; Leathwick, J. The Evaluation Strip: A New and Robust Method for Plotting Predicted Responses from Species Distribution Models. Ecol. Modell. 2005, 186, 280–289. [Google Scholar] [CrossRef]
- ICNF Áreas Protegidas Que Integram a Rede Nacional de Áreas Protegidas. Available online: https://www.icnf.pt/conservacao/rnapareasprotegidas (accessed on 21 May 2025).
- ICNF Rede Natura 2000. Available online: https://www.icnf.pt/conservacao/redenatura2000 (accessed on 23 May 2025).
- MITECO Red Natura 2000. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/espacios-protegidos/red-natura-2000.html (accessed on 23 May 2025).
- MITECO Reservas de La Biosfera. Available online: https://www.miteco.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/mab_descargas.html (accessed on 21 May 2025).
- ICNF Informação Geográfica. Available online: https://geocatalogo.icnf.pt/catalogo_tema1.html (accessed on 23 May 2025).
- MITECO Lista Ramsar y Aportación Española. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/ecosistemas-y-conectividad/conservacion-de-humedales/ch_hum_ramsar_esp_lista.html (accessed on 23 May 2025).




| Evaluation Metric | Sensitivity | Specificity | Calibration |
|---|---|---|---|
| TSS | 0.896 | 0.841 | 0.740 |
| AUCroc | 0.868 | 0.870 | 0.955 |
| BOYCE | 0.843 | 0.895 | 0.975 |
| BIAS | 0.591 | 0.982 | 0.936 |
| CSI | 0.498 | 0.991 | 0.411 |
| Modelling Algorithms | Abbreviation | Description |
|---|---|---|
| Generalized linear models [93] | GLM | GLM is a statistical regression model that assumes a linear relationship between the response variability and predictors [92]. |
| Generalized additive models [94] | GAM | An extension of GLM, but uses nonparametric smooth functions [91]. |
| Random forests [95] | RF | RF is an ensemble of multiple decision trees [92]. |
| Generalised boosted models [96] | GBM | GBM is a machine learning model that sequentially combines single decision trees [97]. |
| Classification tree analysis [98] | CTA | A simple decision tree model [99]. |
| Multivariate adaptive regression splines [100] | MARS | Mars employs piecewise linear regression to model complex relationships [101]. |
| Evaluation Metrics | Abbreviation | Description |
|---|---|---|
| Area under the receiver operating characteristic curve [103] | AUCroc | Measures the model’s ability to distinguish between presence and absence [103]. |
| Bias score (frequency bias) [104] | BIAS | Measures the ratio of the frequency of predicted events to observed events [104]. |
| Boyce Index [105] | BOYCE | Evaluates the performance of presence-only models within the known presence-only [106]. |
| Critical Success Index (threat score) [104] | CSI | Indicates the fraction of correctly predicted presences (hits) out of all actual and predicted presences [104]. |
| Directive | Designation of Area | Database | Collection |
|---|---|---|---|
| Natura 2000 Network [45] | Rede Nacional de Áreas Protegidas (RNAP) | [110] | Natura 2000 |
| Sites of Community Importance (SCI) | [111,112] | ||
| Special Areas of Conservation (SAC) | |||
| Man and the Biosphere Programme (MAB) | Biosphere Reserves | [113,114] | |
| Convention on Wetlands [7] | Wetlands of International Importance (RAMSAR) | [115,116] | RAMSAR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, T.R.; Martins, S.; Jesus, J.; Campos, J.; Fernandes, A.; Menz, C.; Maravalhas, E.; Fraga, H.; Santos, J.A. Bio-Ecological Indicators for Gentiana pneumonanthe L. Climatic Suitability in the Iberian Peninsula. Plants 2025, 14, 2857. https://doi.org/10.3390/plants14182857
Freitas TR, Martins S, Jesus J, Campos J, Fernandes A, Menz C, Maravalhas E, Fraga H, Santos JA. Bio-Ecological Indicators for Gentiana pneumonanthe L. Climatic Suitability in the Iberian Peninsula. Plants. 2025; 14(18):2857. https://doi.org/10.3390/plants14182857
Chicago/Turabian StyleFreitas, Teresa R., Sílvia Martins, Joaquim Jesus, João Campos, António Fernandes, Christoph Menz, Ernestino Maravalhas, Helder Fraga, and João A. Santos. 2025. "Bio-Ecological Indicators for Gentiana pneumonanthe L. Climatic Suitability in the Iberian Peninsula" Plants 14, no. 18: 2857. https://doi.org/10.3390/plants14182857
APA StyleFreitas, T. R., Martins, S., Jesus, J., Campos, J., Fernandes, A., Menz, C., Maravalhas, E., Fraga, H., & Santos, J. A. (2025). Bio-Ecological Indicators for Gentiana pneumonanthe L. Climatic Suitability in the Iberian Peninsula. Plants, 14(18), 2857. https://doi.org/10.3390/plants14182857











