The tae-miR164-TaNAC6A Module from Winter Wheat Could Enhance Cold Tolerance in Transgenic Arabidopsis thaliana
Abstract
1. Introduction
2. Results
2.1. Analysis of Conserved Motifs and Gene Structures of tae-miR164 and TaNAC6A
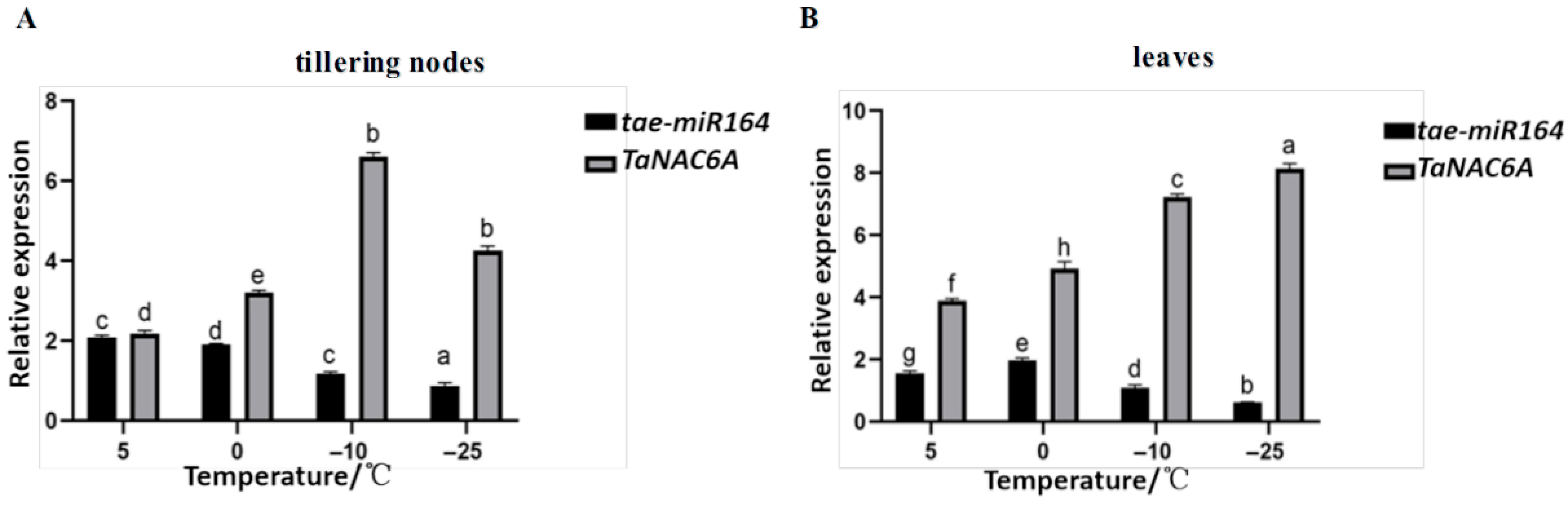
2.2. Expressions of tae-miR164 and TaNAC6A of Dn1 Under Cold Stress
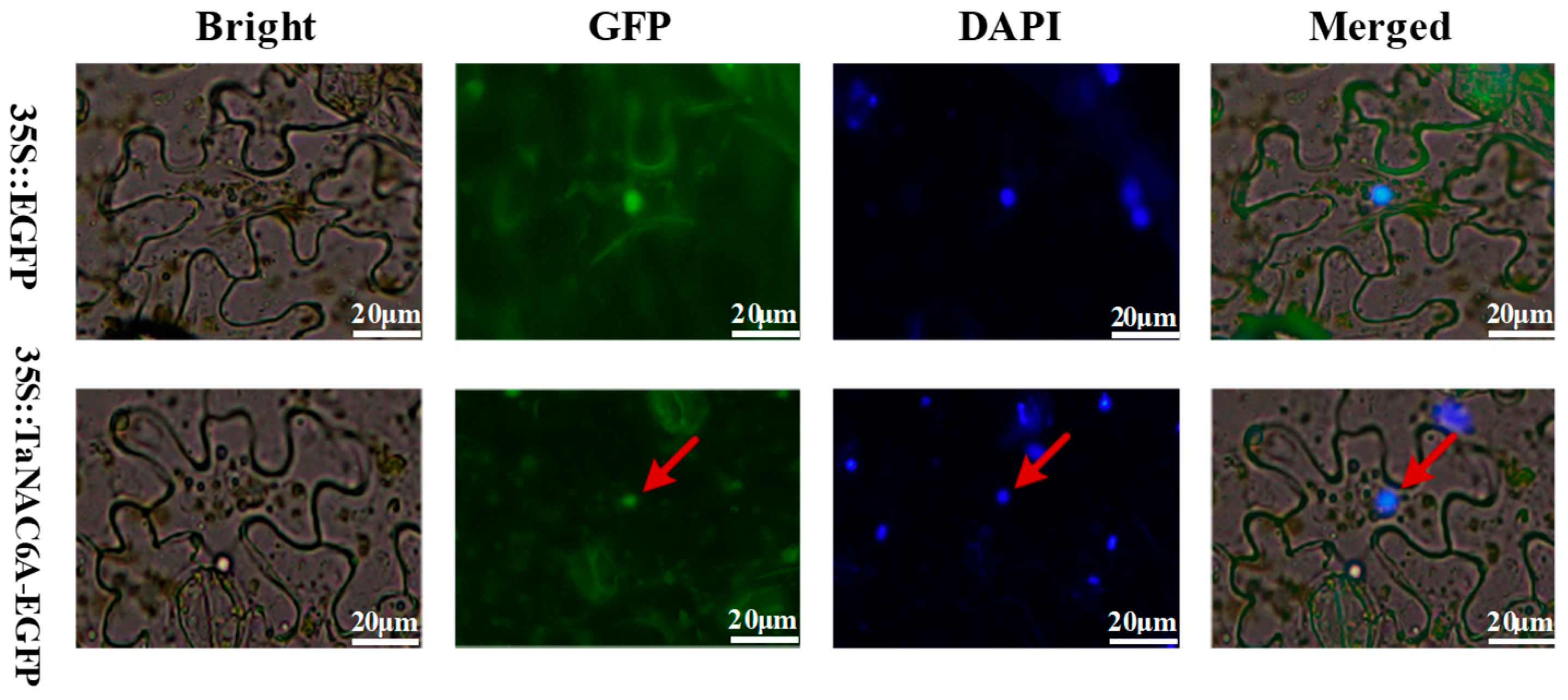
2.3. Subcellular Localization of the TaNAC6A Protein
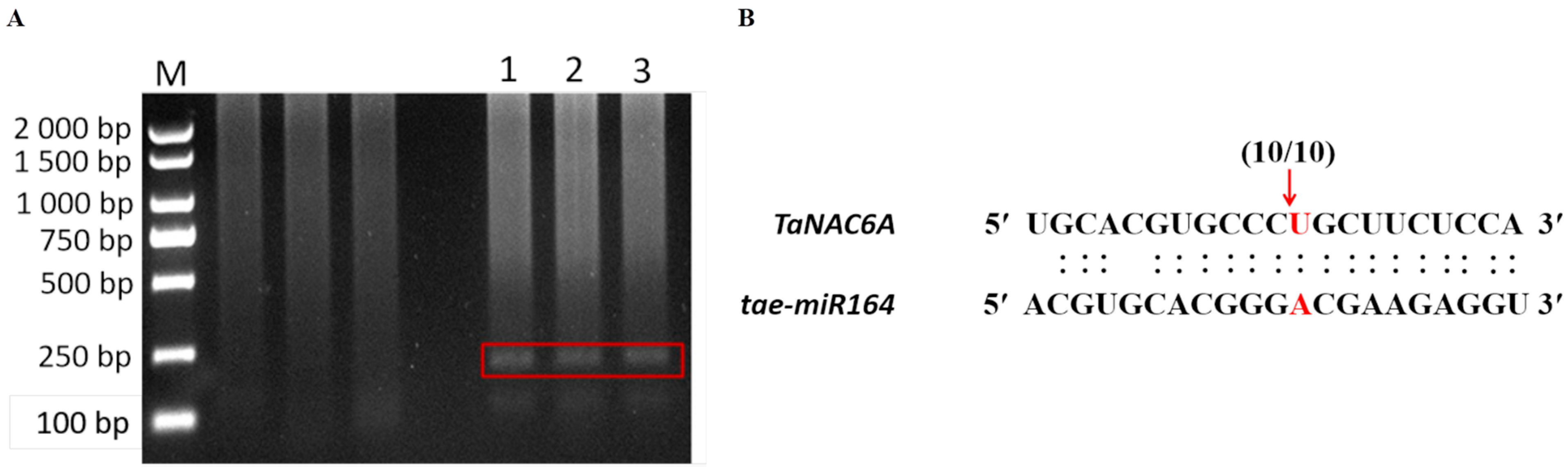
2.4. Verification of Low-Temperature Responsiveness of Pre-tae-miR164 and TaNAC6A
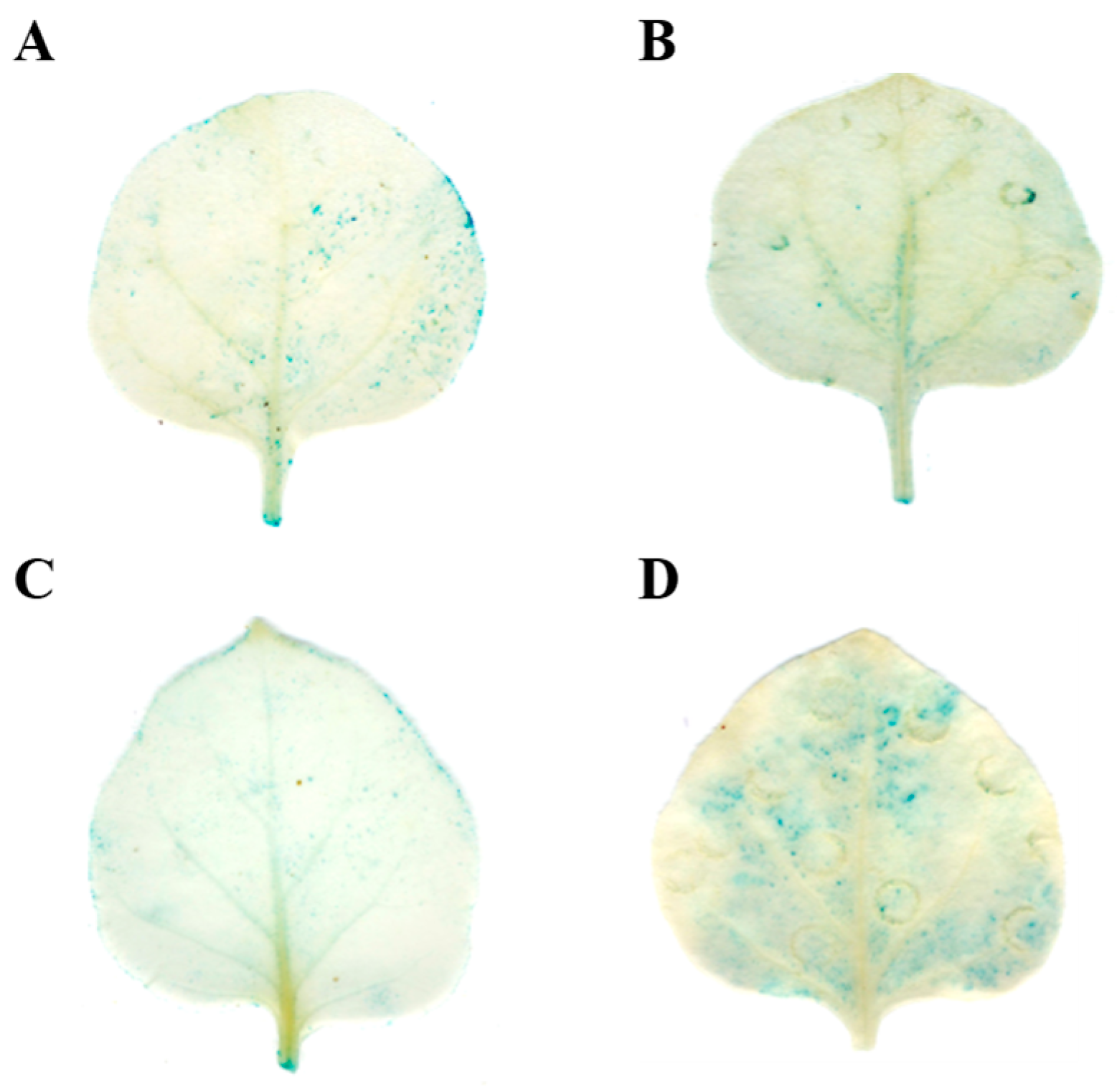
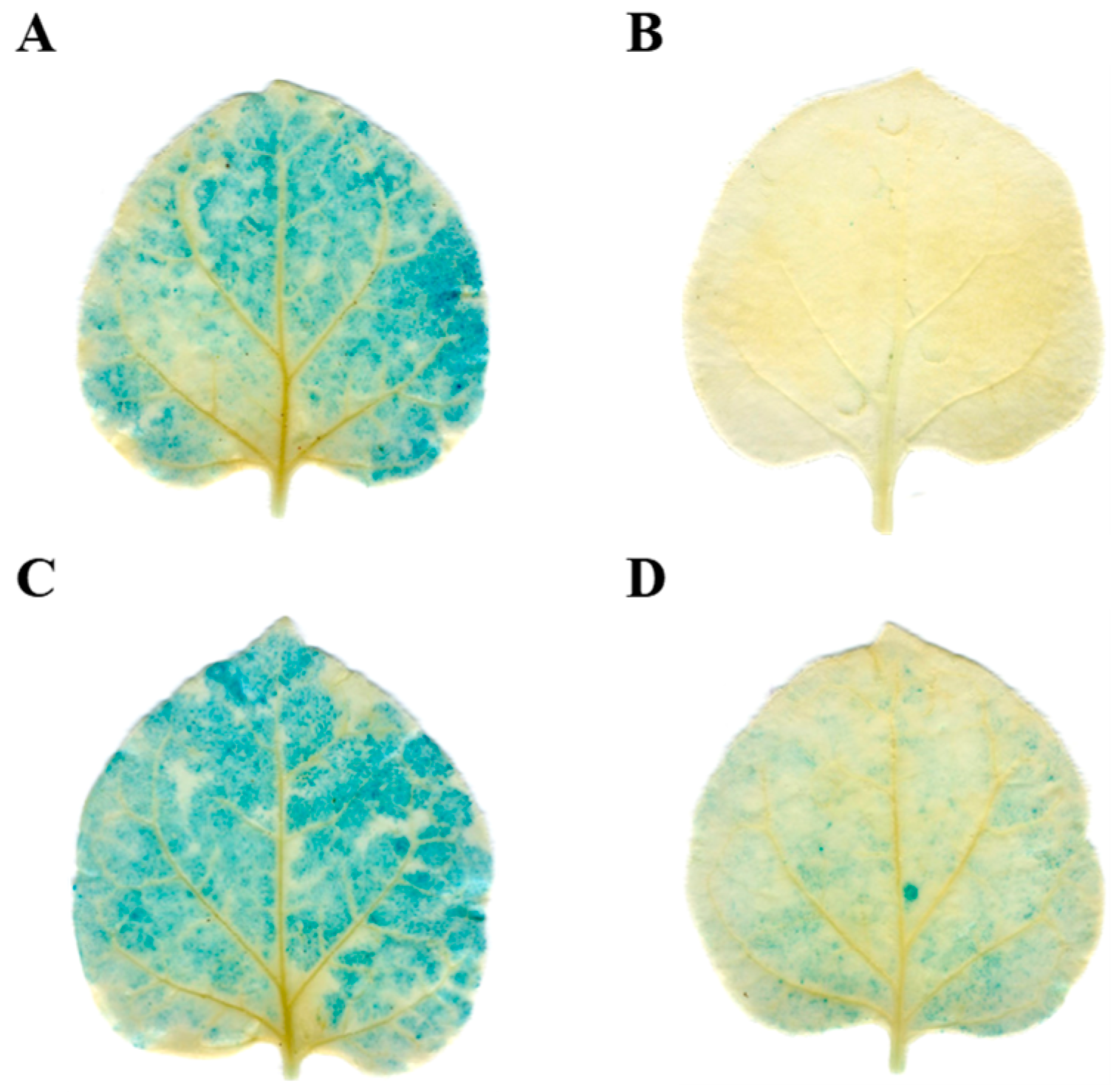
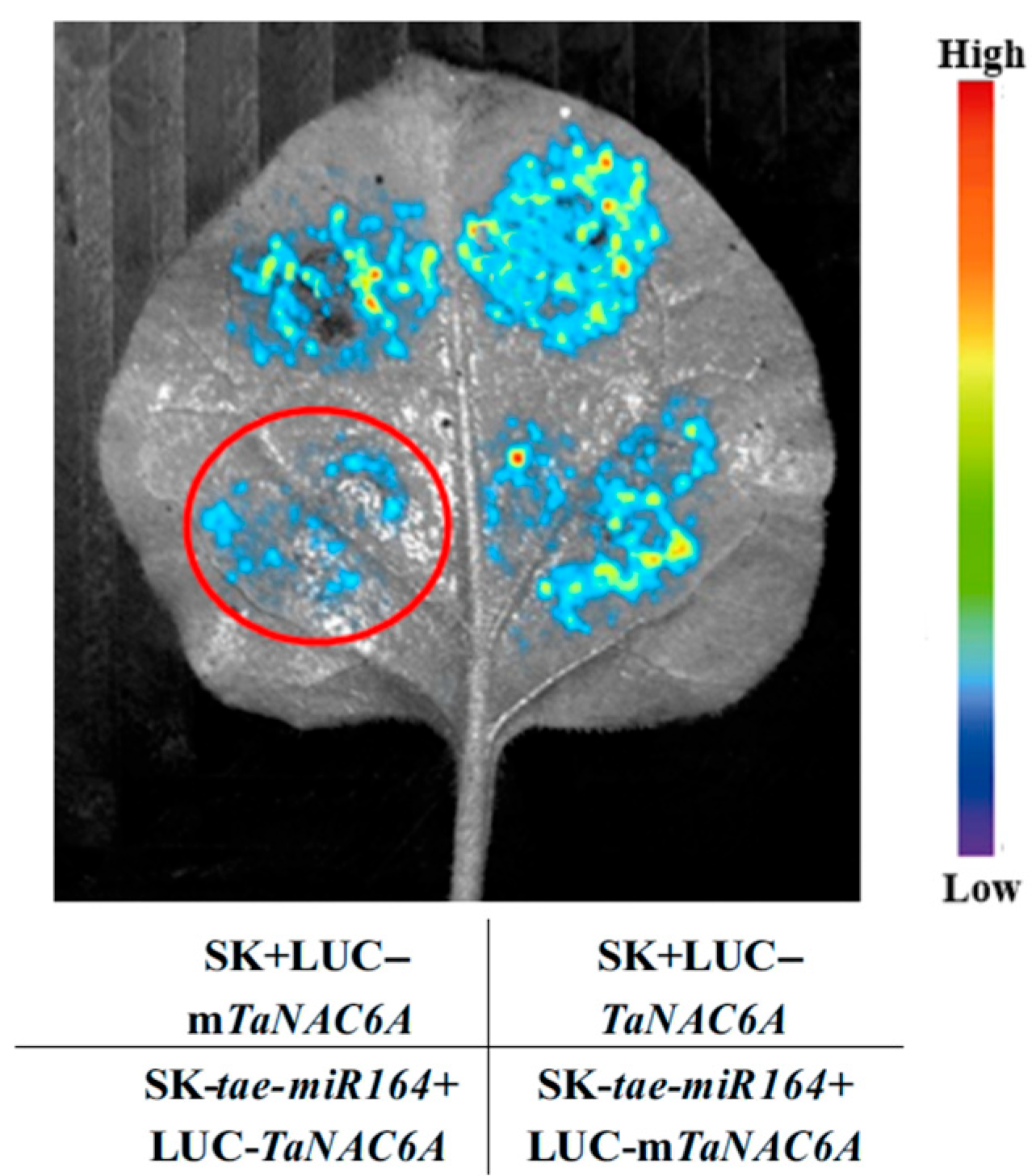
2.5. Validation of the tae-miR164-TaNAC6A Regulatory Relationship
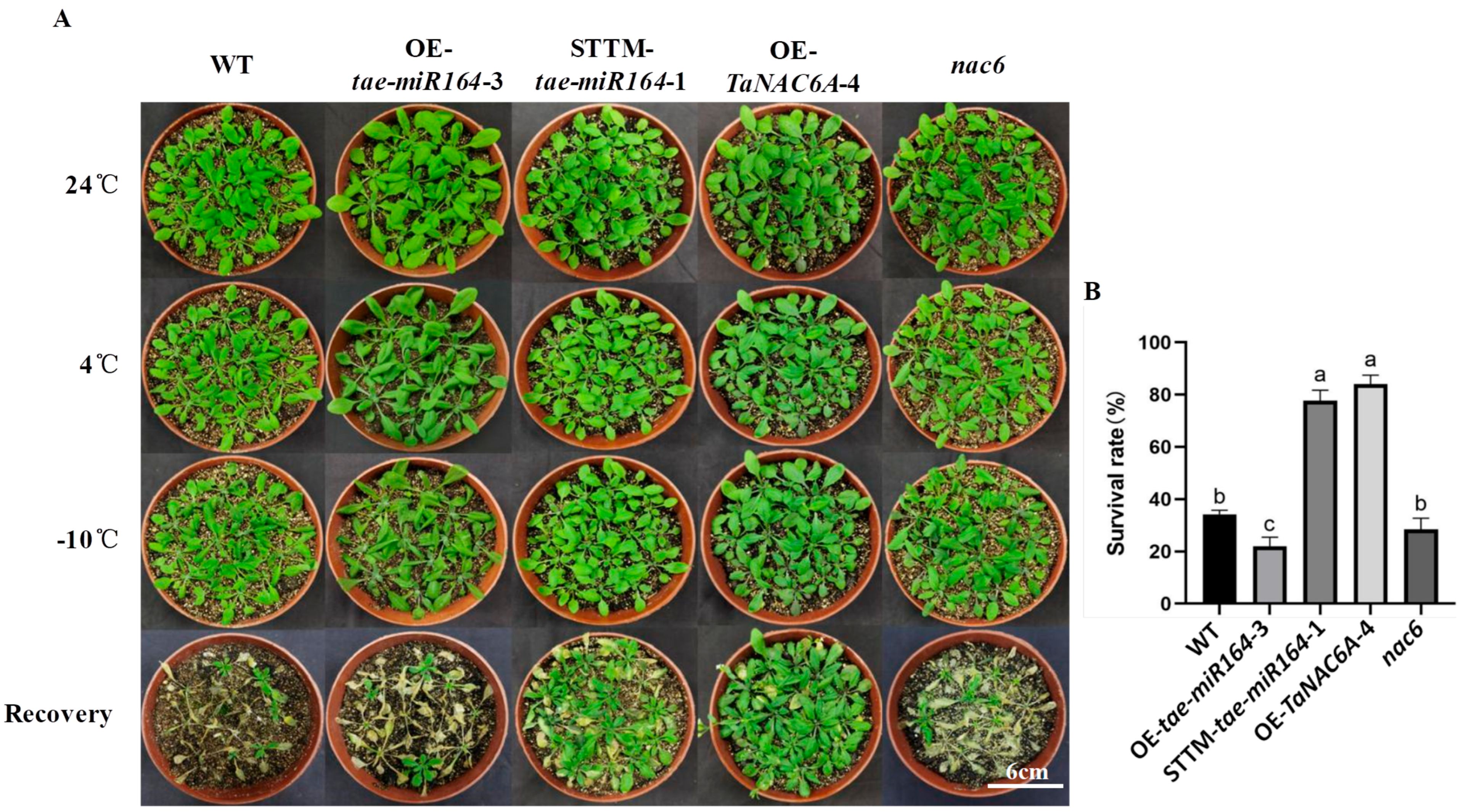
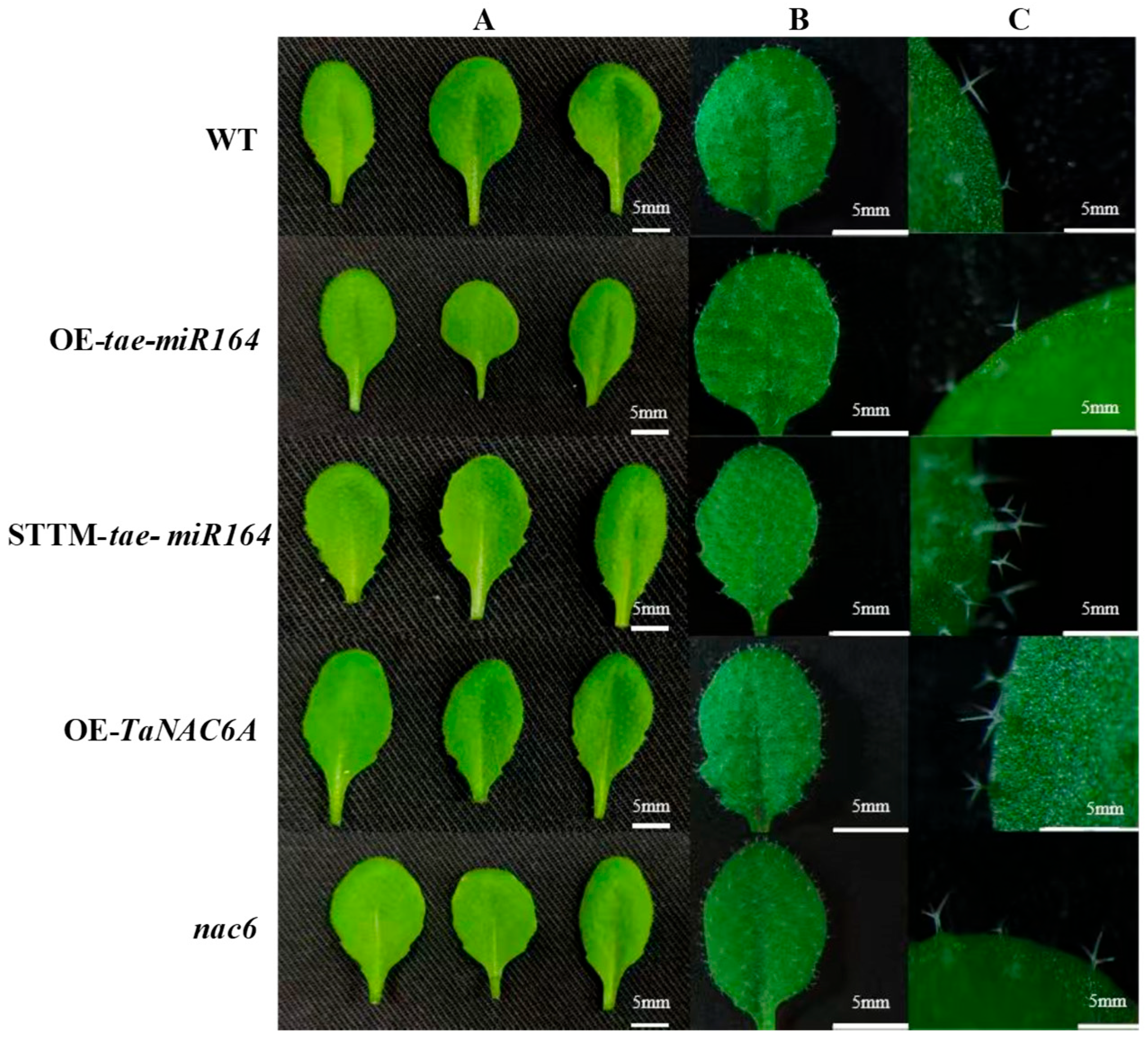
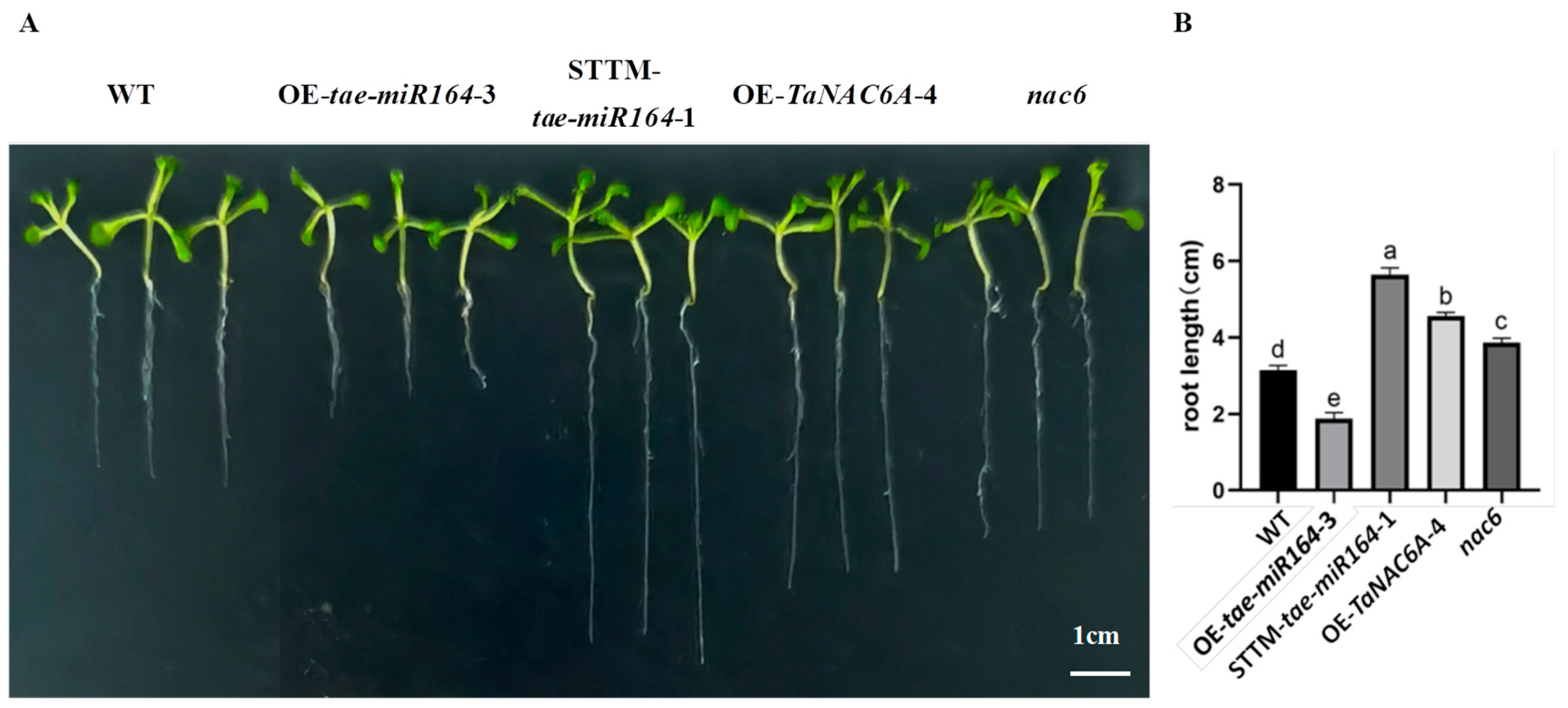
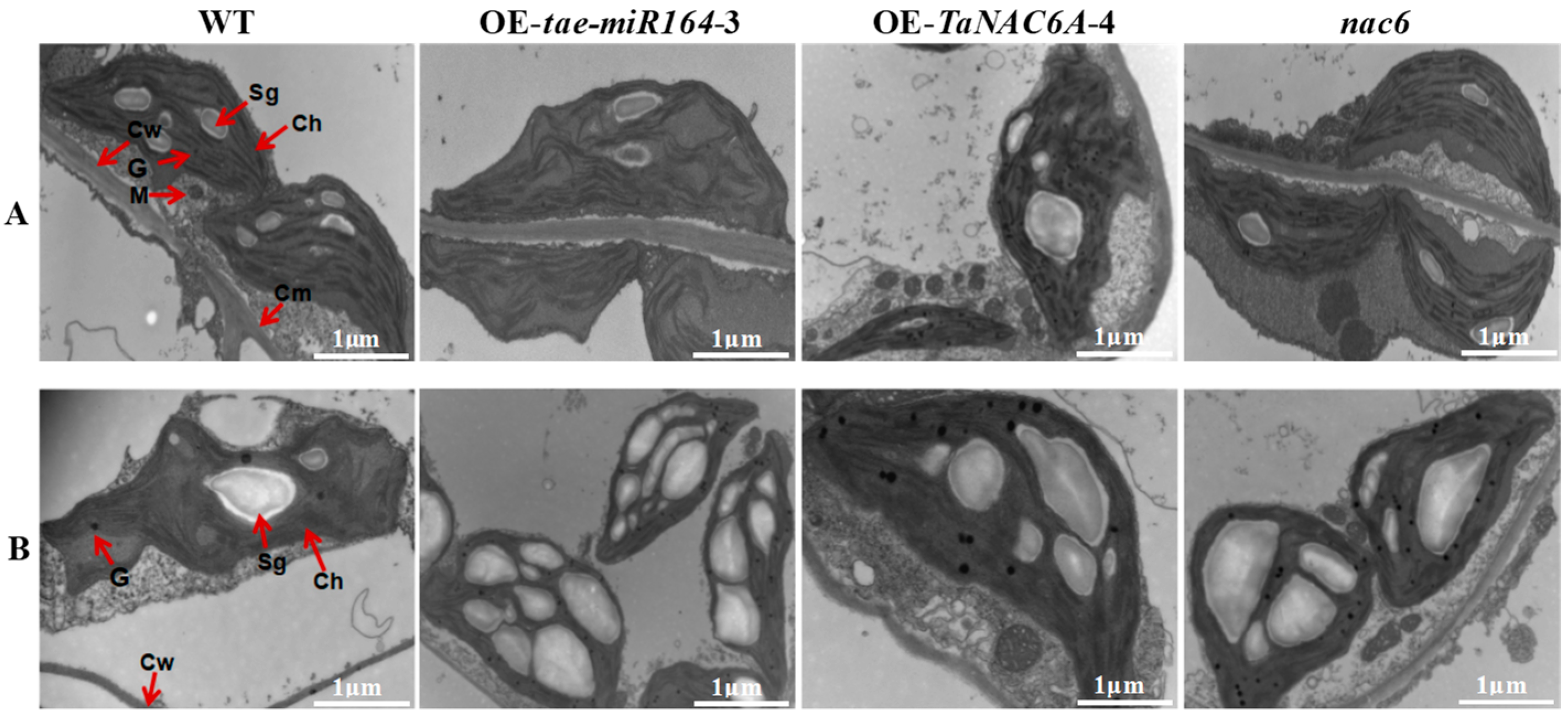
2.6. Phenotypic Changes in Transgenic Arabidopsis Plants
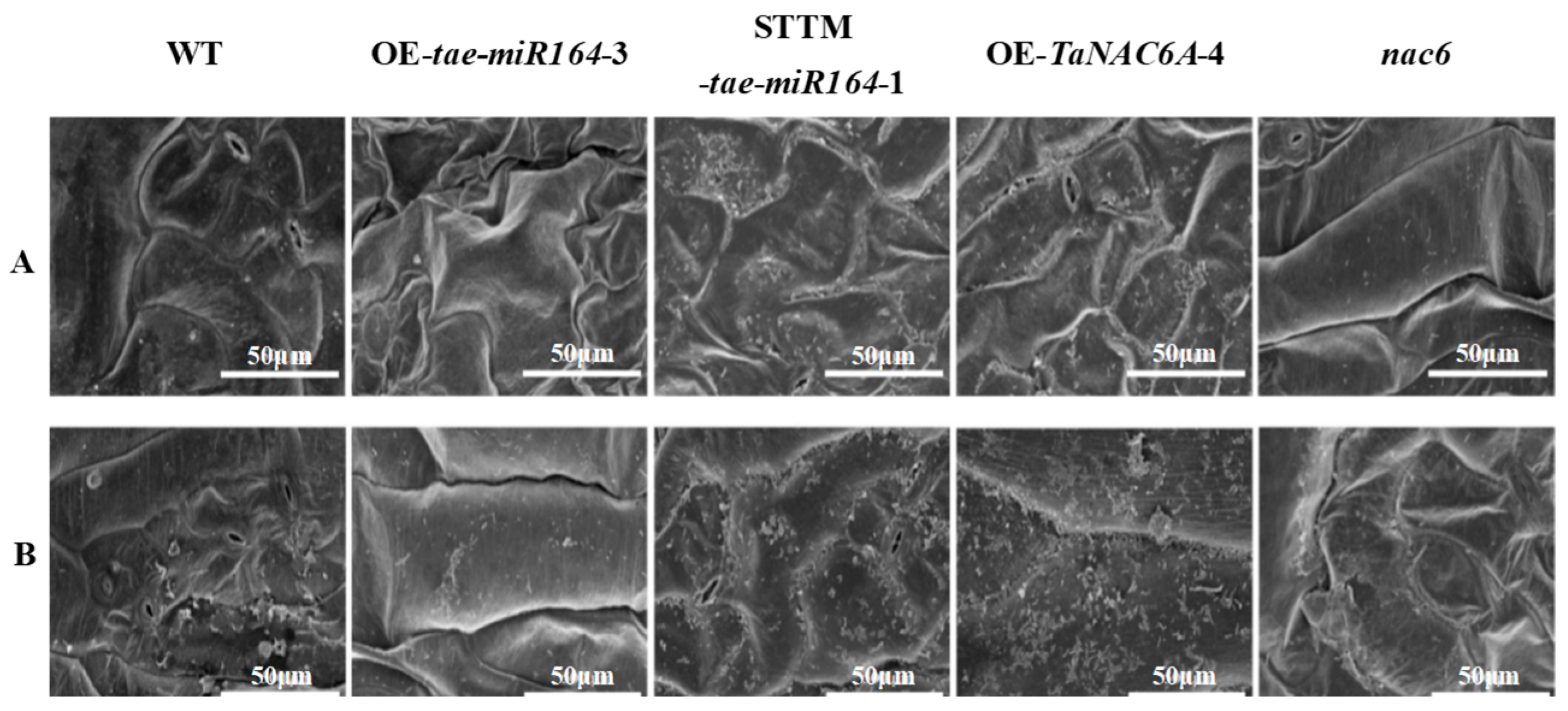
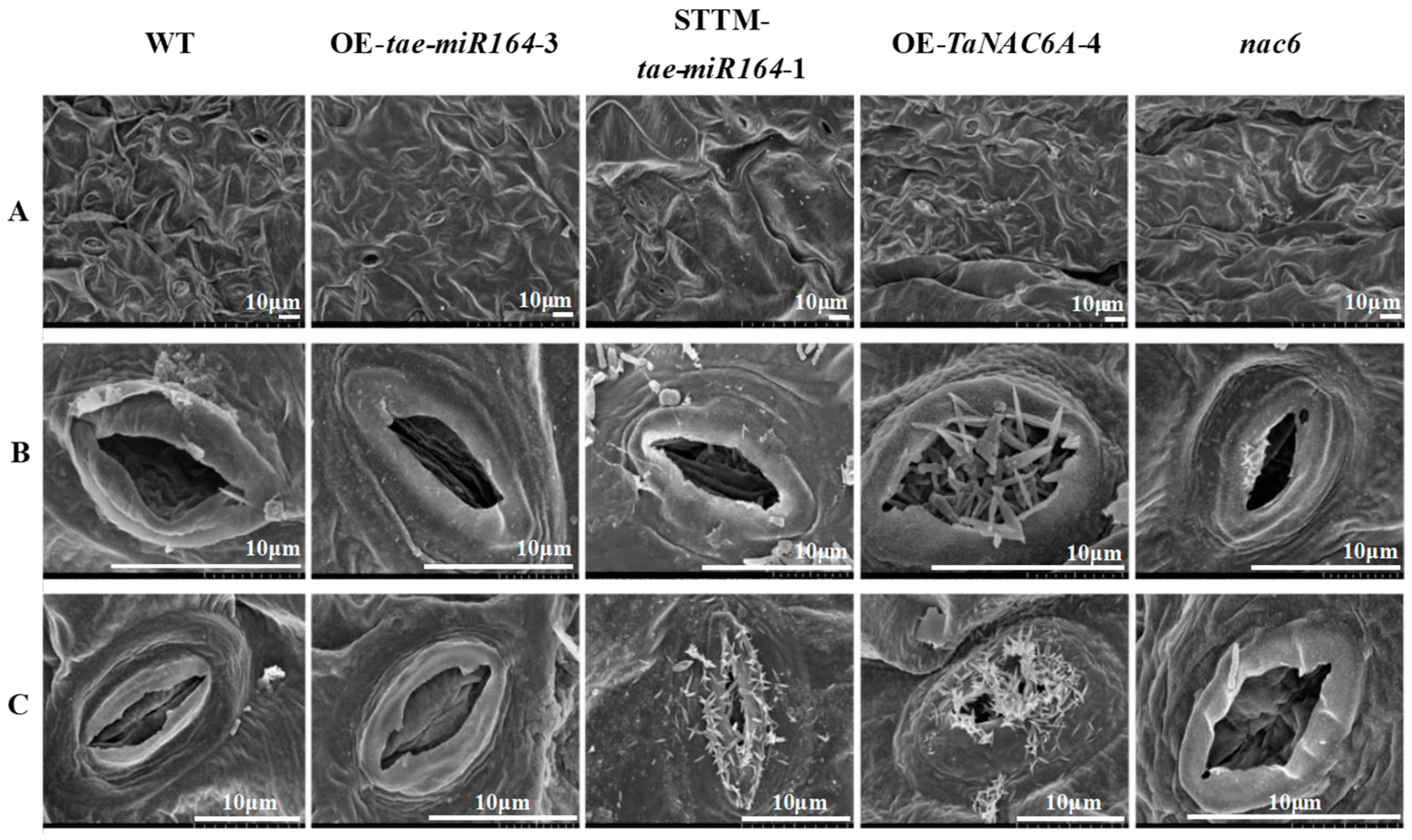
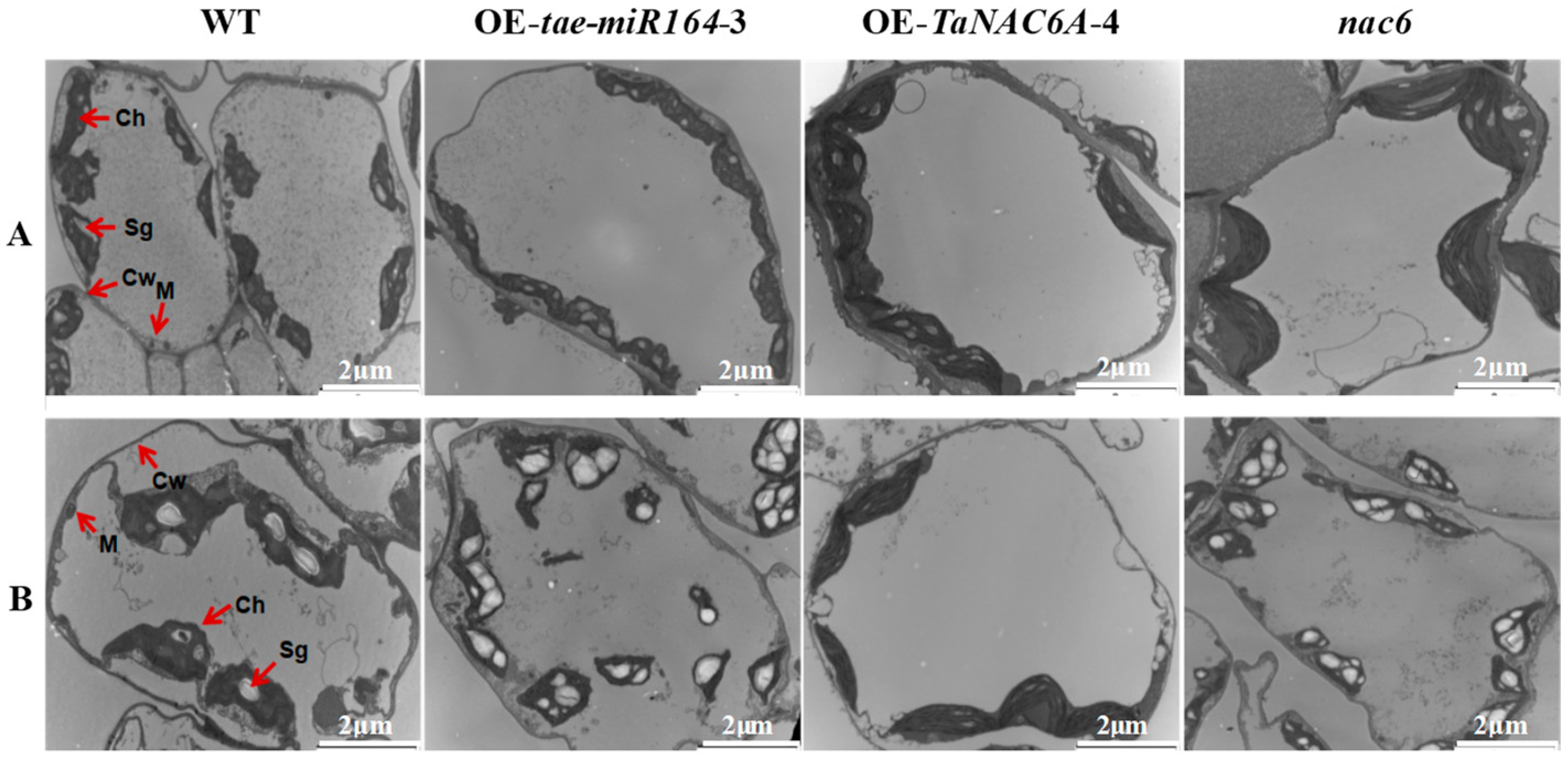
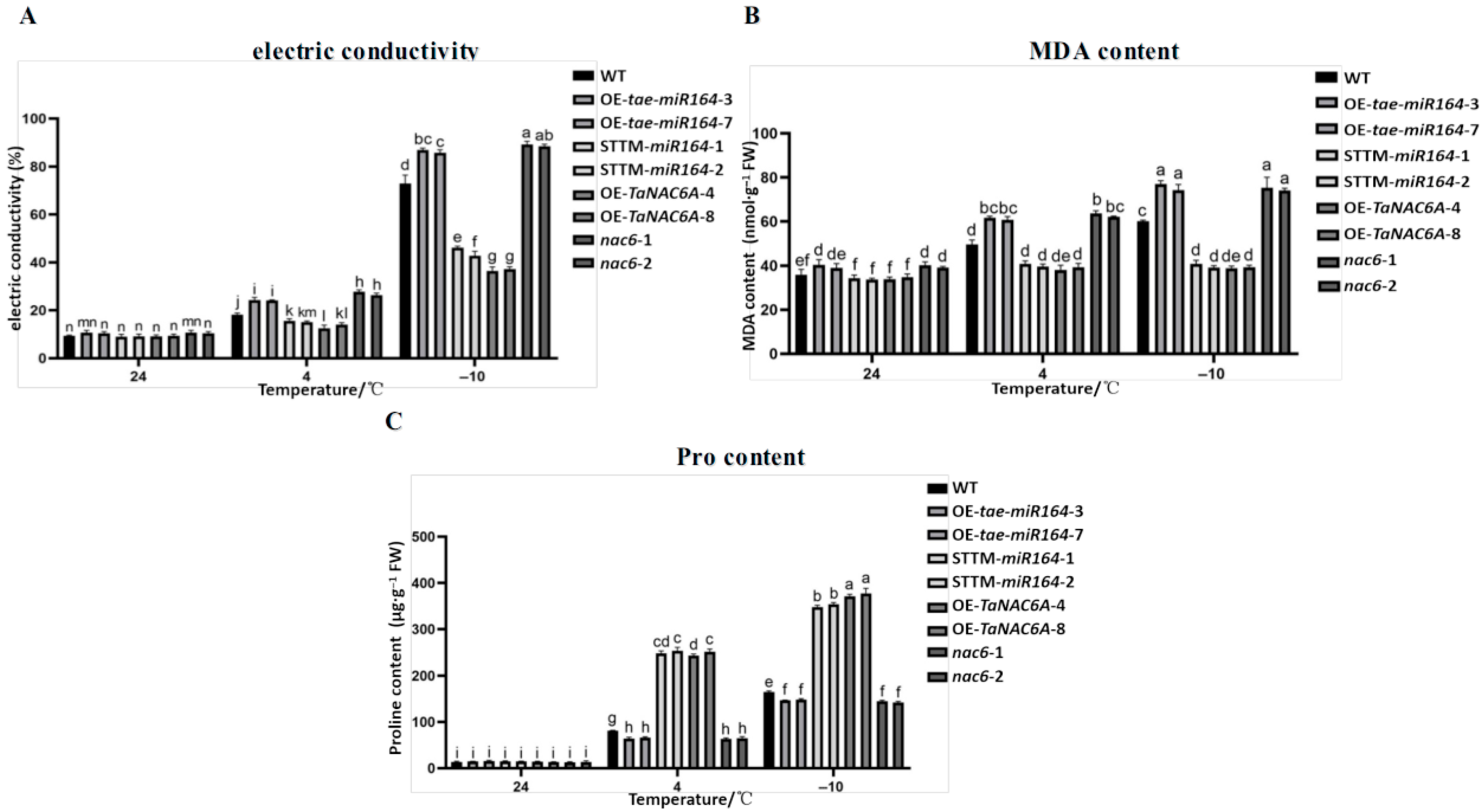
2.7. Physiological Markers of Cold Resistance in Transgenic Arabidopsis Plants
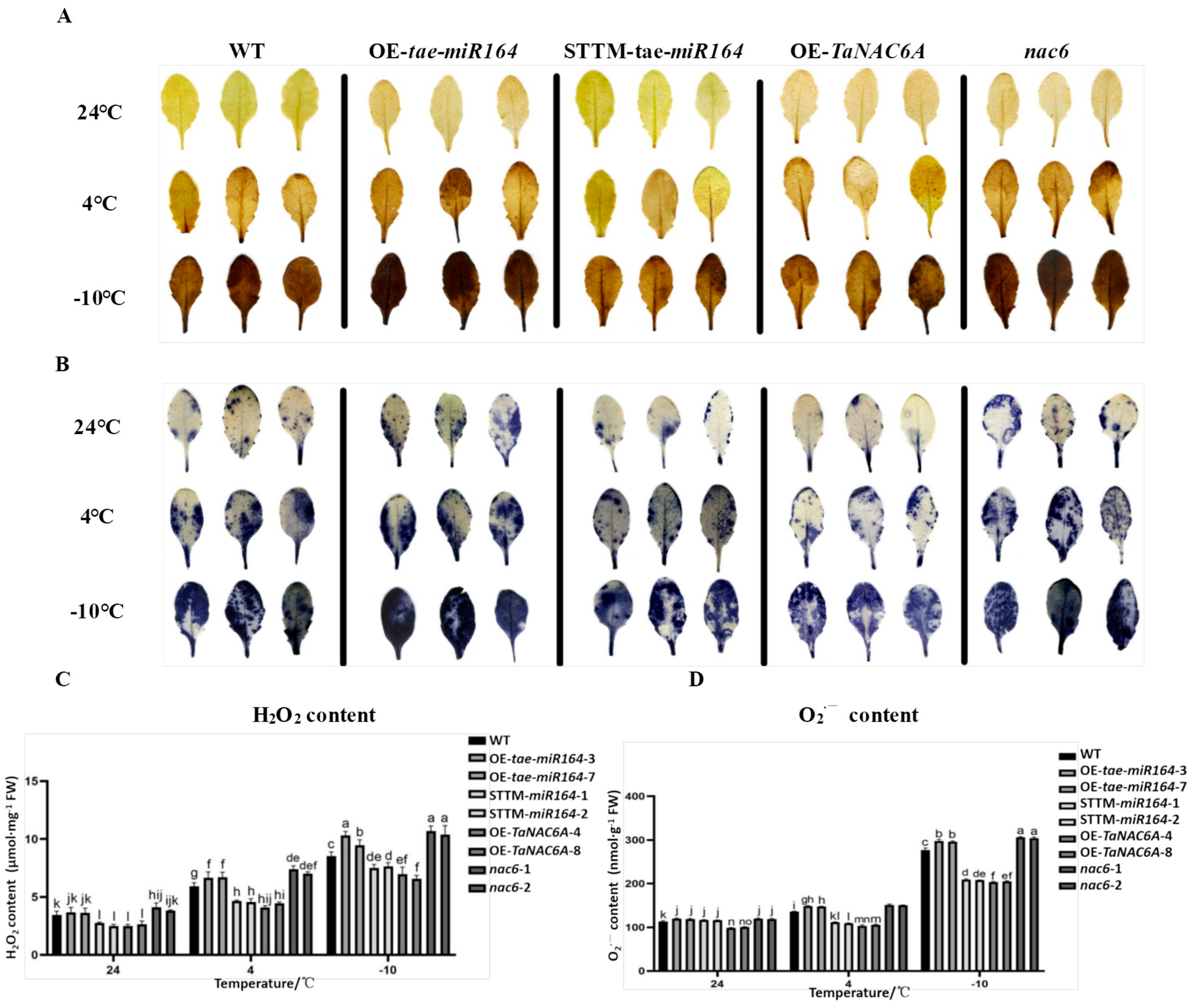
2.8. Cold-Induced ROS Production in Transgenic Arabidopsis Plants
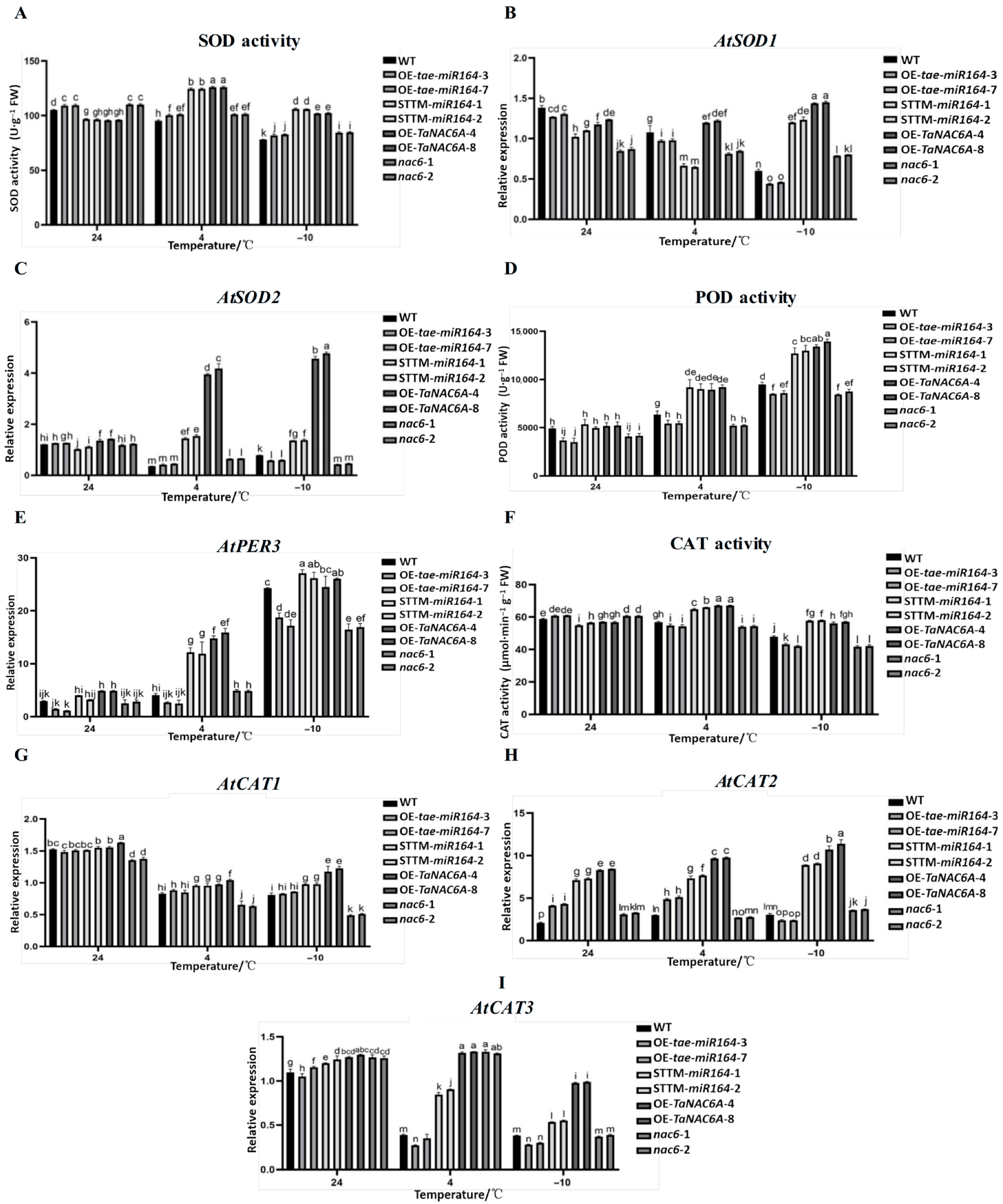
2.9. Changes in Antioxidant Enzyme Activities and Gene Expression Levels in Transgenic Arabidopsis Plants Under Cold Stress
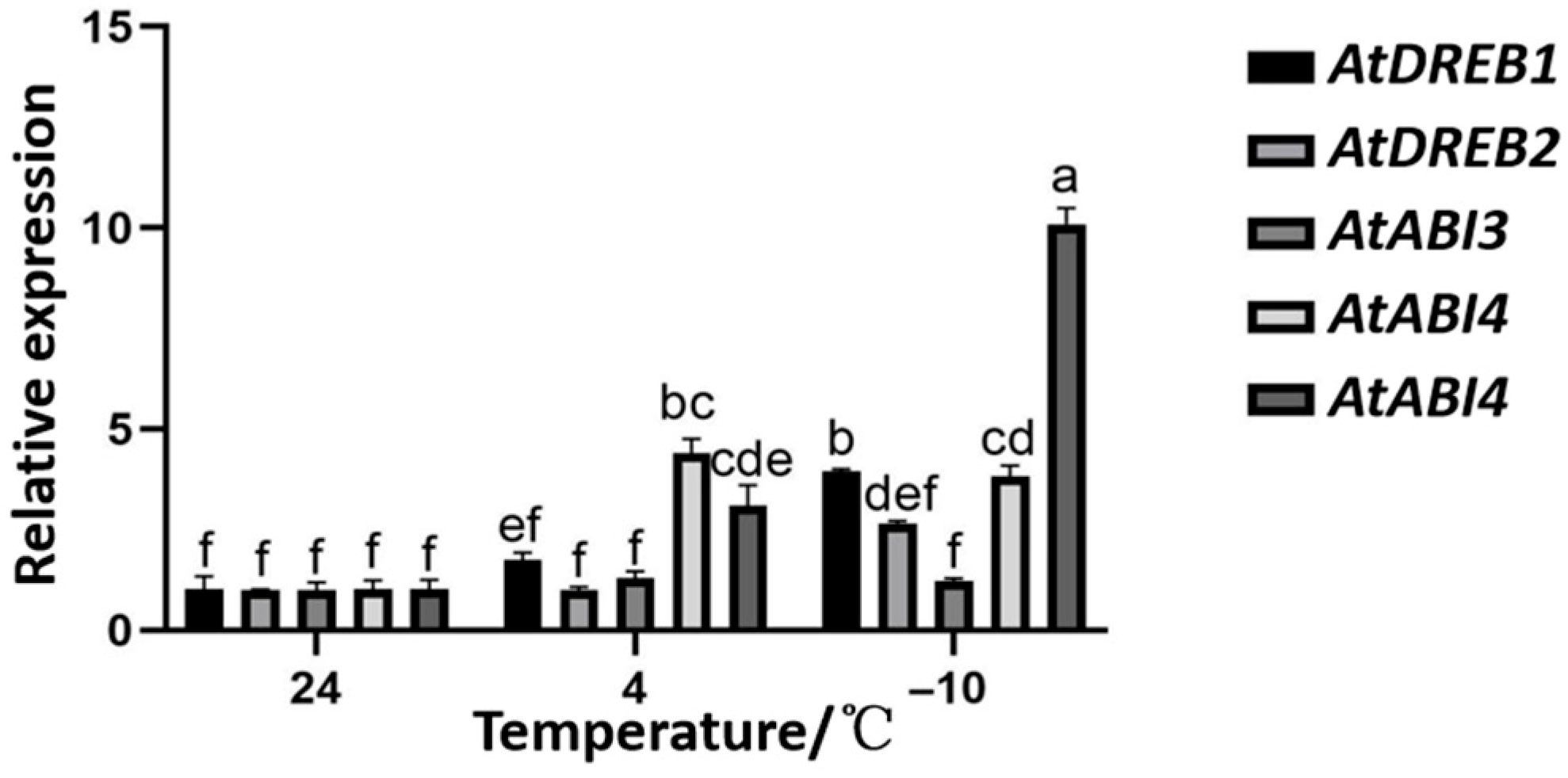
2.10. Alterations in the Expression of Cold-Signaling-Related Genes in OE-TaNAC6A Plants
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Gene Expression Analysis
4.3. Bioinformatics Analysis
4.4. Subcellular Localization Analysis
4.5. Transient Expression Assay in Tobacco
4.6. 5′RACE Assay
4.7. Dual-Luciferase Assay
4.8. Overexpression Vector Construction and Arabidopsis Transformation
4.9. Phenotyping and Physiological Index Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant. 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop Production under Cold Stress: An Understanding of Plant Responses, Acclimation Processes, and Management Strategie. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Anjum, N.A.; Sopory, S.K.; Akram, N.A.; Ashraf, M. Some Key Physiological and Molecular Processes of Cold Acclimation. Biol. Plantarum. 2016, 60, 603–618. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Hydrogen Peroxide is Involved in the Cold Acclimation Induced Chilling Tolerance of Tomato Plants. Plant Physiol. Biochem. 2012, 60, 141–149. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ya, H.U.; Chen, B.H.; Zhu, Y.F.; Dawuda, M.M.; Svetla, S. Physiological Mechanisms of Resistance to Cold Stress Associated with 10 Elite Apple Rootstocks. J. Integr. Agric. 2018, 17, 857–866. [Google Scholar] [CrossRef]
- Hussain, N.; Yasmeen, A.; Afzal Muhammad, A. Exogenously Applied Growth Promoters Modulate the Antioxidant Enzyme System to Improve the Cotton Productivity under Water Stress Conditions. Ital. J. Agron. 2020, 15, 165. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, L.; Liu, C.; Chen, Z.; Guan, S.; Ma, W.; Pan, G. Low temperature Stress Affects Reactive Oxygen Species, Osmotic Adjustment Substances, and Antioxidants in Rice (Oryza sativa L.) at the Reproductive Stage. Sci. Rep. 2022, 12, 6224. [Google Scholar] [CrossRef]
- Sun, X.; Lin, L.; Sui, N. Regulation Mechanism of MicroRNA in Plant Response to Abiotic Stress and Breeding. Mol. Biol. Rep. 2019, 46, 1447–1457. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.H.; Chiang, V.L. Stress-responsive microRNAs in Populus. Plant J. Cell Mol. Biol. 2008, 5, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Figueroa, B.E.; Gao, L.; Wu, Z.; Zhou, X.; Zhu, J.; Jin, H.; Liu, R.; Zhu, J.-K. High Throughput Sequencing Reveals Novel and Abiotic Stress-Regulated MicroRNAs in the Inflorescences of Rice. BMC Plant Biol. 2012, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, L.; Xu, C.; Yuan, S.; Zhang, F.; Zheng, Y.; Zhao, C. Uncovering Small RNA-mediated Responses to Cold Stress in a Wheat Thermosensitive Genic Male-Sterile Line by Deep Sequencing. Plant Physiol. 2012, 159, 721–738. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Z.; Jiang, F.; Zhou, R.; Yang, Z. Identification of Chilling Stress-Responsive Tomato MicroRNAs and Their Target Genes by High-Throughput Sequencing and Degradome Analysis. BMC Genom. 2014, 15, 1130. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Shi, H.; Wang, Y.; Ren, F. Small RNA and Degradome Deep Sequencing Reveal Respective Roles of Cold-Related MicroRNAs Across Chinese Wild Grapevine and Cultivated Grapevine. BMC Genom. 2019, 20, 740. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, N.; Yang, X.; Tu, L.; Zhang, X. Small RNA-Mediated Responses to Low and High-Temperature Stresses in Cotton. Sci. Rep. 2016, 6, 35558. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, F.; Wen, J.; Wu, Z. Overexpression of Solanum Habrochaites microRNA319d (sha-miR319d) Confers Chilling and Heat Stress Tolerance in Tomato (S. lycopersicum). BMC Plant Biol. 2019, 19, 214. [Google Scholar] [CrossRef]
- Peng, K.; Tian, Y.; Sun, X.; Song, C.; Ren, Z.; Bao, Y.; Xing, J.; Li, Y.; Xu, Q.; Yu, J.; et al. Tae-miR399-UBC24 Module Enhances Freezing Tolerance in Winter Wheat via a CBF Signaling Pathway. J. Agric. Food Chem. 2021, 69, 13398–13415. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.A.; Sung, P.H.; Kuo, Y.W.; Chen, M.-C.; Jeng, S.-T.; Lin, J.-S. Involvement of microRNA164 in Responses to Heat Stress in Arabidopsis. Plant Sci. 2023, 329, 111598. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, K.; Xiong, L. Conserved miR164-targeted NAC Genes Negatively Regulate Drought Resistance in Rice. J. Exp. Bot. 2014, 65, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhu, X.; Qi, B.; Gao, Z.; Tian, P.; Li, Z.; Lin, Z.; Zhang, Y.; Huang, T. SlmiR164A Regulates Fruit Ripening and Quality by Controlling SlNAM2 and SlNAM3 in Tomato. Plant Biotechnol. J. 2022, 20, 1456–1469. [Google Scholar] [CrossRef]
- Qing, C.; Du, L.; Wen, M.; Ruo, N.; Bao, W.; Li, G.; Meng, M.; Xiang, L.; Hui, Z. The MiR164-TaNAC14 Module Regulates Root Development and Abiotic-stress Tolerance of Wheat Seedlings. J. Integr. Agric. 2023, 22, 981–998. [Google Scholar]
- Diao, P.; Chen, C.; Zhang, Y.; Meng, Q.; Lv, W.; Ma, N. The Role of NAC Transcription Tactor in Plant Cold Response. Plant Signal. Behav. 2020, 15, 1785668. [Google Scholar] [CrossRef]
- Hou, X.M.; Zhang, H.F.; Liu, S.Y.; Wang, X.-K.; Zhang, Y.-M.; Meng, Y.-C.; Luo, D.; Chen, R.-G. The NAC Transcription Factor CaNAC064 is a Regulator of Cold Stress Tolerance in Peppers. Plant Sci. 2020, 291, 110346. [Google Scholar] [CrossRef]
- Ju, Y.L.; Yue, X.F.; Min, Z.; Wang, X.-H.; Fang, Y.-L.; Zhang, J.-X. VvNAC17, a Novel Stress-responsive Grapevine (Vitis vinifera L.) NAC Transcription Factor, Increases Sensitivity to Abscisic Acid and Enhances Salinity, Freezing, and Drought Tolerance in Transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef]
- Luo, P.; Chen, Y.; Rong, K.; Lu, Y.; Wang, N.; Xu, Z.; Pang, B.; Zhou, D.; Weng, J.; Li, M.; et al. ZmSNAC13, a Maize NAC Transcription Factor Conferring Enhanced Resistance to Multiple Abiotic Stresses in Transgenic Arabidopsis. Plant Physiol. Biochem. 2022, 170, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cang, J.; Yu, J.; Wang, X.; Huang, R.; Wang, J.; Lu, B. Effects of Exogenous Abscisic Acid on Carbohydrate Metabolism and the Expression Levels of Correlative Key Enzymes in Winter Wheat Under Low Temperature. Biosci. Biotechnol. Biochem. 2013, 77, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.N.; Jamshed, M.; Samuel, M.A. Abiotic Stress Signaling in Wheat-An Inclusive Overview of Hormonal Interactions During Abiotic Stress Responses in Wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, F.; Zhou, B. The Characters of Non-Coding RNAs and Their Biological Roles in Plant Development and Abiotic Stress Response. Int. J. Mol. Sci. 2022, 23, 4124. [Google Scholar] [CrossRef]
- Huang, R.; Cang, J.; Yu, J.; Lu, B.; Liu, L.; Wang, J.; Guo, R.; Xu, C. Solexa Sequencing and Bioinformatics Analysis of Small RNA in Winter Wheat. Chin. Bull. Bot. 2014, 49, 8–18. [Google Scholar] [CrossRef]
- Spychała, J.; Tomkowiak, A.; Noweiska, A.; Bobrowska, R.; Bocianowski, J.; Sobiech, A.; Kwiatek, M.T. Diversity of Expression Patterns of Lr34, Lr67, and Candidate Genes towards Lr46 with Analysis of Associated miRNAs in Common Wheat Hybrids in Response to Puccinia triticina Fungus. Curr. Issues Mol. Biol. 2024, 46, 5511–5529. [Google Scholar] [CrossRef]
- Wang, B.; Song, N.; Zhang, Q.; Wang, N.; Kang, Z. TaMAPK4 Acts as a Positive Regulator in Defense of Wheat Stripe-Rust Infection. Front. Plant Sci. 2018, 15, 152. [Google Scholar] [CrossRef]
- Feng, H.; Duan, X.; Zhang, Q.; Li, X.; Wang, B.; Huang, L.; Wang, X.; Kang, Z. The Target Gene of tae-miR164, a Novel NAC Transcription Factor from the NAM Subfamily, Negatively Regulates Resistance of Wheat to Stripe rust. Mol. Plant Pathol. 2014, 15, 284–296. [Google Scholar] [CrossRef]
- Zhou, W.; Qian, C.; Li, R.; Zhou, S.; Zhang, R.; Xiao, J.; Wang, X.; Zhang, S.; Xing, L.; Cao, A. TaNAC6s are Involved in the Basal and Broad-spectrum Resistance to Powdery Mildew in Wheat. Plant Sci. 2018, 277, 218–228. [Google Scholar] [CrossRef]
- Rodríguez-García, D.R.; Rondón Guerrero, Y.D.C.; Ferrero, L.; Rossi, A.H.; Miglietta, E.A.; Aptekmann, A.A.; Marzol, E.; Pacheco, J.M.; Carignani, M.; Gabarain, V.B.; et al. Transcription Factor NAC1 Activates Expression of Peptidase-encoding AtCEPs in Roots to Limit Root Hair Growth. Plant Physiol. 2023, 194, 81–93. [Google Scholar] [CrossRef]
- Xie, C.; Li, C.; Wang, F.; Zhang, F.; Liu, J.; Wang, J.; Zhang, X.; Kong, X.; Ding, Z. NAC1 Regulates Root Ground Tissue Maturation by Coordinating with the SCR/SHR-CYCD6;1 Module in Arabidopsis. Mol. Plant 2023, 16, 709–725. [Google Scholar] [CrossRef]
- Xie, Q.; Frugis, G.; Colgan, D.; Chua, N.-H. Arabidopsis NAC1 Transduces Auxin Signal Downstream of TIR1 to Promote Lateral Root Development. Genes Dev. 2000, 14, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tang, M.; Huang, Z.; Song, J.; Xu, J.; Ahammed, G.J.; Yu, J.; Zhou, Y. The miR164a-NAM3 Module Confers Cold Tolerance by Inducing Ethylene Production in Tomato. Plant J. 2022, 111, 440–456. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhu, J.; Shen, Y.; Zeng, N.; Liu, S.; Wang, H.; Wang, J.; Zhan, X. Role of miR164 in the Growth of Wheat New Adventitious Roots Exposed to Phenanthrene. Environ. Pollut. 2021, 284, 117204. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Mitra, M.; Banerjee, S.; Roy, S. MYB4 Transcription Factor, a Member of R2R3-subfamily of MYB Domain Protein, Regulates Cadmium Tolerance via Enhanced Protection Against Oxidative Damage and Increases Expression of PCS1 and MT1C in Arabidopsis. Plant Sci. 2020, 297, 110501. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, B.; Li, C.; Guo, H.; Bao, A.-K. Transcriptomic Analysis Provides Insight into the ROS Scavenging System and Regulatory Mechanisms in Atriplex canescens Response to Salinity. Int. J. Mol. Sci. 2022, 24, 242. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Repkina, N.; Ignatenko, A.; Holoptseva, E.; MiszalskI, Z.; Kaszycki, P.; Talanova, V. Exogenous Methyl Jasmonate Improves Cold Tolerance with Parallel Induction of Two Cold-Regulated (COR) Genes Expression in Triticum aestivum L. Plants 2021, 10, 1421. Plants 2021, 10, 1421. [Google Scholar] [CrossRef]
- Reeves, W.M.; Lynch, T.J.; Mobin, R.; Finkelstein, R.R. Direct Targets of the Transcription Factors ABA-Insensitive (ABI4 and ABI5) Reveal Synergistic Action by ABI4 and Several bZIP ABA Response Factors. Plant Mol. Biol. 2011, 75, 347–363. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB Transcription Factors in Abiotic and Biotic Stress Tolerance in Plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef]
- Arvidsson, S.; Pérez-Rodríguez, P.; Mueller-Roeber, B. A Growth Phenotyping Pipeline for Arabidopsis Thaliana Integrating Image Analysis and Rosette Area Modeling for Robust Quantification of Genotype Effects. New Phytol. 2011, 191, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M. Thermodynamics of the Water Permeability of Plant Cuticles: Characterization of the Polar Pathway. J. Exp. Bot. 2006, 57, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Zhou, D.M.; Yuan, X.Y.; Zhang, X.H.; Li, Y. Modeling the Interaction and Toxicity of Cu Cd Mixture to Wheat Roots Affected by Humic Acids, in Terms of Cell Membrane Surface Characteristics. Chemosphere 2018, 199, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Xing, J.; Liang, Y.; Ren, Z.; Fu, L.; Yu, J.; Wang, D.; Zhang, D.; Xu, Q.; Cang, J. Analysis of Overwintering Indexes of Winter Wheat in Alpine Regions and Establishment of A Cold Resistance Model. Field Crops Res. 2022, 275, 108347. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, K.; Bao, Y.; Zhang, D.; Meng, J.; Wang, D.; Wang, X.; Cang, J. Glucose-6-phosphate Dehydrogenase and 6-phosphogluconate Dehydrogenase Genes of Winter Wheat Enhance the Cold Tolerance of Transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 161, 86–97. [Google Scholar] [CrossRef]
- Peng, K.; Tian, Y.; Cang, J.; Yu, J. Overexpression of TaFBA-A10 from Winter Wheat Enhances Freezing Tolerance in Arabidopsis thaliana. J. Plant Growth Regul. 2022, 41, 314–326. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Yang, X.; Shan, W.; Hao, Y.; Zhang, D.; Peng, K.; Xu, Q. The tae-miR164-TaNAC6A Module from Winter Wheat Could Enhance Cold Tolerance in Transgenic Arabidopsis thaliana. Plants 2025, 14, 2849. https://doi.org/10.3390/plants14182849
Dai Z, Yang X, Shan W, Hao Y, Zhang D, Peng K, Xu Q. The tae-miR164-TaNAC6A Module from Winter Wheat Could Enhance Cold Tolerance in Transgenic Arabidopsis thaliana. Plants. 2025; 14(18):2849. https://doi.org/10.3390/plants14182849
Chicago/Turabian StyleDai, Ziyao, Xiaoyan Yang, Wenwang Shan, Yiou Hao, Da Zhang, Kankan Peng, and Qinghua Xu. 2025. "The tae-miR164-TaNAC6A Module from Winter Wheat Could Enhance Cold Tolerance in Transgenic Arabidopsis thaliana" Plants 14, no. 18: 2849. https://doi.org/10.3390/plants14182849
APA StyleDai, Z., Yang, X., Shan, W., Hao, Y., Zhang, D., Peng, K., & Xu, Q. (2025). The tae-miR164-TaNAC6A Module from Winter Wheat Could Enhance Cold Tolerance in Transgenic Arabidopsis thaliana. Plants, 14(18), 2849. https://doi.org/10.3390/plants14182849





