OsLAC12, a Laccase-Encoding Gene, Contributes to Rice Heat Tolerance Through Regulation of Antioxidant Defense and Secondary Metabolism
Abstract
1. Introduction
2. Results
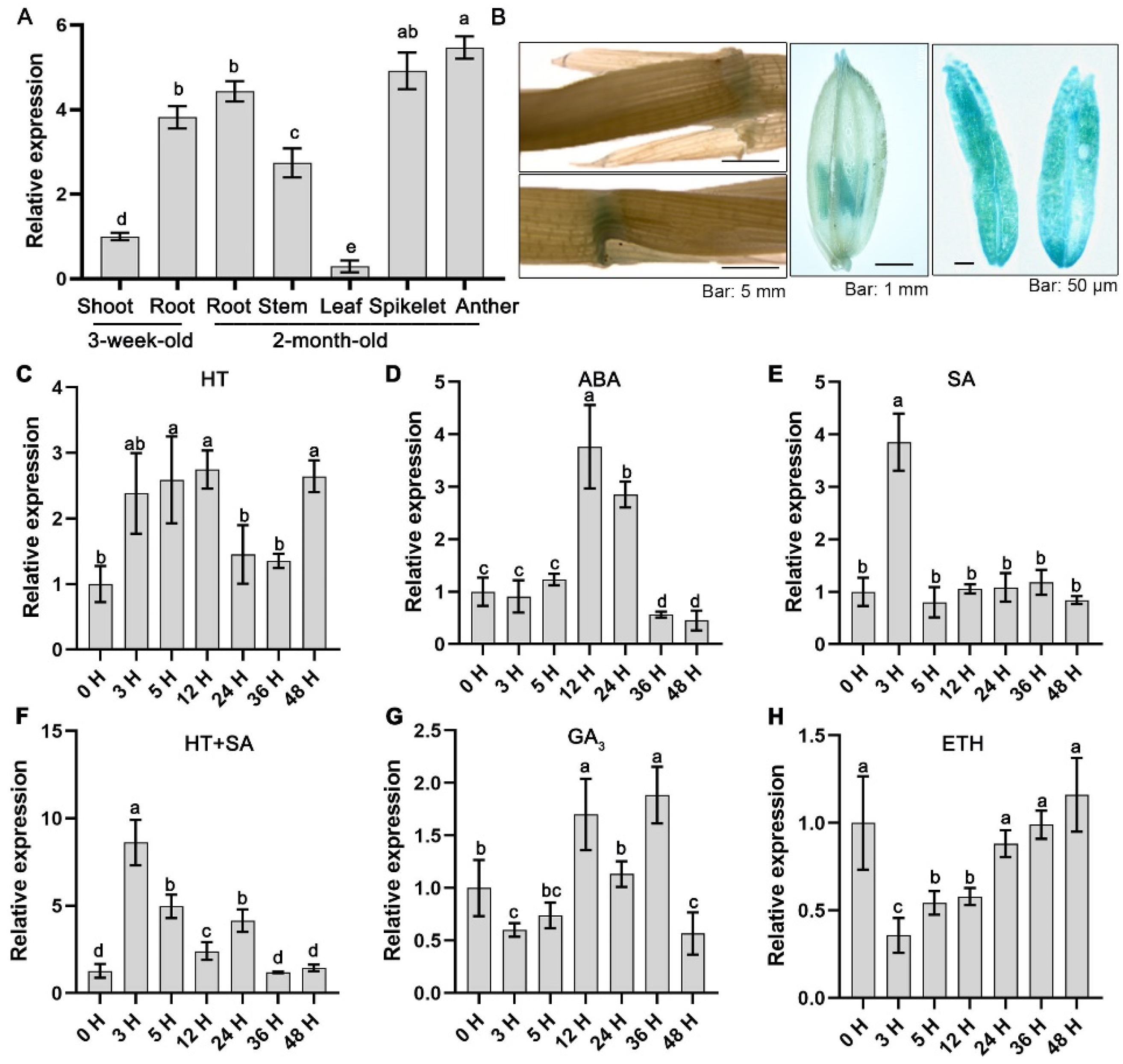
2.1. OsLAC12 Is SA, ABA, and Heat Stress Inducibale
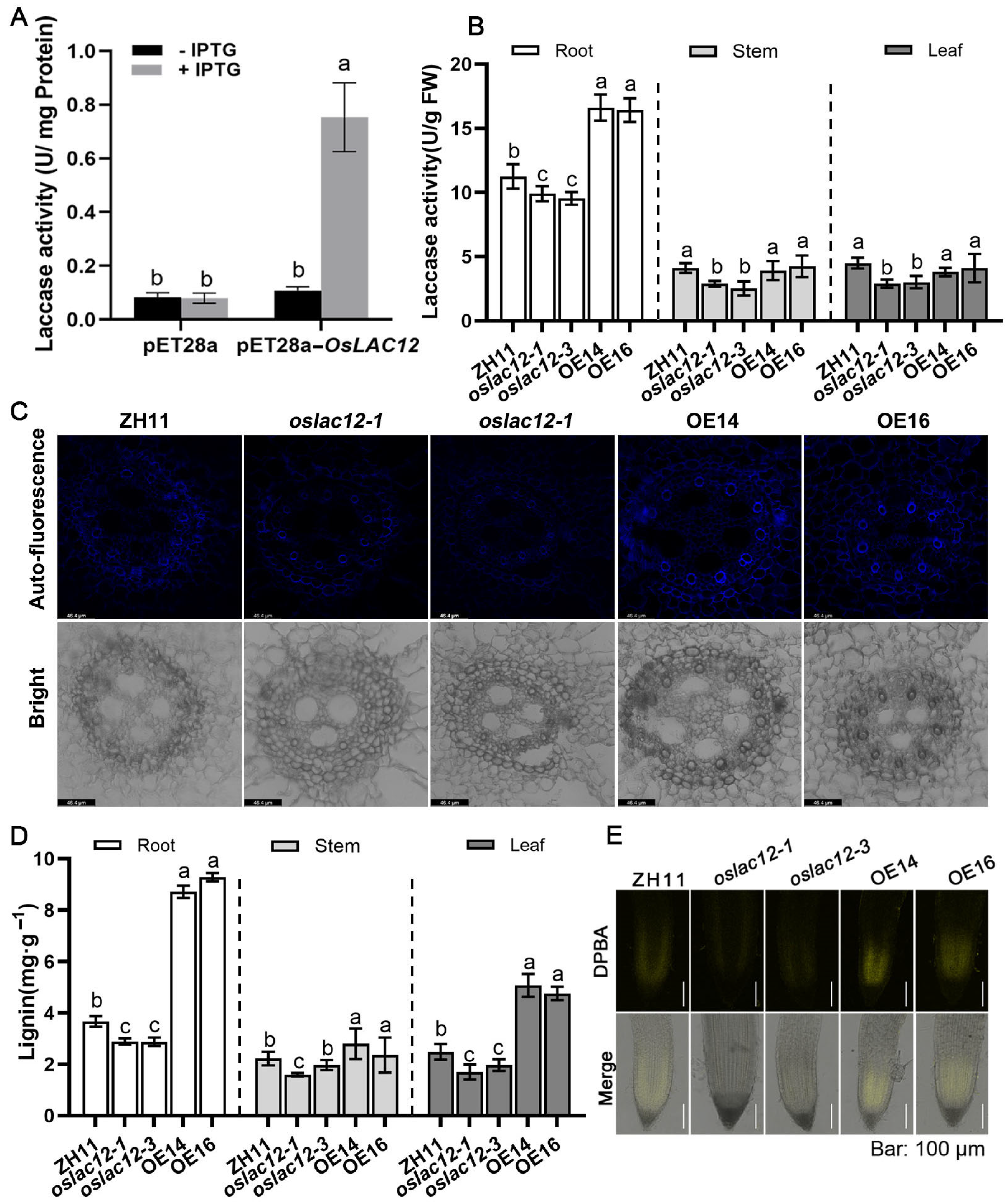
2.2. OsLAC12 Overexpression Increases Laccase Activity as Well as Lignin Biosynthesis
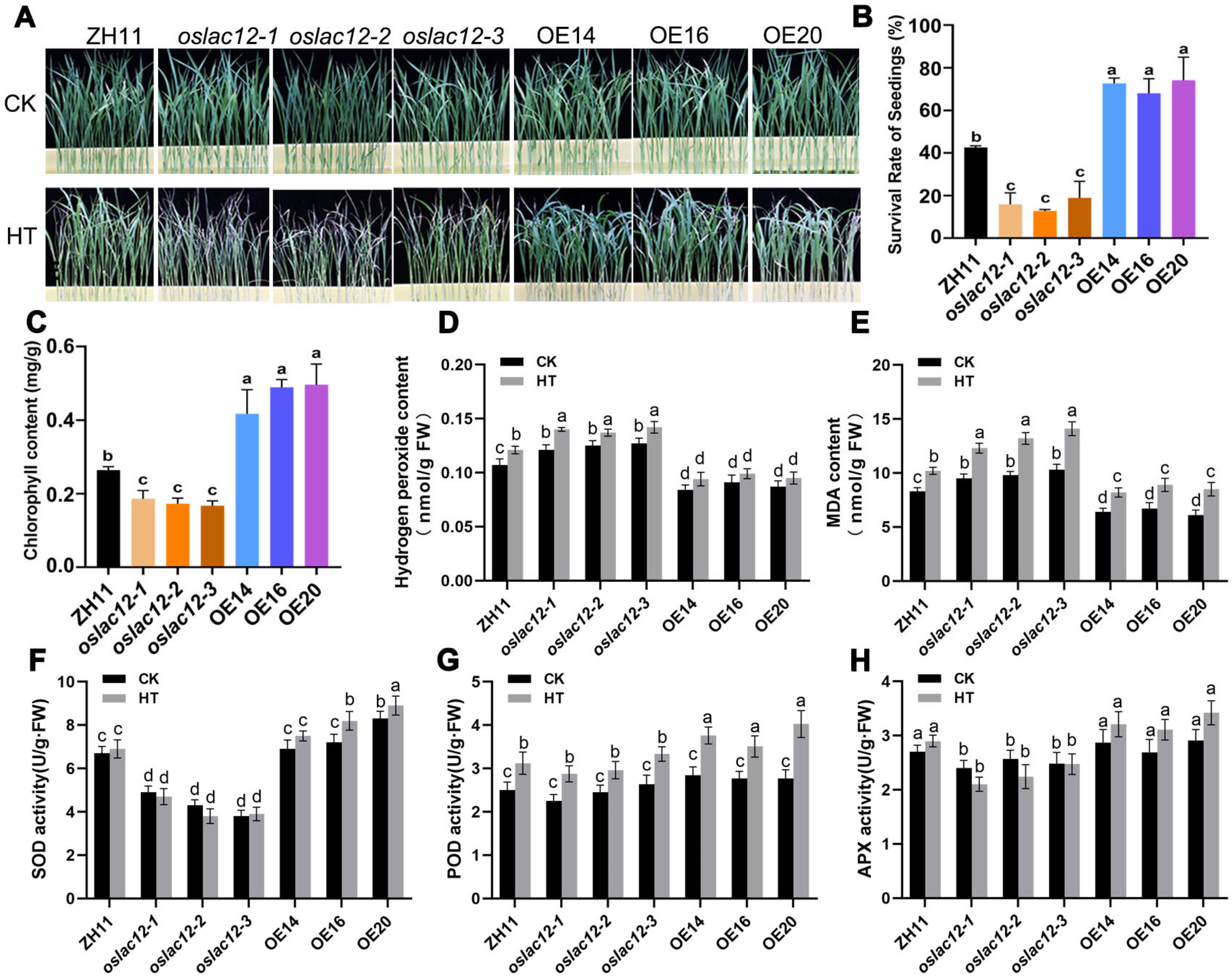
2.3. OsLAC12 Positively Enhances Themotolerance in Seedling Stage
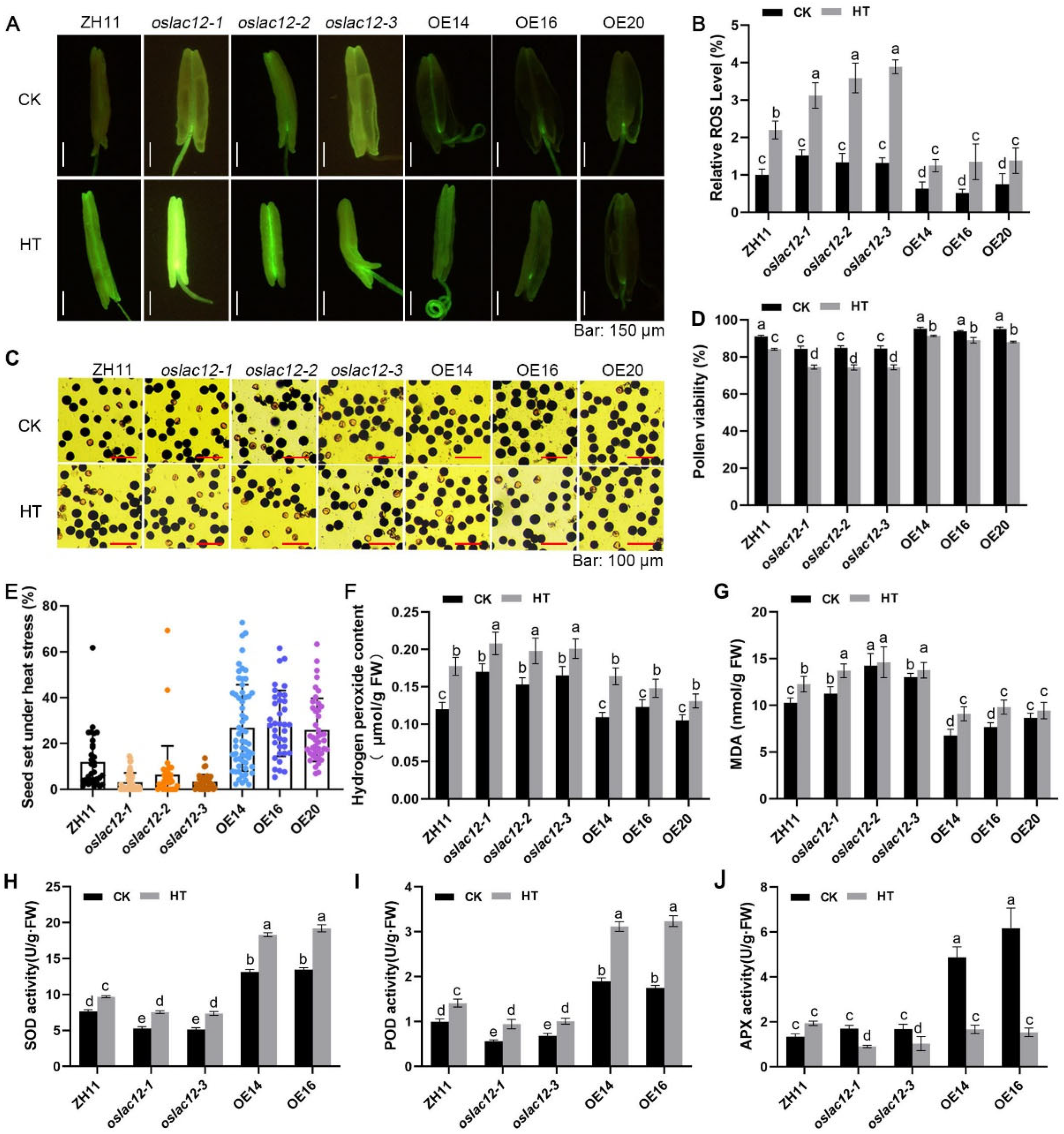
2.4. OsLAC12 Alleviates HT-Induced ROS Brust, Pollen Viability and Spikelet Sterility
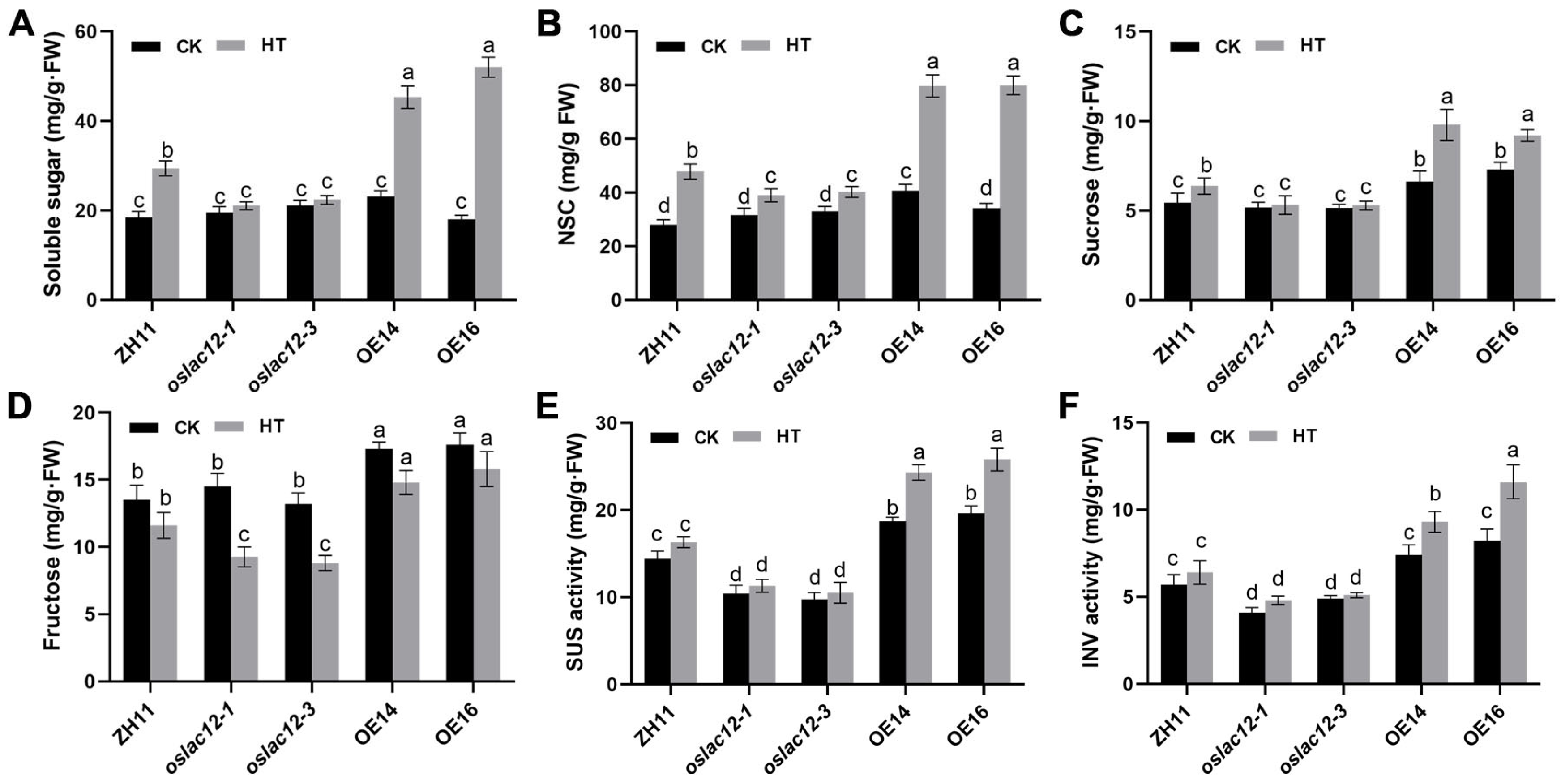
2.5. OsLAC12 Involved in Carbohydrate Metabolism in Response to HT
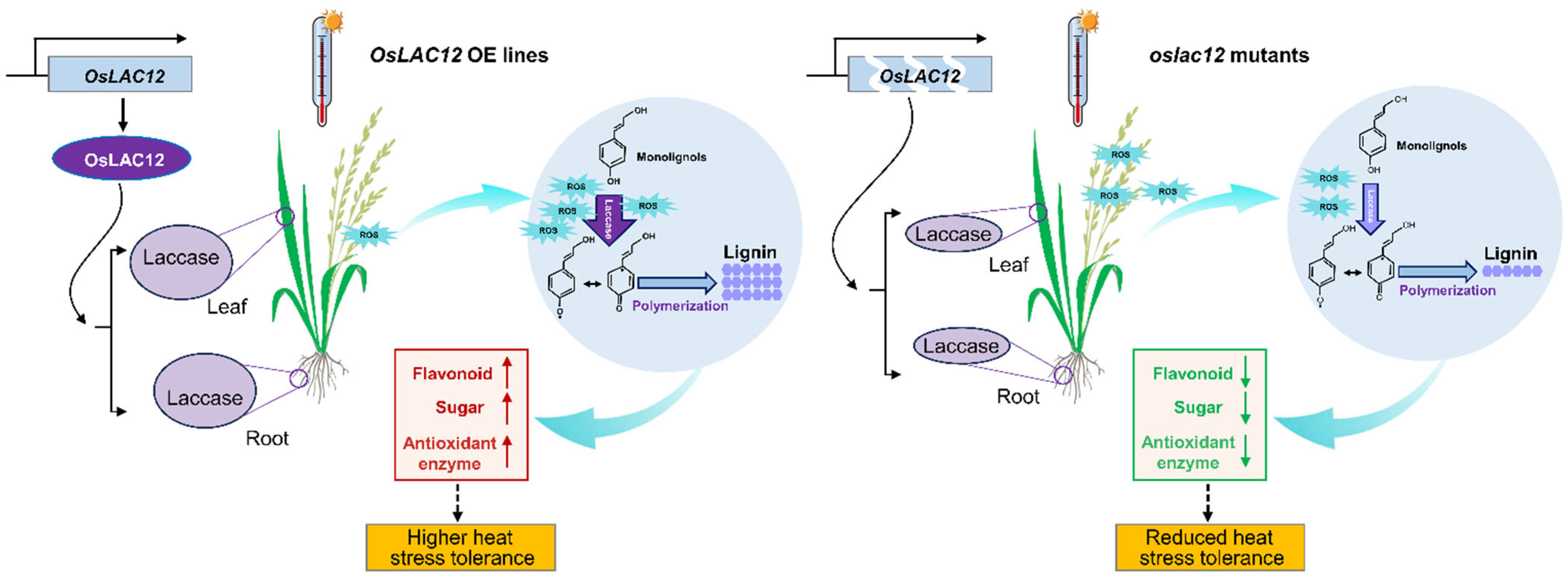
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Stress Treatments and qRT-PCR Analysis
4.3. GUS Staining
4.4. Heat Stress Application at Seedling Stage
4.5. Determination of SOD, POD, APX, H2O2, and MDA
4.6. Heat Treatment on the Reproductive Stage
4.7. Detection of ROS via DCFH-DA
4.8. Determination of Pollen Viability and Seed-Setting Rate
4.9. Determination of Laccase Activity Assay
4.10. Determination of Lignin Content
4.11. Lignin Autofluorescence Analysis
4.12. DPBA Staining
4.13. Determination of Carbohydrate Content, SUS and INV Activity
4.14. Determination of SUS and INV Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Xu, J.; Henry, A.; Sreenivasulu, N. Rice yield formation under high day and night temperatures-a prerequisite to ensure future food security. Plant Cell Environ. 2020, 43, 1595–1608. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Sheehy, J.E.; Thomas, J.M.G. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Endo, M.; Tsuchiya, T.; Hamada, K.; Kawamura, S.; Yano, K.; Ohshima, M.; Higashitani, A.; Watanabe, M.; Kawagishi-Kobayashi, M. High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 2009, 50, 1911–1922. [Google Scholar] [CrossRef]
- Altenbach, S.B.; DuPont, F.M.; Kothari, K.M.; Chan, R.; Johnson, E.L.; Lieu, D. Temperature, water and fertilizer influence the timing of key events during grain development in a US spring wheat. J. Cereal Sci. 2003, 37, 9–20. [Google Scholar] [CrossRef]
- Jagadish, S.V.; Muthurajan, R.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, S.; Xiao, L.; WU, X.; Xiao, Y.; Chen, L. Effect of high temperature stress on physiological characteristics of anther and pollen traits of rice at flowering stage. Acta Agron. Sin. 2013, 39, 177. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox signaling in plant heat stress response. Antioxidants 2023, 12, 605. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Lu, C.; Li, W.; Feng, X.; Chen, J.; Hu, S.; Tan, Y.; Wu, L. The dynamic remodeling of plant cell wall in response to heat stress. Genes 2025, 16, 628. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Ji, Y.; Wang, C.; Wang, S.; Shi, Y.; Le, J.; Zhang, M. The heat shock factor 20-HSF4-cellulose synthase A2 module regulates heat stress tolerance in maize. Plant Cell 2024, 36, 2652–2667. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Silaghi-Dumitrescu, R. Laccases: Complex architectures for one-electron oxidations. Biochemistry 2012, 77, 1395–1407. [Google Scholar] [CrossRef]
- Reiss, R.; Ihssen, J.; Richter, M.; Eichhorn, E.; Schilling, B.; Thöny-Meyer, L. Laccase versus laccase-like multi-copper oxidase: A comparative study of similar enzymes with diverse substrate spectra. PLoS ONE 2013, 8, e65633. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.T.; Torres, J. Reactions of nitric oxide with copper containing oxidases; cytochrome c oxidase and laccase. IUBMB Life 2004, 56, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Davis, E.J.; Ballif, J.; Liang, M.; Bushman, E.; Haroldsen, V.; Torabinejad, J.; Wu, Y. Mutant identification and characterization of the laccase gene family in Arabidopsis. J. Exp. Bot. 2006, 57, 2563–2569. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive analysis of rice laccase gene (OsLAC) family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Jia, W.; Fan, P.; Bao, H.; Li, S.; Li, Y. Genome-wide identification of Sorghum bicolor laccases reveals potential targets for lignin modification. Front. Plant Sci. 2017, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, Y.; Hu, Y.; Jiang, N.; Hu, S.; Li, L.; Li, T. Identification of wheat LACCASEs in response to Fusarium graminearum as potential deoxynivalenol trappers. Front. Plant Sci. 2022, 13, 832800. [Google Scholar] [CrossRef]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cézard, L.; Le Bris, P.; Borrega, N.; Hervé, J.; Blondet, E.; Balzergue, S.; et al. Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- Wang, X.; Zhuo, C.; Xiao, X.; Wang, X.; Docampo-Palacios, M.; Chen, F.; Dixon, R.A. Substrate specificity of LACCASE8 facilitates polymerization of caffeyl alcohol for C-lignin biosynthesis in the seed coat of Cleome hassleriana. Plant Cell 2020, 32, 3825–3845. [Google Scholar] [CrossRef]
- Zhuo, C.; Wang, X.; Docampo-Palacios, M.; Sanders, B.C.; Engle, N.L.; Tschaplinski, T.J.; Hendry, J.I.; Maranas, C.D.; Chen, F.; Dixon, R.A. Developmental changes in lignin composition are driven by both monolignol supply and laccase specificity. Sci. Adv. 2022, 8, eabm8145. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.F.; Zhang, J.P.; Zhang, F.; Zhou, Y.F.; Feng, Y.Z.; Chen, Y.Q.; Zhang, Y.C. Laccase-13 regulates seed setting rate by affecting hydrogen peroxide dynamics and mitochondrial integrity in rice. Front. Plant Sci. 2017, 8, 1324. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Ming, Y.; Zhou, S.; Dong, X.; Liu, S.; Zhang, X.; Zhang, X.; Hu, Q.; Zhu, L. A GhLac1-centered transcriptional regulatory cascade mediates cotton resistance to Verticillium dahliae through the lignin biosynthesis pathway. Int. J. Biol. Macromol. 2024, 279, 135042. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, X.; Yang, J.; Liu, W.; Du, Q.; Wang, H.; Fu, C.; Li, W.X. MicroRNA528 affects lodging resistance of maize by regulating lignin biosynthesis under nitrogen-luxury conditions. Mol. Plant 2018, 11, 806–814. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, C.; Hwang, S.G.; Park, Y.C.; Lim, H.L.; Jang, C.S. Overexpression of the OsChI1 gene, encoding a putative laccase precursor, increases tolerance to drought and salinity stress in transgenic Arabidopsis. Gene 2014, 552, 98–105. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Liang, M.; Kong, W.; Liu, J. The citrus laccase gene CsLAC18 contributes to cold tolerance. Int. J. Mol. Sci. 2022, 23, 14509. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Wang, B.; Ding, L.; Wang, Y.; Luo, L.; Zhang, Y.; Kong, W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene 2019, 689, 114–123. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Cai, Z.; He, F.; Feng, X.; Liang, T.; Wang, H.; Ding, S.; Tian, X. Transcriptomic analysis reveals important roles of lignin and flavonoid biosynthetic pathways in rice thermotolerance during reproductive stage. Front. Genet. 2020, 11, 562937. [Google Scholar] [CrossRef]
- Suzuki, N.; Katano, K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gong, L.; Zhang, X.Y.; Ren, A.; Gao, T.; Zhao, M.W. The regulation of methyl jasmonate on hyphal branching and GA biosynthesis in Ganoderma lucidum partly via ROS generated by NADPH oxidase. Fungal Genet. Biol. 2015, 81, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Wang, G.D.; Li, Q.J.; Luo, B.; Chen, X.Y. Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat. Biotechnol. 2004, 22, 893–897. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, N.; He, B.; Igarashi, Y.; Luo, F. Transcriptome analysis of differential gene expression in Dichomitus squalens during interspecific mycelial interactions and the potential link with laccase induction. J. Microbiol. 2019, 57, 127–137. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, Y.; Liu, F.; Zhang, X.; Li, X.; Zhang, J.; Sun, X. The laccase gene family mediate multi-perspective trade-offs during tea plant (Camellia sinensis) development and defense processes. Int. J. Mol. Sci. 2021, 22, 12554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, H.; Huang, K.; Guo, R.; Zhao, J.; Xie, H.; Zhu, J.; Gu, H.; Chen, H.; Li, G.; et al. Comprehensive analysis of the laccase gene family in tea plant highlights its roles in development and stress responses. BMC Plant Biol. 2023, 23, 129. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Kamble, N.U. Decoding the role of flavonoids in ROS management during heat stress in tomato pollen. Plant Cell 2024, 36, 4285–4286. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.P.; Mérillon, J.M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, N.; Yao, S.B.; Zhuang, J.; Fu, Z.; Ma, J.; Yin, S.; Jiang, X.; Liu, Y.; Gao, L.; et al. CsHCT-mediated lignin synthesis pathway involved in the response of tea plants to biotic and abiotic stresses. J. Agric. Food Chem. 2021, 69, 10069–10081. [Google Scholar] [CrossRef]
- Li, S.S.; Chang, Y.; Li, B.; Shao, S.L.; Zhen-Zhu, Z. Functional analysis of 4-coumarate: CoA ligase from Dryopteris fragrans in transgenic tobacco enhances lignin and flavonoids. Genet. Mol. Biol. 2020, 43, e20180355. [Google Scholar] [CrossRef]
- Li, S.; Chang, Y.; Lin Teoh, P.; Wang, D.; Mo, J.; Li, B.; Shao, S. Overexpression of Df4CL1 from Dryopteris fragrans enhances flavonoids and lignin production in transgenic Tobacco. Russ. J. Plant Physiol. 2021, 68, 110–117. [Google Scholar] [CrossRef]
- Jiao, J.; Zheng, H.; Zhou, X.; Huang, Y.; Niu, Q.; Ke, L.; Tang, S.; Liu, H.; Sun, Y. The functions of laccase gene GhLAC15 in fiber colouration and development in brown-colored cotton. Physiol. Plant 2024, 176, e14415. [Google Scholar] [CrossRef]
- Lima, R.B.; dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; Petkowicz, C.L.d.O. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef]
- Barnes, W.J.; Anderson, C.T. Release, Recycle, Rebuild: Cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant 2018, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, B.; Qin, F. Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2.6-mediated phosphorylation. Plant Cell 2015, 27, 3228–3244. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guan, X.; Zhou, L.; Asad, M.A.; Xu, Y.; Pan, G.; Cheng, F. ABA-triggered ROS burst in rice developing anthers is critical for tapetal programmed cell death induction and heat stress-induced pollen abortion. Plant Cell Environ. 2023, 46, 1453–1471. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Feng, B.; Chen, T.; Fu, W.; Zhang, C.; Tao, L.; Fu, G. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plantarum. 2018, 165, 644–663. [Google Scholar]
- Jiang, N.; Yu, P.; Fu, W.; Li, G.; Feng, B.; Chen, T.; Li, H.; Tao, L.; Fu, G. Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ. 2020, 43, 1273–1287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Gao, Z.; Chen, Y.; Liu, J.; Xue, Y.; Teng, Y.; Qin, S.; Tian, X.; Wang, H. OsLAC12, a Laccase-Encoding Gene, Contributes to Rice Heat Tolerance Through Regulation of Antioxidant Defense and Secondary Metabolism. Plants 2025, 14, 2846. https://doi.org/10.3390/plants14182846
Ding S, Gao Z, Chen Y, Liu J, Xue Y, Teng Y, Qin S, Tian X, Wang H. OsLAC12, a Laccase-Encoding Gene, Contributes to Rice Heat Tolerance Through Regulation of Antioxidant Defense and Secondary Metabolism. Plants. 2025; 14(18):2846. https://doi.org/10.3390/plants14182846
Chicago/Turabian StyleDing, Shuangcheng, Zihui Gao, Yuhang Chen, Jiaxin Liu, Yuxin Xue, Yulu Teng, Simin Qin, Xiaohai Tian, and Hongwei Wang. 2025. "OsLAC12, a Laccase-Encoding Gene, Contributes to Rice Heat Tolerance Through Regulation of Antioxidant Defense and Secondary Metabolism" Plants 14, no. 18: 2846. https://doi.org/10.3390/plants14182846
APA StyleDing, S., Gao, Z., Chen, Y., Liu, J., Xue, Y., Teng, Y., Qin, S., Tian, X., & Wang, H. (2025). OsLAC12, a Laccase-Encoding Gene, Contributes to Rice Heat Tolerance Through Regulation of Antioxidant Defense and Secondary Metabolism. Plants, 14(18), 2846. https://doi.org/10.3390/plants14182846





