Identification of Candidate Genes for Low Phosphorus Tolerance in Maize Seedling Stage Based on GWAS and Transcriptome
Abstract
1. Introduction
2. Results
2.1. Phenotypic Analysis of Maize Under Phosphorus Stress at Seedling Stage
2.2. GWAS Analysis of Low Phosphorus Tolerance Traits in Maize Seedling Stage
2.3. Gene Function Annotation in Candidate Intervals
2.4. Transcriptome Analysis of Low Phosphorus Tolerance in Maize Seedling Stage
2.4.1. Transcriptome Sequencing Results and Quality Assessment and Genome Comparison Rate
2.4.2. Differential Gene Analysis
2.4.3. GO Enrichment Analysis of DEGs in Maize Seedlings Under Phosphorus Stress
2.4.4. KEGG Enrichment Analysis of DEGs in Maize Seedlings Under Phosphorus Stress
2.4.5. WGCNA Analysis of Maize Seedlings Under Phosphorus Stress
2.5. Integration Analysis of Candidate Gene Results Under Phosphorus Stress in Maize Seedling Stage
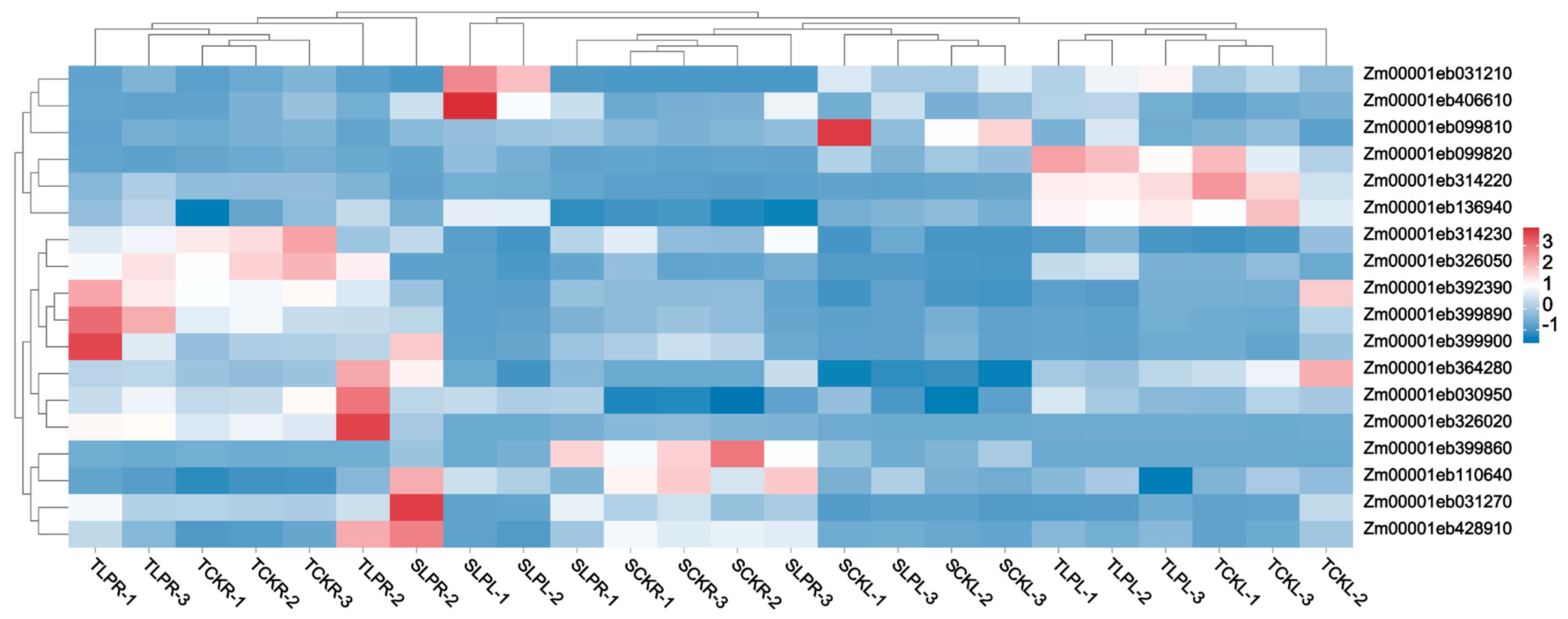
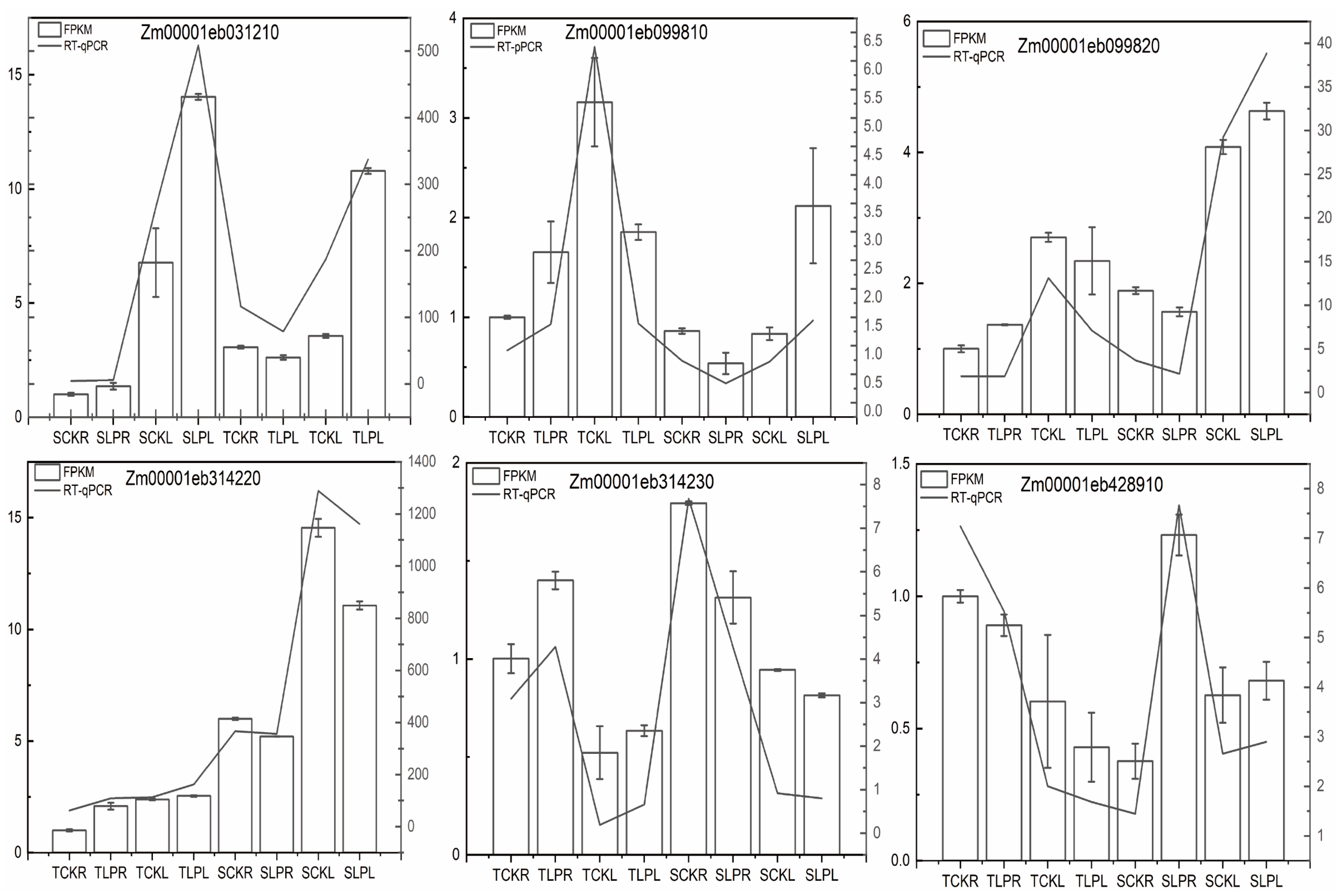
2.6. RT-qPCR Validation
3. Discussion
3.1. Analysis of Low Phosphorus Tolerance Phenotype of Maize
3.2. Genome-Wide Association Analysis of Low Phosphorus Tolerance in Maize Seedling Stage
3.3. Differential Gene Expression and Weighted Gene Co-Expression Network Analysis
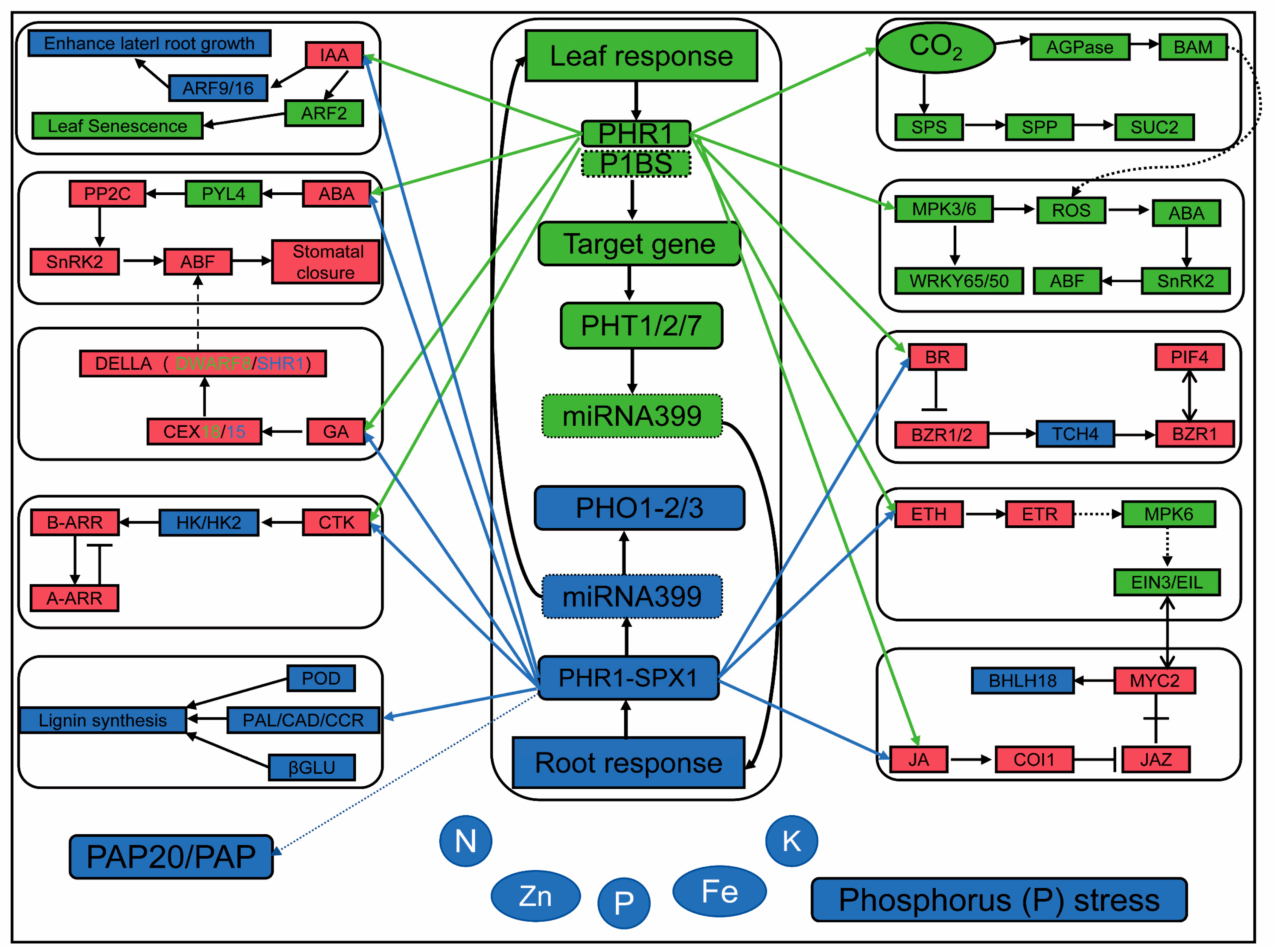
3.4. Candidate Genes and Molecular Regulatory Network of Low Phosphorus Tolerance in Maize
4. Materials and Methods
4.1. Experimental Materials and Culture Conditions
4.2. Determination of Related Indicators
4.3. Statistical Analysis of Phenotypic Data
4.4. Genome-Wide Association (GWAS) Analysis
4.5. Gene Analysis of Related Loci
4.6. Transcriptome Analysis
4.6.1. Total RNA Extraction and Illumina Deep Sequencing
4.6.2. Quality Assessment of Sequencing Results
4.6.3. Analysis of Differentially Expressed Genes
4.6.4. Gene Co-Expression Network Analysis
4.7. DEG Real-Time Fluorescence Quantitative PCR (RT-qPCR) Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Macdonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Vazquez, C.; Ibarra-Laclette, E.; Caballero-Perez, J.; Herrera-Estrella, L. Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant- and species-specific levels. J. Exp. Bot. 2008, 59, 2479–2497. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Peng, S.; Rillig, M.C.; Camenzind, T.; Delgado-Baquerizo, M.; Terrer, C.; Xu, X.; Feng, M.; Wang, M.; Fang, L.; et al. Global patterns of nutrient limitation in soil microorganisms. Proc. Natl. Acad. Sci. USA 2025, 122, e2424552122. [Google Scholar] [CrossRef]
- Dissanayaka, D.; Plaxton, W.C.; Lambers, H.; Siebers, M.; Marambe, B.; Wasaki, J. Molecular mechanisms underpinning phosphorus-use efficiency in rice. Plant Cell Environ. 2018, 41, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Hanyabui, E.; Apori, S.O.; Frimpong, K.A.; Atiah, K.; Byalebeka, J. Phosphorus sorption in tropical soils. AIMS Agric. Food 2020, 5, 599–616. [Google Scholar] [CrossRef]
- Chatterjee, N.; Margenot, A. Crop growth is increased by arbuscular mycorrhizae for both phosphate rock and soluble phosphorus fertilizers, but fertilizer solubility primarily determines crop growth. Biol. Fertil. Soils 2023, 59, 843–862. [Google Scholar] [CrossRef]
- Sen, A.; Banerjee, S.; Poddar, R.; Balo, S. Effectiveness of Three Organic Acids on Phosphorus Solubilization in Some Acid Soils of Eastern India. Commun. Soil Sci. Plant Anal. 2023, 54, 992–1004. [Google Scholar]
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Jeyasingh, P.D.; Neiman, M.; Richardson, A.E.; Soltis, P.S.; et al. Impacts of nitrogen and phosphorus: From genomes to natural ecosystems and agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef]
- Chen, X.-X.; Liu, Y.-M.; Zhao, Q.-Y.; Cao, W.-Q.; Chen, X.-P.; Zou, C.-Q. Health risk assessment associated with heavy metal accumulation in wheat after long-term phosphorus fertilizer application. Environ. Pollut. 2020, 262, 114348. [Google Scholar] [CrossRef]
- Kumar, S.; Pallavi; Chugh, C.; Seem, K.; Kumar, S.; Vinod, K.; Mohapatra, T. Characterization of contrasting rice (Oryza sativa L.) genotypes reveals the Pi-efficient schema for phosphate starvation tolerance. BMC Plant Biol. 2021, 21, 282. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Li, D.; Wang, N.; Sun, H.; Zhang, Y.; Zhang, K.; Li, A.; Bai, Z.; Li, C. Root Phenotypes of Cotton Seedlings Under Phosphorus Stress Revealed Through RhizoPot. Front. Plant Sci. 2021, 12, 716691. [Google Scholar] [CrossRef]
- Wu, L.; Kobayashi, Y.; Wasaki, J.; Koyama, H. Organic acid excretion from roots: A plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. Soil Sci. Plant Nutr. 2018, 64, 697–704. [Google Scholar] [CrossRef]
- Renshan, Z.; Hua, Q.; Yuzhe, S.; Shi, X.; Leong, L.B.; Haitao, S. Transgenic Arabidopsis thaliana containing increased levels of ATP and sucrose is more susceptible to Pseudomonas syringae. PLoS ONE 2017, 12, e0171040. [Google Scholar]
- Zamani, K.; Sabet, M.S.; Lohrasebi, T.; Mousavi, A.; Malboobi, M.A. Improved phosphate metabolism and biomass production by overexpression of AtPAP18 in tobacco. Biologia 2012, 67, 713–720. [Google Scholar] [CrossRef]
- Del Vecchio, H.A.; Ying, S.; Park, J.; Knowles, V.L.; Kanno, S.; Tanoi, K.; She, Y.M.; Plaxton, W.C. The cell wall-targeted purple acid phosphatase AtPAP25 is critical for acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. Plant J. Cell Mol. Biol. 2015, 80, 569–581. [Google Scholar] [CrossRef]
- Prathap, V.; Kumar, A.; Maheshwari, C.; Tyagi, A. Phosphorus homeostasis: Acquisition, sensing, and long-distance signaling in plants. Mol. Biol. Rep. 2022, 49, 8071–8086. [Google Scholar] [CrossRef] [PubMed]
- Sega, P.; Pacak, A. Plant PHR Transcription Factors: Put on A Map. Genes 2019, 10, 1018. [Google Scholar] [CrossRef]
- Qi, W.; Manfield, I.W.; Muench, S.P.; Baker, A. AtSPX1 affects the AtPHR1-DNA binding equilibrium by binding monomeric AtPHR1 in solution. Biochem. J. 2017, 474, 3675–3687. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Du, Q.; Wang, K.; Zou, C.; Li, W.X. The long non-coding RNA PILNCR2 increases low phosphate tolerance in maize by interfering with miRNA399-guided cleavage of ZmPHT1s. Mol. Plant 2023, 16, 1146–1159. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Jiang, H.; Jiang, C.; Du, Y.; Gong, C.; Wang, W.; Zhu, S.; Han, G.; Cheng, B. Systematic identification, evolution and expression analysis of the Zea mays PHT1 gene family reveals several new members involved in root colonization by arbuscular mycorrhizal fungi. Int. J. Mol. Sci. 2016, 17, 930. [Google Scholar] [CrossRef] [PubMed]
- Sahito, J.H.; Zhang, H.; Gishkori, Z.G.N.; Ma, C.; Wang, Z.; Ding, D.; Zhang, X.; Tang, J. Advancements and prospects of genome-wide association studies (GWAS) in maize. Int. J. Mol. Sci. 2024, 25, 1918. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Hu, W.; Li, Z.; Li, J.; Xie, G.; Wang, J.; Wang, L. Appraising the genetic architecture of kernel traits in hexaploid wheat using GWAS. Int. J. Mol. Sci. 2020, 21, 5649. [Google Scholar] [CrossRef]
- Wei, X.; Yao, S.; Di, J.; Guan, J.; Wang, A.; Yang, J.; Zhang, L.; Liu, Y.; Liang, M.; Niu, Z. Single-and Multi-Locus GWAS Unravels Novel Genomic Regions Related to Low-Phosphate Stress in Cotton Seedlings. Plants 2025, 14, 1803. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, W.; Cao, H.; Zhai, L.; Song, Q.; Xu, J. Identification of candidate genes for cold tolerance at seedling stage by GWAS in rice (Oryza sativa L.). Biology 2024, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Raman, H.; Raman, R.; Qiu, Y.; Yadav, A.S.; Sureshkumar, S.; Borg, L.; Rohan, M.; Wheeler, D.; Owen, O.; Menz, I. GWAS hints at pleiotropic roles for FLOWERING LOCUS T in flowering time and yield-related traits in canola. BMC Genom. 2019, 20, 636. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Wang, M.; Li, G.; Liu, W.J. Genetic Dissection of Phosphorus Use Efficiency in a Maize Association Population under Two P Levels in the Field. Int. J. Mol. Sci. 2021, 22, 9311. [Google Scholar] [CrossRef]
- Gowda, M.; Das, B.; Makumbi, D.; Babu, R.; Semagn, K.; Mahuku, G.; Olsen, M.S.; Bright, J.M.; Beyene, Y.; Prasanna, B.; et al. Genome-wide association and genomic prediction of resistance to maize lethal necrosis disease in tropical maize germplasm. Theor. Appl. Genet. 2015, 128, 1957–1968. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Shi, H.; Hu, H.; Yi, L.; Hou, J. Time-course transcriptome and WGCNA analysis revealed the drought response mechanism of two sunflower inbred lines. PLoS ONE 2022, 17, e0265447. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Wang, H.; Pan, T.; Wang, Y.; Xu, Y.; Xu, C.; Yang, Z.; Genetics, A. Genetic control of root plasticity in response to salt stress in maize. Theor. Appl. Genet. 2021, 134, 1475–1492. [Google Scholar] [CrossRef]
- Zhi, N.; Bowen, L.; Xiao, Z.; Ling, W.; Dan, L.; Jialei, G.; Xuan, H.; Duojiang, G.; Shiqiang, G.; Shibin, G. Combined Transcriptome and Proteome Analysis of Maize (Zea mays L.) Reveals A Complementary Profile in Response to Phosphate Deficiency. Curr. Issues Mol. Biol. 2021, 43, 1142–1155. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Guo, Y.; Du, Y.; Luo, Z.; Guo, Y.; Liu, W. Genome-Wide Identification and Expression Analysis of the Phosphate Transporter Gene Family in Zea mays Under Phosphorus Stress. Int. J. Mol. Sci. 2025, 26, 1445. [Google Scholar] [CrossRef]
- Luo, B.; Zhang, H.; Han, Z.; Zhang, X.; Guo, J.; Zhang, S.; Luo, X.; Zhao, J.; Wang, W.; Yang, G.; et al. Exploring the phosphorus–starch content balance mechanisms in maize grains using GWAS population and transcriptome data. Theor. Appl. Genet. 2024, 137, 158. [Google Scholar] [CrossRef]
- Liang, L.; Liu, B.; Huang, D.; Kuang, Q.; An, T.; Liu, S.; Liu, R.; Xu, B.; Zhang, S.; Deng, X. Arbuscular mycorrhizal fungi alleviate low phosphorus stress in maize genotypes with contrasting root systems. Plants 2022, 11, 3105. [Google Scholar] [CrossRef]
- Wu, F.; Yahaya, B.S.; Gong, Y.; He, B.; Gou, J.; He, Y.; Li, J.; Kang, Y.; Xu, J.; Wang, Q.; et al. ZmARF1 positively regulates low phosphorus stress tolerance via modulating lateral root development in maize. PLoS Genet. 2024, 20, e1011135. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Akter, F.; Azam, M.G.; Bagum, S.A.; Hossain, N.; Billah, M.; Biswas, P.L.; Hasibuzzaman, A.; Khaldun, A.; Alsuhaibani, A. Evaluation of Inbred Maize (Zea mays L.) for tolerance to low phosphorus at the seedling stage. Plants 2023, 12, 2520. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, J.; Han, Z.; Yang, J.; Ge, C.; Wu, Q. Revealing new insights into different phosphorus-starving responses between two maize (Zea mays) inbred lines by transcriptomic and proteomic studies. Sci. Rep. 2017, 7, 44294. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhang, G.; Yu, T.; Zhang, C.; Yang, G.; Luo, X.; Zhang, S.; Guo, J.; Zhang, H.; Zheng, H.; et al. Genome-wide association studies dissect low-phosphorus stress response genes underling field and seedling traits in maize. Theor. Appl. Genet. 2024, 137, 172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Yuan, Y.; Liao, Z.; Jiang, Y.; Lu, Y.J. Genome-Wide Association Study of 13 Traits in Maize Seedlings under Low Phosphorus Stress. Plant Genome 2019, 12, 190039. [Google Scholar] [CrossRef]
- Fernández-Marcos, M.; Desvoyes, B.; Manzano, C.; Liberman, L.M.; Benfey, P.N.; Del Pozo, J.C.; Gutierrez, C. Control of Arabidopsis lateral root primordium boundaries by MYB36. New Phytol. 2017, 213, 105–112. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Chen, W.T.; Zhao, M.Z.; Li, Y.C.; Li, M.; Hu, X. Genome-Wide Characterization and Evolutionary Analyses of Purple Acid Phosphatase (PAP) Gene Family with Their Expression Profiles in Response to Low Phosphorus Stresses in Moso Bamboo (Phyllostachys edulis). Forests 2021, 12, 326. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, G.; Wijewardene, I.; Cai, Y.; Esmaeili, N.; Sun, L.; Zhang, H. The B’ζ subunit of protein phosphatase 2A negatively regulates ethylene signaling in Arabidopsis. Plant Physiol. Biochem. PPB 2021, 169, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, W.; Huang, M. Overexpression of PeRHD3 alters the root architecture in Populus. Biochem. Biophys. Res. Commun. 2012, 424, 239–244. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57. [Google Scholar] [CrossRef]
- Seem, K.; Selvan, T.; Kaur, S.; Kumar, S.; Mohapatra, T. Pup1QTL-Mediated Tissue-Specific Differential Expression of Transcription Factors Help Mitigate Deleterious Effects of Phosphorus-Starvation in Rice (Oryza sativa L.). Plant Mol. Biol. Report. 2025, 43, 794–814. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, Z.; Zhang, X.; Huang, P.; Fan, C.; Yu, G.; Chen, F.; Xu, K.; Chen, Q.; Miao, Y. Spatial Divergence of PHR-PHT1 Modules Maintains Phosphorus Homeostasis in Soybean Nodules1. Plant Physiol. 2020, 184, 236–250. [Google Scholar] [CrossRef]
- Ribot, C.; Wang, Y.; Poirier, Y. Expression analyses of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 2008, 227, 1025–1036. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cao, B.L.; Zhang, B.B.; Zhao, D.; Gao, Z.Y.; Xia, T.C.; Zhang, Y.C.; Zhu, Y.F.; Gong, B. Open stomata 1 and phosphate starvation response 1 regulate tomato root system architecture during heterogeneous phosphate availability. Int. J. Biol. Macromol. 2025, 316, 144611. [Google Scholar] [CrossRef]
- Nilsson, L.; Müller, R.; Nielsen, T.H. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ. 2007, 30, 1499–1512. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Liu, D. Comparative functional analyses of PHR1, PHL1, and PHL4 transcription factors in regulating Arabidopsis responses to phosphate starvation. Front. Plant Sci. 2024, 15, 1379562. [Google Scholar] [CrossRef] [PubMed]
- Matías, B.; Manuel, D.J.; Antonella, F.; Palatnik, J.F. ARF2 represses expression of plant GRF transcription factors in a complementary mechanism to microRNA miR396. Plant Physiol. 2021, 185, 1798–1812. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Lei, P.; Feng, X.H.; Xu, X.J.; Xu, H.; Yang, H.B.; Tang, W.Q. Effect of poly(γ-glutamic acid) on microbial community and nitrogen pools of soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 657–668. [Google Scholar] [CrossRef]
- Rajput, P.; Urfan, M.; Sharma, S.; Hakla, H.R.; Nandan, B.; Das, R.; Roychowdhury, R.; Chowdhary, S.P. Natural variation in root traits identifies significant SNPs and candidate genes for phosphate deficiency tolerance in Zea mays L. Physiol. Plant. 2024, 176, e14396. [Google Scholar]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhao, Y.; Hu, J.T.; Wang, L.L.; Li, L.Y.; Zhang, X.Y.; Zhou, Z.J.; Chen, L.L.; Wang, H.; Wang, J.Y.; et al. The OsBZR1-OsSPX1/2 module fine-tunes the growth-immunity trade-off in adaptation to phosphate availability in rice. Mol. Plant 2024, 17, 258–276. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, S.H.; Cheng, L.H.; Wu, J.J.; Lin, Y.C.; Tsay, Y.F. Potential Transceptor AtNRT1.13 Modulates Shoot Architecture and Flowering Time in a Nitrate-Dependent Manner. Plant Cell 2021, 33, 1492–1505. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, J.H.; Baek, W.; Kim, B.S.; Lee, S.C. Functional roles of the protein phosphatase 2C, AtAIP1, in abscisic acid signaling and sugar tolerance in Arabidopsis. Plant Sci. 2012, 187, 83–88. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhao, X.S.; Wu, Q.; Shen, R.F. Abscisic acid is involved in root cell wall phosphorus remobilization independent of nitric oxide and ethylene in rice (Oryza sativa). Ann. Bot. 2018, 121, 1361–1368. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Sun, J.; Guo, Z.; Zou, C.; Li, W.-X.; Xie, C.; Huang, C.; Xu, R.; Liao, H.; et al. Genome-wide association study dissects yield components associated with low-phosphorus stress tolerance in maize. Theor. Appl. Genet. 2018, 131, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- To, H.T.M.; Le, K.Q.; Van Nguyen, H.; Duong, L.V.; Kieu, H.T.; Chu, Q.A.T.; Mai, N.T. A genome-wide association study reveals the quantitative trait locus and candidate genes that regulate phosphate efficiency in a Vietnamese rice collection. Physiol. Mol. Biol. Plants 2020, 26, 2267–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, B.; Liu, J.; Jin, X.; Zhang, H.; Zhong, H.; Li, B.; Hu, H.; Wang, Y.; Ali, A.; et al. Functional analysis of ZmG6PE reveals its role in responses to low-phosphorus stress and regulation of grain yield in maize. Front. Plant Sci. 2023, 14, 1286699. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, N.; Tang, S.; Qin, T.; Huang, J. Roles of glutamate receptor-like channels (GLRs) in plant growth and response to environmental stimuli. Plants 2022, 11, 3450. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ma, M.; Liu, H.; Tao, D.; Salam, S.A.; Han, X.; Liu, Y.; Yong, J. Exogenous GABA-Ca Alleviates Growth Inhibition Induced by a Low-P Environment in Peanuts (Arachis hypogaea). Antioxidants 2024, 13, 1414. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Geilfus, C.-M.; Mühling, K.-H. Glutamine synthetase activity in leaves of Zea mays L. as influenced by magnesium status. Planta 2015, 242, 1309–1319. [Google Scholar] [CrossRef]
- Wei, N.; Chen, A.; Guo, X.; Zhang, S.; Song, L.; Gan, N.; Zheng, L.; Jia, Y.; Li, J. Changes in nitrogen metabolism of phosphorus-starved bloom-forming cyanobacterium Microcystis aeruginosa: Implications for nutrient management. Sci. Total Environ. 2023, 903, 166832. [Google Scholar] [CrossRef]
- Han, H.; Wang, C.; Yang, X.; Wang, L.; Ye, J.; Xu, F.; Liao, Y.; Zhang, W. Role of bZIP transcription factors in the regulation of plant secondary metabolism. Planta 2023, 258, 13. [Google Scholar] [CrossRef]
- Wu, R.; Liu, Z.; Sun, S.; Qin, A.; Liu, H.; Zhou, Y.; Li, W.; Liu, Y.; Hu, M.; Yang, J. Identification of bZIP Transcription Factors That Regulate the Development of Leaf Epidermal Cells in Arabidopsis thaliana by Single-Cell RNA Sequencing. Int. J. Mol. Sci. 2024, 25, 2553. [Google Scholar] [CrossRef]
- Tang, D.; Christiansen, K.M.; Innes, R.W. Regulation of Plant Disease Resistance, Stress Responses, Cell Death, and Ethylene Signaling in Arabidopsis by the EDR1 Protein Kinase. Plant Physiol. 2005, 138, 1018–1026. [Google Scholar] [CrossRef]
- Zaheer, U.; Munir, F.; Salum, Y.M.; He, W. Function and regulation of plant ARGONAUTE proteins in response to environmental challenges: A review. PeerJ 2024, 12, e17115. [Google Scholar] [PubMed]
- Li, Y.; Yang, Y.; Liu, Y.; Li, D.; Liu, Z. Overexpression of OsAGO1b Induces Adaxially Rolled Leaves by Affecting Leaf Abaxial Sclerenchymatous Cell Development in Rice. Rice 2019, 12, 60. [Google Scholar] [CrossRef]
- Li, T.; Zhu, S.; Li, Y.; Yao, J.; Wang, C.; Fang, S.; Pan, J.; Chen, W.; Zhang, Y. Characteristic of GEX1 genes reveals the essential roles for reproduction in cotton. Int. J. Biol. Macromol. 2023, 253, 127645. [Google Scholar] [CrossRef]
- Li, H.; Peng, Z.; Yang, X.; Wang, W.; Fu, J.; Wang, J.; Han, Y.; Chai, Y.; Guo, T.; Yang, N. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 2013, 45, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Lu, Y.; Yang, X.; Huang, J.; Zhou, Y.; Ali, F.; Wen, W.; Liu, J.; Li, J.; Yan, J. Genome wide association studies using a new nonparametric model reveal the genetic architecture of 17 agronomic traits in an enlarged maize association panel. PLoS Genet. 2014, 10, e1004573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, M.; Zhang, H.; Liu, X.; Zhang, W.; Wang, Q.; Jia, Q.; Xu, D.; Chen, H.; Su, C. A genome-wide association analysis for salt tolerance during the soybean germination stage and development of KASP markers. Front. Plant Sci. 2024, 15, 1352465. [Google Scholar] [CrossRef]
- Harshitha, R.; Arunraj, D. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. A Bimon. Publ. Int. Union Biochem. Mol. Biol. 2021, 49, 800–812. [Google Scholar] [CrossRef] [PubMed]


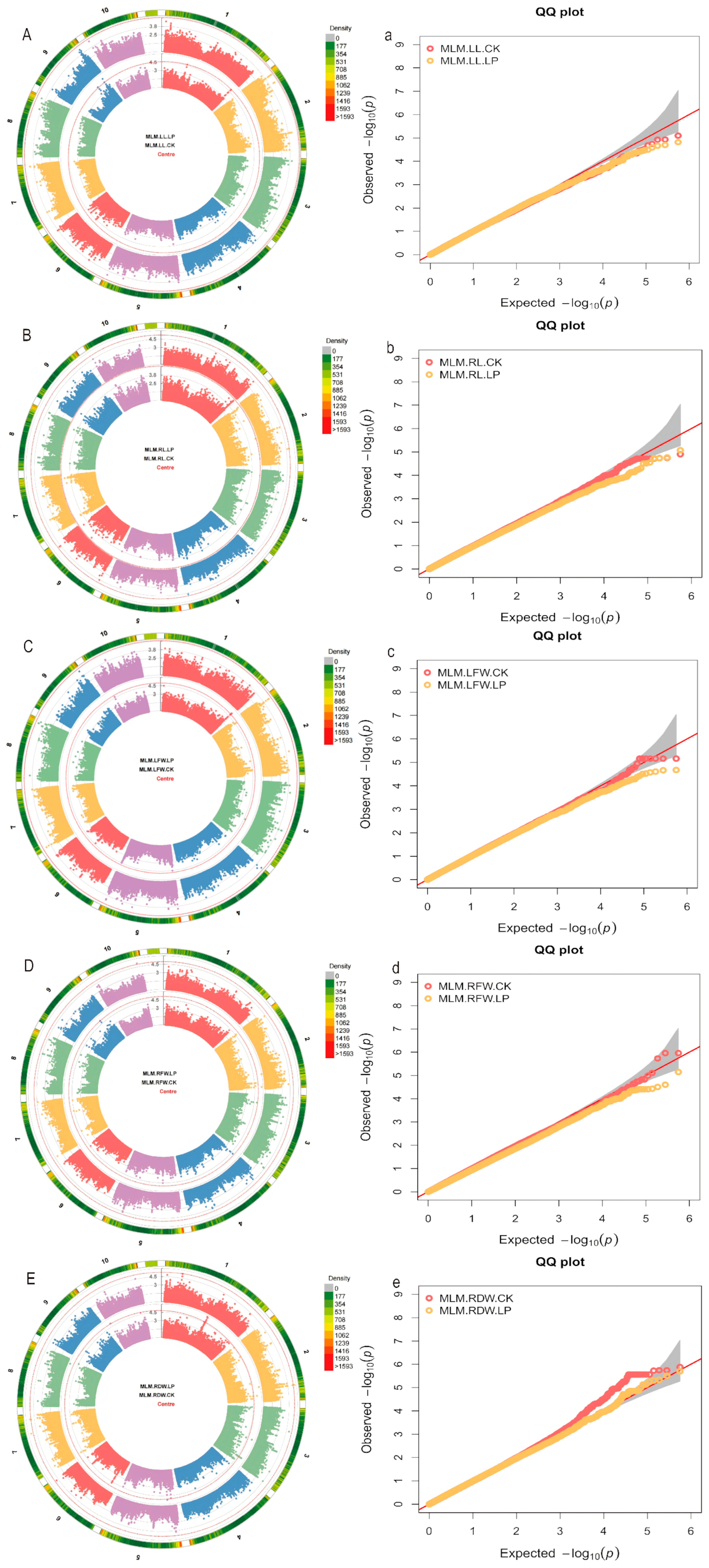
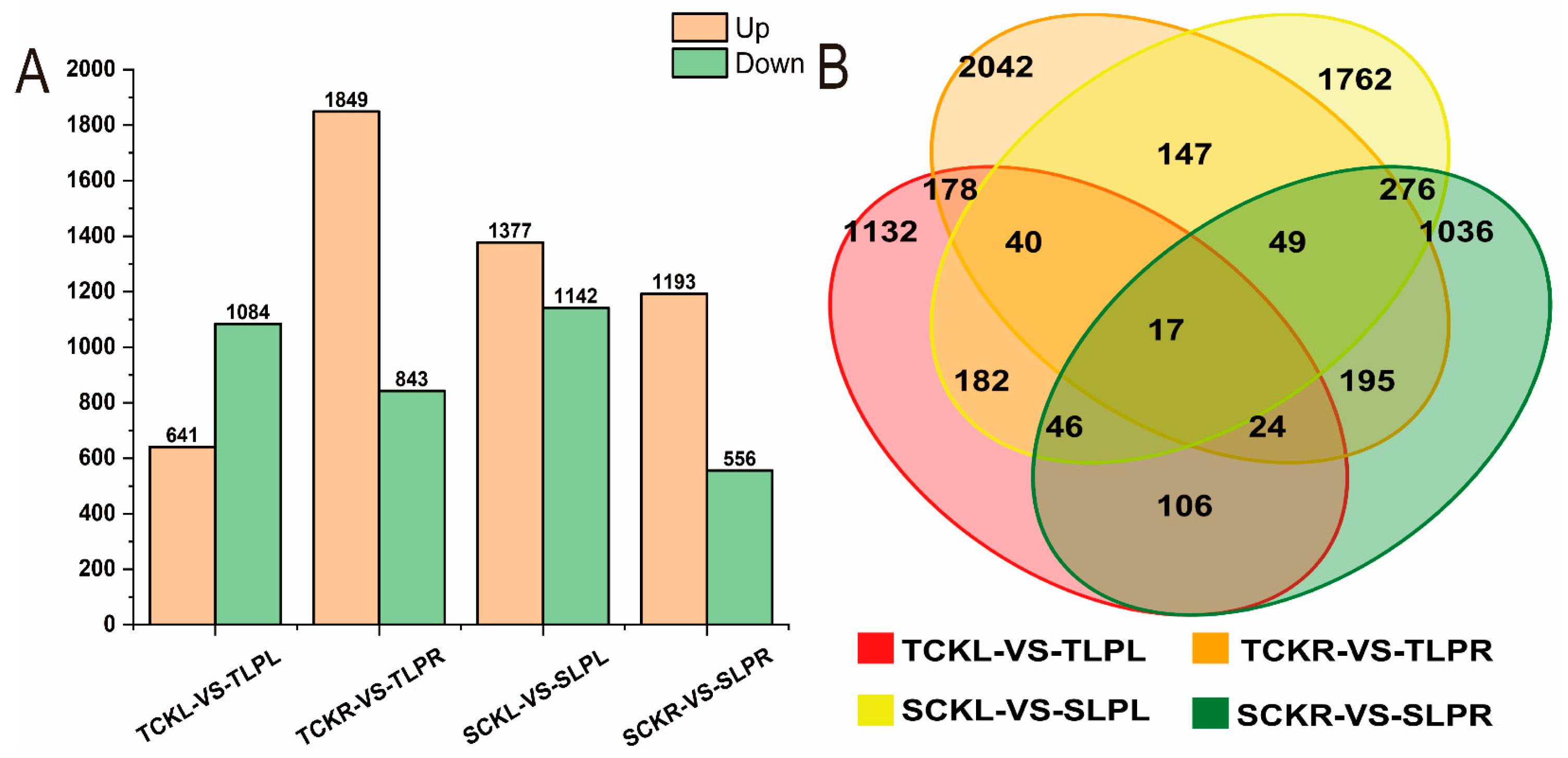
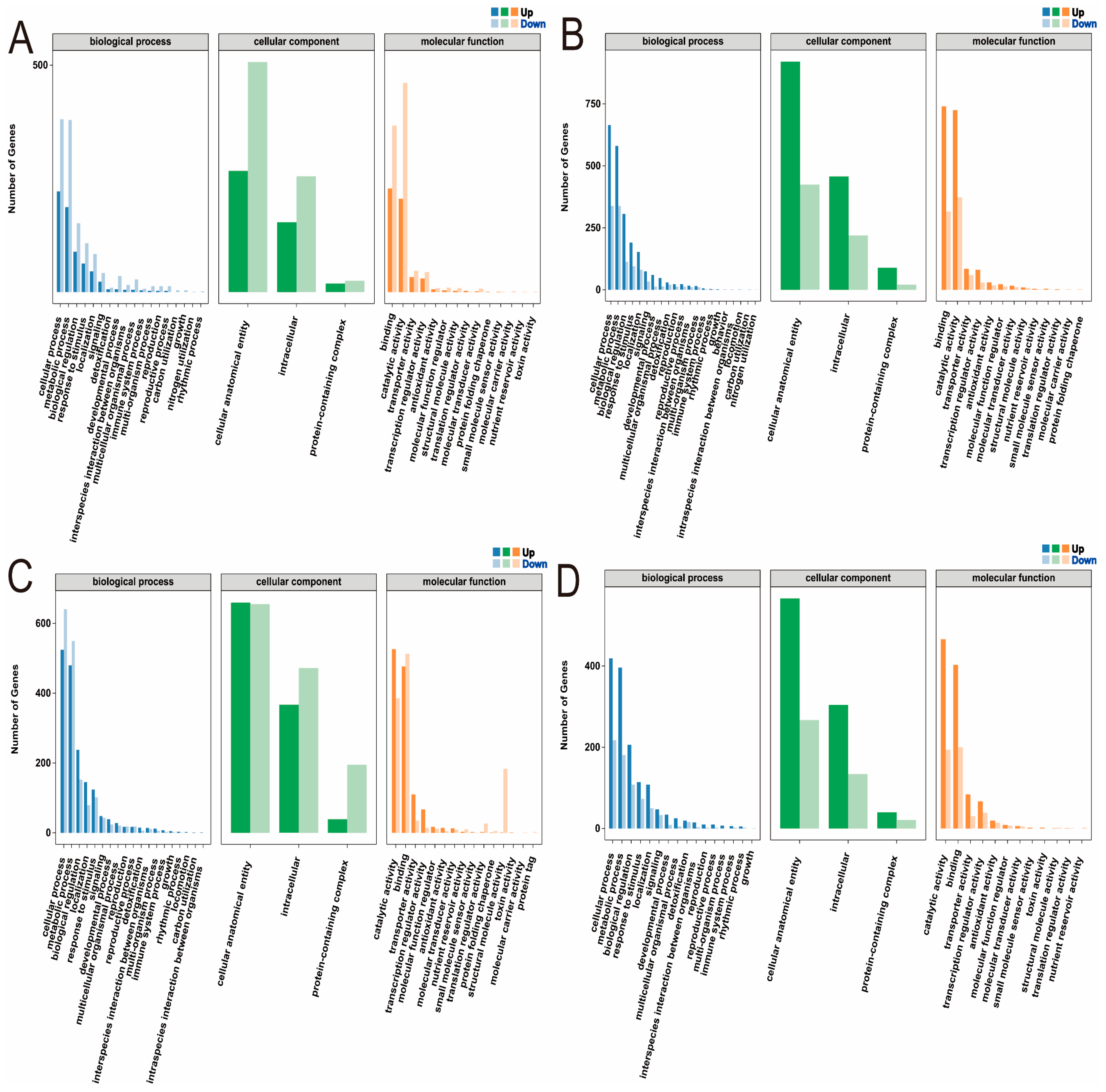
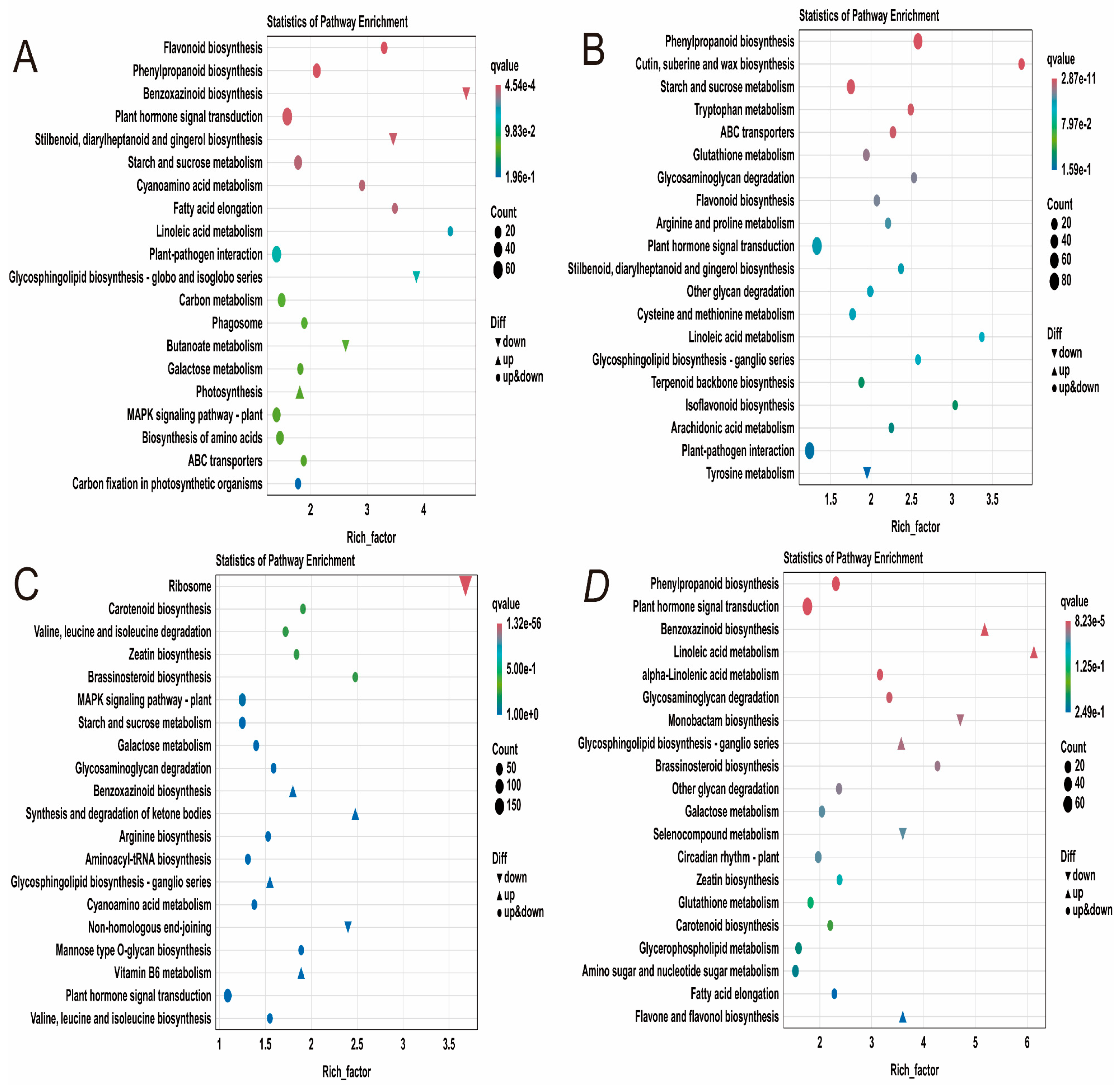

| Trait | P-Level | Mean ± SD | Rage | CV (%) | G | E | G × E | H2 | Rd (%) |
|---|---|---|---|---|---|---|---|---|---|
| LL | CK | 34.99 ± 7.23 | 15.17 to 61.5 | 20.66 | ** | ns | ** | 0.85 | 0.03 |
| LP | 35 ± 7.28 | 16.7 to 57.75 | 20.81 | ||||||
| RL | CK | 28.67 ± 6.98 | 11.5 to 51.77 | 24.33 | ** | ** | ** | 0.62 | 3.28 |
| LP | 27.73 ± 7.01 | 11.1 to 53.85 | 25.27 | ||||||
| LFW | CK | 1.63 ± 0.52 | 0.4 to 3.28 | 32.03 | ** | ns | ** | 0.80 | 0.61 |
| LP | 1.62 ± 0.52 | 0.52 to 3.55 | 31.96 | ||||||
| RFW | CK | 1.06 ± 0.36 | 0.36 to 2.49 | 34.43 | ** | ** | ** | 0.72 | 5.66 |
| LP | 1 ± 0.36 | 0.24 to 2.33 | 36.27 | ||||||
| SDW | CK | 0.15 ± 0.09 | 0.03 to 1.32 | 60.97 | ** | ns | ** | 0.89 | 6.67 |
| LP | 0.14 ± 0.05 | 0.04 to 0.33 | 37.53 | ||||||
| RDW | CK | 0.07 ± 0.03 | 0.02 to 0.26 | 35.49 | ** | ** | ** | 0.76 | 0 |
| LP | 0.07 ± 0.02 | 0.01 to 0.17 | 33.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Lei, G.; Zhong, Z.; Zhuang, Z.; Bian, J.; Zhang, L.; Ta, W.; Ren, Z.; Peng, Y. Identification of Candidate Genes for Low Phosphorus Tolerance in Maize Seedling Stage Based on GWAS and Transcriptome. Plants 2025, 14, 2836. https://doi.org/10.3390/plants14182836
Hao X, Lei G, Zhong Z, Zhuang Z, Bian J, Zhang L, Ta W, Ren Z, Peng Y. Identification of Candidate Genes for Low Phosphorus Tolerance in Maize Seedling Stage Based on GWAS and Transcriptome. Plants. 2025; 14(18):2836. https://doi.org/10.3390/plants14182836
Chicago/Turabian StyleHao, Xiaojia, Gonxin Lei, Zhiming Zhong, Zelong Zhuang, Jianwen Bian, Lei Zhang, Wanling Ta, Zhenping Ren, and Yunling Peng. 2025. "Identification of Candidate Genes for Low Phosphorus Tolerance in Maize Seedling Stage Based on GWAS and Transcriptome" Plants 14, no. 18: 2836. https://doi.org/10.3390/plants14182836
APA StyleHao, X., Lei, G., Zhong, Z., Zhuang, Z., Bian, J., Zhang, L., Ta, W., Ren, Z., & Peng, Y. (2025). Identification of Candidate Genes for Low Phosphorus Tolerance in Maize Seedling Stage Based on GWAS and Transcriptome. Plants, 14(18), 2836. https://doi.org/10.3390/plants14182836





