Stoichiometric Homeostasis and Functional Group Divergence Jointly Enhance Alpine Plant Adaptation to Environmental Stress
Abstract
1. Introduction
2. Results
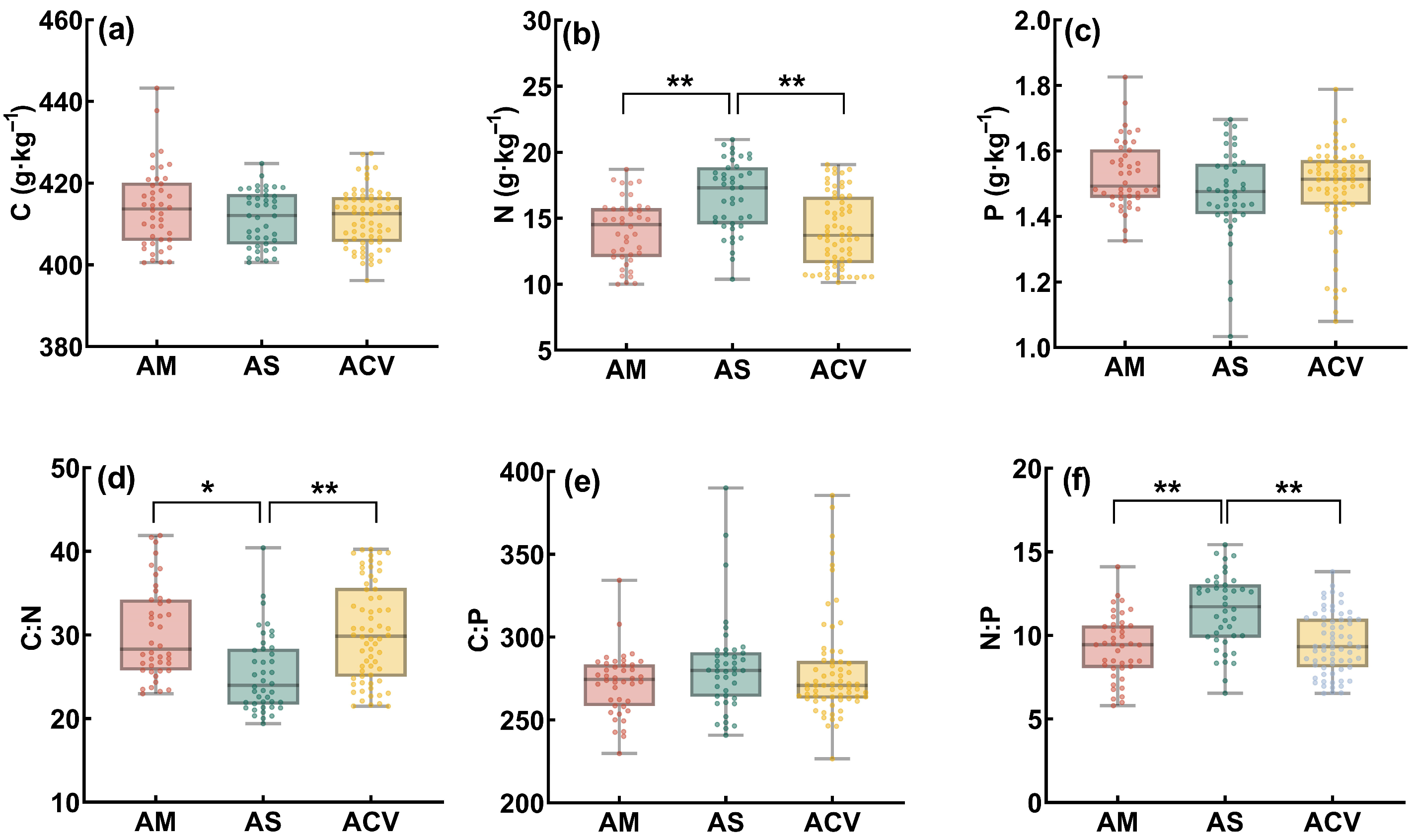
2.1. Soil Stoichiometric Characteristics
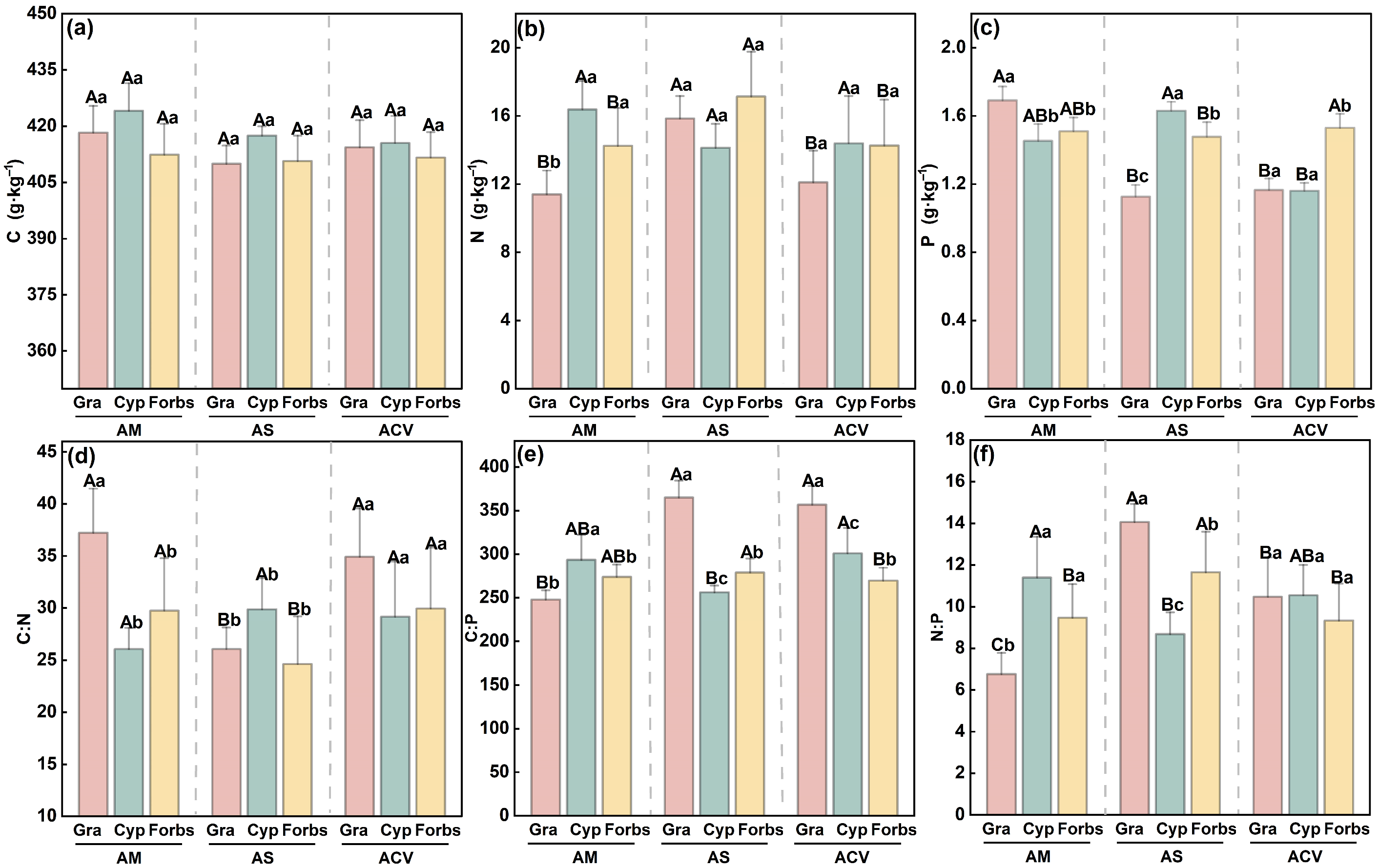
2.2. Plant Stoichiometric Characteristics
2.3. Homeostasis of Plant Functional Groups
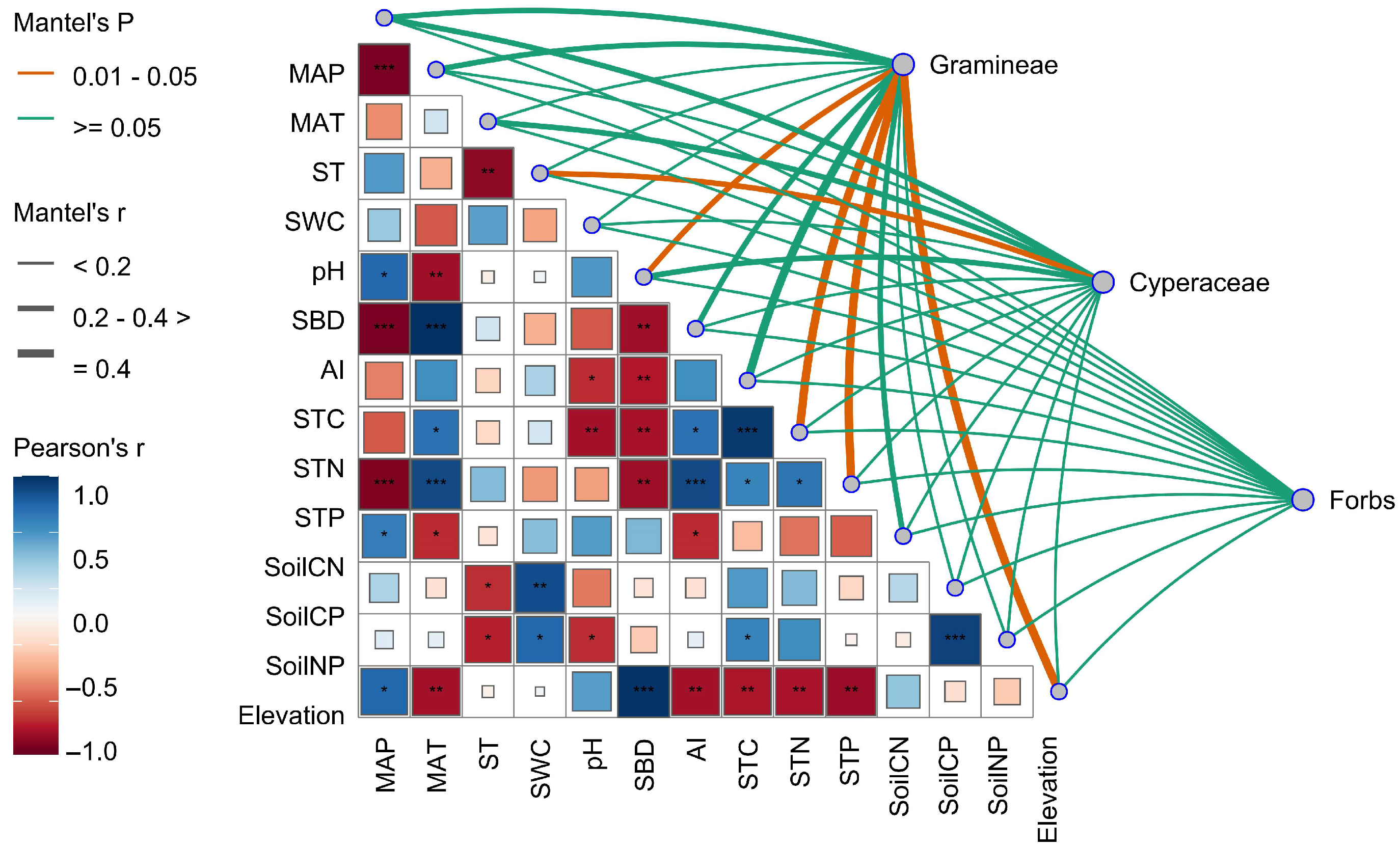
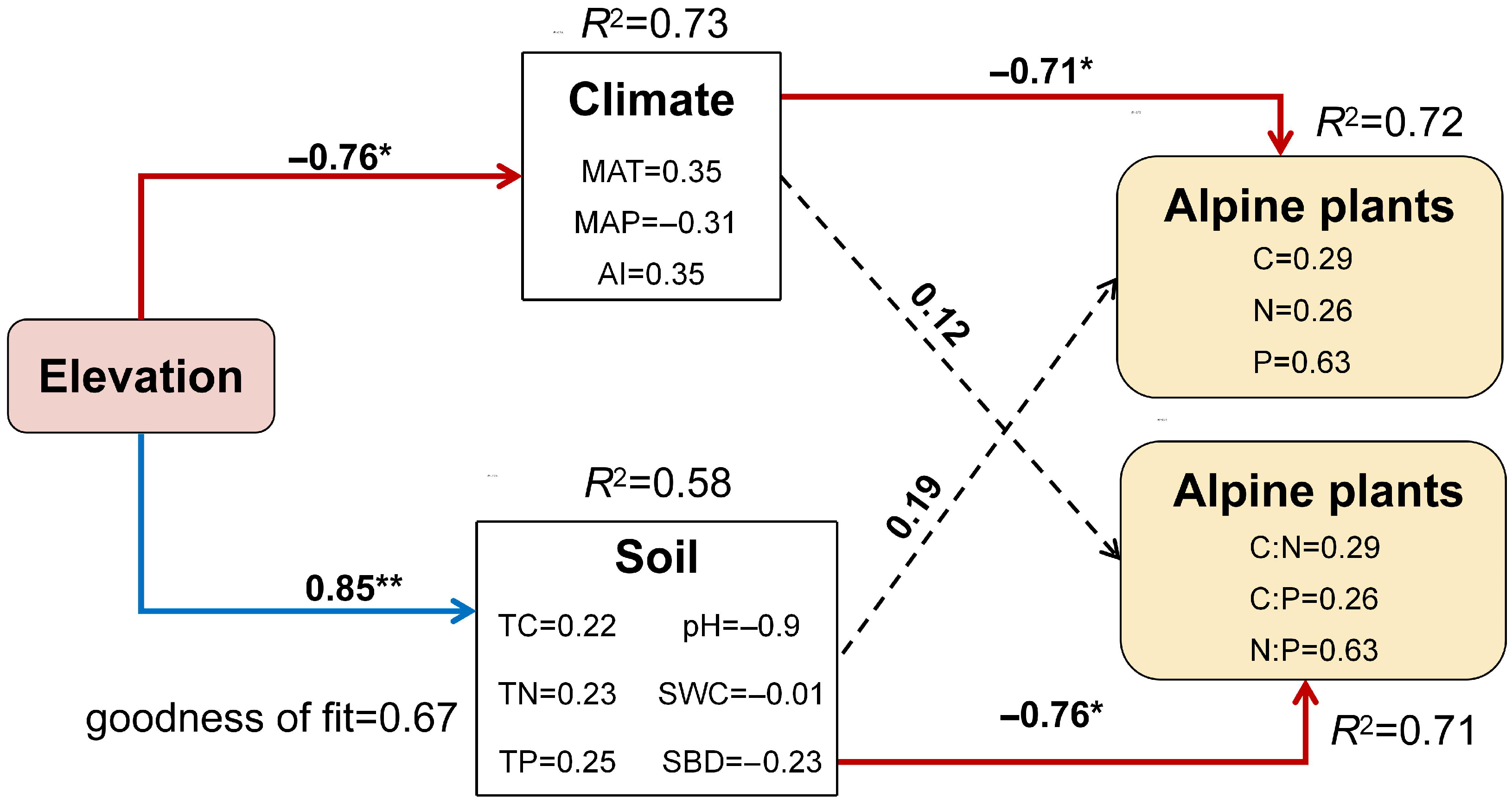
2.4. Environmental Drivers of Stoichiometric Variation in Functional Groups
3. Discussion
3.1. Stoichiometric Traits of Plant Functional Groups Across Altitudinal Vegetation Zones
3.2. Environmental Response Strategies of Different Plant Functional Groups
3.3. Drivers of Stoichiometric Variation in Alpine Grassland Plants
4. Materials and Methods
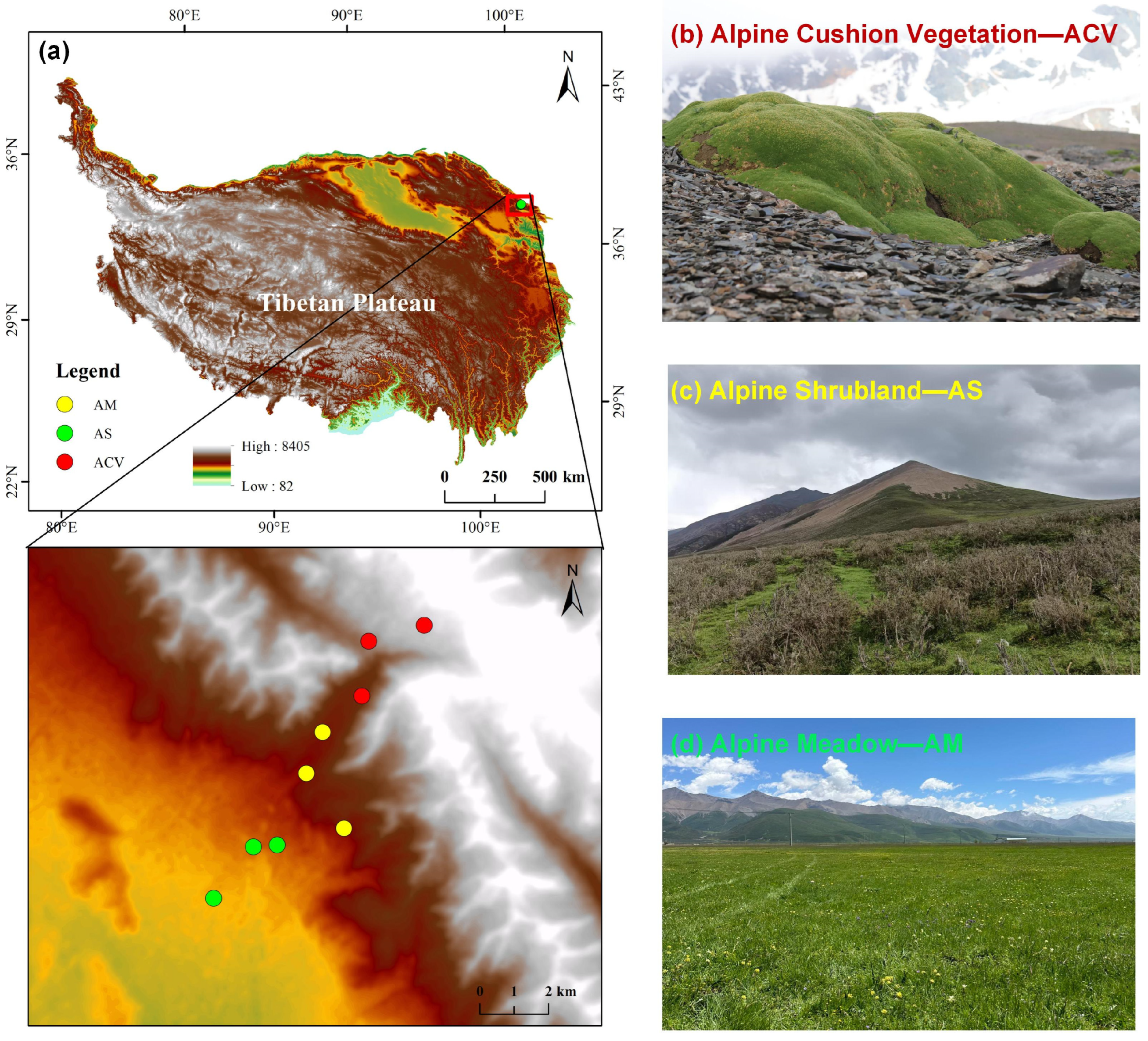
4.1. Study Area
4.2. Experimental Design
4.3. Environmental Measurements and Sample Determinations
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Fernandez-Martinez, M. From atoms to ecosystems: Elementome diversity meets ecosystem functioning. New Phytol. 2022, 234, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Julian, P.; Gerber, S.; Bhomia, R.K.; King, J.; Osborne, T.Z.; Wright, A.L.; Powers, M.; Dombrowski, J. Evaluation of nutrient stoichiometric relationships among ecosystem compartments of a subtropical treatment wetland. Do we have “Redfield wetlands”? Ecol. Process. 2019, 8, 20. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Mayor, J.R.; Sanders, N.J.; Classen, A.T.; Bardgett, R.D.; Clément, J.C.; Fajardo, A.; Lavorel, S.; Sundqvist, M.K.; Bahn, M.; Chisholm, C.; et al. Elevation alters ecosystem properties across temperate treelines globally. Nature 2017, 542, 91–95. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Wardle, D.A.; Vincent, A.; Giesler, R. Contrasting nitrogen and phosphorus dynamics across an elevational gradient for subarctic tundra heath and meadow vegetation. Plant Soil 2014, 383, 387–399. [Google Scholar] [CrossRef]
- Broadbent, A.A.D.; Newbold, L.K.; Pritchard, W.J.; Michas, A.; Goodall, T.; Cordero, I.; Giunta, A.; Snell, H.S.K.; Pepper, V.; Grant, H.K.; et al. Climate change disrupts the seasonal coupling of plant and soil microbial nutrient cycling in an alpine ecosystem. Glob. Change Biol. 2024, 30, e17245. [Google Scholar] [CrossRef]
- Pan, J.X.; Zhang, X.Y.; Liu, S.; Liu, N.; Liu, M.J.; Chen, C.; Zhang, X.Y.; Niu, S.L.; Wang, J.S. Precipitation alleviates microbial C limitation but aggravates N and P limitations along a 3000-km transect on the Tibetan Plateau. Catena 2024, 247, 108535. [Google Scholar] [CrossRef]
- Rousk, K.; Degboe, J.; Michelsen, A.; Bradley, R.; Bellenger, J.-P. Molybdenum and phosphorus limitation of moss-associated nitrogen fixation in boreal ecosystems. New Phytol. 2017, 214, 97–107. [Google Scholar] [CrossRef]
- Zheng, L.; Cao, X.; Yang, Z.; Wang, H.; Zang, Q.; Song, W.; Shen, M.; Xiao, C.; Tian, Q. Effects of warming conditions on plant nitrogen-phosphorus stoichiometry and resorption of three plant species in alpine meadows on the Tibetan Plateau. J. Plant Ecol. 2024, 17, rtae032. [Google Scholar] [CrossRef]
- He, J.-S.; Fang, J.; Wang, Z.; Guo, D.; Flynn, D.F.B.; Geng, Z. Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 2006, 149, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.B.; Niklas, K.J.; Han, W.X.; Kattge, J.; Reich, P.B.; Luo, Y.K.; Chen, Y.H.; Tang, Z.Y.; Hu, H.F.; et al. Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent. Natl. Sci. Rev. 2018, 5, 728–739. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ji, C.J.; Datta, A.; Li, P.; Ma, W.H.; Mohammat, A.; Shen, H.H.; Hu, H.F.; Knapp, B.O.; et al. Stoichiometric shifts in surface soils over broad geographical scales: Evidence from China’s grasslands. Glob. Ecol. Biogeogr. 2014, 23, 947–955. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.X.; Seabloom, E.W.; Sardans, J.; Penuelas, J.; Zhang, H.Y.; Wei, C.Z.; Han, X.G. Multiple Nutrient Additions Homogenize Multidimensional Plant Stoichiometry in a Meadow Steppe. Glob. Change Biol. 2025, 31, e70123. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1221–1226. [Google Scholar] [CrossRef]
- Prager, C.M.; Classen, A.T.; Sundqvist, M.K.; Barrios-Garcia, M.N.; Cameron, E.K.; Chen, L.T.; Chisholm, C.; Crowther, T.W.; Deslippe, J.R.; Grigulis, K.; et al. Integrating natural gradients, experiments, and statistical modeling in a distributed network experiment: An example from the WaRM Network. Ecol. Evol. 2022, 12, e9396. [Google Scholar] [CrossRef] [PubMed]
- Guiz, J.; Hillebrand, H.; Borer, E.T.; Abbas, M.; Ebeling, A.; Weigelt, A.; Oelmann, Y.; Fornara, D.; Wilcke, W.; Temperton, V.M.; et al. Long-term effects of plant diversity and composition on plant stoichiometry. Oikos 2016, 125, 613–621. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Fanin, N.; Han, X.; Du, X.; Liu, H.; Li, Y.; Li, Q. Adaptation of soil micro-food web to elemental limitation: Evidence from the forest-steppe ecotone. Soil Biol. Biochem. 2022, 170, 108698. [Google Scholar] [CrossRef]
- Jin, L.; Wu, Q.H.; Xie, S.J.; Chen, W.W.; Duan, C.Q.; Sun, C.Q.; Pan, Y.; Lauridsen, T.L. Phosphorus stoichiometric homeostasis of submerged macrophytes and associations with interspecific interactions and community stability in Erhai Lake, China. Water Res. 2024, 256, 121575. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, Q.; Elser, J.J.; He, N.; Wu, H.; Zhang, G.; Wu, J.; Bai, Y.; Han, X. Linking stoichiometric homeostasis with ecosystem structure, functioning, and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, L.; Qin, S.Q.; Kou, D.; Wang, S.Y.; Zheng, Z.H.; Peñuelas, J.; Yang, Y.H. Microbial nitrogen and phosphorus co-limitation across permafrost region. Glob Change Biol. 2023, 29, 3910–3923. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, X.; Tang, X.; Wang, W.; Zhou, G.; Xu, S.; Ma, Y. Patterns and controlling factors of plant nitrogen and phosphorus stoichiometry across China’s forests. Biogeochemistry 2019, 143, 191–205. [Google Scholar] [CrossRef]
- Convey, P.; Gibson, J.A.E.; Hillenbrand, C.; Hodgson, D.A.; Pugh, P.J.A.; Smellie, J.L.; Stevens, M.I. Antarctic terrestrial life—Challenging the history of the frozen continent? Biol. Rev. 2010, 83, 103–117. [Google Scholar] [CrossRef]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above- and belowground linkages shape responses of mountain vegetation to climate change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.G. Mountain Weather and Climate, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Güsewell, S. N : P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.X.; Song, C.C.; Zhang, X.H.; Mao, R.; Guo, Y.D.; Gao, F.Y. Plant zonation patterns reflected by the differences in plant growth, biomass partitioning and root traits along a water level gradient among four common vascular plants in freshwater marshes of the Sanjiang Plain, Northeast China. Ecol. Eng. 2015, 81, 158–164. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, L.; Zhou, Y.; Sang, W.; Zhao, J.; Liu, L.; Li, C.; Xiao, C.; Deng, Y. Changes in soil microbial community structure and function following degradation in a temperate grassland. J. Plant Ecol. 2020, 14, 384–397. [Google Scholar] [CrossRef]
- Ganjurjav, H.; Gao, Q.; Gornish, E.S.; Schwartz, M.W.; Liang, Y.; Cao, X.; Zhang, W.; Zhang, Y.; Li, W.; Wan, Y.; et al. Differential response of alpine steppe and alpine meadow to climate warming in the central Qinghai–Tibetan Plateau. Agric. For. Meteorol. 2016, 223, 233–240. [Google Scholar] [CrossRef]
- Zhang, C.; An, Y.; Yun, J.; Wang, L.L.; Zhou, Z.L.; Wang, L.P.; Yang, Y.P.; Duan, Y. Processes on reproductive ecology of plant species in the Qinghai-Xizang Plateau and adjacent highlands. Chin. J. Plant Ecol. 2020, 44, 1–21. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology-A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Walker, L.R.; del Moral, R. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Cao, Z.Y.; Xu, L.; Zong, N.; Zhang, J.J.; He, N.P. Impacts of Climate Warming on Soil Phosphorus Forms and Transformation in a Tibetan Alpine Meadow. J. Soil. Sci. Plant Nutr. 2022, 22, 2545–2556. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. The global nitrogen-phosphorus imbalance. Science 2022, 375, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2012, 196, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Wright, S.J. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Wang, J.; Luo, P.; Yang, H.; Mou, C.; Mo, L. Different responses of alpine plants to nitrogen addition: Effects on plant-plant interactions. Sci Rep. 2016, 6, 38320. [Google Scholar] [CrossRef]
- Hong, S.; Chen, J.; Biswas, A.; Cao, J.; Dong, X.; Han, W. Leaf stoichiometry of common species along altitude gradients in the Qilian Mountains, China. J. Plant Ecol. 2023, 17, rtad044. [Google Scholar] [CrossRef]
- Niu, Q.C.; Jin, G.F.; Yin, S.X.; Gan, L.; Yang, Z.Y.; Dorji, T.; Shen, M.G. Transcriptional Changes Underlying the Degradation of Plant Community in Alpine Meadow Under Seasonal Warming Impact. Plant Cell Environ. 2025, 48, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.R.; Jones, V.L. Ten Ways That Weed Evolution Defies Human Management Efforts Amidst a Changing Climate. Agronomy 2021, 11, 284. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen—Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. Chinese Soil Taxonomy, 3rd ed.; Science Press: Beijing, China; Springer: Dordrecht, The Netherlands, 2022. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Li, Y.; Ma, J.; Xiao, C.; Li, Y. Effects of climate factors and soil properties on soil nutrients and elemental stoichiometry across the Huang–Huai–Hai River Basin, China. J. Soils Sediments 2020, 20, 1970–1982. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M.L. Linkages of C: N: P stoichiometry between soil and leaf and their response to climatic factors along altitudinal gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- National Earth System Science Data Center. Monthly Gridded Precipitation Data (1 km Resolution, 1901–2024). Available online: https://www.geodata.cn/data/datadetails.html?dataguid=xxxxxxx (accessed on 16 August 2025).
- China Meteorological Data Service Center. Temperature and Precipitation Data. Available online: http://data.cma.cn (accessed on 16 August 2025).
- National Tibetan Plateau Data Center. Radiation Data. Available online: https://data.tpdc.ac.cn/ (accessed on 28 August 2025).
- Hargreaves, G.H.; Samani, Z.A. Reference crop evapotranspiration from temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Milly, P.C.D. Climate, soil water storage, and the average annual water balance. Water Resour. Res. 1994, 30, 2143–2156. [Google Scholar] [CrossRef]
- Persson, J.; Fink, P.; Goto, A.; Hood, J.M.; Jonas, J.; Kato, S. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2012, 119, 741–751. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Cha, X.; Zhou, T.; Pang, X.; Zhao, F.; Han, X.; Yang, G.; Wei, G.; Ren, C. The biogeography of soil microbiome potential growth rates. Nat. Commun. 2024, 15, 9472. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. Imeta 2023, 2, e83. [Google Scholar] [CrossRef] [PubMed]


| Lg(x) | Lg(y) | Functional Group | AM | AS | ACV | |||
|---|---|---|---|---|---|---|---|---|
| p | H−1 | p | H−1 | p | H−1 | |||
| Csoil | Cplant | Gra | 0.224 | 0.6363 | 0.431 | 0.8291 | 0.474 | 0.3393 |
| Cyp | 0.250 | 3.5840 | 0.765 | 0.0611 | 0.468 | 0.2606 | ||
| Forbs | 0.137 | 1.9380 | 0.449 | 0.7253 | 0.961 | 0.0148 | ||
| Nsoil | Nplant | Gra | 0.549 | 3.6400 | 0.958 | 0.0433 | 0.480 | 3.8840 |
| Cyp | 0.590 | 2.5110 | 0.873 | 0.1254 | 0.361 | 2.2430 | ||
| Forbs | 0.736 | 2.2090 | 0.079 | 0.4085 | 0.504 | 2.5940 | ||
| Psoil | Pplant | Gra | 0.173 | 0.9155 | 0.283 | 1.0240 | 0.187 | 0.1243 |
| Cyp | 0.669 | 0.6885 | 0.026 * | 0.5526 | 0.322 | 0.4667 | ||
| Forbs | 0.107 | 0.5235 | 0.767 | 0.3049 | 0.686 | 0.0627 | ||
| Csoil:Nsoil | Cplant:Nplant | Gra | 0.270 | 4.2270 | 0.988 | 0.0110 | 0.446 | 6.3700 |
| Cyp | 0.075 | 2.8380 | 0.908 | 0.0879 | 0.297 | 3.4280 | ||
| Forbs | 0.928 | 0.4615 | 0.093 | 0.4598 | 0.423 | 4.8640 | ||
| Csoil:Psoil | Cplant:Pplant | Gra | 0.213 | 1.2840 | 0.290 | 0.7289 | 0.107 | 0.2193 |
| Cyp | 0.282 | 1.7440 | 0.002 ** | 0.4669 | 0.561 | 0.2812 | ||
| Forbs | 0.088 | 1.1360 | 0.575 | 0.4886 | 0.295 | 0.1270 | ||
| Nsoil:Psoil | Nplant:Pplant | Gra | 0.173 | 3.2710 | 0.411 | 0.6936 | 0.207 | 0.8989 |
| Cyp | 0.290 | 2.9550 | 0.549 | 0.7550 | 0.947 | 0.0431 | ||
| Forbs | 0.433 | 2.3870 | 0.470 | 0.6913 | 0.267 | 0.6203 | ||
| Vegetation Type | Dominant Species | Plot ID | Elevation (m) | Geographic Coordinates | Slope (°) |
|---|---|---|---|---|---|
| Alpine Meadow | Carex alatauensis, Elymus nutans | 1 | 3283 | E 101°24′9.37″ N 37°37′42.20″ | 0 |
| 2 | 3361 | E 101°24′55.10″ N 37°38′33.62″ | 0 | ||
| 3 | 3410 | E 101°25′23.86″ N 37°38′51.23″ | 11 | ||
| Alpine Shrubland | Dasiphora fruticosa, Caragana jubata, Salix oritrepha | 4 | 3504 | E 101°28′43.50″ N 37°38′51.23″ | 18 |
| 5 | 3600 | E 101°25′56.03″ N 37°39′40.70″ | 15 | ||
| 6 | 3666 | E 101°26′13.62″ N 37°40′19.35″ | 25 | ||
| Alpine Cushion Vegetation | Thylacospermum caespitosum, Sibbaldia tetrandra | 7 | 3868 | E 101°26′59.5″ N 37°40′54.12″ | 36 |
| 8 | 4104 | E 101°27′5.33″ N 37°41′44.95″ | 9 | ||
| 9 | 4371 | E 101°28′11.76″ N 37°42′1.56″ | 17 |
| Vegetation Type | pH | Temperature (°C) | Water Content (%) | Bulk Density (g·cm−3) |
|---|---|---|---|---|
| Alpine Meadow | 6.51 ± 0.02 a | 14.78 ± 0.03 a | 15.04 ± 0.01 a | 0.58 ± 0.01 a |
| Alpine Shrubland | 6.23 ± 0.37 a | 11.02 ± 0.89 c | 33.19 ± 0.02 a | 0.83 ± 0.04 a |
| Alpine Cushion Vegetation | 6.91 ± 0.15 a | 13.07 ± 1.57 b | 21.90 ± 0.05 b | 1.37 ± 0.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.; Chen, Z.; Jing, X.; Chen, Y.; Guan, J.; Wang, S.; Wang, W.; Zhou, H.; Sun, J.; Mao, X.; et al. Stoichiometric Homeostasis and Functional Group Divergence Jointly Enhance Alpine Plant Adaptation to Environmental Stress. Plants 2025, 14, 2835. https://doi.org/10.3390/plants14182835
Ma A, Chen Z, Jing X, Chen Y, Guan J, Wang S, Wang W, Zhou H, Sun J, Mao X, et al. Stoichiometric Homeostasis and Functional Group Divergence Jointly Enhance Alpine Plant Adaptation to Environmental Stress. Plants. 2025; 14(18):2835. https://doi.org/10.3390/plants14182835
Chicago/Turabian StyleMa, Aihui, Zhe Chen, Xin Jing, Yu Chen, Jinhong Guan, Shixiong Wang, Wenying Wang, Huakun Zhou, Jian Sun, Xufeng Mao, and et al. 2025. "Stoichiometric Homeostasis and Functional Group Divergence Jointly Enhance Alpine Plant Adaptation to Environmental Stress" Plants 14, no. 18: 2835. https://doi.org/10.3390/plants14182835
APA StyleMa, A., Chen, Z., Jing, X., Chen, Y., Guan, J., Wang, S., Wang, W., Zhou, H., Sun, J., Mao, X., & Jin, Y. (2025). Stoichiometric Homeostasis and Functional Group Divergence Jointly Enhance Alpine Plant Adaptation to Environmental Stress. Plants, 14(18), 2835. https://doi.org/10.3390/plants14182835








