Ionomic Signatures of Olive Trees Affected by Quick Decline Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Farm, Agronomic Management, and Leaf Sampling
- No wilted branches;
- Slightly wilted branches (up to 25% of the canopy);
- Moderately wilted branches (26–50% of the canopy);
- Severely wilted branches (51–75% of the canopy);
- Highly severe wilted branches (over 76% of the canopy).
2.2. ICP-OES Analysis
2.3. Non-Protein Thiol Extraction and Determination
2.4. Enzyme Extraction and Assay and Protein Quantification
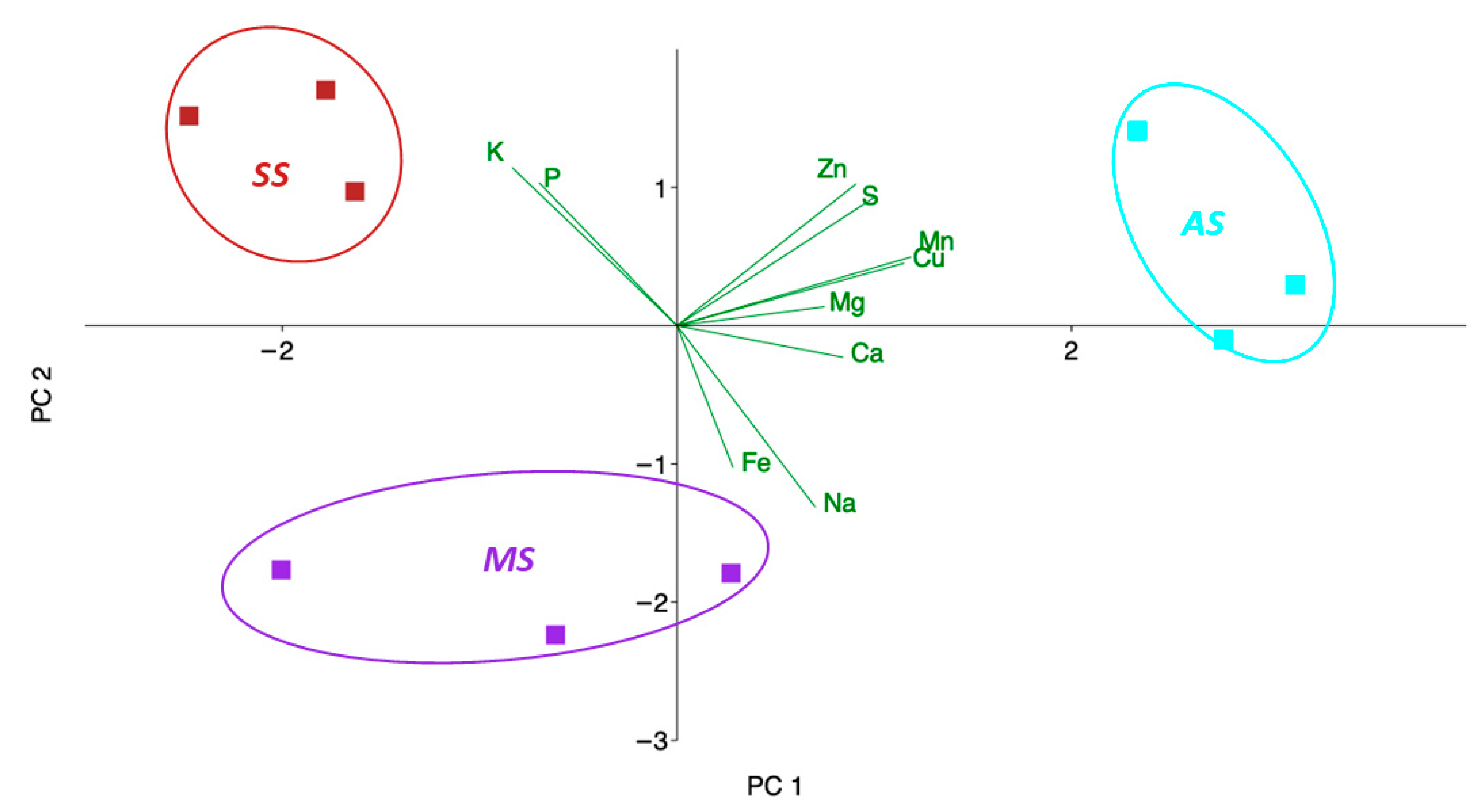
2.5. Statistical and Principal Component Analysis
3. Results
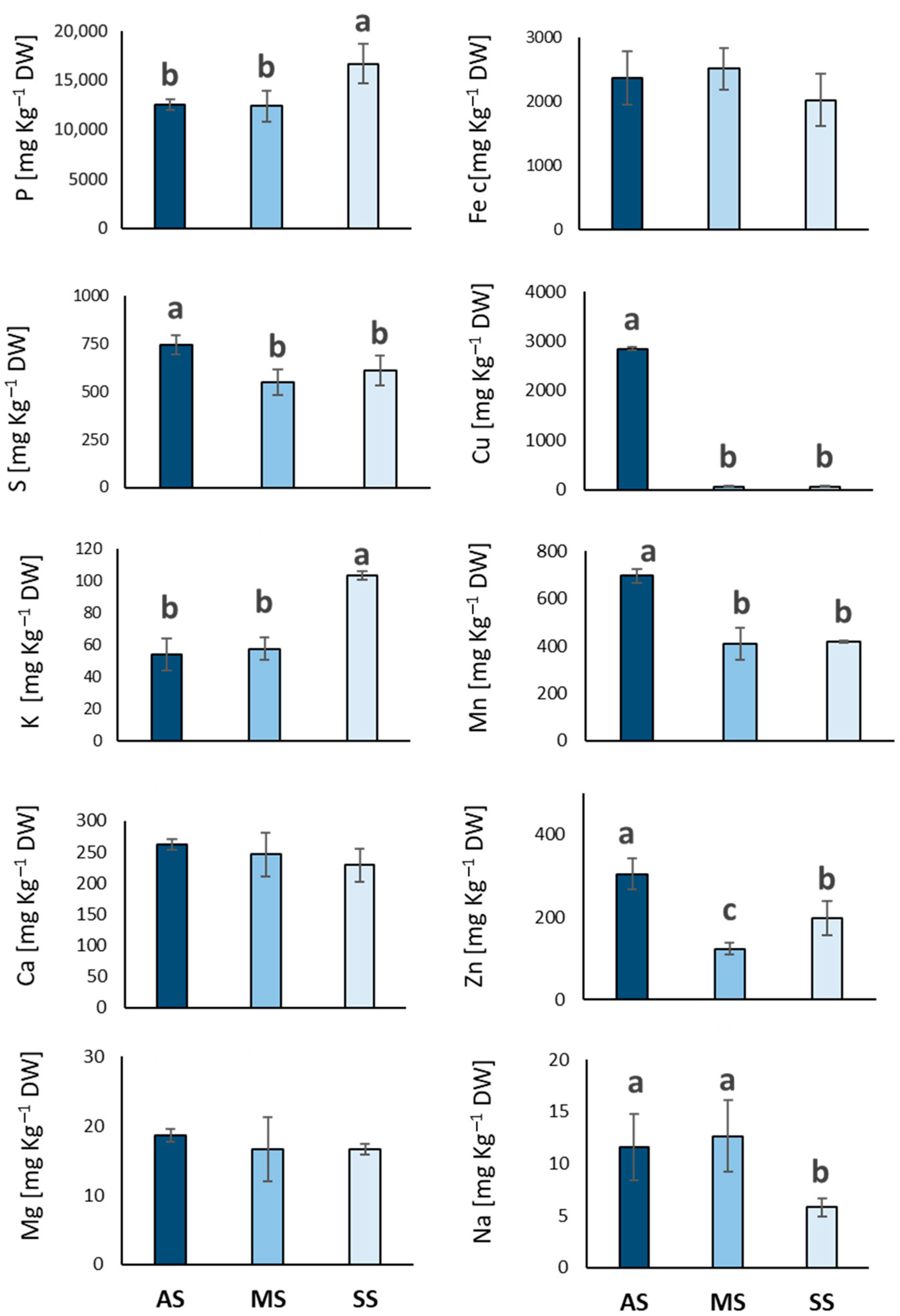
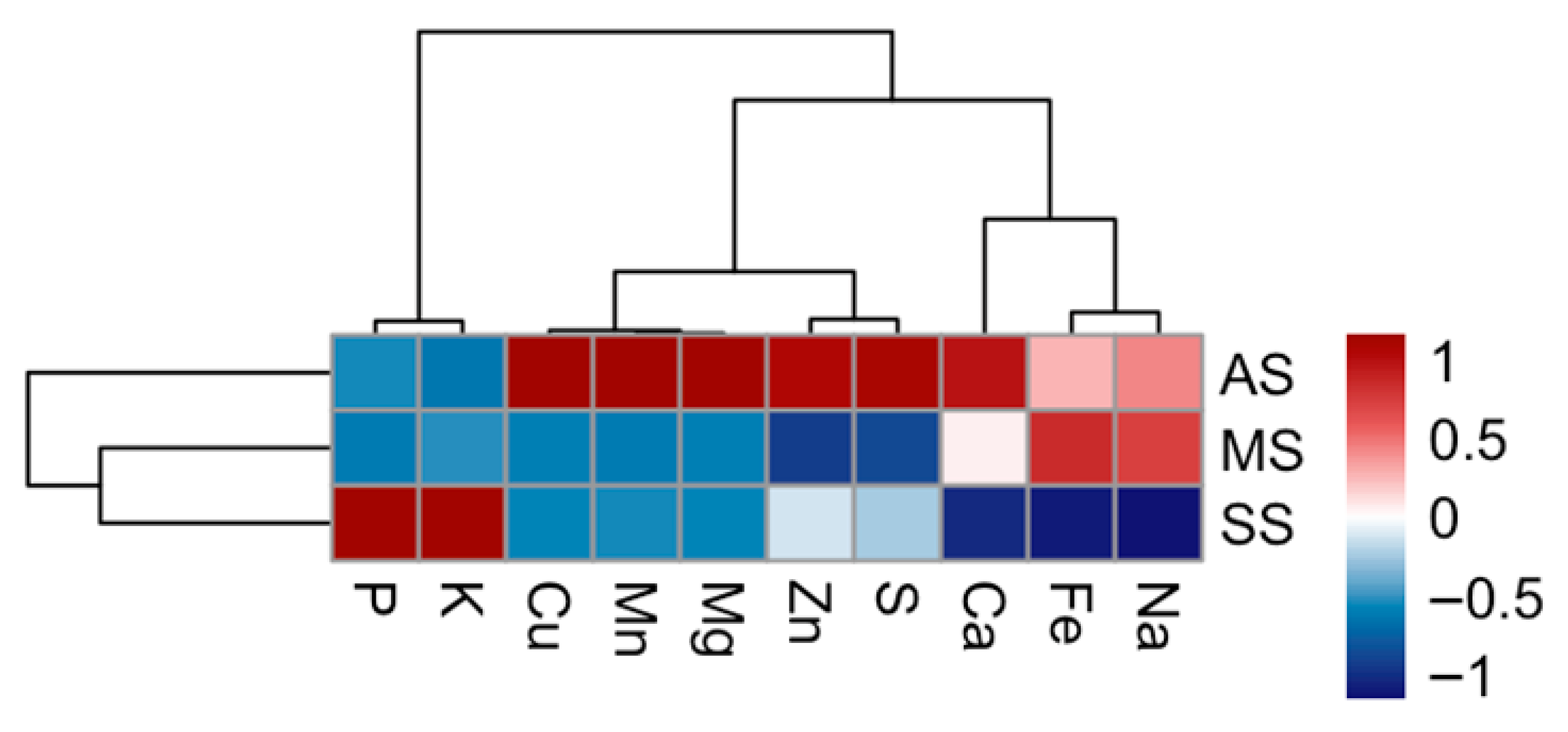
3.1. Elemental Composition of Leaves (Macro and Micro Elements)
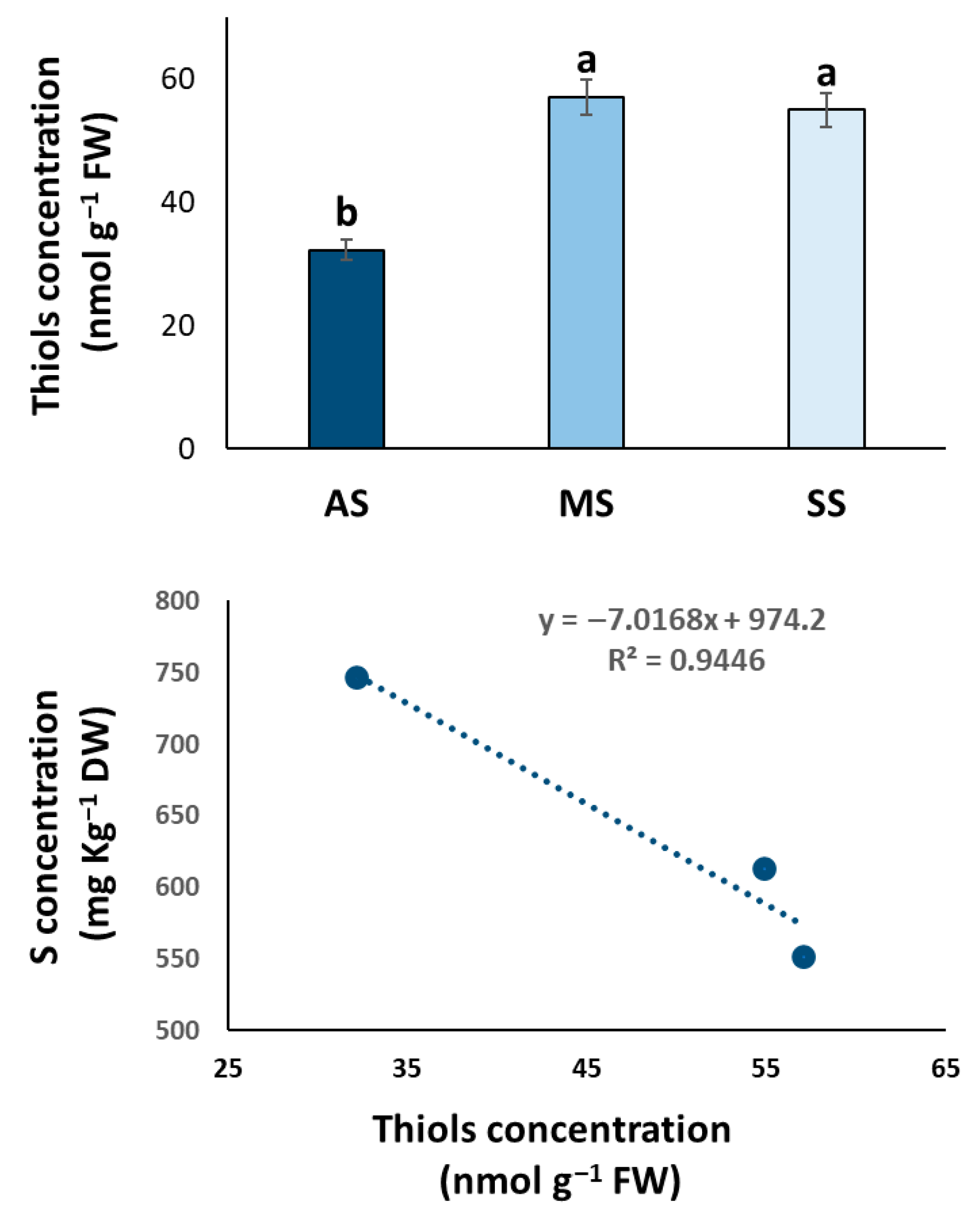
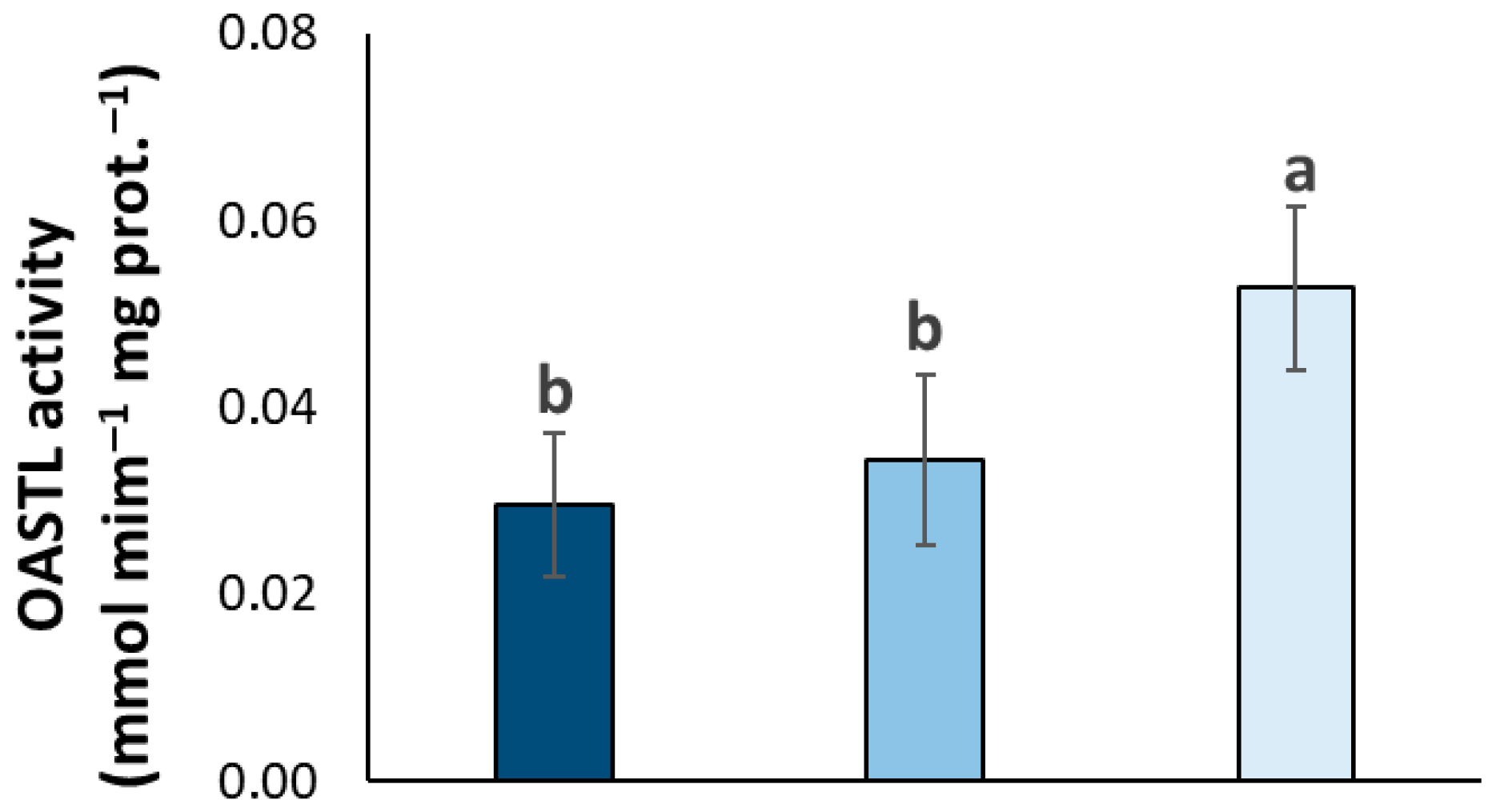
3.2. Thiol Dynamics and S Assimilation Rate in Olive Trees with Varying OQDS Symptoms
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2016, 144, 235–243. [Google Scholar] [CrossRef]
- Bollettino Ufficiale Della Regione Puglia n. 160 del 05/12/2013. Available online: https://burp.regione.puglia.it/documents/20135/782515/DETERMINAZIONE+DEL+DIRIGENTE+SERVIZIO+AGRICOLTURA+20+novembre+2013%2C+n.+521+%28id+4904411%29.pdf/ba50807a-2ad8-3185-1c35-7d054ae6c10f?version=1.0&t=1622793336572 (accessed on 24 May 2025).
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Evol. Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- EFSA. 2018. Available online: https://www.efsa.europa.eu/it/topics/topic/xylella-fastidiosa (accessed on 24 May 2025).
- Fierro, A.; Liccardo, A.; Porcelli, F. A lattice model to manage the vector and the infection of the Xylella fastidiosa on olive trees. Sci. Rep. 2019, 9, 8723. [Google Scholar] [CrossRef]
- D’Attoma, G.; Morelli, M.; Saldarelli, P.; Saponari, M.; Giampetruzzi, A.; Boscia, D.; Savino, V.N.; De La Fuente, L.; Cobine, P.A. Ionomic Differences between Susceptible and Resistant Olive Cultivars Infected by Xylella fastidiosa in the Outbreak Area of Salento, Italy. Pathogens 2019, 8, 272. [Google Scholar] [CrossRef]
- Landa, B.B.; Saponari, M.; Feitosa-Junior, O.R.; Giampetruzzi, A.; Vieira, F.J.D.; Mor, E.; Robatzek, S. Xylella fastidiosa’s relationships: The bacterium, the host plants, and the plant microbiome. New Phytol. 2022, 234, 1598–1605. [Google Scholar] [CrossRef]
- EPPO. Update of the Situation of Xylella fastidiosa in Italy. EPPO Reporting Service 2022. Available online: https://gd.eppo.int/reporting/article-7342 (accessed on 29 July 2025).
- Gilioli, G.; Simonetto, A.; Colturato, M.; Bazarra, N.; Fernández, J.R.; Naso, M.G.; Donato, B.; Bosco, D.; Dongiovanni, C.; Maiorano, A.; et al. An eco-epidemiological model supporting rational disease management of Xylella fastidiosa. An application to the outbreak in Apulia (Italy). Ecol. Model. 2023, 476, 110226. [Google Scholar] [CrossRef]
- Delbianco, A.; Gibin, D.; Pasinato, L.; Boscia, D.; Morelli, M.; European Food Safety Authority (EFSA). Update of the Xylella spp. host plant database—Systematic literature search up to 30 June 2022. EFSA J. 2023, 21, 7726. [Google Scholar]
- Cornara, D.; Boscia, D.; D’aTtoma, G.; Digiaro, M.; Ligorio, A.M.; Loconsole, G.; Minutillo, S.A.; Montilon, V.; Palmisano, F.; Ragone, G.; et al. An integrated strategy for pathogen surveillance unveiled Xylella fastidiosa ST1 outbreak in hidden agricultural compartments in the Apulia region (Southern Italy). Eur. J. Plant Pathol. 2025, 171, 277–285. [Google Scholar] [CrossRef]
- La Notte, P.L.; Saponari, M.; Mousavi, S.; Mariotti, R.; Abou Kubaa, R.; Nikbakht, R.; Melcarne, G.; Specchia, F.; Altamura, G.; Ligorio, A.; et al. A survey in natural olive resources exposed to high inoculum pressure indicates the presence of traits of resistance to Xylella fastidiosa in Leccino offspring. Front. Plant Sci. 2024, 15, 1457831. [Google Scholar] [CrossRef]
- De La Fuente, L.; Navas-Cortés, J.A.; Landa, B.B. Ten challenges to understanding and managing the insect-transmitted, xylem-limited bacterial pathogen Xylella fastidiosa. Phytopathology 2024, 114, 869–884. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Ciarroni, S.; Tagliavento, V.; De Stradis, A.; Vergaro, V.; Suranna, G.P.; Balestra, G.M.; Ciccarella, G. Thymol-Nanoparticles as Effective Biocides against the Quarantine Pathogen Xylella fastidiosa. Nanomaterials 2023, 13, 1285. [Google Scholar] [CrossRef]
- Tatulli, G.; Baldassarre, F.; Schiavi, D.; Tacconi, S.; Cognigni, F.; Costantini, F.; Balestra, G.M.; Dini, L.; Pucci, N.; Rossi, M. Chitosan-Coated Fosetyl-Al Nanocrystals’ Efficacy on Nicotiana tabacum Colonized by Xylella fastidiosa. Phytopathology 2024, 114, 1466–1479. [Google Scholar] [CrossRef]
- Sabri, M.; El Handi, K.; Cara, O.; De Stradis, A.; Valentini, F.; Elbeaino, T. Xylella phage MATE 2: A novel bacteriophage with potent lytic activity against Xylella fastidiosa subsp. pauca. Front. Microbiol. 2024, 15, 1412650. [Google Scholar] [CrossRef] [PubMed]
- Moll, L.; Badosa, E.; De La Fuente, L.; Montesinos, E.; Planas, M.; Bonaterra, A.; Feliu, L. Mitigation of Almond Leaf Scorch by a Peptide that Inhibits the Motility of Xylella fastidiosa. Plant Dis. 2025, 109, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Del Grosso, C.; Saponari, M.; Saldarelli, P.; Palmieri, D.; Altamura, G.; Abou Kubaa, R.; De Curtis, F.; Lima, G. Use of commercial fertilizers in an IPDM protocol to mitigate Olive Quick Decline Syndrome caused by Xylella fastidiosa subsp. pauca in Southern Italy. Plant Dis. 2025, 109, 1657–1667. [Google Scholar] [CrossRef]
- Ahmed, M.; Hasanuzzaman, M.; Raza, M.A.; Malik, A.; Ahmad, S. Plant nutrients for crop growth, development and stress tolerance. Sustain. Agric. Era Clim. Chang. 2020, 1, 43–92. [Google Scholar]
- Ellingboe, A.H. Plant—Pathogen interactions: Genetic and comparative analyses. Eur. J. Plant Pathol. 2001, 107, 79–84. [Google Scholar] [CrossRef]
- Lahner, B.; Gong, J.; Mahmoudian, M.; Smith, E.L.; Abid, K.B.; Rogers, E.E.; Guerinot, M.L.; Harper, J.F.; Ward, J.M.; McIntyre, L.; et al. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 1215–1221. [Google Scholar] [CrossRef]
- Surano, A.; Del Grosso, C.; Musio, B.; Todisco, S.; Giampetruzzi, A.; Altamura, G.; Saponari, M.; Gallo, V.; Mastrorilli, P.; Boscia, D.; et al. Exploring the xylem-sap to unravel biological features of Xylella fastidiosa subspecies pauca ST53 in immune, resistant and susceptible crop species through metabolomics and in vitro studies. Front. Plant Sci. 2024, 14, 1343876. [Google Scholar] [CrossRef]
- Goodwin, S.; Conrad, R.; Zeikus, J.G. Influence of pH on microbial hydrogen metabolism in diverse sedimentary ecosystems. Appl. Environ. Microbiol. 1988, 54, 590–593. [Google Scholar] [CrossRef]
- Silva-Stenico, M.E.; Pacheco, F.T.H.; Pereira-Filho, E.R.; Rodrigues, J.L.M.; Souza, A.N.; Etchegaray, A.; Gomes, J.E.; Tsai, S.M. Nutritional deficiency in citrus with symptoms of citrus variegated chlorosis disease. Braz. J. Biol. 2009, 69, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, R.; Dubiella, U.; Barbacci, A.; Genin, S.; Raffaele, S.; Roby, D. Advances on plant–pathogen interactions from molecular toward systems biology perspectives. Plant J. 2017, 90, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.S.; Aggarwal, A. Plant Pathology; Tata McGraw-Hill: New Delhi, India, 2003. [Google Scholar]
- Jones, L.R. The relation of environment to disease in plants. Am. J. Bot. 1924, 11, 601–609. [Google Scholar] [CrossRef]
- Matta, A.; Buonaurio, R.; Favaron, F.; Scala, A.; Scala, F. Fondamenti di Patologia Vegetale; Pàtron Editore: Bologna, Italy, 2017. [Google Scholar]
- Scortichini, M.; Cesari, G. An evaluation of the monitoring surveys of the quarantine bacterium Xylella fastidiosa performed in containment and buffer areas of Apulia, southern Italy. Appl. Biosaf. 2019, 24, 96–99. [Google Scholar] [CrossRef]
- Haneklaus, S.; Bloem, E.; Schnug, E. Plant disease control by nutrient management: Sulphur. In Disease Control in Crops: Biological and Environmentally Friendly Approaches; APS Press: Minneapolis, MN, USA, 2009; pp. 221–236. [Google Scholar]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Kolozsváriné Nagy, J.; Király, L. The Versatile Roles of Sulfur-Containing Biomolecules in Plant Defense—A Road to Disease Resistance. Plants 2020, 9, 1705. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Significance of sulfur compounds in the protection of plants against pests and diseases. J. Plant Nutr. 2005, 28, 763–784. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Olive Cultivars Susceptible or Tolerant to Xylella fastidiosa subsp. pauca Exhibit Mid-Term Different Metabolomes upon Natural Infection or a Curative Treatment. Plants 2021, 10, 772. [Google Scholar]
- Bollettino Ufficiale Della Regione Puglia n. 52 del 16/04/2014. Available online: https://burp.regione.puglia.it/documents/20135/830449/DELIBERAZIONE+DELLA+GIUNTA+REGIONALE+2+aprile+2014%2C+n.+580+%28id+4800216%29.pdf/89820ac0-3d2f-adfd-7a56-6535936deccc?version=1.0&t=1622787529778 (accessed on 24 May 2025).
- Bollettino Ufficiale Della Regione Puglia n. 15 del 20/02/2015. Available online: https://burp.regione.puglia.it/documents/20135/2603465/DET_22_15_2_2025.pdf/16df0dec-959b-15a4-c3eb-2d841cd29081?version=1.0&t=1740065778697 (accessed on 24 May 2025).
- Bollettino Ufficiale Della Regione Puglia n. 147 del 12/11/2015. Available online: https://burp.regione.puglia.it/documents/20135/953945/DETERMINAZIONE+DEL+DIRIGENTE+SERVIZIO+AGRICOLTURA+5+novembre+2015%2C+n.+571+%28id+4773814%29.pdf/f94b3e3e-bed3-439d-6313-2028cd70f236?version=1.0&t=1622801405333 (accessed on 24 May 2025).
- Bardsley, C.E.; Lancaster, J.D. Determination of reserve sulfur and soluble sulfates in soils. Soil Sci. Soc. Am. Proc. 1960, 24, 265–268. [Google Scholar]
- Quagliata, G.; Abdirad, S.; Celletti, S.; Sestili, F.; Astolfi, S. Screening of Triticum turgidum genotypes for tolerance to drought stress. Plant Physiol. Biochem. 2023, 194, 271–280. [Google Scholar] [CrossRef]
- Coppa, E.; Quagliata, G.; Molina, M.D.G.; Maghrebi, M.; Vigani, G.; Sestili, F.; Astolfi, S. Sulphur-mediated iron homeostasis in four tetraploid wheats (Triticum turgidum L.). Plant Biol. 2025; early view. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Del Coco, L.; Migoni, D.; Girelli, C.R.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Soil and Leaf Ionome Heterogeneity in Xylella fastidiosa subsp. Pauca-Infected, Non-Infected and Treated Olive Groves in Apulia, Italy. Plants 2020, 9, 760. [Google Scholar]
- Luvisi, A.; Aprile, A.; Sabella, E.; Vergine, M.; Nicolì, F.; Nutricati, E.; Miceli, A.; Negro, C.; De Bellis, L. Xylella fastidiosa subsp. pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: Profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathol. Mediterr. 2017, 56, 259–273. [Google Scholar]
- Veresoglou, S.D.; Barto, E.K.; Menexes, G.; Rillig, M.C. Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol. 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Val-Torregrosa, B.; Bundó, M.; Martín-Cardoso, H.; Bach-Pages, M.; Chiou, T.J.; Flors, V.; Segundo, B.S. Phosphate-induced resistance to pathogen infection in Arabidopsis. Plant J. 2022, 110, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Campos-Soriano, L.; Bundó, M.; Bach-Pages, M.; Chiang, S.F.; Chiou, T.J.; San Segundo, B. Phosphate excess increases susceptibility to pathogen infection in rice. Mol. Plant Pathol. 2020, 21, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, Z.; Xiao, S.; Ye, Z.; Nie, X.; Zhang, X.; Kong, J.; Zhu, L. Phosphate deficiency enhances cotton resistance to Verticillium dahliae through activating jasmonic acid biosynthesis and phenylpropanoid pathway. Plant Sci. 2021, 302, 110724. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Vogiatzaki, E.; Glauser, G.; Poirier, Y. Phosphate deficiency induces the jasmonate pathway and enhances resistance to insect herbivory. Plant Physiol. 2016, 171, 632–644. [Google Scholar] [CrossRef]
- Ortel, C.C.; Roberts, T.L.; Rupe, J.C. A review of the interaction between potassium nutrition and plant disease control. Agrosyst. Geosci. Environ. 2024, 7, e20489. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; de Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Shi, X.; Long, Y.; He, F.; Zhang, C.; Wang, R.; Zhang, T.; Wu, W.; Hao, Z.; Wang, Y.; Wang, G.L.; et al. The fungal pathogen Magnaporthe oryzae suppresses innate immunity by modulating a host potassium channel. PLoS Pathog. 2018, 14, e1006878. [Google Scholar] [CrossRef]
- Mann, R.L.; Kettlewell, P.S.; Jenkinson, P. Effect of foliar-applied potassium chloride on septoria leaf blotch of winter wheat. Plant Pathol. 2004, 53, 653–659. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Reddy, P.R.; Sridhar, R. Effect of major nutrients on the susceptibility of rice plants to bacterial leaf blight. J. Plant Dis. Prot. 1983, 90, 50–54. [Google Scholar]
- Tang, E.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Bhar, A.; Chakraborty, A.; Roy, A. The captivating role of calcium in plant-microbe interaction. Front. Plant Sci. 2023, 14, 1138252. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H.M. Plant-growth-promoting rhizobacteria emerging as an effective bioinoculant to improve the growth, production and stress tolerance of vegetable crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Mishra, B.; Mukhtar, M.S.; McDowell, J.M. Sparking a sulfur war between plants and pathogens. Trends Plant Sci. 2022, 27, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.M.; Williams, J.S. Elemental sulphur as an induced antifungal substance in plant defence. J. Exp. Bot. 2004, 55, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Bloem, E.; Haneklaus, S.; Salac, I.; Wickenhäuser, P.; Schnug, E. Facts and fiction about sulfur metabolism in relation to plant-pathogen interactions. Plant Biol. 2007, 9, 596–607. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Ye, Y.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotoxicol. Environ. Saf. 2019, 184, 109671. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef]
- Ge, Q.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. The copper-binding protein CutC is involved in copper homeostasis and affects virulence in the xylem-limited pathogen Xylella fastidiosa. Phytopathology 2022, 112, 1620–1629. [Google Scholar] [CrossRef] [PubMed]




| AS | MS | SS | ||
|---|---|---|---|---|
| Sand (2.0–0.05 mm) | % | 66 | 73 | 69 |
| Silt (0.05–0.002 mm) | % | 16 | 14 | 14 |
| Clay (<0.002) | % | 18 | 13 | 17 |
| pH | 7.8 | 7.5 | 7.6 | |
| Cation Exchange Capacity (CEC) | meq/100 g | 13.70 | 10.19 | 13.45 |
| Organic Matter | % | 2.18 | 1.41 | 1.34 |
| Total Nitrogen | % | 0.13 | 0.090 | 0.077 |
| Electrical Conductivity (EC) | mS/cm | 0.303 | 0.363 | 0.330 |
| Practice | AS | MS | SS |
|---|---|---|---|
| Pruning | Every two years | Every four years | Every two years |
| Soil fertilization | N-P (25-15) (every two years); organic amendments (every two years) | Urea (every two years) | NPK (15-5-5) + Fe-Zn (every two years) |
| Foliar fertilization | Zn-Mn-Cu; protein hydrolysate | Mg-B-Cu-Fe-Mn-Zn | - |
| Chemical weeding (Glyphosate) | - | Every two years | Every year |
| Chemical weeding (Oxyfluorfen) | - | Every year | - |
| Antifungal treatments | - | Copper Oxychloride | Copper Sulfate |
| Pesticide treatments | - | Pyraclostrobin; Dimethoate | Dimethoate |
| Sampled Tree | Wilted Branches | Severity Class |
Average Severity
(Corresponding Class) |
|---|---|---|---|
| AS1 | 4% | 2 | 4% (2) |
| AS2 | 0% | 1 | |
| AS3 | 13% | 2 | |
| AS4 | 1% | 2 | |
| AS5 | 2% | 2 | |
| MS1 | 58% | 4 | 27% (3) |
| MS2 | 36% | 3 | |
| MS3 | 28% | 3 | |
| MS4 | 11% | 2 | |
| MS5 | 2% | 2 | |
| SS1 | 75% | 4 | 81% (5) |
| SS2 | 84% | 5 | |
| SS3 | 87% | 5 | |
| SS4 | 76% | 5 | |
| SS5 | 83% | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestra, G.M.; Giordani, M.; Coppa, E.; Schiavi, D.; Astolfi, S. Ionomic Signatures of Olive Trees Affected by Quick Decline Syndrome. Plants 2025, 14, 2834. https://doi.org/10.3390/plants14182834
Balestra GM, Giordani M, Coppa E, Schiavi D, Astolfi S. Ionomic Signatures of Olive Trees Affected by Quick Decline Syndrome. Plants. 2025; 14(18):2834. https://doi.org/10.3390/plants14182834
Chicago/Turabian StyleBalestra, Giorgio Mariano, Mauro Giordani, Eleonora Coppa, Daniele Schiavi, and Stefania Astolfi. 2025. "Ionomic Signatures of Olive Trees Affected by Quick Decline Syndrome" Plants 14, no. 18: 2834. https://doi.org/10.3390/plants14182834
APA StyleBalestra, G. M., Giordani, M., Coppa, E., Schiavi, D., & Astolfi, S. (2025). Ionomic Signatures of Olive Trees Affected by Quick Decline Syndrome. Plants, 14(18), 2834. https://doi.org/10.3390/plants14182834








