Predicting Potential Habitats of the Endangered Mangrove Species Acanthus ebracteatus Under Current and Future Climatic Scenarios Based on MaxEnt and OPGD Models
Abstract
1. Introduction
2. Results and Analysis
2.1. Model Optimization and Accuracy Evaluation
2.2. Key Environmental Factors That Are Drivers of Habitat Distribution
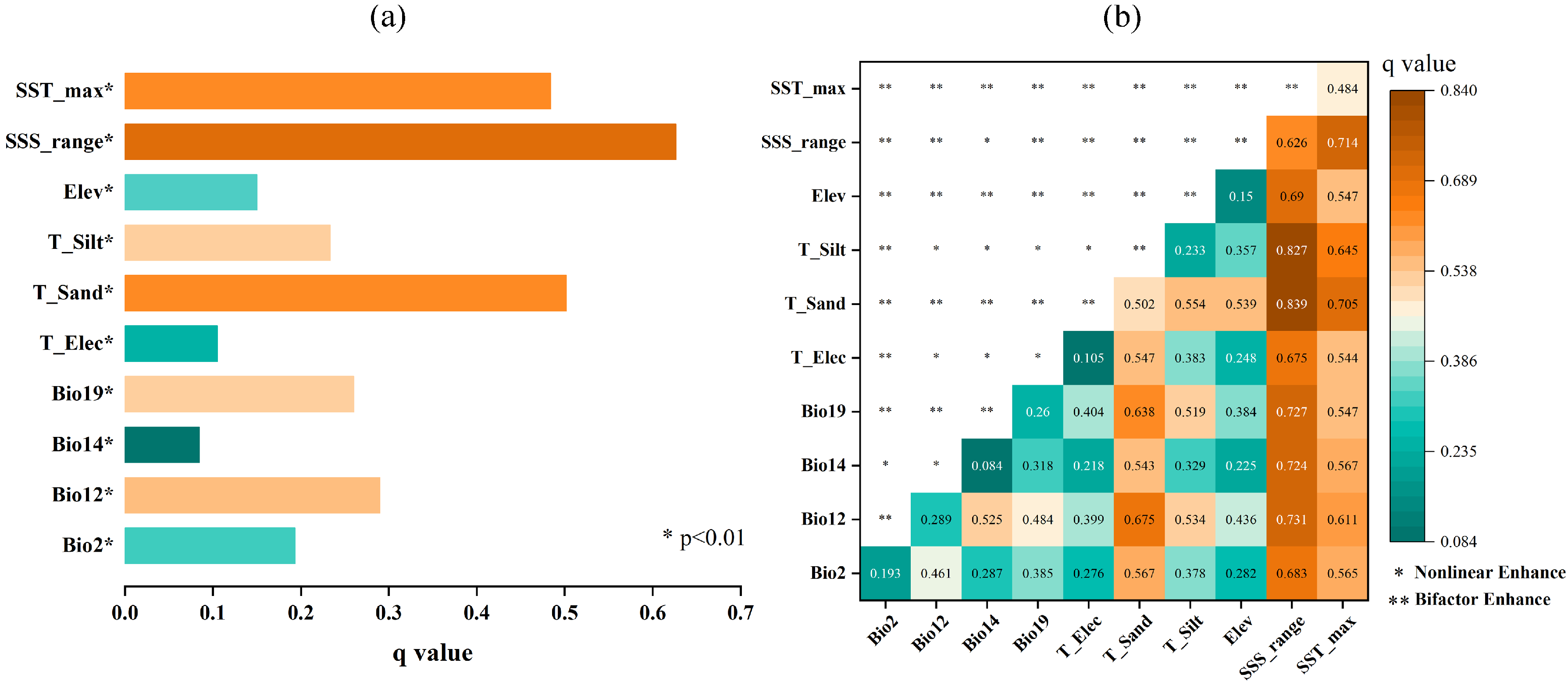
2.2.1. Identification and Ranking of Dominant Factors
2.2.2. Ecological Response Mechanisms of Dominant Factors
2.2.3. Interaction Effects of Environmental Factors Revealed by OPGD Analysis
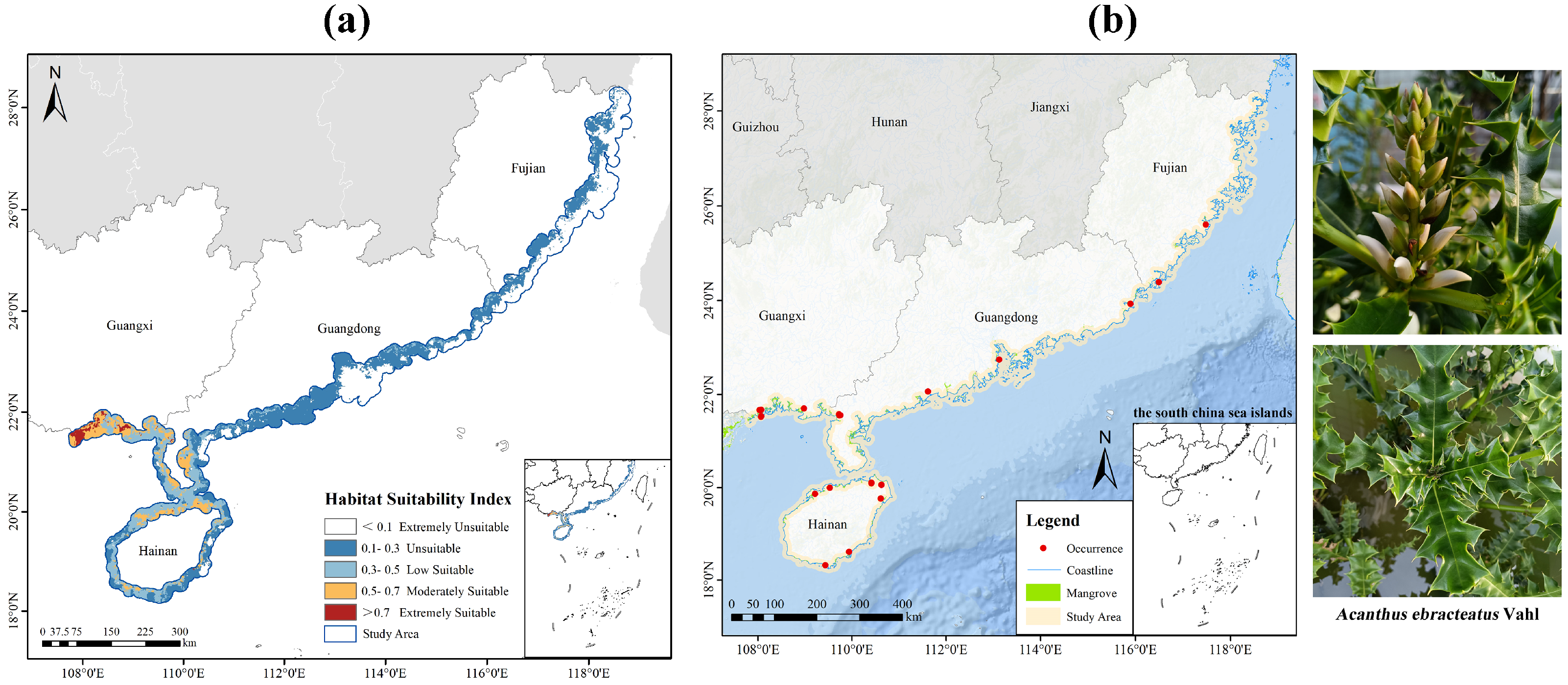
2.3. Current Distribution Pattern of Potentially Suitable Habitats
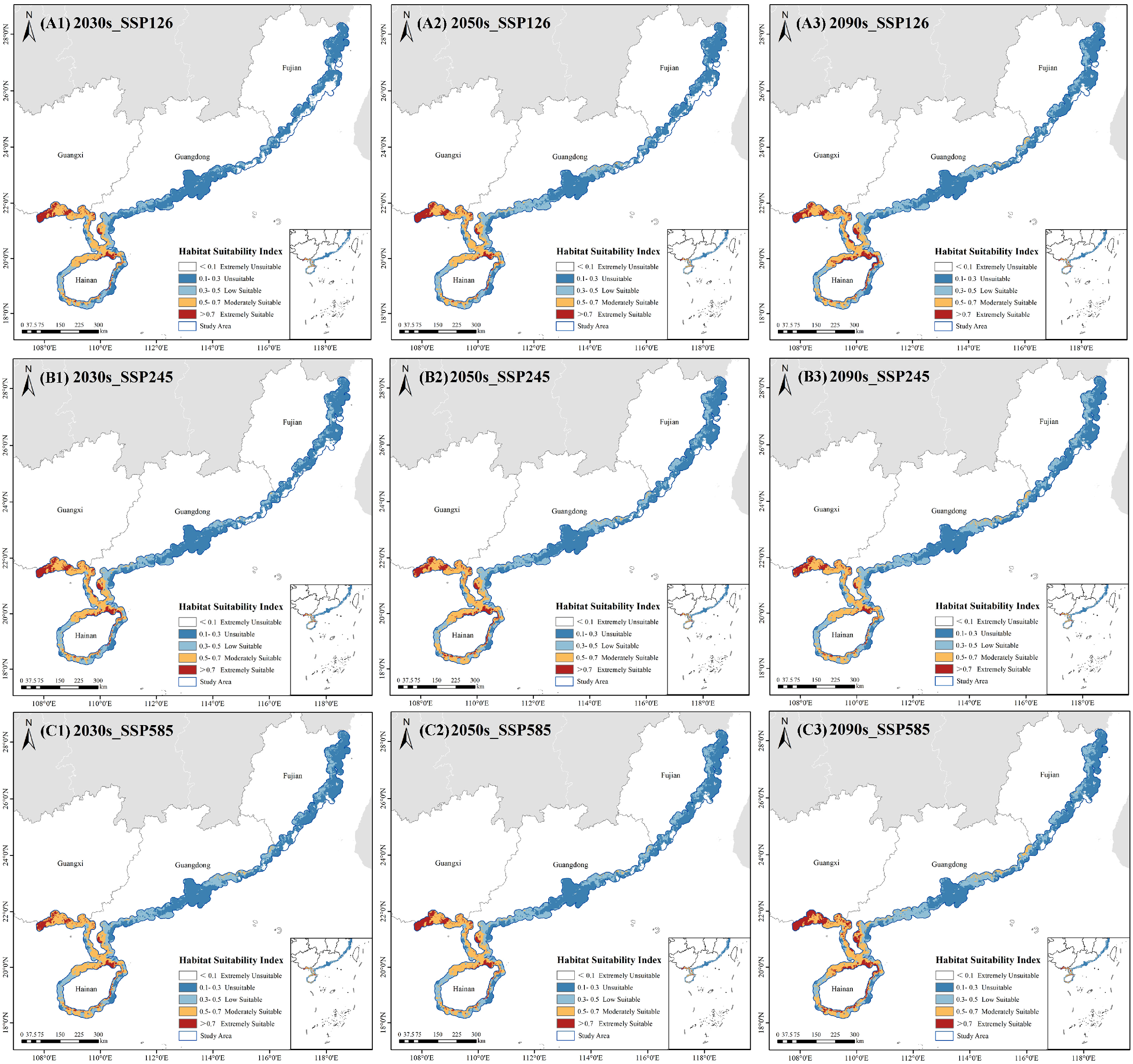
2.4. Spatiotemporal Dynamics of Future Habitats
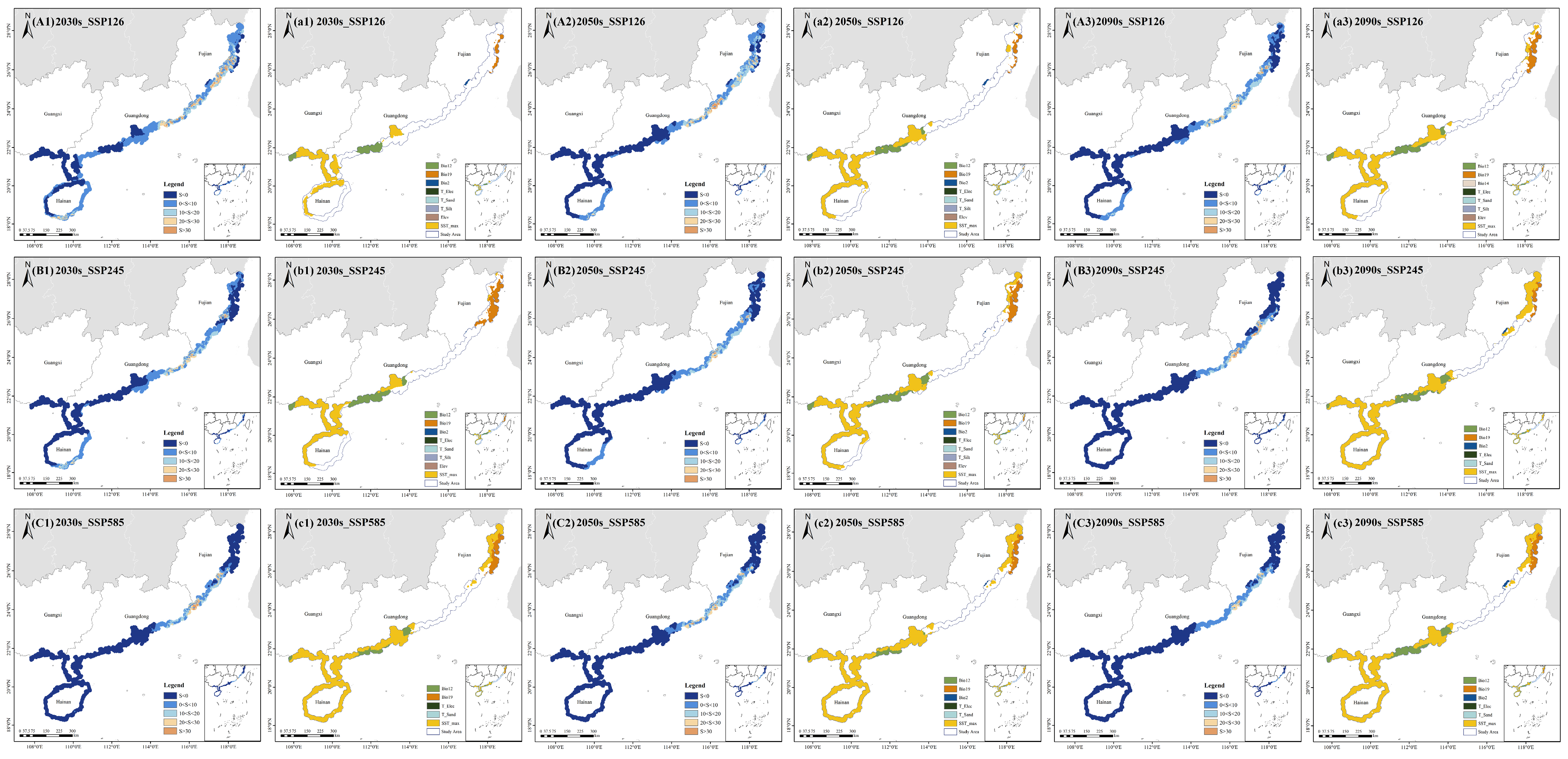
2.4.1. Distribution Patterns of Potential Suitable Habitats Under Future Climate Scenarios
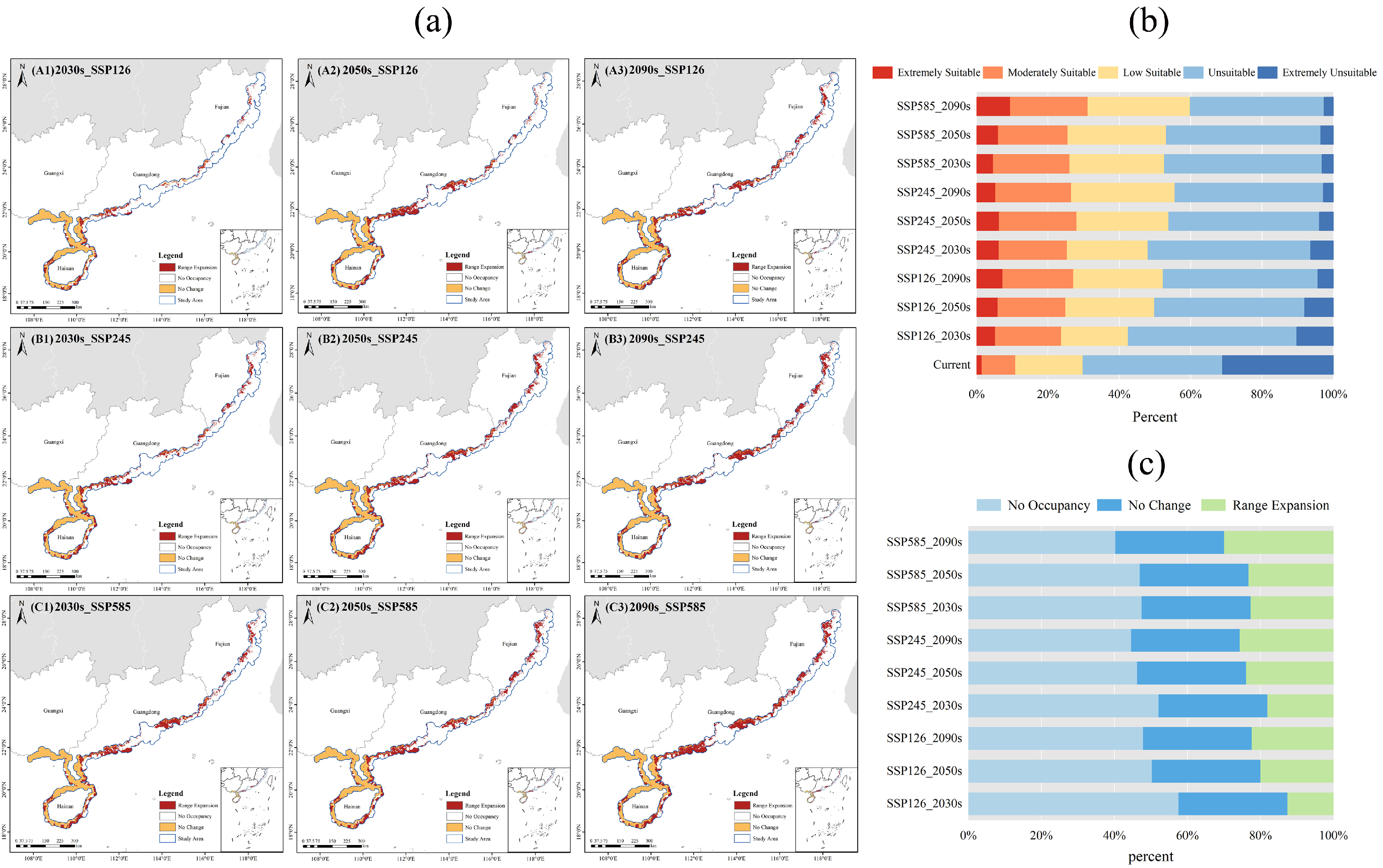
2.4.2. Spatial–Temporal Dynamics of Potential Suitable Areas
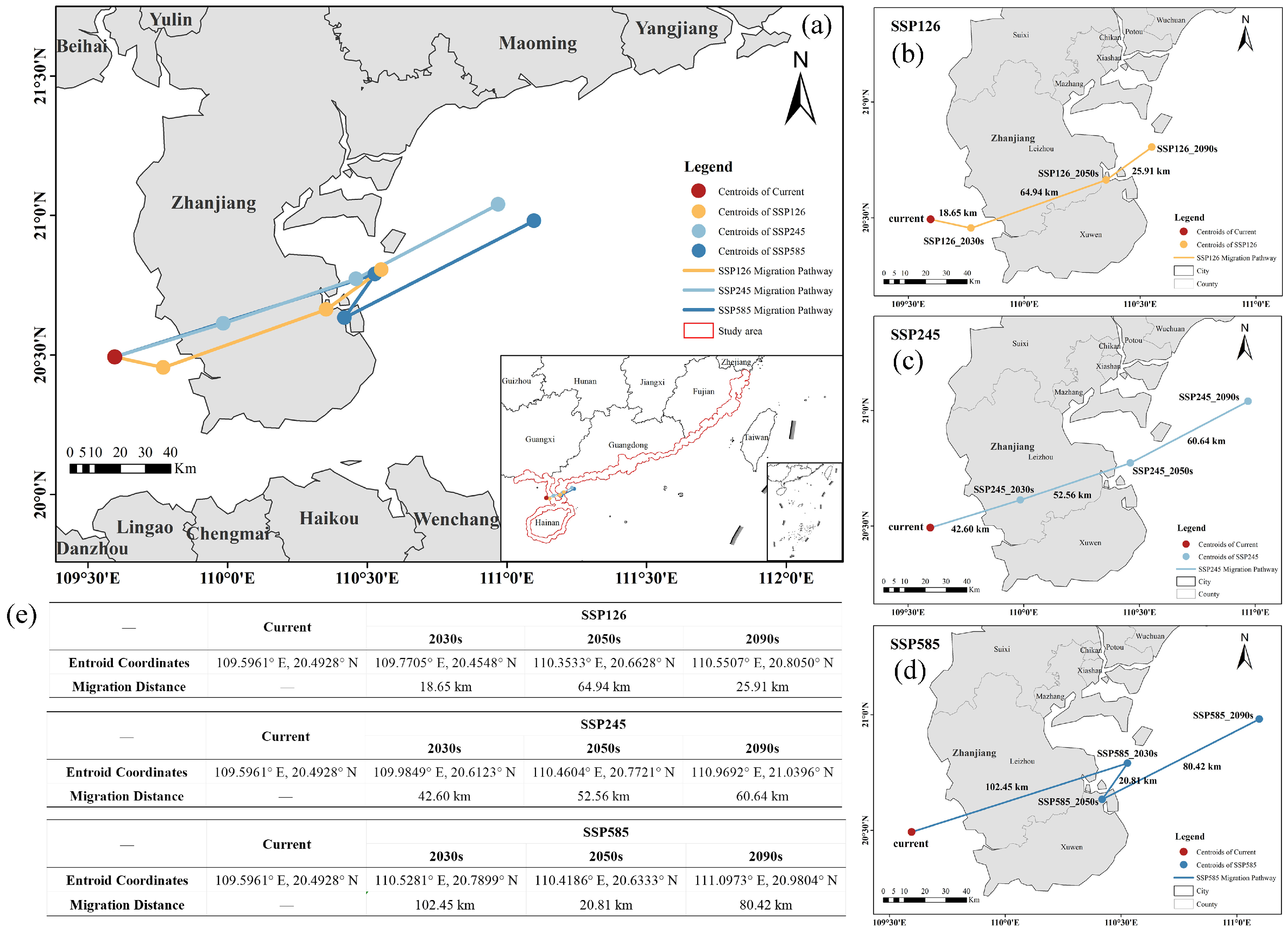
2.4.3. Shifts in the Geographic Centroid of Suitable Habitats
2.5. Novelty and Uncertainty in Future Climate Projections
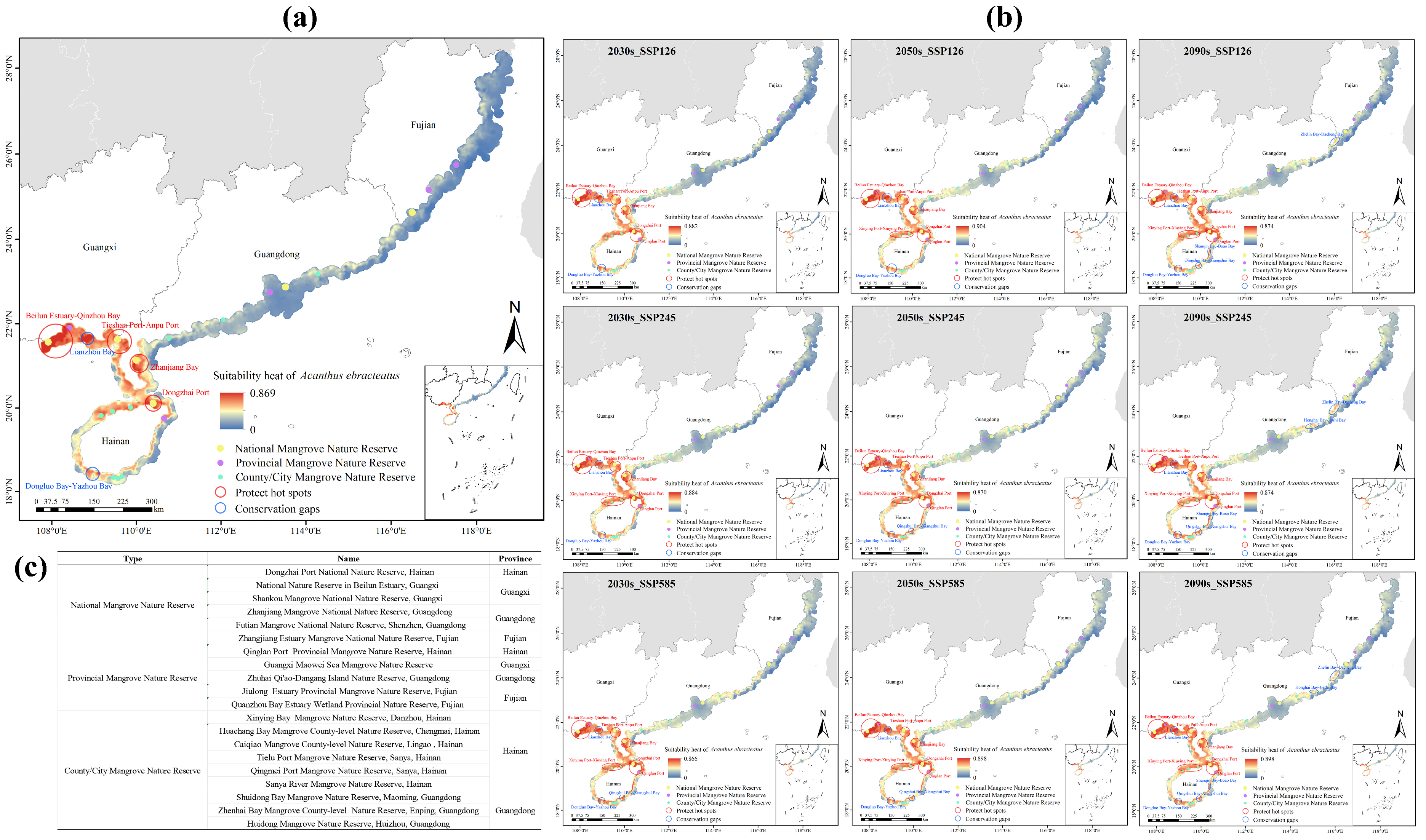
2.6. Conservation Priorities and Gaps Analysis
3. Discussion
3.1. Effects of Key Environmental Factors on the Distribution of A. ebracteatus
3.2. Response of A. ebracteatus Distribution to Future Climate Change
3.3. Conservation and Management Recommendations for A. ebracteatus
3.4. Potential Limitations and Future Research Directions
4. Materials and Methods
4.1. Species Occurrence Data and Study Area
4.2. Environment Variables and Processing
4.3. Model Parameter Optimization and Evaluation
4.4. Identifying Key Environmental Drivers and Interactions
4.5. Spatiotemporal Dynamics of Habitat Suitability
4.5.1. Habitat Suitability Mapping and Classification
4.5.2. Quantification of Habitat Dynamics: Changes in Area and Centroid Shift
4.5.3. Assessment of Climate Novelty in Future Projections
4.6. Analysis of Conservation Priorities and Gaps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friess, D.A.; Adame, M.F.; Adams, J.B.; Lovelock, C.E. Mangrove Forests under Climate Change in a 2 °C World. WIREs Clim. Change 2022, 13, e792. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove Forests: Resilience, Protection from Tsunamis, and Responses to Global Climate Change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The State of the World’s Mangrove Forests: Past, Present, and Future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef]
- Fan, H.Q.; Wang, W.Q. Important Issues in the Conservation of Mangroves in China. J. Xiamen Univ. Nat. Sci. 2017, 56, 323–330. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The Habitat Function of Mangroves for Terrestrial and Marine Fauna: A Review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.L.; Zhu, J.H.; Hu, W.J.; Wang, Q.Z.; Huang, S.S.; Pan, X.L. Study on Culturable Bacterial Diversity and Biological Activity of Acanthus ebracteatus. Guihaia 2023, 43, 635–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, G.C.; Zhong, C.R. Research and Restoration Status of Endangered Mangrove Plants in China. J. Appl. Oceanogr. 2021, 40, 142–153. [Google Scholar] [CrossRef]
- Huang, L.Y.; Shi, X.F.; Mo, Z.C.; Yan, B.; Pan, L.H. Distribution and Population Characteristics of the Rare and Endangered True Mangrove Plant Acanthus ebracteatus in Guangxi. J. Guangxi Acad. Sci. 2020, 36, 353–360. [Google Scholar] [CrossRef]
- Lin, G.X.; Zhu, Y.J.; Guo, J.; Xu, W.G.; Chen, Y.C.; He, T.; Xu, F.H. Resource Status and Conservation Strategies of the Rare Mangrove Plant Acanthus ebracteatus in Leizhou Peninsula. Wetl. Sci. Manag. 2022, 18, 62–65. [Google Scholar] [CrossRef]
- Zhang, T.X.; Zheng, C.F.; Luo, F.P.; He, J.H.; Li, D.F.; Li, W.J.; Lin, S.Q.; Zhang, Y. Study on the Propagation of Medicinal Mangrove Plant Acanthus ebracteatus. Seed 2023, 42, 38–48. [Google Scholar] [CrossRef]
- Elith, J. Species Distribution Modeling. In Ecology; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Qiao, H.; Feng, X.; Escobar, L.E.; Peterson, A.T.; Soberón, J.; Zhu, G.; Papeş, M. An Evaluation of Transferability of Ecological Niche Models. Ecography 2018, 42, 521–534. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Paul, S.; Samant, S.S. Population Ecology and Habitat Suitability Modelling of an Endangered and Endemic Medicinal Plant Meconopsis aculeata Royle under Projected Climate Change in the Himalaya. Environ. Exp. Bot. 2024, 225, 105837. [Google Scholar] [CrossRef]
- Fan, Y.; Yao, W.H.; Wang, Z.H.; Fan, X.Y.; Hu, S.Y.; Wang, H.F.; Ou, J. Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the MaxEnt Model. Plants 2025, 14, 506. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Hu, W.J.; Wang, Y.Y.; Zhang, D.; Yu, W.W.; Chen, G.C.; Xie, T.; Liu, Z.H.; Ma, Z.Y.; Du, J.G.; Chao, B.X.; et al. Mapping the Potential of Mangrove Forest Restoration Based on Species Distribution Models: A Case Study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Sacristán, J.; Johansen, K.; Duarte, C.M.; Daffonchio, D.; Hoteit, I.; McCabe, M.F. Mangrove Distribution and Afforestation Potential in the Red Sea. Sci. Total Environ. 2022, 843, 157098. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.L.; Ai, B.; Jian, Z.K.; Zhao, J.; Sun, S.J. Simulation of Mangrove Suitable Habitat in the Guangdong-Hong Kong-Macao Area under the Background of Climate Change. J. Environ. Manag. 2024, 351, 119678. [Google Scholar] [CrossRef]
- Song, Y.Z.; Wang, J.F.; Ge, Y.; Xu, C.D. An Optimal Parameters-Based Geographical Detector Model Enhances Geographic Characteristics of Explanatory Variables for Spatial Heterogeneity Analysis: Cases with Different Types of Spatial Data. GISci. Remote Sens. 2020, 57, 593–610. [Google Scholar] [CrossRef]
- ESRI. How Kernel Density Works. ArcGIS Desktop 10.8 Documentation. 2020. Available online: https://desktop.arcgis.com/en/arcmap/10.8/tools/spatial-analyst-toolbox/how-kernel-density-works.htm (accessed on 7 April 2025).
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Ahmed, S.; Sarker, S.K.; Friess, D.A.; Kamruzzaman, M.; Jacobs, M.; Islam, M.A.; Alam, M.A.; Suvo, M.J.; Sani, M.N.H.; Dey, T.; et al. Salinity Reduces Site Quality and Mangrove Forest Functions. From Monitoring to Understanding. Sci. Total Environ. 2022, 853, 158662. [Google Scholar] [CrossRef]
- Tong, L.H.; Gao, T.W.; Lan, J.N.; Quan, J.H.; Zhong, Y.X.; Yan, B. Effects of Salinity on Growth and Physiology of Endangered Mangrove Plant Acanthus ebracteatus Seedlings. J. Guangxi Acad. Sci. 2024, 40, 267–276. [Google Scholar] [CrossRef]
- Hu, W.J.; Wang, Y.Y.; Dong, P.; Zhang, D.; Yu, W.W.; Ma, Z.Y.; Chen, G.C.; Liu, Z.H.; Du, J.G.; Chen, B.; et al. Predicting Potential Mangrove Distributions at the Global Northern Distribution Margin Using an Ecological Niche Model: Determining Conservation and Reforestation Involvement. For. Ecol. Manag. 2020, 478, 118517. [Google Scholar] [CrossRef]
- Hu, W.J.; Chao, B.X.; Wang, Y.Y.; Dong, P.; Zhang, D.; Yu, W.W.; Chen, G.C.; Chen, B. Assessment of Potential Suitable Areas for Mangroves in Fujian Province Based on Maxent Model. China Environ. Sci. 2020, 40, 4029–4038. [Google Scholar] [CrossRef]
- Constance, A.; Oehri, J.; Bunbury, N.; Wiesenberg, G.L.B.; Pennekamp, F.; A’Bear, L.; Fleischer-Dogley, F.; Schaepman-Strub, G. Soil Nutrient Content and Water Level Variation Drive Mangrove Forest Aboveground Biomass in the Lagoonal Ecosystem of Aldabra Atoll. Ecol. Indic. 2022, 143, 109292. [Google Scholar] [CrossRef]
- Li, L.F.; Li, W.A.; Wang, M.; Chen, S.J.; Li, F.Q.; Xu, X.L.; Tan, Y.C.; Xu, Y.H.; Jiang, W.G. Analysis of Mangrove Distribution and Suitable Habitat in Beihai, China, Using Optimized MaxEnt Modeling: Improving Mangrove Restoration Efficiency. Front. For. Glob. Change 2024, 7, 1293366. [Google Scholar] [CrossRef]
- Ying, B.K.; Tian, K.; Guo, H.Y.; Yang, X.L.; Li, W.Y.; Li, Q.; Luo, Y.C.; Zhang, X.M. Predicting Changes in Potential Suitable Habitat of Mangrove Kandelia obovata in China under Future Climate Change Scenarios Based on MaxEnt Model. Acta Ecol. Sin. 2024, 44, 224–234. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K.; et al. Synthesis Report of the IPCC Sixth Assessment Report (AR6); Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar]
- Wang, B.; Jin, C.H.; Liu, J. Understanding Future Change of Global Monsoons Projected by CMIP6 Models. J. Clim. 2020, 33, 6471–6489. [Google Scholar] [CrossRef]
- Xu, T.Y.; Li, R.L.; Wang, W.Q.; Tang, L.L. Subtropical Mangroves Poleward Shift to the Yangtze Estuary under Different Carbon Emission Scenarios. J. Hydrol. 2024, 637, 131356. [Google Scholar] [CrossRef]
- Peng, Y.S.; Zheng, M.X.; Zheng, Z.X.; Wu, G.C.; Chen, Y.C.; Xu, H.L.; Tian, G.H.; Peng, S.H.; Chen, G.Z.; Lee, S.Y. Virtual Increase or Latent Loss? A Reassessment of Mangrove Populations and Their Conservation in Guangdong, Southern China. Mar. Pollut. Bull. 2016, 109, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.G.; Dong, M.; Li, J.Q.; Chen, X.Y.; Zeng, S.J.; Jiang, M.X.; Li, Z.Q.; Huang, J.H. Research on Conservation and Restoration Techniques for Typical Plant Species with Extremely Small Populations. Acta Ecol. Sin. 2016, 36, 7130–7135. [Google Scholar] [CrossRef]
- Chao, B.X.; Wang, Y.Y.; Yu, W.W.; Ma, Z.Y.; Chen, G.C.; Chen, B.; Hu, W.J. Prediction of Potential Mangrove Habitat Driven by Land Use in Guangdong Province. China Environ. Sci. 2021, 41, 5282–5291. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, G.Q.; Bu, W.J.; Gao, Y.B. The Principle of Ecological Niche Models and Their Application in Biodiversity Conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Sun, Y.C.; Ye, M.Z.; Ai, B.; Lai, Z.L.; Zhao, J.; Jian, Z.K.; Qi, X.Y. Annual Change in the Distribution and Landscape Health of Mangrove Ecosystems in China from 2016 to 2023 with Sentinel Imagery. Glob. Ecol. Conserv. 2025, 57, e03355. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chao, B.X.; Dong, P.; Zhang, D.; Yu, W.W.; Hu, W.J.; Ma, Z.Y.; Chen, G.C.; Liu, Z.H.; Chen, B. Simulating Spatial Change of Mangrove Habitat under the Impact of Coastal Land Use: Coupling MaxEnt and Dyna-CLUE Models. Sci. Total Environ. 2021, 788, 147914. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Saha, A.K.; Roy, A. Spatio-Temporal Pattern of Change in Mangrove Populations along the Coastal West Bengal, India. Environ. Chall. 2021, 5, 100306. [Google Scholar] [CrossRef]
- Ximenes, A.C.; Maeda, E.E.; Arcoverde, G.F.B.; Dahdouh-Guebas, F. Spatial Assessment of the Bioclimatic and Environmental Factors Driving Mangrove Tree Species’ Distribution along the Brazilian Coastline. Remote Sens. 2016, 8, 451. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Hijmans, R.J. raster: Geographic Data Analysis and Modeling; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wei, T.; Simko, V. R Package ‘corrplot’: Visualization of a Correlation Matrix, R package version 0.92; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Ma, Q.; Yan, J.H.; Wei, M.; Xin, X.G.; Zhang, L.; Zhang, F.; Wu, T.W. BCC Data Sharing Platform for CMIP6 and Its Applications. J. Appl. Meteorol. Sci. 2022, 33, 617–627. [Google Scholar] [CrossRef]
- Yang, P.R.; Zhao, Z.P.; Nie, L.S.; Xu, W.H.; Li, Y.H.; Wang, L.G. Spatiotemporal Dynamics of Ecological Plantations of Camellia sinensis var. assamica: Synergistic Effects of Climate Change and Policy Constraints in Yunnan, China. Ind. Crops Prod. 2025, 231, 121138. [Google Scholar] [CrossRef]
- Solka, J.; Wegman, E.; Priebe, C.; Poston, W.; Rogers, G. A Method to Determine the Structure of an Unknown Mixture Using the Akaike Information Criterion and the Bootstrap. Commun. Stat.-Theory Methods 1994, 23, 1419–1436. [Google Scholar]
- Zhao, Y.; Deng, X.W.; Xiang, W.H.; Chen, L.; Ouyang, S. Predicting Potential Suitable Habitats of Chinese Fir under Current and Future Climatic Scenarios Based on Maxent Model. Ecol. Inf. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking Receiver Operating Characteristic Analysis Applications in Ecological Niche Modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Wang, J.F.; Xu, C.D. Geodetector: Principle and Prospective. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
| Variable | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|
| SSS_range | 36.4 | 40.0 |
| SST_max | 19.0 | 11.7 |
| T_Silt | 17.2 | 17.6 |
| T_Sand | 10.0 | 3.8 |
| Bio12 | 8.3 | 7.3 |
| Bio14 | 3.4 | 6.2 |
| T_Elec | 2.5 | 3.0 |
| Bio2 | 1.5 | 3.5 |
| Bio19 | 1.0 | 5.2 |
| Elev | 0.6 | 1.7 |
| Type | Code | Factor Description | Unit |
|---|---|---|---|
| Bioclimate | Bio2 | Mean Diurnal Range | °C |
| Bio12 | Annual Precipitation | mm | |
| Bio14 | Precipitation of Driest Month | mm | |
| Bio19 | Precipitation of Coldest Quarter | mm | |
| Topographic | Elev | Elevation | m |
| Soil | T_Sand | Topsoil Sand | % |
| T_Silt | Topsoil Silt | % | |
| T_Elec | Topsoil Electric Conductivity | dS/m | |
| Sea Surface Temperature | SST_max | Max Sea Surface Temperature | °C |
| Sea Surface Salinity | SSS_range | Range of Sea Surface Salinity | PSU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wu, L.; Yang, C.; Qiu, Q.; Wang, Y.; Li, Z.; Xia, C. Predicting Potential Habitats of the Endangered Mangrove Species Acanthus ebracteatus Under Current and Future Climatic Scenarios Based on MaxEnt and OPGD Models. Plants 2025, 14, 2827. https://doi.org/10.3390/plants14182827
Chen J, Wu L, Yang C, Qiu Q, Wang Y, Li Z, Xia C. Predicting Potential Habitats of the Endangered Mangrove Species Acanthus ebracteatus Under Current and Future Climatic Scenarios Based on MaxEnt and OPGD Models. Plants. 2025; 14(18):2827. https://doi.org/10.3390/plants14182827
Chicago/Turabian StyleChen, Jiaqi, Liuping Wu, Chongcheng Yang, Qiongzhen Qiu, Yi Wang, Zhixin Li, and Chunhua Xia. 2025. "Predicting Potential Habitats of the Endangered Mangrove Species Acanthus ebracteatus Under Current and Future Climatic Scenarios Based on MaxEnt and OPGD Models" Plants 14, no. 18: 2827. https://doi.org/10.3390/plants14182827
APA StyleChen, J., Wu, L., Yang, C., Qiu, Q., Wang, Y., Li, Z., & Xia, C. (2025). Predicting Potential Habitats of the Endangered Mangrove Species Acanthus ebracteatus Under Current and Future Climatic Scenarios Based on MaxEnt and OPGD Models. Plants, 14(18), 2827. https://doi.org/10.3390/plants14182827







