Abstract
Cicerbita alpina (L.) Wallroth and Peucedanum ostruthium W.D.J. Koch occur in megaphorb communities in alpine and subalpine areas; both species often share the same habitats. P. ostruthium is used as a spice for spirits, while young shoots of C. alpina are collected in the northeastern regions of Italy as a local delicacy. In the present study, we isolated eleven known coumarins and one chromone from subaerial parts of P. ostruthium; two furanocoumarins were found for the first time in this species. Using UHPLC-HRMS, we analyzed the furanocoumarin content of two P. ostruthium accessions, one commercially purchased and one collected in the wild. These samples were compared to six rootstock samples of Cicerbita alpina collected in the wild. Though the furanocoumarins imperatorin, isoimperatorin, oxypeucedanin, and ostruthol had been reported from C. alpina before, we were not able to detect any of these compounds in our samples of C. alpina. Therefore, and due to the occurrence of both taxa in the same habitat, we assume that the original report of furanocoumarins in C. alpina was based on a mixed collection of C. alpina and P. ostruthium. This hypothesis seems plausible, because furanocoumarins have not been reported from any other taxon of the Cichorieae tribe of the Asteraceae family.
1. Introduction
Furanocoumarins are a class of specialized natural products occurring in some families of higher plants, which are, however, not closely related. Furanocoumarins are classified into linear and angular furanocoumarins, based on the angle between furane ring and coumarin moiety. Furanocoumarins are found inter alia, in the Apiaceae, Fabaceae, Moraceae, and Rutaceae families. Furanocoumarins serve important ecological functions for the taxa producing them, e.g., as chemical defenses against herbivores and pathogens, but also as phytoalexins in response to biotic stress [1,2,3].
Peucedanum ostruthium W.D.J. Koch (vernacular name “masterwort”, German “Meisterwurz”) is a perennial herb native to the mountainous regions of Europe. The taxon has traditionally been used in the preparation of liqueurs, bitters, and herbal teas [4,5,6,7]. Peucedanum ostruthium, and the genus Peucedanum in general, are known to contain furanocoumarins. The genus Peucedanum comprises approximately 120 species and furanocoumarins have been detected in many of them [8].
Cicerbita alpina (L.) Wallroth is a member of the Cichorieae tribe of the Asteraceae family. C. alpina is, like P. ostruthium, distributed in the mountainous regions of Europe. It is locally used as a delicacy in some alpine regions of Italy [9]. Sesquiterpene lactones and in particular sesquiterpene lacone glucosides are the most characteristic compounds found in C. alpina. Sesquiterpene glucosides are excellent chemophenetic markers within and for the Cichorieae tribe of the Asteraceae family [10]. Compounds identified in the roots of C. alpina include bitter tasting lactucin derivatives and sonchusides [11,12]. Phytochemical analysis of extracts of young shoots of C. alpina revealed a diverse array of phenolic constituents, exhibiting both significant antioxidant activity and anthelmintic effects [13,14,15]. C. alpina is currently the only species within the Cichorioieae tribe of the Asteraceae family for which furanocoumarins have been reported [13,16]. Furanocoumarins have previously been reported only for a few species of the Asteraceae, belonging to the subfamily Mutisioideae as well as in “Artemisia reticulata”. However, the latter report is hard to assess, as the name “Artemisia reticulata” seems never to have been validly published and does not appear in any of the major taxonomic online databases [17,18].
As Cicerbita alpina sprouts are locally eaten as a vegetable collected from the wild in parts of Northern Italy, we aimed to determine whether furanocoumarins actually occur in C. alpina, or whether the previous report was based on mixed collection of C. alpina with P. ostruthium. Roots of P. ostruthium are traditionally used in medicine and known to contain high amounts of furanocoumarins. Since furanocoumarins are pharmacologically active, but may also pose potential health risks, clarifying their presence in C. alpina is of considerable interest to guarantee the safety of its traditional dietary use. This regards in particular the known phototoxicty of furanocoumarins.
C. alpina and P. ostruthium frequently co-occur (Figure 1) in parts of their distribution range, in particular in the Alps. We therefore hypothesized that the furanocoumarins previously reported from roots of C. alpina in fact originated from a mixed collection of C. alpina and P. ostruthium. Roots of P. ostruthium are known to contain high amounts of furanocoumarins. To support our hypothesis, we isolated twelve coumarins from P. ostruthium and carried out a comparative UHPLC-HRMS analysis of acetone extracts from six accessions of C. alpina and two samples of P. ostruthium.

Figure 1.
Cicerbita alpina and Peucedanum ostruthium growing together in a subalpine megaphorb community (5th of August 2024 near Passo del Tonale, Vermiglio/Trentino/Italy; N 46°15′12.0″, E 10°36′18.5″ E, 2000 m. a.m.s.l.; CZ).
2. Results
2.1. Isolation and Identification
Exhaustive maceration, followed by repeated chromatographic separation led to the isolation of one prenylated chromone (1), three prenylated coumarins (2 to 4) and eight linear furanocoumarins (5 to 12) (Figure 2). After comparison of mass spectrometry (MS) and nuclear magnetic resonance (NMR) data, these compounds were identified as peucenin (1) [19], osthole (2) [20], ostruthin (3) [21], auraptene (4) [22], xanthotoxin (5) [23], imperatorin (6) [24], isoimperatorin (7) [25], phellopterin (8) [26], oxypeucedanin hydrate (9) [27], oxypeucedanin methanolate (10) [28], ostruthol (11) [29], and oxypeucedanin (12) [30]. To the best of our knowledge, phellopterin (8) and xanthotoxin (5) are reported here for the first time from P. ostruthium. NMR and MS data for these compounds are provided in the Supplementary Material.
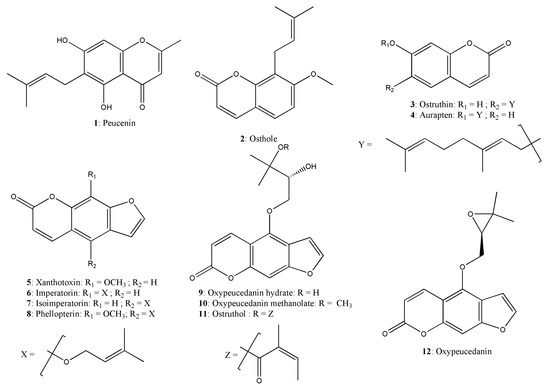
Figure 2.
Chemical structures of isolated compounds from P. ostruthium.
2.2. Comparative HPLC Analysis
Acetone extracts from six accessions of Cicerbita alpina were analyzed and compared with those from two samples of Peucedanum ostruthium using a modified version of the chromatographic method of Vogl et al. [4]. In the base peak chromatogram of the commercial sample of Peucedanum ostruthium (P2) shown in Figure 3, all previously isolated furanocoumarins were detected. The corresponding high-resolution accurate mass spectrometric data for these compounds are summarized in Table 1.
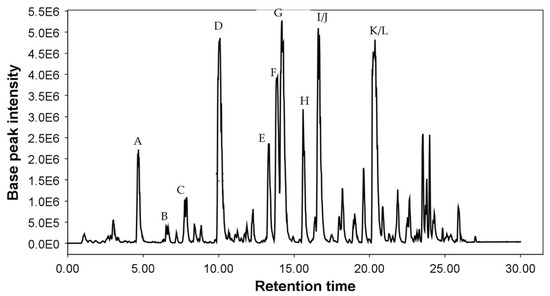
Figure 3.
Base peak chromatogram of P. ostruthium acetone extract. Peak A = oxypeucedanin hydrate (9), B = xanthotoxin (5), C = oxypeucedanin methanolate (10), D = oxypeucedanin (12), E = peucenin (1), F = ostruthol (11), G = imperatorin (6), H = phellopterin (8), I = isoimperatorin (7), J = osthole (2), K = ostruthin (3), L = auraptene (4).

Table 1.
List of compounds 1–12 and their high-resolution accurate mass spectrometric data in P. ostruthium.
UHPLC-HRMS data sets were processed using MZmine, applying a noise threshold of 5.0 × 102 [31]. All isolated compounds were detected in both P. ostruthium samples (P1 and P2). In contrast, none of the furanocoumarins, including the ones previously described from C. alpina, were detected in any of the six samples of C. alpina (C1–C6). Figure 4 indicates the absence of apolar compounds, including furanocoumarins, in the extracts of C. alpina.
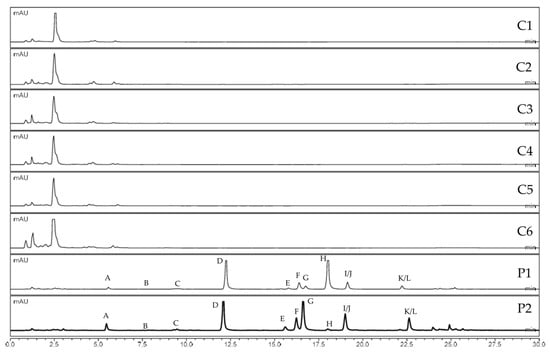
Figure 4.
Comparison of UV chromatograms (254 nm) of acetone extracts from C. alpina accessions (C1–C6) and P. ostruthium samples (P1 and P2).
Interestingly, the sample of Peucedanum ostruthium collected in the wild, contained higher amounts of phellopterin (8), but markedly lower amounts of isoimperatorin (9) than the commercial sample.
3. Discussion
Furanocoumarins were not detectable in rootstocks of Cicerbita alpina. This fact conforms with existing chemophenetic data for other members of the Cichorieae tribe of the Asteraceae family. In fact, the discrepancy between the reported occurrence of furanocoumarins in C. alpina and the lack of reports of furanocoumarins from any other member of the Cichorieae tribe was one of the main motivations for this study. Within the Asteraceae family, the subfamily Mutisioideae has been reported to contain furanocoumarins. However, this subfamily is phylogenetically quite distant from the Cichorieae [18,32,33]. The detection of ostruthol (11) by Zheleva-Dimitrova et al. through LC-MS/MS analysis underscores the critical role of reference standards in the accurate identification of natural products [13].
The assignment of compound structures based solely on MS/MS spectral comparison, presents significant perils for erroneous positive reports (in particular, when “only” confirming previous—in this case erroneous—studies. Zheleva-Dimitrova et al. [13] adopted the identification confidence framework proposed by Schymanski et al., which ranks compound annotations by confidence levels—Level 1 indicating confirmation via comparison with an authentic reference standard, and Level 2 representing a probable structure based on spectral library matching [34]. Çiçek et al. proposed a new ranking system of confidence levels for the identification of phytochemical metabolites [35]. According to this classification system, only NMR data combined with an additional technique providing comprehensive structural information—such as high-resolution mass spectrometry (HR-MS) or X-ray crystallography—is considered sufficient to achieve the highest confidence level in structural annotation (Level A). In contrast, the sole reliance on MS data from spectral libraries, without the support of authentic standards or retention indices, is deemed inadequate for confirming the presence of natural products with a very high confidence level (Level C). False positive reports based on LC-MS data are a growing problem in phytochemical analysis. Inaccurate compound identification easily leads to the erroneous re-reporting of the same compound in subsequent studies, based to the expected presence. Another persistent challenge in comparative natural product chemistry is the frequent lack of adequate control materials, including the proper storage of voucher specimens in recognized institutions (and not simply in laboratory collections which, due to relocation/retirement of their reference person cannot guarantee constant availability). This issue is particularly pronounced for studies published in the last century, for which verification of the original samples is often no longer feasible. As a result, inaccurate reports may be accepted as valid and perpetuated in subsequent publications, thereby perpetuating the issue and making retrospective corrections increasingly challenging. In cases of sample contamination with material from a different species, accurate and detailed information regarding the collection site, the taxonomic identification procedures, and associated plant species is essential to determine whether the sample may represent a mixture of two species [36]. However, apart from exercising due diligence during plant collection and ideally taking pictures of the collection site during collection, this kind of error seems to be particularly hard to avoid and even more difficult to detect in retrospect.
The occurrence of furanocoumarins has been extensively investigated in the Apiaceae family, with significant progress made in elucidating their biosynthetic pathways and the underlying genetic mechanisms [37,38,39,40,41]. This study constitutes the first report of xanthotoxin (5) and phellopterin (8) in Peucedanum ostruthium. Within the genus Peucedanum, xanthotoxin (5) has previously been identified in P. formosanum Hayata, P. galbanum Benth. & Hook.f., P. grande C.B. Clarke, P. harry-smithii var. subglabrum (R.H. Shan & Sheh) R.H. Shan & M.G. Sheh, P. japonicum Thunb., P. luxurians Tamamsch., P. palustre (L.) Moench, and P. praeruptorum Dunn [42,43,44,45,46,47,48]. Phellopterin 8 has been reported from P. medicum var. gracile Dunn ex R.H. Shan & M.L. Sheh P. palustre (L.) Moench, and P. zenkeri Engl. [45,49,50]. Within the genus Peucedanum, the presence of furanocoumarins is a well-documented chemophenetic trait.
In general, the presence or absence of particular secondary metabolite may also be related to environmental or developmental factors, which all influence the array of compounds contained in an individual plant at a specific point in time. Also, the isolation procedure significantly affects the array of compounds detectable in an extract. Therefore, it is essential to include accessions from multiple regions and to apply identical sample preparation methods in comparative phytochemical studies.
In conclusion, furanocoumarins were absent in all six accessions of C. alpina investigated. Given the frequent co-occurrence of C. alpina and P. ostruthium, the previous report of furanocoumarins by Appendino et al. [16] is most easily explained by a mixed collection of these two species. The subsequent detection of ostruthol 11 by UHPLC-HRMS in aerial parts and flowering heads of C. alpina from Bulgaria with a confidence level of 2 at the Schimansky scale [34], is probably a false positive report, induced by the fact that ostruthol 11 had already been reported from C. alpina and was thus also to be expected in the analyzed extracts.
4. Materials and Methods
4.1. Plant Material, Reagents and Experimental Procedures
Two kilograms of dried root stock of Peucedanum ostruthium (L.) W.D.J.Koch were purchased from Alfred Galke GmbH, Am Bahnhof 1, 37539 Bad Grund, Germany. Three accessions of Cicerbita alpina (L.) Wallr. were collected in May 2025 from the Harz Mountains, Lower Saxonia, Germany. The collection sites were as follows: C1, south of Altenau/Goslar/Lower Saxonia/Germany, N 51°47′20.4″, E 10°26′54.6″, 586 m a.m.s.l. (CMC-202504524-3), C2, north of Clausthal-Zellerfeld/Goslar/Lower Saxonia/Germany, N 51°50′05.4″, E 10°20′26.4″, 529 m a.m.s.l. (CMC-20250524-2), and C3, northwest of Braunlage/Goslar/Lower Saxonia/Germany, N 51°44′29.1″, E 10°36′21.2″, 637 m a.m.s.l. (CMC-20250524-6). C4 was collected in May 2025 W Žacléř/Trutnov/Hradec Kralove/Czech Republic, N 50°39′57.0″, E 15°51′52.7″, 950 m a.m.s.l. (CZ-20250531B-1). C5 was collected E Karpacz Górny/Jelenia Góra/Dolnoślaskie/Poland, N 50°46′12.4″, E 15°43′50.9″, 790 m a.m.s.l. (CZ-20250529B-1). C6 was collected in Vitosha Mountains/Sofia/Oblast Sofia/Bulgaria, near Hotel Moreni, N 42°35′28.7″, E 23°17′35.0″, 1780 m. a.m.s.l. Voucher specimens are deposited under the herbarium of Kiel University (KIEL0005021-KIEL0005026) and the Bulgarian Academy of Sciences (SOM179541). P1 was collected S Karpacz Górny/Jelenia Góra/Dolnoślaskie/Poland, N 50°46′53.6″, E 15°43′19.4″, 860 m a.m.s.l. (CZ-20250529A-1).
LC-MS-grade acetonitrile and water, as well as other (analytical grade) solvents and reagents, were purchased from VWR International GmbH (Darmstadt, Germany). LC-MS-grade formic acid was purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). The water used in the semi-preparative HPLC was double-distilled in-house. LC-MS Grade formic acid was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). TLC was performed on silica gel 60 F254 plates (VWR International, Darmstadt, Germany) using Dichlormethane and Petrolether (1:1) as the mobile phase and 366 nm UV-Light for detection. Column chromatography was performed using a Sephadex LH-20 column (GE Healthcare AB, Uppsala, Sweden) and Silica gel 60 (Carl Roth GmbH + Co. KG, Karlsruhe, Germany). Semi-preparative HPLC was performed using an Ultimate 3000 instrument equipped with an HPG-3400SD pump, WPS-3000SL autosampler, TCC-3000SD column heater, and VWD-3400RS variable wavelength detector (Thermo Fisher Scientific Inc., Waltham, MA, USA) using a Phenomenex Aqua column (5 µm, 250 × 10 mm). Extracts, fractions and pure compounds were analyzed on a Shimadzu Nexera 2 liquid chromatograph connected to a LC-MS triple quadrupole mass spectrometer with electrospray ionization (Shimadzu, Kyoto, Japan). The comparative HPLC-MS analysis was performed using a Shimadzu Nexaera 4 liquid chromatograph connected to a SciEx X500r qTOF (AB Sciex LLC, Marlborough, MA, USA). For separation a Phenomenex Luna Omega polar C18 column (100 × 2.1 mm, 1.6 µm particle size, Phenomenex, Aschaffenburg, Germany) was used. 1D (1H and 13C) and 2D (HSQC, HMBC, and COSY) NMR spectra were recorded on a Bruker Avance III 400 NMR spectrometer operating at 400 MHz for the proton channel and 100 MHZ for the 13C channel with a 5 mm PABBO broadband probe with a z-gradient unit at 298 K (Bruker BioSpin GmbH, Rheinstetten, Germany). Reference Values were 7.26 (1H) and 77.16 (13C) for Chloroform. Structure elucidation was performed on Topspin 3.6 software (Bruker Biospin GmbH, Rheinstetten, Germany). 5 mm NMR sample tubes were obtained from Rototec-Spintec GmbH, Griesheim, Germany.
4.2. Extraction and Isolation
Powdered roots of P. ostruthium (1.00 kg) were extracted by maceration, after sonification for 15 min, at room temperature for two weeks. The solvents used were acetone (10.5 L) for 7 days, followed by methanol–acetone–water (3:1:1) (10.5 L) for additional seven days, yielding 112.9 g and 167.1 g of crude extract, respectively. The acetone extract (112.9 g) was further processed by vacuum liquid chromatography (VLC), using a stepwise elution with 800 mL of hexane, followed by 500 mL each of increasing mixtures of hexane/dichloromethane (1, 2, 5, 25, 50 and 100%), then dichloromethane/methanol (1, 2, 5, 25, 50 and 100%) yielding thirteen fractions (1–13). The first three fractions were combined (Fraction A, 20.3 g) and subjected to further separation by silica gel column chromatography (ID 50 mm, 205 g of silica) using the same gradient solvent system as previously described yielding 80 fractions (A1–A80) of which fraction A52 consisted of 1.00 g of 7 and the methanol insoluble part of A54 126 mg of 6. Sephadex LH-20 column chromatography of fraction A49 afforded eleven subfractions (A49A–A49K). Subfraction A49F was further purified by semi-preparative HPLC, yielding the isolation of 2 (7 mg), 4 (32 mg) and 9 (6 mg). Similarly, fractions A55 and A62 were subjected to semi-preparative HPLC, resulting in the isolation of 1 (1 mg), 3 (3 mg), 5 (2 mg), 8 (6 mg), 10 (2 mg), 12 (2 mg) and 11 (2 mg).
4.3. Sample Preparation for HPLC Analysis
A total of 0.500 g of dried plant material from each accession of C. alpina and P. ostruthium was extracted three times for 15 min each using 10 mL of acetone in an ultrasonic bath. The combined extracts were evaporated to dryness under reduced pressure using a Büchi rotavapor R300 and re-dissolved in 1.5 mL of methanol for further analysis.
4.4. HPLC Analysis
HPLC-MS analyses were performed using a modified gradient based on the method described by Vogl et al. [4], employing 0.1% formic acid in water as solvent A and 0.1% formic acid in acetonitrile as solvent B. The gradient profile was as follows: 25% B at 0 min; a linear increase to 37% B from 0 to 5.72 min; 45% B from 5.72 to 12.74 min; 65% B from 12.74 to 21.51 min; 95% B from 21.51 to 22.39 min; and maintained at 95% B until 30 min. The chromatographic separation was performed at a flow rate of 0.300 mL/min. The column oven temperature was maintained at 38 °C. Detection was carried out using a UV detector (254 nm) and a quadrupole time-of-flight mass spectrometer (qTOF-MS) operated in positive ion mode, with a mass range of m/z 100–1000.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14182815/s1.
Author Contributions
Conceptualization, C.Z.; investigation, C.M.C. and G.M.F.G.; resources, C.M.C., D.Z.-D., and C.Z. writing—original draft preparation, C.M.C.; writing—review and editing, D.Z.-D., G.A., and C.Z.; supervision, C.Z.; project administration, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Original data will be shared on reasonably justified request.
Acknowledgments
The authors would like to thank Ulrich Girreser for performing the NMR measurements, Mayra Galarza Pérez for her support with the laboratory work, and Thomas Stegemann for his assistance with the MS analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Peroutka, R.; Schulzová, V.; Botek, P.; Hajšlová, J. Analysis of furanocoumarins in vegetables (Apiaceae) and citrus fruits (Rutaceae). J. Sci. Food Agric. 2007, 87, 2152–2163. [Google Scholar] [CrossRef]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Vogl, S.; Zehl, M.; Picker, P.; Urban, E.; Wawrosch, C.; Reznicek, G.; Saukel, J.; Kopp, B. Identification and quantification of coumarins in Peucedanum ostruthium (L.) Koch by HPLC-DAD and HPLC-DAD-MS. J. Agric. Food Chem. 2011, 59, 4371–4377. [Google Scholar] [CrossRef]
- Cisowski, W.; Sawicka, U.; Mardarowicz, M.; Asztemborska, M.; Luczkiewicz, M. Essential oil from herb and rhizome of Peucedanum ostruthium (L. Koch.) ex DC. Z. Naturforsch. C J. Biosci. 2001, 56, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-Inflammatory and wound healing properties of leaf and rhizome extracts from the medicinal plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef] [PubMed]
- Hiermann, A.; Schantl, D. Antiphlogistic and antipyretic activity of Peucedanum ostruthium. Planta Med. 1998, 64, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Sarkhail, P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar] [CrossRef] [PubMed]
- Fusani, P.; Zidorn, C. Phenolics and a sesquiterpene lactone in the edible shoots of Cicerbita alpina (L.) Wallroth. J. Food Compos. Anal. 2010, 23, 658–663. [Google Scholar] [CrossRef]
- Shulha, O.; Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae revisited: An update (2008–2017). Phytochemistry 2019, 163, 149–177. [Google Scholar] [CrossRef]
- Djordjević, I.; Tešević, V.; Janaćković, P.; Milosavljević, S.; Vajs, V. Sesquiterpene lactones from Cicerbita alpina. Biochem. Syst. Ecol. 2004, 32, 209–210. [Google Scholar] [CrossRef]
- Zidorn, C.; Schwaha, R.; Ellmerer, E.; Stuppner, H. On the occurrence of sonchuside A in Cicerbita alpina and its chemosystematic significance. J. Serb. Chem. Soc. 2005, 70, 171–175. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Petrova, A.; Zengin, G.; Sinan, K.I.; Balabanova, V.; Joubert, O.; Zidorn, C.; Voynikov, Y.; Simeonova, R.; Gevrenova, R. Metabolite profiling and bioactivity of Cicerbita alpina (L.) Wallr. (Asteraceae, Cichorieae). Plants 2023, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulou, I.; Horgan, M.J.; Siewert, B.; Siller, M.; Palmieri, L.; Martinidou, E.; Martens, S.; Fusani, P.; Temml, V.; Stuppner, H.; et al. In vitro evaluation of the effects of methanolic plant extracts on the embryonation rate of Ascaridia galli eggs. Vet. Res. Commun. 2023, 47, 409–419. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Petrova, A.; Savov, Y.; Gevrenova, R.; Balabanova, V.; Momekov, G.; Simeonova, R. Protective potential of Cicerbita alpina leaf extract on metabolic disorders and oxidative stress in model animals. Int. J. Mol. Sci. 2024, 25, 10851. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Tettamanzi, P.; Gariboldi, P. Sesquiterpene lactones and furanocoumarins from Cicerbita alpina. Phytochemistry 1991, 30, 1319–1320. [Google Scholar] [CrossRef]
- Bauri, A.K.; Foro, S.; Nguyen Do, N.Q. Crystal structure of a photobiologically active furanocoumarin from Artemisia reticulata. Acta Crystallogr. E Crystallogr. Commun. 2016, 72, 463–466. [Google Scholar] [CrossRef]
- Vestena, A.S.; Meirelles, G.d.C.; Zuanazzi, J.A.; von Poser, G.L. Taxonomic significance of coumarins in species from the subfamily Mutisioideae, Asteraceae. Phytochem. Rev. 2023, 22, 85–112. [Google Scholar] [CrossRef]
- Okorie, D.A. Chromones and limonoids from Harrisonia abyssinica. Phytochemistry 1982, 21, 2424–2426. [Google Scholar] [CrossRef]
- Kerimli, E.H.; Kerimov, Y.B.; Mamedov, A.M. Constituents of the EtOH extract of Ferula persica roots. Chem. Nat. Compd. 2023, 59, 1171–1172. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Zhang, M.; Zhang, Z.; Zhang, B.; Wang, X.; Wang, J.; Tu, P.; Jiang, Y.; Shi, S.-P. Characterization of a coumarin C-/O-prenyltransferase and a quinolone C-prenyltransferase from Murraya exotica. Org. Biomol. Chem. 2022, 20, 5535–5542. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Zarubaev, V.V.; Orshanskaya, I.R.; Kadyrova, R.A.; Sannikova, V.A.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. Anti-influenza activity of monoterpene-containing substituted coumarins. Bioorg. Med. Chem. Lett. 2017, 27, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Parmar, A.; Pandey, N.; Bhardwaj, N.; Chakrabarty, S.; Sarkar, R.; Kumar, H.; Jain, S.K. Isolation, cytotoxicity, and in-silico screening of coumarins from Psoralea corylifolia Linn. Chem. Biodivers. 2024, 21, e202301841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-T.; Yang, J.; Chen, Q.-F. A new coumarin glucoside from Angelica pubescens. Nat. Prod. Res. 2025, 39, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Juraev, K.S.; Komilov, B.J.; Eshbakova, K.A.; Turgunov, K.K.; Tashkhodzhaev, B. Furocoumarins from Ferula lehmannii. Chem. Nat. Compd. 2023, 59, 909–911. [Google Scholar] [CrossRef]
- Yang, S.-J.; Liu, M.-C.; Liang, N.; Xiang, H.-M.; Yang, S. Chemical constituents of Cyrtomium fortumei (J.) Smith. Nat. Prod. Res. 2013, 27, 2066–2068. [Google Scholar] [CrossRef]
- Furumi, K.; Fujioka, T.; Fujii, H.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M.; Mihashi, K. Novel antiproliferative falcarindiol furanocoumarin ethers from the root of Angelica japonica. Bioorg. Med. Chem. Lett. 1998, 8, 93–96. [Google Scholar] [CrossRef]
- Bergendorff, O.; Dekermendjian, K.; Nielsen, M.; Shan, R.; Witt, R.; Ai, J.; Sterner, O. Furanocoumarins with affinity to brain benzodiazepine receptors in vitro. Phytochemistry 1997, 44, 1121–1124. [Google Scholar] [CrossRef]
- Naseri, M.; Monsef-Esfehani, H.R.; Saeidnia, S.; Dastan, D.; Gohari, A.R. Antioxidative coumarins from the roots of Ferulago subvelutina. Asian J. Chem. 2013, 25, 1875–1878. [Google Scholar] [CrossRef]
- Tavakoli, S.; Delnavazi, M.-R.; Hadjiaghaee, R.; Jafari-Nodooshan, S.; Khalighi-Sigaroodi, F.; Akhbari, M.; Hadjiakhoondi, A.; Yassa, N. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran. Nat. Prod. Res. 2018, 32, 2724–2728. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef] [PubMed]
- Katinas, L.; Funk, V.A. An updated classification of the basal grade of Asteraceae (= Compositae): From Cabrera’s 1977 tribe Mutisieae to the present. N. Z. J. Bot. 2020, 58, 67–93. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Çiçek, S.S.; Mangoni, A.; Hanschen, F.S.; Agerbirk, N.; Zidorn, C. Essentials in the acquisition, interpretation, and reporting of plant metabolite profiles. Phytochemistry 2024, 220, 114004. [Google Scholar] [CrossRef]
- Zidorn, C. Guidelines for consistent characterisation and documentation of plant source materials for studies in phytochemistry and phytopharmacology. Phytochemistry 2017, 139, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Shtratnikova, V.Y. Furanocoumarins: History of research, diversity, synthesis, physiological role in the plant, and medical application. Russ. J. Plant Physiol. 2023, 70, 169. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef]
- Schulzová, V.; Hajšlová, J.; Botek, P.; Peroutka, R. Furanocoumarins in vegetables: Influence of farming system and other factors on levels of toxicants. J. Sci. Food Agric. 2007, 87, 2763–2767. [Google Scholar] [CrossRef]
- Stanjek, V.; Piel, J.; Boland, W. Biosynthesis of furanocoumarins: Mevalonate-independent prenylation of umbelliferone in Apium graveolens (Apiaceae). Phytochemistry 1999, 50, 1141–1146. [Google Scholar] [CrossRef]
- Huang, X.-C.; Tang, H.; Wei, X.; He, Y.; Hu, S.; Wu, J.-Y.; Xu, D.; Qiao, F.; Xue, J.-Y.; Zhao, Y. The gradual establishment of complex coumarin biosynthetic pathway in Apiaceae. Nat. Commun. 2024, 15, 6864. [Google Scholar] [CrossRef]
- Campbell, W.E.; Mathee, S.; Wewers, F. Phytochemical studies on the Blister Bush, Peucedanum galbanum. Planta Med. 1994, 60, 586–587. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Tu, L.-F.; Liu, D.-P.; Luo, Y.-M. A new coumarin derivative from the roots of Peucedanum praeruptorum Dunn. Biochem. Syst. Ecol. 2023, 108, 104624. [Google Scholar] [CrossRef]
- Chen, I.S.; Chang, C.T.; Sheen, W.S.; Teng, C.M.; Tsai, I.L.; Duh, C.Y.; Ko, F.N. Coumarins and antiplatelet aggregation constituents from Formosan Peucedanum japonicum. Phytochemistry 1996, 41, 525–530. [Google Scholar] [CrossRef]
- Ojala, T.; Vuorela, P.; Kiviranta, J.; Vuorela, H.; Hiltunen, R. A bioassay using Artemia salina for detecting phototoxicity of plant coumarins. Planta Med. 1999, 65, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Chinou, I.; Widelski, J.; Fokialakis, N.; Magiatis, P.; Glowniak, K. Coumarins from Peucedanum luxurians. Fitoterapia 2007, 78, 448–449. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, P.-Y.; Wu, C.-C.; Tsai, I.-L.; Chen, I.-S. Chemical constituents and anti-platelet aggregation activity from the root of Peucedanum formosanum. J. Food Drug Anal. 2008, 16, 10. [Google Scholar] [CrossRef]
- Aslam, M.; Ali, M.; Dayal, R.; Javed, K. Coumarins and a naphthyl labdanoate diarabinoside from the fruits of Peucedanum grande C. B. Clarke. Z. Naturforsch. C J. Biosci. 2012, 67, 580–586. [Google Scholar] [CrossRef][Green Version]
- Huang, P.; Zheng, X.Z.; Lai, M.X.; Rao, W.Y.; Nishi, M.; Nakanishi, T. Studies on chemical constituents of Peucedanum medicum Dunn var. garcile Dunn ex Shan at Sheh. Zhongguo Zhong Yao Za Zhi 2000, 25, 222–224. [Google Scholar][Green Version]
- Ngwendson, J.N.; Bedir, E.; Efange, S.M.N.; Okunji, C.O.; Iwu, M.M.; Schuster, B.G.; Khan, I.A. Constituents of Peucedanum zenkeri seeds and their antimicrobial effects. Pharmazie 2003, 58, 587–589. [Google Scholar][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).