Influence of Biotic and Abiotic Elicitors on Rosmarinic Acid Accumulation in Hairy Root Cultures of Dracocephalum kotschyi Boiss.
Abstract
1. Introduction
2. Results
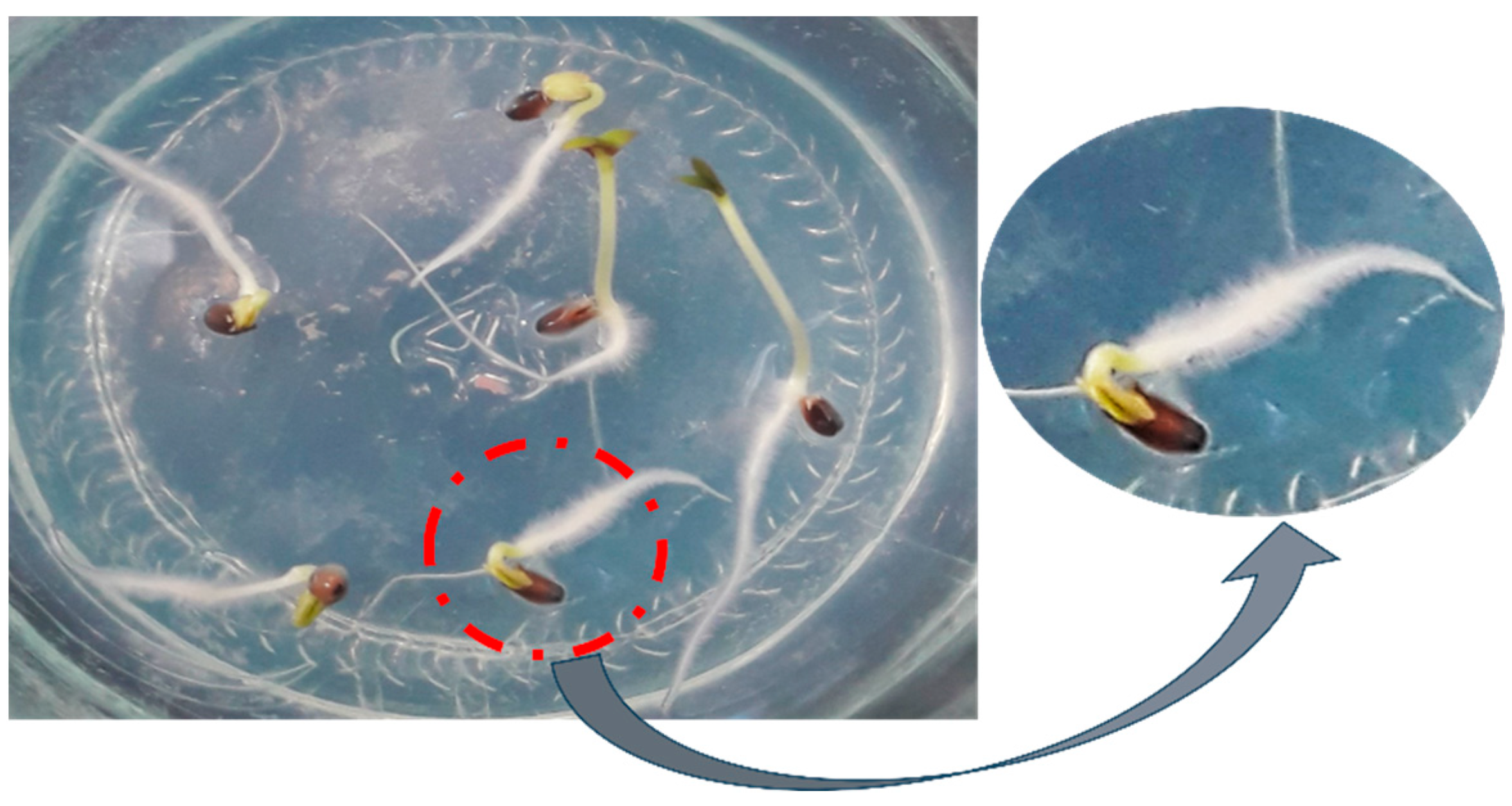
2.1. Short-Term Moist Stratification Promotes Dormancy Release in D. kotschyi Seeds
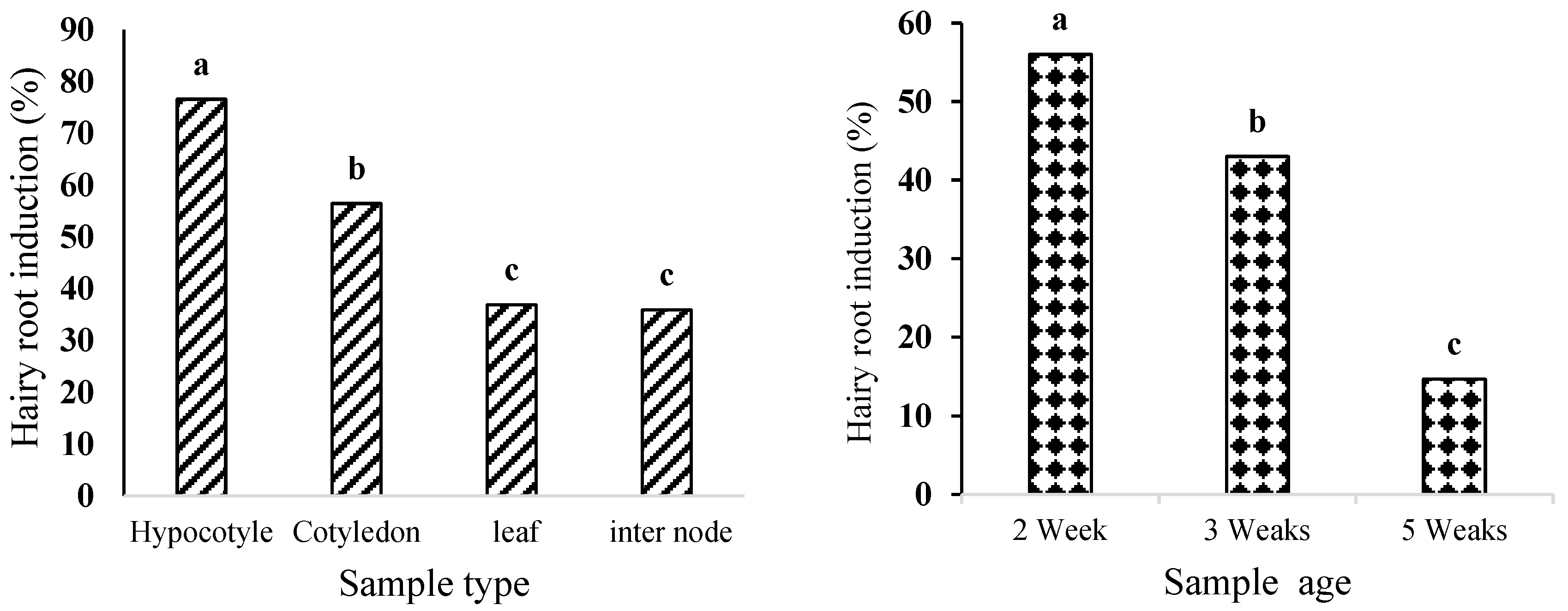
2.2. The Effect of Explant Type and Age on Hairy Root Induction
2.3. The Effect of L-Arginine on Hairy Root Induction
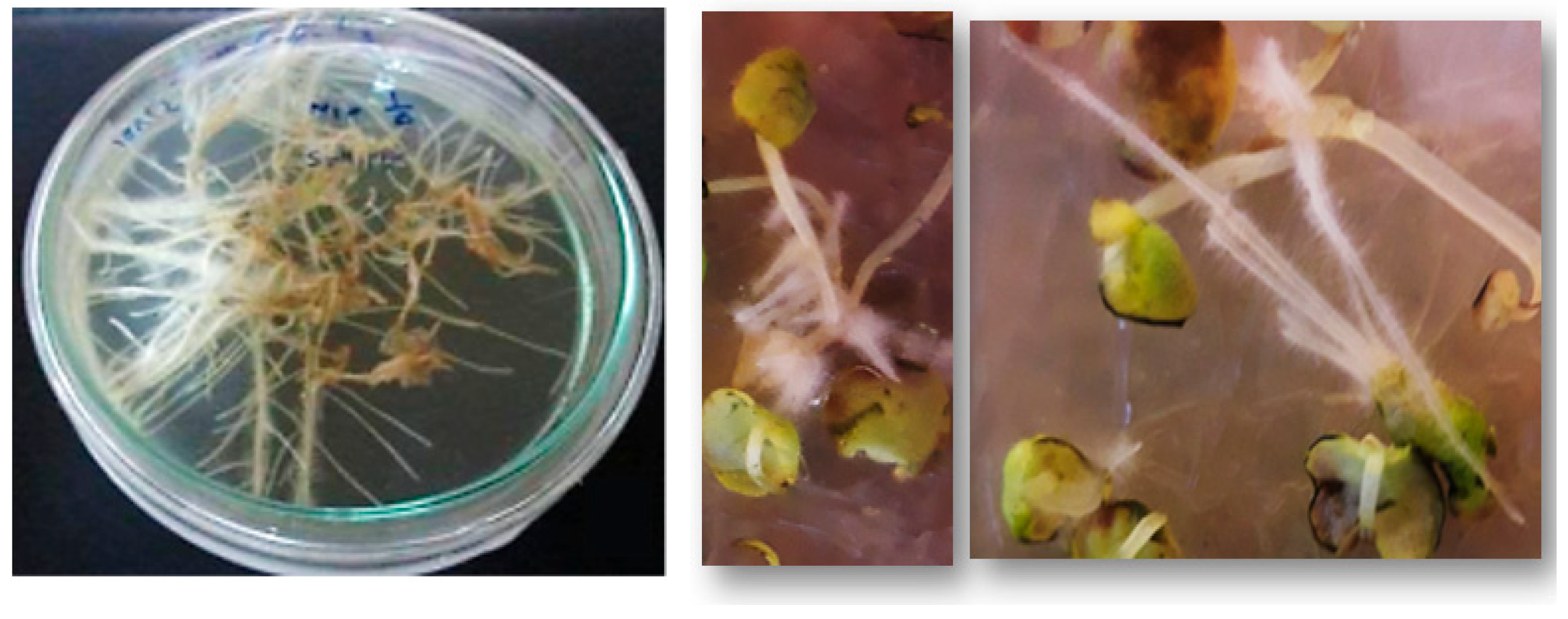
2.4. Hairy Root Growth and Development
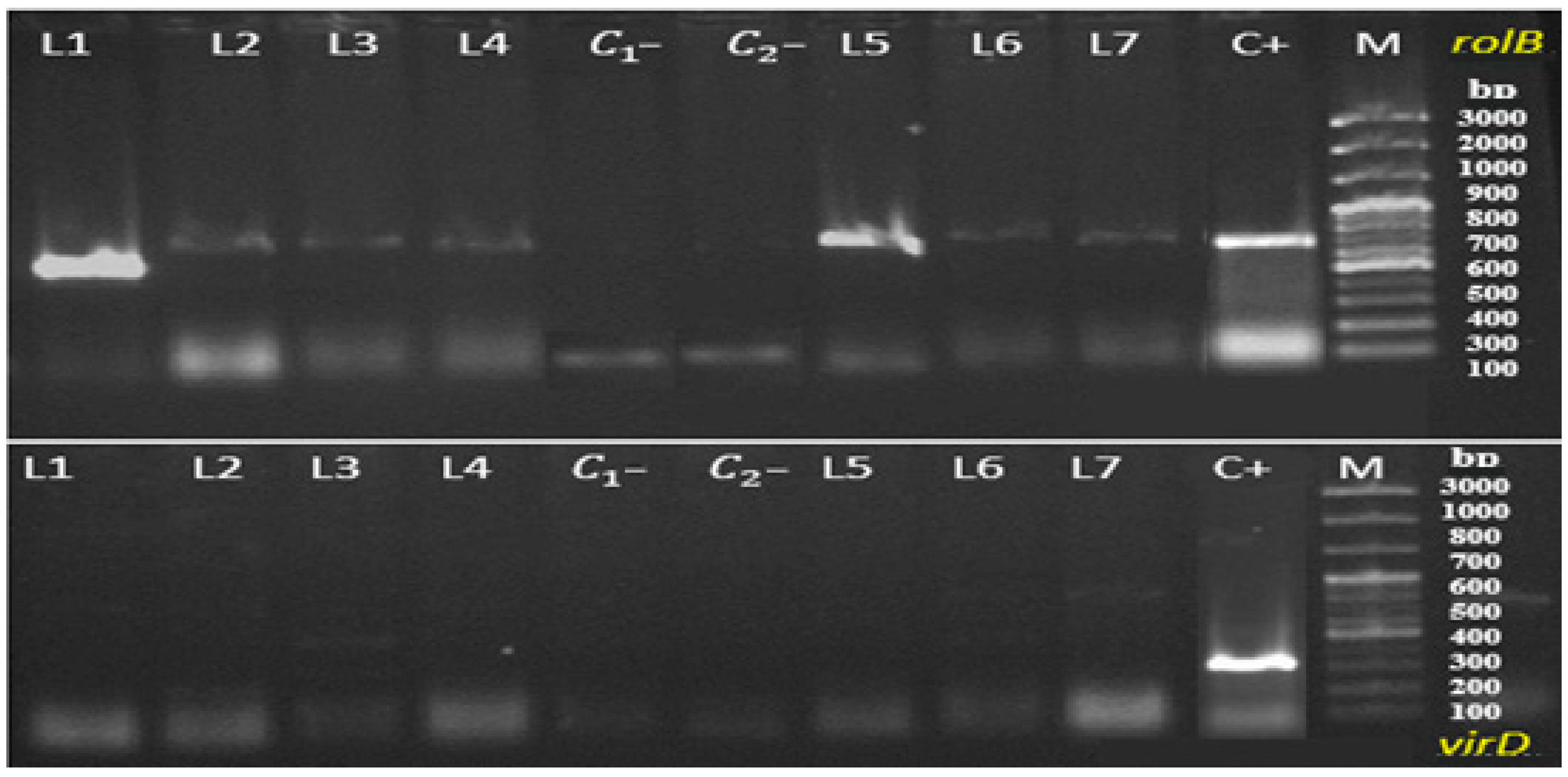
2.5. Molecular Verification of the Transgenic Nature of Hairy Roots
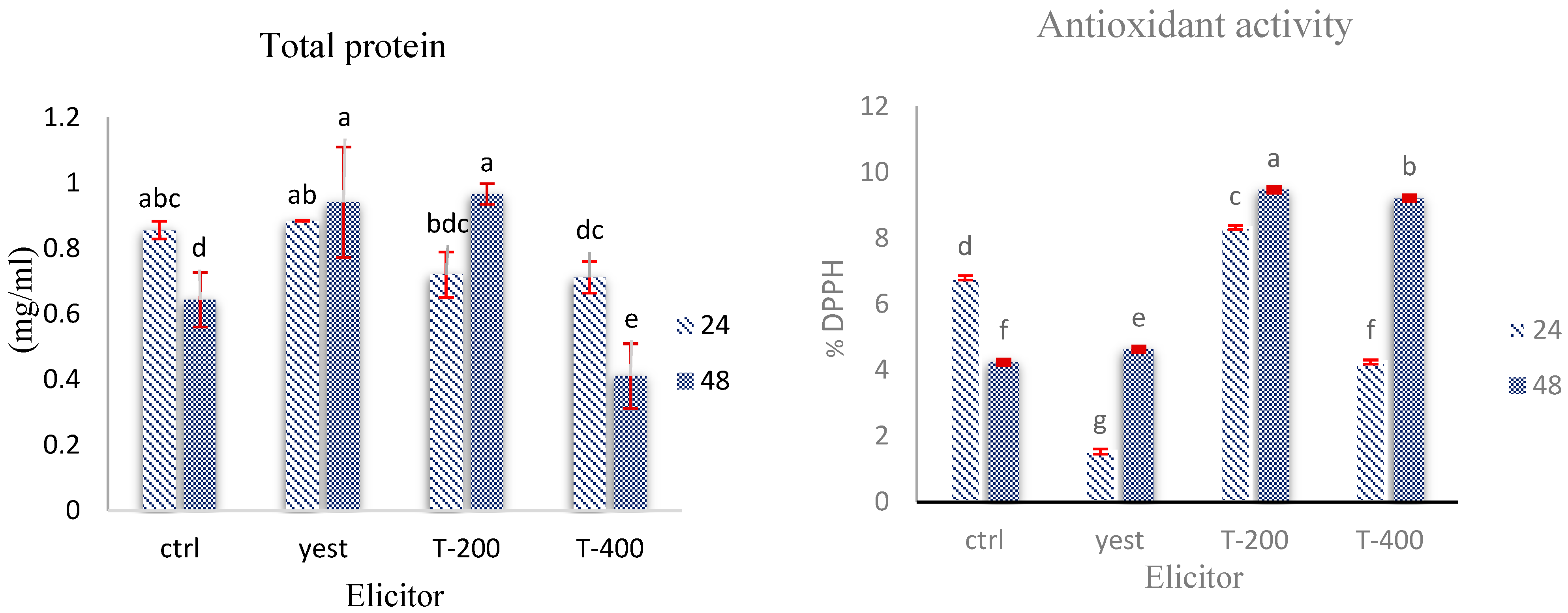
2.6. Effects of Biotic and Abiotic Elicitors on Biochemical Traits in D. kotschyi Hairy Roots
Determination of Total Protein Concentration
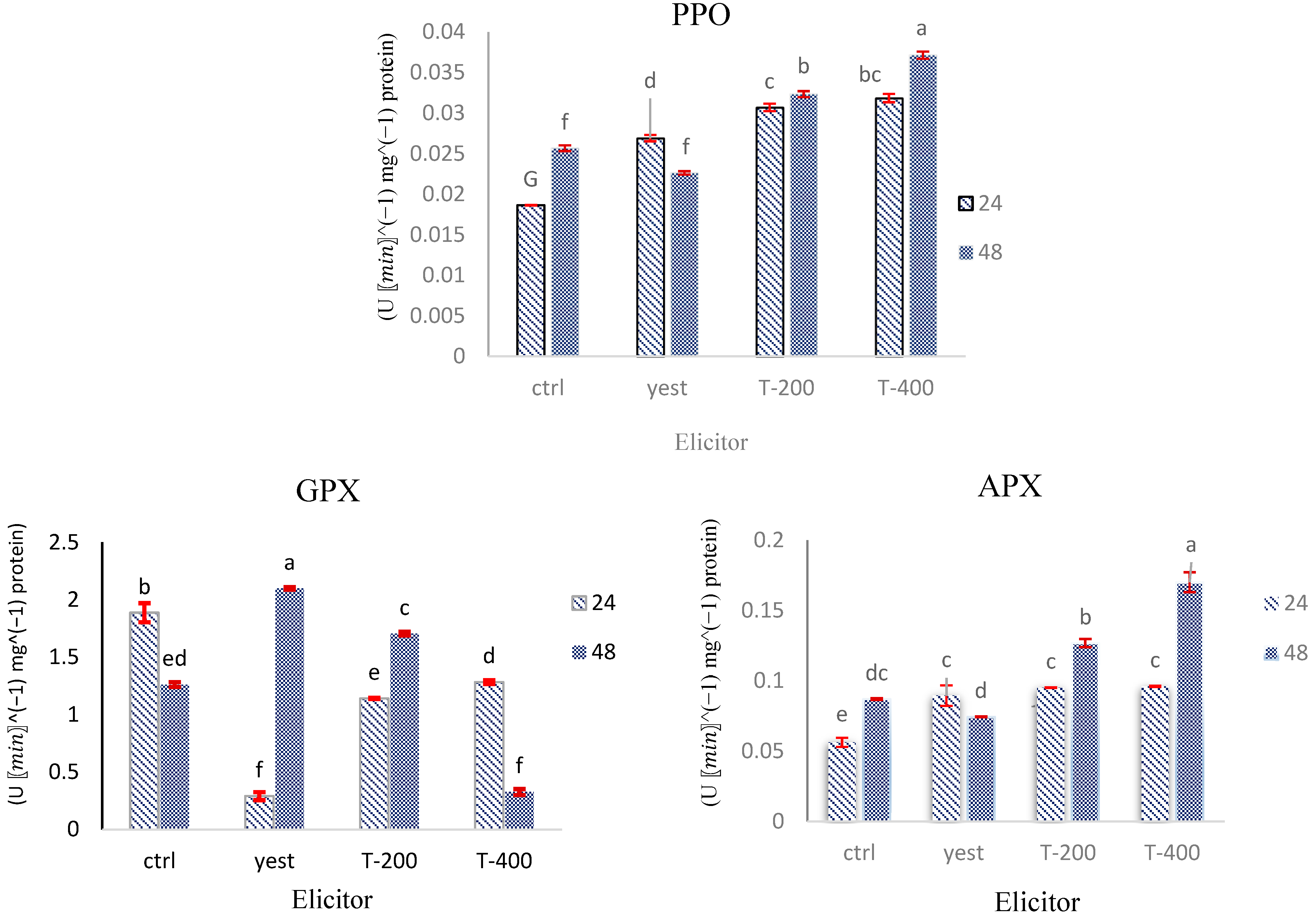
2.7. Assessment of Antioxidant Enzyme Dynamics in D. kotschyi Hairy Roots Under TiO2 NPs and Yeast Extract Treatments
2.7.1. GPX Activity
2.7.2. APX Activity
2.7.3. PPO Activity
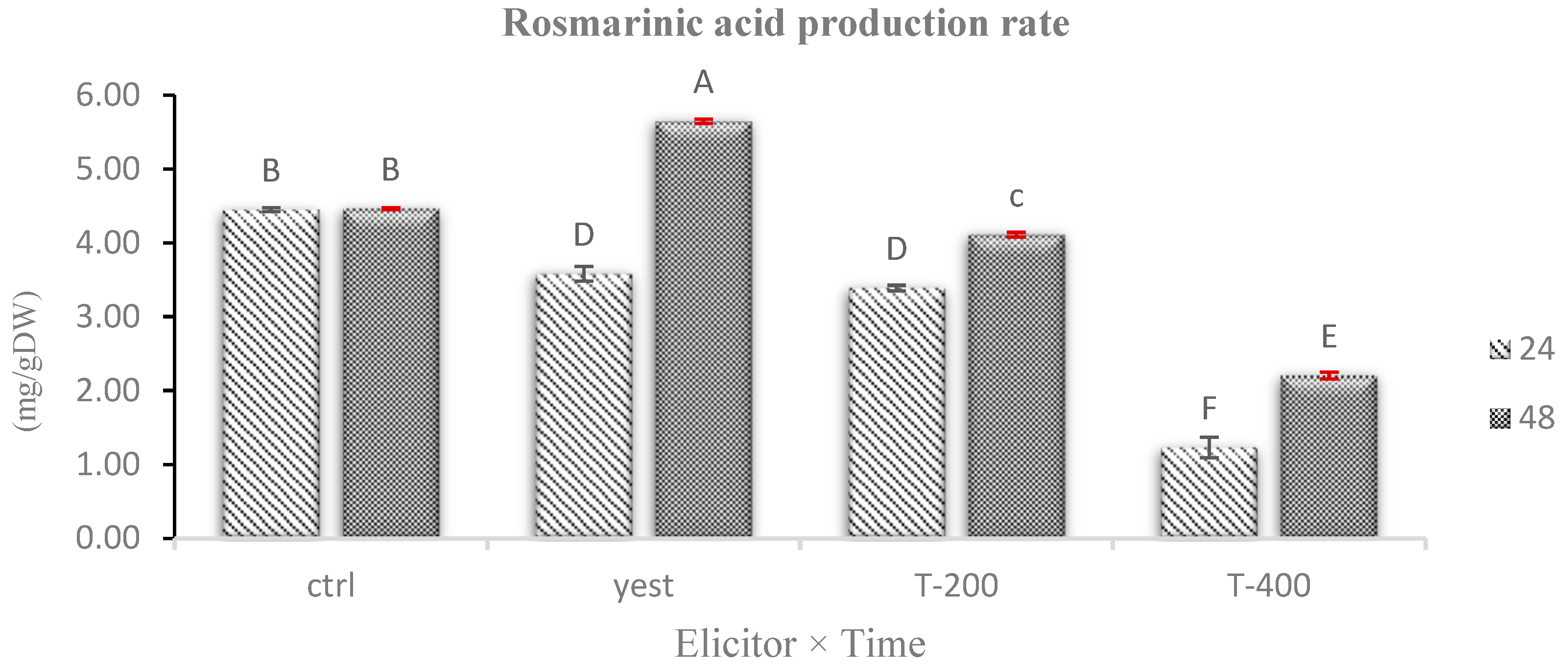
2.8. Effects of Biotic and Abiotic Elicitors on Rosmarinic Acid Accumulation in Hairy Roots of D. kotschyi
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Effect of Explant Type and Age on Hairy-Root Induction
4.3. Bacterial Strain and Culture Conditions
4.4. Preparation of Co-Cultivation Medium
4.5. Explant Inoculation and Bacterial Elimination
4.6. Molecular Confirmation of Transgenic Hairy Roots
4.6.1. Primer Design
4.6.2. Genomic and Plasmid DNA Isolation
4.6.3. PCR Amplification of rolB and virD Genes
4.7. Establishment and Growth of Hairy-Root Cultures
4.7.1. Initiation and Maintenance of Clonal Lines
4.7.2. Preparation of Elicitors
4.8. Determination of Total Protein Content and Antioxidant Enzyme Activities
4.8.1. Preparation of Extraction Buffer and Crude Enzyme Extract
4.8.2. Quantification of Total Protein
4.8.3. PPO Activity Assay
4.8.4. GPX Activity Assay
4.8.5. APX Activity Assay
4.9. Methanolic Extraction for Antioxidant Assays
4.10. DPPH Radical Scavenging Activity Assay
4.11. Determination of Rosmarinic Acid Content in Hairy Root Samples of D. kotschyi
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agatha, O.; Mutwil-Anderwald, D.; Tan, J.Y.; Mutwil, M. Plant sesquiterpene lactones. Philos. Trans. B 2024, 379, 20230350. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Foroozandeh, E.; Asadi Gharooneh, H.A. Dracocephalum kotschyi Boiss.: An Iranian endemic medicinal plant; A review. J. Med. Herbs 2021, 12, 9–17. [Google Scholar]
- Kazempour, M.; Shahangian, S.S.; Sariri, R. Dracocephalum kotschyi: Inhibition of critical enzyme relevant to type-2 diabetes, essential oil composition, bactericidal and anti-oxidant activity. Casp. J. Environ. Sci. 2024, 22, 289–303. [Google Scholar]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy root culture: A potent method for improved secondary metabolite production of Solanaceous plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Wang, B.; Liang, Z.; Liu, Y.; Liu, F.; Qi, Z. Selective responses of enzymes in the two parallel pathways of the rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J. Biosci. Bioeng. 2014, 117, 645–651. [Google Scholar] [CrossRef]
- Abraham, J.; Thomas, T.D. Hairy root culture for the production of valuable secondary metabolites. In Biotechnology and Production of Anti-Cancer Compounds; Springer: Cham, Switzerland, 2017; pp. 201–230. [Google Scholar]
- Choi, M.; Yoon, J.; Yang, S.H.; Kim, J.K.; Park, S.U. Production of phenolic compounds and antioxidant activity in hairy root cultures of Salvia plebeia. Plants 2023, 12, 3840. [Google Scholar] [CrossRef]
- Shariatmadari, Z.; Zarezadeh, S.; Riahi, H.; Ghotbi-Ravandi, A.A.; Seyed Hashtroudi, M.; Shahroudi, E. Cyanobacterial elicitor enhances the biomass of Mentha piperita L. and improves the production of high-value rosmarinic acid under in vitro culture of apical meristem. BMC Plant Biol. 2024, 24, 190. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent advances in the analysis of rosmarinic acid from herbs in the Lamiaceae family. Nat. Prod. Commun. 2019, 14, 1934578X19864216. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Zhu, C.; Jing, B.; Shi, K.; Yu, J.; Hu, Z. Exogenous rosmarinic acid application enhances thermotolerance in tomatoes. Plants 2022, 11, 1172. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I.; Frydrych, B.; Hnatuszko-Konka, K.; Gerszberg, A.; Wysokińska, H. Rosmarinic acid accumulation and antioxidant potential of Dracocephalum moldavica L. cell suspension culture. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 215–219. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful plant antioxidants: A new biosustainable approach to the production of rosmarinic acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.; Warchoł, M.; Skrzypek, E. Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives. Cells 2025, 14, 850. [Google Scholar] [CrossRef]
- Liu, C.; Ahmad, N.; Tao, Y.; Hussain, H.; Chang, Y.; Umar, A.W.; Liu, X. Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis. Plants 2025, 14, 1928. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, H.; Cao, Y.; Ma, G.; Zheng, X.; Zhu, H.; Song, X.; Sui, S. Reducing costs and shortening the cetyltrimethylammonium bromide (CTAB) method to improve DNA extraction efficiency from wintersweet and some other plants. Sci. Rep. 2025, 15, 13441. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Samadi, M.; Khanizadeh, P. The effect of different treatments on breaking seed dormancy and stimulating germination in dragonhead (Dracocephalum kotschyi Boiss.). Iran. J. Range Desert Res. 2019, 26, 918–931. [Google Scholar]
- Cheng, J.; Huang, H.; Liu, W.; Zhou, Y.; Han, W.; Wang, X.; Zhang, Y. Unraveling the effects of cold stratification and temperature on the seed germination of invasive Spartina alterniflora across latitude. Front. Plant Sci. 2022, 13, 911804. [Google Scholar] [CrossRef]
- Rawat, B.S.; Khanduri, V.P.; Sharma, C.M. Beneficial effects of cold-moist stratification on seed germination behaviors of Abies pindrow and Picea smithiana. J. For. Res. 2008, 19, 125–130. [Google Scholar] [CrossRef]
- Asl, K.R.; Hosseini, B.; Sharafi, A.; Palazon, J. Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng. Life Sci. 2019, 19, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.; Ghogare, R.; Morton, L.B.; Dhingra, A.; Potlakayala, S.; Rudrabhatla, S.; Dhir, S.K. Evaluation of parameters affecting Agrobacterium-mediated transient gene expression in industrial hemp (Cannabis sativa L.). Plants 2024, 13, 664. [Google Scholar] [CrossRef]
- Yan, H.; Ma, D.; Yi, P.; Sun, G.; Chen, X.; Yi, Y.; Huang, X. Highly efficient Agrobacterium rhizogenes-mediated transformation for functional analysis in woodland strawberry. Plant Methods 2023, 19, 99. [Google Scholar] [CrossRef]
- Dinkeloo, K.; Boyd, S.; Pilot, G. Update on amino acid transporter functions and possible amino acid sensing mechanisms in plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Tian, Y.; Farooq, T.H.; Li, M.; Ma, X.; Wu, P. L-Arginine enhances stress resilience against P deficiency of Chinese fir in root system: Physiological and proteomics analysis. Environ. Exp. Bot. 2024, 225, 105864. [Google Scholar] [CrossRef]
- Hamdeni, I.; Louhaichi, M.; Slim, S.; Boulila, A.; Bettaieb, T. Incorporation of organic growth additives to enhance in vitro tissue culture for producing genetically stable plants. Plants 2022, 11, 3087. [Google Scholar] [CrossRef]
- Wang, M.; Qin, Y.-Y.; Wei, N.-N.; Xue, H.-Y.; Dai, W.-S. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation in citrus seeds and its application in gene functional analysis. Front. Plant Sci. 2023, 14, 1293374. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, Y.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. Soybean hairy roots produced in vitro by Agrobacterium rhizogenes-mediated transformation. Crop J. 2018, 6, 162–171. [Google Scholar] [CrossRef]
- Habibi, P.; De Sa, M.F.G.; Makhzoum, A.; Malik, S.; da Silva, A.L.L.; Hefferon, K.; Soccol, C.R. Bioengineering hairy roots: Phytoremediation, secondary metabolism, molecular pharming, plant-plant interactions and biofuels. In Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2017; pp. 213–251. [Google Scholar]
- Ma, H.; Meng, X.; Xu, K.; Li, M.; Gmitter Jr, F.G.; Liu, N.; Gai, Y.; Huang, S.; Wang, M.; Wang, M. Highly efficient hairy root genetic transformation and applications in citrus. Front. Plant Sci. 2022, 13, 1039094. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Fattahi, M.; Nazemiyeh, H. Optimization of induction and hairy root culture establishment in two mullein species, Verbascum erianthum and Verbascum stachydiforme. Sci. Rep. 2024, 14, 5636. [Google Scholar] [CrossRef]
- Lin, K.; Lu, L.-X.; Pan, B.-Z.; Chai, X.; Fu, Q.-T.; Geng, X.-C.; Mo, Y.; Fei, Y.-C.; Xu, J.-J.; Li, M. Agrobacterium rhizogenes-Mediated Hairy Root Genetic Transformation Using Agrobacterium Gel Inoculation and RUBY Reporter Enables Efficient Gene Function Analysis in Sacha Inchi (Plukenetia volubilis). Int. J. Mol. Sci. 2025, 26, 2496. [Google Scholar] [CrossRef]
- Babaei, M.J.; Ebrahimi, A.; Heidari, P.; Azadvari, E.; Gharanjik, S.; Chaghakaboodi, Z. Titanium dioxide-mediated regulation of enzymatic and non-enzymatic antioxidants, pigments, and diosgenin content promotes cold stress tolerance in Trigonella foenum-graecum L. Sci. Rep. 2025, 15, 1837. [Google Scholar] [CrossRef]
- Lescano, L.; Cziáky, Z.; Custódio, L.; Rodrigues, M.J. Yeast extract elicitation enhances growth and metabolite production in Limonium algarvense callus cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2025, 160, 1–45. [Google Scholar] [CrossRef]
- Khanizadeh, P.; Mumivand, H.; Morshedloo, M.R.; Maggi, F. Application of Fe2O3 nanoparticles improves the growth, antioxidant power, flavonoid content, and essential oil yield and composition of Dracocephalum kotschyi Boiss. Front. Plant Sci. 2024, 15, 1475284. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Adabavazeh, F.; Mosayebi, Z. Titanium dioxide nanoparticles improve element uptake, antioxidant properties, and essential oil productivity of Melissa officinalis L. seedlings under in vitro drought stress. Environ. Sci. Pollut. Res. 2023, 30, 98020–98033. [Google Scholar] [CrossRef] [PubMed]
- Mueangnak, K.; Kitwetcharoen, H.; Thanonkeo, S.; Klanrit, P.; Apiraksakorn, J.; Klanrit, P.; Klanrit, P.; Thanonkeo, P. Enhancing betalains production and antioxidant activity in Celosia argentea cell suspension cultures using biotic and abiotic elicitors. Sci. Rep. 2025, 15, 376. [Google Scholar] [CrossRef]
- Imtiaz, H.; Arif, Y.; Shiraz, M.; Hasan, S.A.; Faizan, M.; Alam, P.; Hayat, S. Effect of SiO2 and TiO2 nanoparticles through foliar and root dipping treatments: A comprehensive comparison of growth, photosynthesis, secondary metabolites, redox status, and enzyme responses in Ocimum sanctum. Discov. Sustain. 2025, 6, 1–29. [Google Scholar] [CrossRef]
- Petrova, M.; Geneva, M.; Trendafilova, A.; Miladinova-Georgieva, K.; Dimitrova, L.; Sichanova, M.; Nikolova, M.; Ivanova, V.; Dimitrova, M.; Sozoniuk, M. Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid. Plants 2025, 14, 967. [Google Scholar] [CrossRef] [PubMed]
- Machanuru, R.; Shrivastava, M.; Singh, R.; Singh, B.; Chakraborty, D.; Ramalingappa, P.L.; Narayan, M. Plant enzymatic activity as an indicator of nano-TiO2 exposure in rice ecosystems. Plant Nano Biol. 2024, 10, 100117. [Google Scholar] [CrossRef]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, J.-P.; Wang, X.-W.; Li, P. Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications. Antioxidants 2025, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hashmi, S.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef]
- Farahi, S.M.M.; Yazdi, M.E.T.; Einafshar, E.; Akhondi, M.; Ebadi, M.; Azimipour, S.; Mahmoodzadeh, H.; Iranbakhsh, A. The effects of titanium dioxide (TiO2) nanoparticles on physiological, biochemical, and antioxidant properties of Vitex plant (Vitex agnus-Castus L). Heliyon 2023, 9, 1–12. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Singh, V.K. Copper oxide nanomaterial induced genotoxicity and mutagenic potential of an Indian major carp, Labeo rohita after in vivo exposure. J. Exp. Zool. India 2023, 26, 1467–1475. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Salehi, H.; Zhang, L.; Alp-Turgut, F.N.; Arikan, B.; Elbasan, F.; Ozfidan-Konakci, C.; Balcı, M.; Zengin, G.; Yildiztugay, E.; Lucini, L. The exogenous application of naringenin and rosmarinic acid modulates functional traits in Lepidium sativum. J. Sci. Food Agric. 2024, 104, 2761–2771. [Google Scholar] [CrossRef]
- Shaw, R.; Kumar, H.; Kapoor, M. Recent Studies on the Effect of TiO2-NPS on Marine Bivalves: Unveiling Potential Threats and Ecotoxicological Implications. Int. J. Marit. Eng. 2024, 1, 419–426. [Google Scholar]
- Xu, W.; Shu, M.; Yuan, C.; Dumat, C.; Zhang, J.; Zhang, H.; Xiong, T. Lettuce (Lactuca sativa L.) alters its metabolite accumulation to cope with CuO nanoparticles by promoting antioxidant production and carbon metabolism. Environ. Geochem. Health 2024, 46, 371. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Qureshi, M.I.; Alam, T.; Abdin, M. Protocol for isolation of genomic DNA from dry and fresh roots of medicinal plants suitable for RAPD and restriction digestion. Afr. J. Biotechnol. 2007, 6, 175. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Pagariya, M.C.; Devarumath, R.M.; Kawar, P.G. Biochemical characterization and identification of differentially expressed candidate genes in salt-stressed sugarcane. Plant Sci. 2012, 184, 1–13. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Glick, D.; Maehly, A. Assay of catalases and peroxidases. Methods Biochem. Anal 1955, 10, 9780470110171. [Google Scholar]
- Ranieri, A.; Castagna, A.; Baldan, B.; Soldatini, G.F. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef]
- Fattahi, S.; Ardekani, A.M.; Zabihi, E.; Abedian, Z.; Mostafazadeh, A.; Pourbagher, R.; Akhavan-Niaki, H. Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 5317–5323. [Google Scholar] [CrossRef]
- Vieira, T.O.; Seifriz, I.; Charão, C.C.; Oliveira, S.Q.d.; Creczynski-Pasa, T.B. Antioxidant effects of crude extracts from Baccharis species: Inhibition of myeloperoxidase activity, protection against lipid peroxidation, and action as oxidative species scavenger. Rev. Bras. Farmacogn. 2011, 21, 601–607. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]





| Gene | Primer ID | Sequence (5′ → 3′) | Primer Length (nt) | Expected Amplicon Size (bp) |
|---|---|---|---|---|
| robB | RolB F | 5′-GTTCTCGCGAGAAGATGCA 3′ | 20 bp | bp 780 |
| RolB R | 5′-CAGTTTCGCATCTTGACAG-3′ | 20 bp | ||
| virD | virD F | 5′-ATGCCCGATCGAGCTCAAGT-3′ | 20 bp | bp 338 |
| virD R | 5′-CCTGACCCAAACATCTCGGCT-3′ | 20 bp |
| Elicitor | Applied Concentration | Exposure Time (h) |
|---|---|---|
| Yeast extract | 200 mg L−1 | 24, 48 |
| TiO2 | 200 mg L−1; 400 mg L−1 | 24, 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, H.S.; Sabokdast, M.; Dedicova, B. Influence of Biotic and Abiotic Elicitors on Rosmarinic Acid Accumulation in Hairy Root Cultures of Dracocephalum kotschyi Boiss. Plants 2025, 14, 2809. https://doi.org/10.3390/plants14172809
Kiani HS, Sabokdast M, Dedicova B. Influence of Biotic and Abiotic Elicitors on Rosmarinic Acid Accumulation in Hairy Root Cultures of Dracocephalum kotschyi Boiss. Plants. 2025; 14(17):2809. https://doi.org/10.3390/plants14172809
Chicago/Turabian StyleKiani, Hoda Sadat, Manijeh Sabokdast, and Beata Dedicova. 2025. "Influence of Biotic and Abiotic Elicitors on Rosmarinic Acid Accumulation in Hairy Root Cultures of Dracocephalum kotschyi Boiss." Plants 14, no. 17: 2809. https://doi.org/10.3390/plants14172809
APA StyleKiani, H. S., Sabokdast, M., & Dedicova, B. (2025). Influence of Biotic and Abiotic Elicitors on Rosmarinic Acid Accumulation in Hairy Root Cultures of Dracocephalum kotschyi Boiss. Plants, 14(17), 2809. https://doi.org/10.3390/plants14172809






