Exogenous Application of ENOD40 and CEP1 Peptides Boosts Symbiotic Signaling Gene Expression and Productivity in Common Bean
Abstract
1. Introduction
2. Results
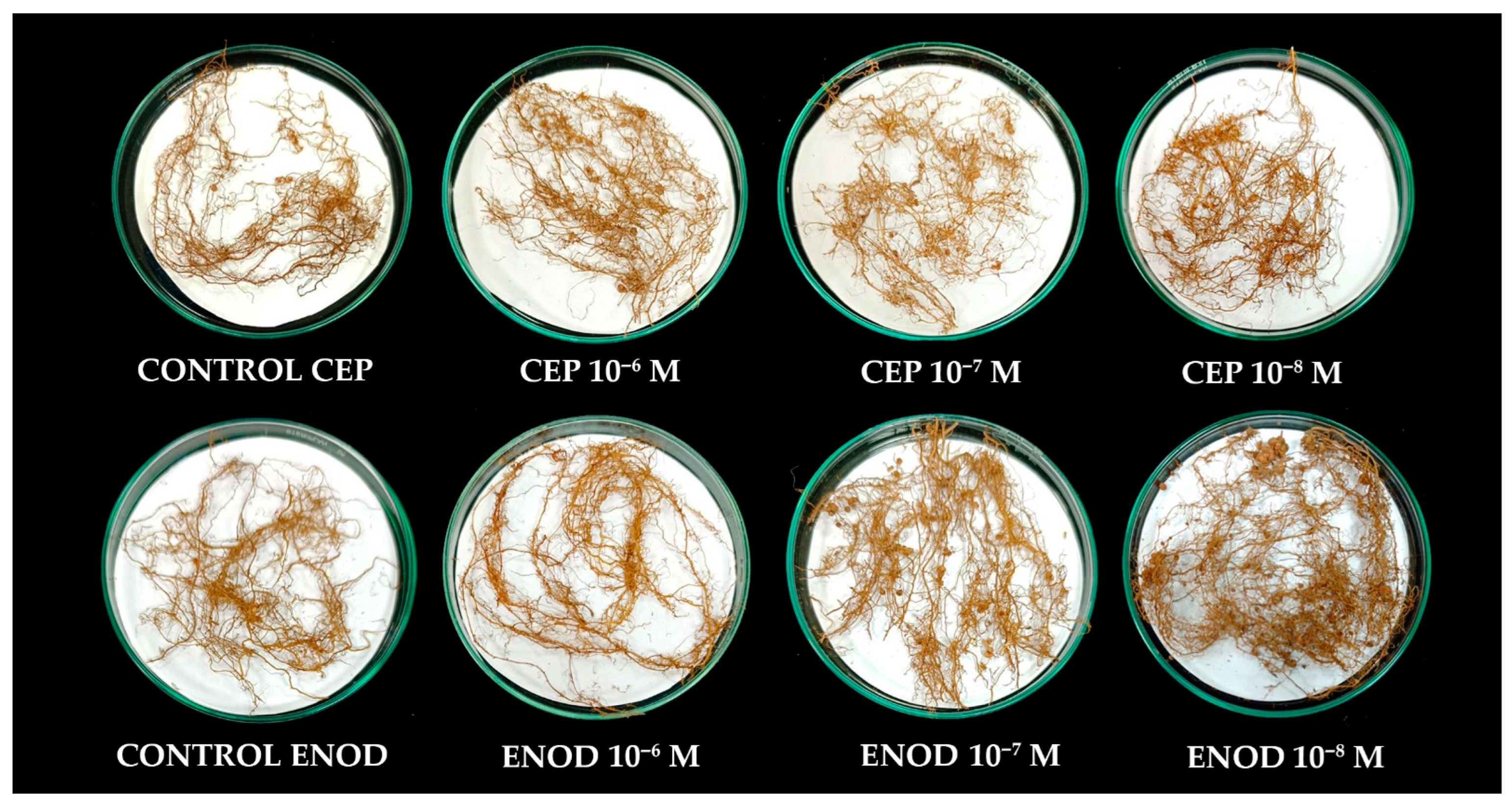
2.1. Exogenous Application of ENOD40 and CEP1 Peptides Activates Symbiotic Signaling Genes in Common Bean Roots
2.2. Peptide Application Suppresses Jasmonate-Associated Defense Gene Expression Without Strongly Affecting Salicylate Pathways
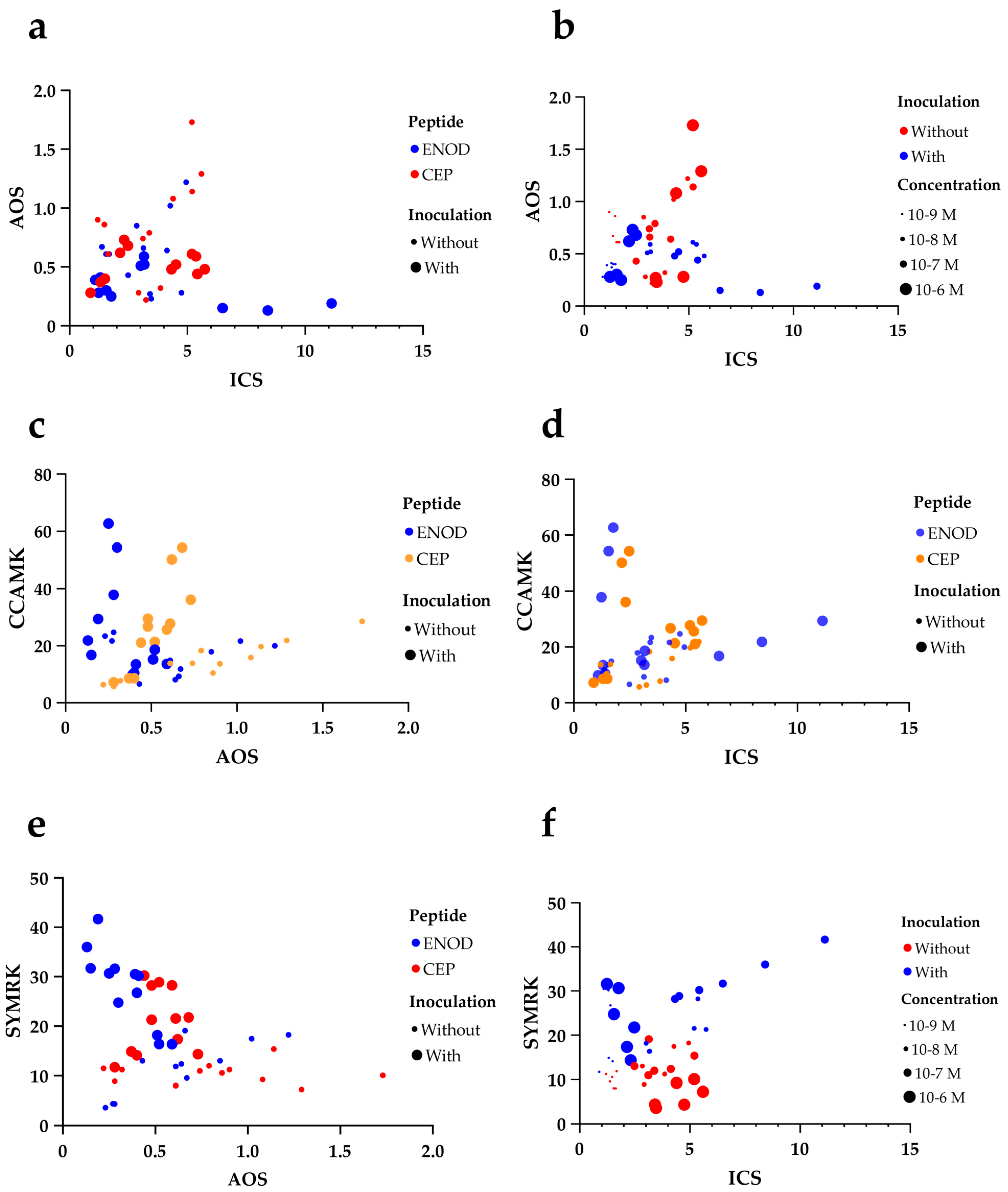
2.3. Interaction Between Peptide Type, Concentration, and Inoculation Reveals Synergistic Activation of Symbiotic Genes
2.4. Correlation and Multivariate Analysis Reveal Co-Regulated Modules Among Symbiotic and Defense Genes
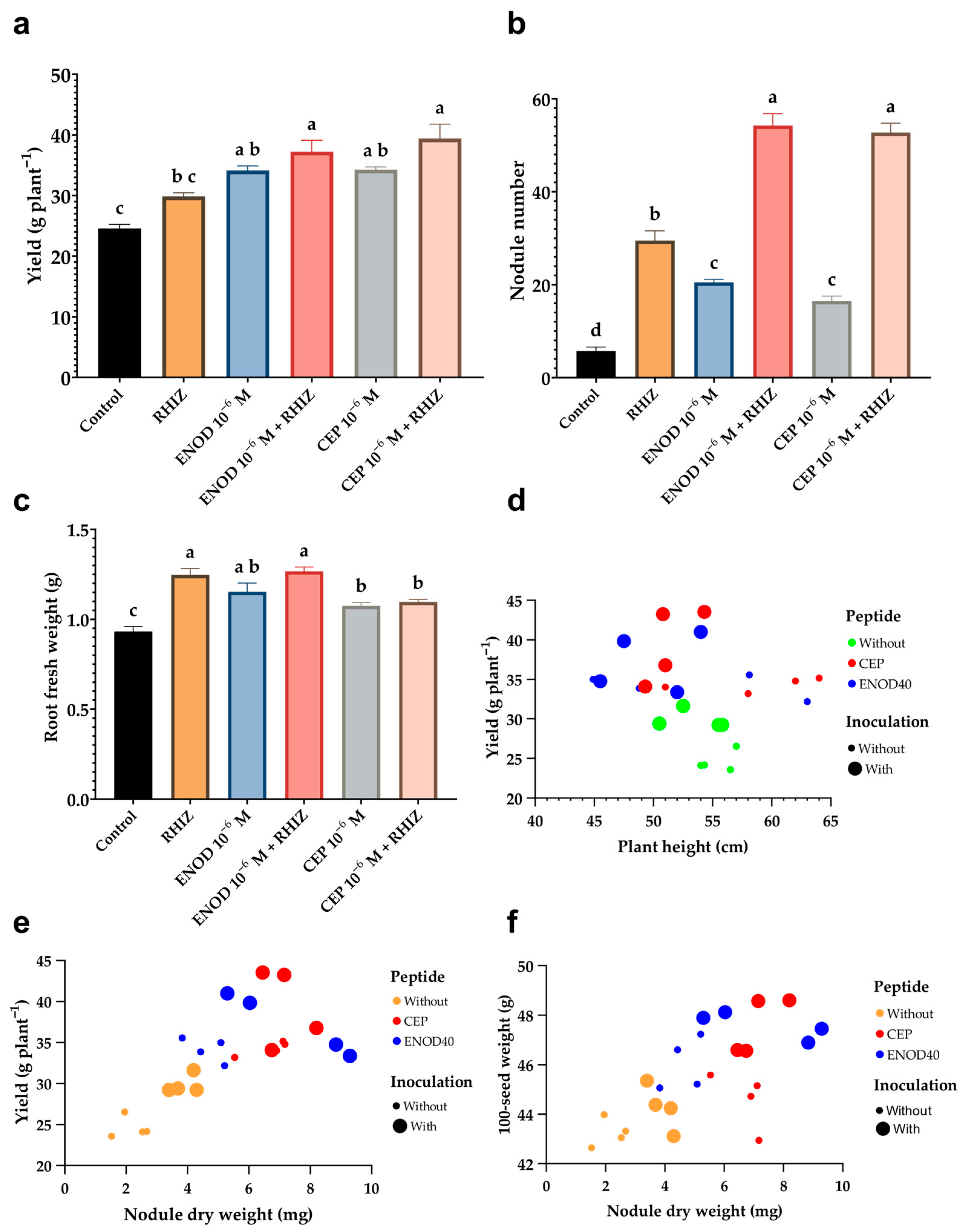
2.5. Foliar Application of Synthetic Peptides Enhances Biomass, Nodulation, and Yield Components Under Greenhouse Conditions
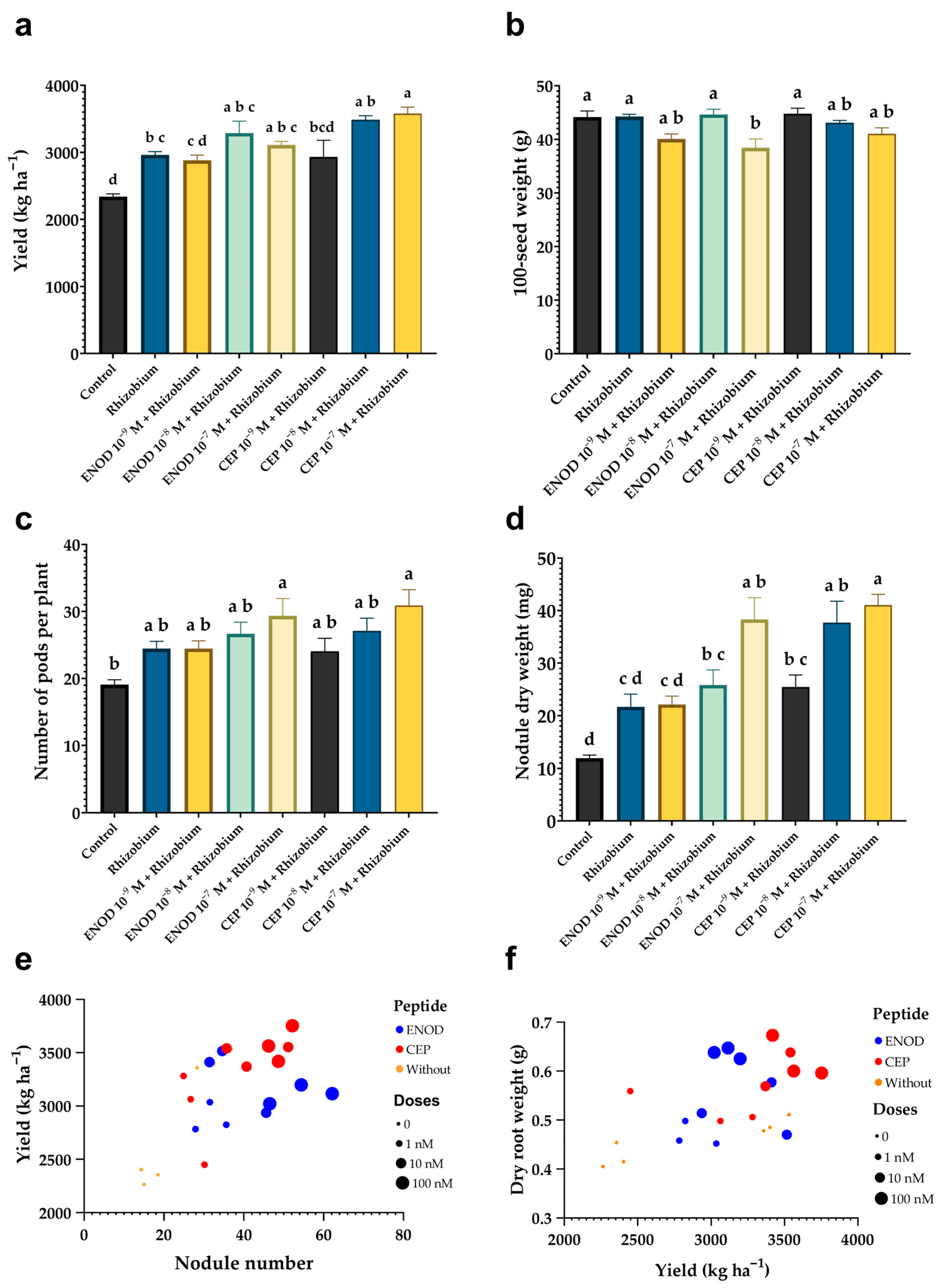
2.6. Field Application of ENOD40 and CEP1 Improves Nodulation and Increases Grain Yield in Common Bean
3. Discussion
3.1. Synthetic Peptides as Modulators of Symbiotic Gene Expression in Common Bean
3.2. Modulation of Defense-Related Gene Expression by Peptide Treatments
3.3. Interactions Between Peptide Type, Concentration, and Rhizobial Inoculation
3.4. Greenhouse Evaluation: Morphological, Symbiotic, and Agronomic Responses to Peptide Treatments
3.5. Field Evaluation: Morphological, Symbiotic, and Agronomic Responses to Peptide Treatments
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Peptide Hormones and Treatments
4.3. Sample Collection and RNA Extraction
4.4. cDNA Synthesis and Quantitative Real-Time PCR
4.5. Greenhouse Assay
4.6. Field Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Wang, T.; Balla, B.; Kovács, S.; Kereszt, A. Varietas Delectat: Exploring Natural Variations in Nitrogen-Fixing Symbiosis Research. Front. Plant Sci. 2022, 13, 856187. [Google Scholar] [CrossRef] [PubMed]
- Flores-Duarte, N.J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Pajuelo, E.; Rodriguez-Llorente, I.D.; Navarro-Torre, S. Role of Nodulation-Enhancing Rhizobacteria in the Promotion of Medicago Sativa Development in Nutrient-Poor Soils. Plants 2022, 11, 1164. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Albrecht, C.; Geurts, R.; Bisseling, T. Legume Nodulation and Mycorrhizae Formation; Two Extremes in Host Specificity Meet. EMBO J. 1999, 18, 281. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ané, J. Plant Hormones and Initiation of Legume Nodulation and Arbuscular Mycorrhization. In Ecological Aspects of Nitrogen Metabolism in Plants; Wiley: Hoboken, NJ, USA, 2011; pp. 354–396. [Google Scholar]
- Mohanta, T.K.; Bae, H. Functional Genomics and Signaling Events in Mycorrhizal Symbiosis. J. Plant Interact. 2015, 10, 21–40. [Google Scholar] [CrossRef]
- Rathna Priya, T.S.; Manickavasagan, A. Common Bean. In Pulses; Springer International Publishing: Cham, Switzerland, 2020; pp. 77–97. [Google Scholar]
- Blair, M.W.; Soler, A.; Cortés, A.J. Diversification and Population Structure in Common Beans (Phaseolus vulgaris L.). PLoS ONE 2012, 7, 49488. [Google Scholar] [CrossRef]
- Razafintsalama, H.; Trap, J.; Rabary, B.; Razakatiana, A.T.E.; Ramanankierana, H.; Rabeharisoa, L.; Becquer, T. Effect of Rhizobium Inoculation on Growth of Common Bean in Low-Fertility Tropical Soil Amended with Phosphorus and Lime. Sustainability 2022, 14, 4907. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic Practices to Increase the Yield and Quality of Common Bean (Phaseolus vulgaris L.): A Systematic Review. Agronomy 2022, 12, 271. [Google Scholar] [CrossRef]
- Horácio, E.H.; Zambrano Gavilanes, F.E.; Feliciano, M.V.; de Moraes, J.G.; Zucareli, C.; Andrade, D.S.; Maddela, N.R.; Prasad, R. Exploring the Interaction Effects between Common Bean Cultivars and Rhizobia Inoculation on Plant Growth and Yield. J. Agric. Food Res. 2024, 15, 100926. [Google Scholar] [CrossRef]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-Inoculation Effect of Rhizobia and Plant Growth Promoting Rhizobacteria on Common Bean Growth in a Low Phosphorus Soil. Front. Plant Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, G.J.; Stougaard, J. Root Nodulation: A Paradigm for How Plant-Microbe Symbiosis Influences Host Developmental Pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef]
- De Bruijn, F.J. The Common Symbiotic Signaling Pathway (CSSP or SYM). In The Model Legume Medicago truncatula; Wiley: New York, NY, USA, 2020. [Google Scholar]
- Kumar, N.; Srivastava, P.; Vishwakarma, K.; Kumar, R.; Kuppala, H.; Maheshwari, S.K.; Vats, S. The Rhizobium–Plant Symbiosis: State of the Art. In Plant Microbe Symbiosis; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–20. ISBN 978-3-030-36248-5. [Google Scholar]
- Radhakrishnan, G.V.; Keller, J.; Rich, M.K.; Vernié, T.; Mbadinga Mbadinga, D.L.; Vigneron, N.; Cottret, L.; Clemente, H.S.; Libourel, C.; Cheema, J.; et al. An Ancestral Signalling Pathway Is Conserved in Intracellular Symbioses-Forming Plant Lineages. Nat. Plants USA 2020, 6, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Gherbi, H.; Markmann, K.; Svistoonoff, S.; Estevan, J.; Autran, D.; Giczey, G.; Auguy, F.; Péret, B.; Laplaze, L.; Franche, C.; et al. SymRK Defines a Common Genetic Basis for Plant Root Endosymbioses with Arbuscular Mycorrhiza Fungi, Rhizobia, and Frankia Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4928–4932. [Google Scholar] [CrossRef]
- Holsters, M. SYMRK, an Enigmatic Receptor Guarding and Guiding Microbial Endosymbioses with Plant Roots. Proc. Natl. Acad. Sci. USA 2008, 105, 4537–4538. [Google Scholar] [CrossRef]
- Gong, X.; Jensen, E.; Bucerius, S.; Parniske, M. A CCaMK/Cyclops Response Element in the Promoter of Lotus Japonicus Calcium-Binding Protein 1 (CBP1) Mediates Transcriptional Activation in Root Symbioses. New Phytol. 2022, 235, 1196–1211. [Google Scholar] [CrossRef]
- Limpens, E.; Bisseling, T. CYCLOPS: A New Vision on Rhizobium-Induced Nodule Organogenesis. Cell Host Microbe 2014, 15, 127–129. [Google Scholar] [CrossRef]
- Capoen, W.; Oldroyd, G. How CYCLOPS Keeps an Eye on Plant Symbiosis. Proc. Natl. Acad. Sci. USA 2008, 105, 20053–20054. [Google Scholar] [CrossRef]
- Murray, J.D.; Muni, R.R.D.; Torres-Jerez, I.; Tang, Y.; Allen, S.; Andriankaja, M.; Li, G.; Laxmi, A.; Cheng, X.; Wen, J.; et al. Vapyrin, a Gene Essential for Intracellular Progression of Arbuscular Mycorrhizal Symbiosis, Is Also Essential for Infection by Rhizobia in the Nodule Symbiosis of Medicago Truncatula. Plant J. 2011, 65, 244–252. [Google Scholar] [CrossRef]
- Shumilina, J.; Soboleva, A.; Abakumov, E.; Shtark, O.Y.; Zhukov, V.A.; Frolov, A. Signaling in Legume–Rhizobia Symbiosis. Int. J. Mol. Sci. 2023, 24, 17397. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwaran, M.; Volkening, J.D.; Sussman, M.R.; Ané, J.M. Symbiosis and the Social Network of Higher Plants. Curr. Opin. Plant Biol. 2013, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Uggerhøj Andersen, S.; Ratet, P. Molecular and Cellular Mechanisms of the Legume-Rhizobia Symbiosis. Front. Plant Sci. 2018, 9, 1839. [Google Scholar] [CrossRef] [PubMed]
- Favery, B.; Quentin, M.; Abad, P. Compatible Plant-Root Knot Nematode Interaction and Parallels with Symbiosis. In Signaling and Communication in Plant Symbiosis; Springer: Berlin/Heidelberg, Germany, 2012; pp. 239–257. [Google Scholar]
- Ghorbani, S.; Fernandez, A.; Hilson, P.; Beeckman, T. Signaling Peptides in Plants. Cell Dev. Biol. 2014, 3, 1000141. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Shi, K. Plant Peptides in Plant Defense Responses. Plant Signal Behav. 2018, 13, e1475175. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, W.; Li, W.; Zhang, Y.; Hajný, J.; Han, H. Small Signaling Peptides Mediate Plant Adaptions to Abiotic Environmental Stress. Planta 2022, 255, 72. [Google Scholar] [CrossRef]
- Schaller, A. Bioactive Peptides as Signal Molecules in Plant Defense, Growth, and Development. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2001; Volume 25, pp. 367–411. [Google Scholar]
- Takahashi, F.; Hanada, K.; Kondo, T.; Shinozaki, K. Hormone-like Peptides and Small Coding Genes in Plant Stress Signaling and Development. Curr. Opin. Plant Biol. 2019, 51, 88–95. [Google Scholar] [CrossRef]
- Wan, X.; Hontelez, J.; Lillo, A.; Guarnerio, C.; Van De Peut, D.; Fedorova, E.; Bisseling, T.; Franssen, H. Medicago Truncatula ENOD40-1 and ENOD40-2 Are Both Involved in Nodule Initiation and Bacteroid Development. J. Exp. Bot. 2007, 58, 2033–2041. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Li, F.; Lu, Y.; Fang, Z.; Fu, M.; Mysore, K.S.; Wen, J.; Gong, J.; Murray, J.D.; et al. The Small Peptide CEP1 and the NIN-like Protein NLP1 Regulate NRT2.1 to Mediate Root Nodule Formation across Nitrate Concentrations. Plant Cell 2023, 35, 776–794. [Google Scholar] [CrossRef]
- Van de Sande, K.; Pawlowski, K.; Czaja, I.; Wieneke, U.; Schell, J.; Schmidt, J.; Walden, R.; Matvienko, M.; Wellink, J.; Van Kammen, A.; et al. Modification of Phytohormone Response by a Peptide Encoded by ENOD40 of Legumes and a Nonlegume. Science 1996, 273, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hirsch, A.M. Studying Early Nodulin Gene ENOD40 Expression and Induction by Nodulation Factor and Cytokinin in Transgenic Alfalfa1. Plant Physiol. 1998, 116, 53–68. [Google Scholar] [CrossRef]
- Kumagai, H.; Kinoshita, E.; Ridge, R.W.; Kouchi, H. RNAi Knock-Down of ENOD40 s Leads to Significant Suppression of Nodule Formation in Lotus Japonicus. Plant Cell Physiol. 2006, 47, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Taleski, M.; Imin, N.; Djordjevic, M.A. CEP Peptide Hormones: Key Players in Orchestrating Nitrogen-Demand Signalling, Root Nodulation, and Lateral Root Development. J. Exp. Bot. 2018, 69, 1829–1836. [Google Scholar] [CrossRef]
- Chapman, K.; Ivanovici, A.; Taleski, M.; Sturrock, C.J.; Ng, J.L.P.; Mohd-Radzman, N.A.; Frugier, F.; Bennett, M.J.; Mathesius, U.; Djordjevic, M.A. CEP Receptor Signalling Controls Root System Architecture in Arabidopsis and Medicago. New Phytol. 2020, 226, 1809–1821. [Google Scholar] [CrossRef]
- Roy, S.; Griffiths, M.; Torres-Jerez, I.; Sanchez, B.; Antonelli, E.; Jain, D.; Krom, N.; Zhang, S.; York, L.M.; Scheible, W.R.; et al. Application of Synthetic Peptide CEP1 Increases Nutrient Uptake Rates Along Plant Roots. Front. Plant Sci. 2022, 12, 793145. [Google Scholar] [CrossRef]
- Laffont, C.; Frugier, F. Rhizobium Symbiotic Efficiency Meets CEP Signaling Peptides. New Phytol. 2024, 241, 24–27. [Google Scholar] [CrossRef]
- Costa, S.R.; Ng, J.L.P.; Mathesius, U. Interaction of Symbiotic Rhizobia and Parasitic Root-Knot Nematodes in Legume Roots: From Molecular Regulation to Field Application. Mol. Plant-Microbe Interact. 2021, 34, 470–490. [Google Scholar] [CrossRef]
- Djordjevic, M.A.; Mohd-Radzman, N.A.; Imin, N. Small-Peptide Signals That Control Root Nodule Number, Development, and Symbiosis. J. Exp. Bot. 2015, 66, 5171–5181. [Google Scholar] [CrossRef]
- Valmas, M.I.; Sexauer, M.; Markmann, K.; Tsikou, D. Plants Recruit Peptides and Micro RNAs to Regulate Nutrient Acquisition from Soil and Symbiosis. Plants 2023, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Pichon, M.; Journet, E.P.; Dedieu, A.; de Billy, F.; Truchet, G.; Barker, D.G. Rhizobium Meliloti Elicits Transient Expression of the Early Nodulin Gene ENOD12 in the Differentiating Root Epidermis of Transgenic Alfalfa. Plant Cell 1992, 4, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Crespi, M.D.; Jurkevitch, E.; Poiret, M.; d’Aubenton-Carafa, Y.; Petrovics, G.; Kondorosi, E.; Kondorosi, A. Enod40, a Gene Expressed during Nodule Organogenesis, Codes for a Non-Translatable RNA Involved in Plant Growth. EMBO J. 1994, 13, 5099–5112. [Google Scholar] [CrossRef]
- Sinvany, G.; Kapulnik, Y.; Wininger, S.; Badani, H.; Jurkevitch, E. The Early Nodulin Enod40 Is Induced by; and Also Promotes Arbuscular Mycorrhizal Root Colonization. Physiol. Mol. Plant Pathol. 2002, 60, 103–109. [Google Scholar] [CrossRef]
- Kouchi, H.; Hata, S. Isolation and Characterization of Novel Nodulin CDNAs Representing Genes Expressed at Early Stages of Soybean Nodule Development. Mol. Gen. Genet. 1993, 238, 106–119. [Google Scholar] [CrossRef]
- Yang, W.; Katinakis, P.; Hendriks, P.; Smolders, A.; de Vries, F.; Spee, J.; van Kammen, A.; Bisseling, T.; Franssen, H. Characterization of GmENOD40, a Gene Showing Novel Patterns of Cell-specific Expression during Soybean Nodule Development. Plant J. 1993, 3, 573–585. [Google Scholar] [CrossRef]
- Staehelin, C.; Charon, C.; Boller, T.; Crespi, M.; Kondorosi, Á. Medicago Truncatula Plants Overexpressing the Early Nodulin Gene ENOD40 Exhibit Accelerated Mycorrhizal Colonization and Enhanced Formation of Arbuscules. Proc. Natl. Acad. Sci. USA 2001, 98, 15366–15371. [Google Scholar] [CrossRef]
- Röhrig, H.; Schmidt, J.; Miklashevichs, E.; Schell, J.; John, M. Soybean ENOD40 Encodes Two Peptides That Bind to Sucrose Synthase. Proc. Natl. Acad. Sci. USA 2002, 99, 1915–1920. [Google Scholar] [CrossRef]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The Peptide-Encoding CEP1 Gene Modulates Lateral Root and Nodule Numbers in Medicago Truncatula. J. Exp. Bot. 2013, 64, 5395–5409. [Google Scholar] [CrossRef]
- Laffont, C.; Ivanovici, A.; Gautrat, P.; Brault, M.; Djordjevic, M.A.; Frugier, F. The NIN Transcription Factor Coordinates CEP and CLE Signaling Peptides That Regulate Nodulation Antagonistically. Nat. Commun. 2020, 11, 3167. [Google Scholar] [CrossRef]
- Kereszt, A.; Mergaert, P.; Montiel, J.; Endre, G.; Kondorosi, É. Impact of Plant Peptides on Symbiotic Nodule Development and Functioning. Front. Plant Sci. 2018, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Batut, J.; Mergaert, P.; Masson-Boivin, C. Peptide Signalling in the Rhizobium–Legume Symbiosis. Curr. Opin. Microbiol. 2011, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Yeun, L.H.; Domonkos, Á.; Halász, G.; Gobbato, E.; Ayaydin, F.; Miró, K.; Hirsch, S.; Sun, J.; Tadege, M.; et al. Medicago Truncatula IPD3 Is a Member of the Common Symbiotic Signaling Pathway Required for Rhizobial and Mycorrhizal Symbioses. Mol. Plant-Microbe Interact. 2011, 24, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Miller, J.B.; Granqvist, E.; Wiley-Kalil, A.; Gobbato, E.; Maillet, F.; Cottaz, S.; Samain, E.; Venkateshwaran, M.; Fort, S.; et al. Activation of Symbiosis Signaling by Arbuscular Mycorrhizal Fungi in Legumes and Rice. Plant Cell 2015, 27, 823–838. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal Symbiosis Modulates the Rhizosphere Microbiota to Promote Rhizobia–Legume Symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef]
- Mulder, L.; Hogg, B.; Bersoult, A.; Cullimore, J.V. Integration of Signalling Pathways in the Establishment of the Legume-rhizobia Symbiosis. Physiol. Plant 2005, 123, 207–218. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, Friend, and Enter: Signalling Systems That Promote Beneficial Symbiotic Associations in Plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Stracke, S.; Kistner, C.; Yoshida, S.; Mulder, L.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; Stougaard, J.; Szczyglowski, K.; et al. A Plant Receptor-like Kinase Required for Both Bacterial and Fungal Symbiosis. Nature 2002, 417, 959–962. [Google Scholar] [CrossRef]
- Dávila-Delgado, R.; Flores-Canúl, K.; Juárez-Verdayes, M.A.; Sánchez-López, R. Rhizobia Induce SYMRK Endocytosis in Phaseolus vulgaris Root Hair Cells. Planta 2023, 257, 83. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Singh, S.; Parniske, M. Activation of Calcium- and Calmodulin-Dependent Protein Kinase (CCaMK), the Central Regulator of Plant Root Endosymbiosis. Curr. Opin. Plant Biol. 2012, 15, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Pimprikar, P.; Carbonnel, S.; Paries, M.; Katzer, K.; Klingl, V.; Bohmer, M.J.; Karl, L.; Floss, D.S.; Harrison, M.J.; Parniske, M.; et al. A CCaMK-CYCLOPS-DELLA Complex Activates Transcription of RAM1 to Regulate Arbuscule Branching. Curr. Biol. 2016, 26, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Cerri, M.R.; Wang, Q.; Stolz, P.; Folgmann, J.; Frances, L.; Katzer, K.; Li, X.; Heckmann, A.B.; Wang, T.L.; Downie, J.A.; et al. The ERN1 Transcription Factor Gene Is a Target of the CCaMK/CYCLOPS Complex and Controls Rhizobial Infection in Lotus Japonicus. New Phytol. 2017, 215, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Soyano, T.; Hayashi, M. Transcriptional Networks Leading to Symbiotic Nodule Organogenesis. Curr. Opin. Plant Biol. 2014, 20, 146–154. [Google Scholar] [CrossRef]
- Rose, C.M.; Venkateshwaran, M.; Volkening, J.D.; Grimsrud, P.A.; Maeda, J.; Bailey, D.J.; Park, K.; Howes-Podoll, M.; den Os, D.; Yeun, L.H.; et al. Rapid Phosphoproteomic and Transcriptomic Changes in the Rhizobia-Legume Symbiosis. Mol. Cell. Proteom. 2012, 11, 724–744. [Google Scholar] [CrossRef]
- Singh, S.; Katzer, K.; Lambert, J.; Cerri, M.; Parniske, M. CYCLOPS, A DNA-Binding Transcriptional Activator, Orchestrates Symbiotic Root Nodule Development. Cell Host Microbe 2014, 15, 139–152. [Google Scholar] [CrossRef]
- Pumplin, N.; Mondo, S.J.; Topp, S.; Starker, C.G.; Gantt, J.S.; Harrison, M.J. Medicago Truncatula Vapyrin Is a Novel Protein Required for Arbuscular Mycorrhizal Symbiosis. Plant J. 2010, 61, 482–494. [Google Scholar] [CrossRef]
- Bapaume, L.; Laukamm, S.; Darbon, G.; Monney, C.; Meyenhofer, F.; Feddermann, N.; Chen, M.; Reinhardt, D. VAPYRIN Marks an Endosomal Trafficking Compartment Involved in Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Feddermann, N.; Duvvuru Muni, R.R.; Zeier, T.; Stuurman, J.; Ercolin, F.; Schorderet, M.; Reinhardt, D. The PAM1 Gene of Petunia, Required for Intracellular Accommodation and Morphogenesis of Arbuscular Mycorrhizal Fungi, Encodes a Homologue of VAPYRIN. Plant J. 2010, 64, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Li, J.; Liu, P.; Wang, Y.; Cao, N.; Fang, X.; Wang, T.; Dong, J. The Jasmonate Pathway Promotes Nodule Symbiosis and Suppresses Host Plant Defense in Medicago Truncatula. Mol. Plant 2024, 17, 1183–1203. [Google Scholar] [CrossRef] [PubMed]
- Samac, D.A.; Graham, M.A. Recent Advances in Legume-Microbe Interactions: Recognition, Defense Response, and Symbiosis from a Genomic Perspective. Plant Physiol. 2007, 144, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Antolín-Llovera, M.; Petutsching, E.K.; Ried, M.K.; Lipka, V.; Nürnberger, T.; Robatzek, S.; Parniske, M. Knowing Your Friends and Foes—Plant Receptor-like Kinases as Initiators of Symbiosis or Defence. New Phytol. 2014, 204, 791–802. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E. Hormone Modulation of Legume-rhizobial Symbiosis. J. Integr. Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kawaguchi, M. Shoot-Applied MeJA Suppresses Root Nodulation in Lotus Japonicus. Plant Cell Physiol. 2006, 47, 176–180. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, H.; Choi, D.; Hwang, I. Plant Hormonal Regulation of Nitrogen-Fixing Nodule Organogenesis. Mol. Cells 2012, 34, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cardoza, V.; Mitchell, D.M.; Bright, L.; Oldroyd, G.; Harris, J.M. Crosstalk between Jasmonic Acid, Ethylene and Nod Factor Signaling Allows Integration of Diverse Inputs for Regulation of Nodulation. Plant J. 2006, 46, 961–970. [Google Scholar] [CrossRef]
- Benjamin, G.; Pandharikar, G.; Frendo, P. Salicylic Acid in Plant Symbioses: Beyond Plant Pathogen Interactions. Biology 2022, 11, 861. [Google Scholar] [CrossRef]
- Lian, B.; Zhou, X.; Miransari, M.; Smith, D.L. Effects of Salicylic Acid on the Development and Root Nodulation of Soybean Seedlings. J. Agron. Crop Sci. 2000, 185, 187–192. [Google Scholar] [CrossRef]
- Delay, C.; Imin, N.; Djordjevic, M.A. CEP Genes Regulate Root and Shoot Development in Response to Environmental Cues and Are Specific to Seed Plants. J. Exp. Bot. 2013, 64, 5383–5394. [Google Scholar] [CrossRef]
- Basile, L.A.; Lepek, V.C. Legume–Rhizobium Dance: An Agricultural Tool That Could Be Improved? Microb. Biotechnol. 2021, 14, 1897–1917. [Google Scholar] [CrossRef] [PubMed]
- Hardarson, G. Methods for Enhancing Symbiotic Nitrogen Fixation. Plant Soil 1993, 152, 1–17. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root Exudates as Mediators of Mineral Acquisition in Low-Nutrient Environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Rodiño, A.P.; De La Fuente, M.; De Ron, A.M.; Lema, M.J.; Drevon, J.J.; Santalla, M. Variation for Nodulation and Plant Yield of Common Bean Genotypes and Environmental Effects on the Genotype Expression. Plant Soil 2011, 346, 349–361. [Google Scholar] [CrossRef]
- Kipe-Nolt, J.A.; Giller, K.E. A Field Evaluation Using the 15N Isotope Dilution Method of Lines of Phaseolus vulgaris L. Bred for Increased Nitrogen Fixation. In Enhancement of Biological Nitrogen Fixation of Common Bean in Latin America; Springer: Dordrecht, The Netherlands, 1993; pp. 107–114. [Google Scholar]
- Nova-Franco, B.; Íñiguez, L.P.; Valdés-López, O.; Alvarado-Affantranger, X.; Leija, A.; Fuentes, S.I.; Ramírez, M.; Paul, S.; Reyes, J.L.; Girard, L.; et al. The Micro-RNA172c-APETALA2-1 Node as a Key Regulator of the Common Bean-Rhizobium etli Nitrogen Fixation Symbiosis. Plant Physiol. 2015, 168, 273–291. [Google Scholar] [CrossRef]
- Oladzad, A.; González, A.; Macchiavelli, R.; de Jensen, C.E.; Beaver, J.; Porch, T.; McClean, P. Genetic Factors Associated with Nodulation and Nitrogen Derived from Atmosphere in a Middle American Common Bean Panel. Front. Plant Sci. 2020, 11, 576078. [Google Scholar] [CrossRef]
- Graham, P.H.; Rosas, J.C. Growth and Development of Indeterminate Bush and Climbing Cultivars of Phaseolus vulgaris L. Inoculated with Rhizobium. J. Agric. Sci. 1977, 88, 503–508. [Google Scholar] [CrossRef]
- Vargas, M.A.T.; Mendes, I.C.; Hungria, M. Response of Field-Grown Bean (Phaseolus vulgaris L.) to Rhizobium Inoculation and Nitrogen Fertilization in Two Cerrados Soils. Biol. Fertil. Soils 2000, 32, 228–233. [Google Scholar] [CrossRef]
- Subramanian, S.; Stacey, G.; Yu, O. Distinct, Crucial Roles of Flavonoids during Legume Nodulation. Trends Plant Sci. 2007, 12, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Radzman, N.A.; Binos, S.; Truong, T.T.; Imin, N.; Mariani, M.; Djordjevic, M.A. Novel MtCEP1 Peptides Produced in Vivo Differentially Regulate Root Development in Medicago Truncatula. J. Exp. Bot. 2015, 66, 5289–5300. [Google Scholar] [CrossRef]
- Cantaro-Segura, H.; Huaringa-Joaquín, A.; Zúñiga-Dávila, D. Symbiotic Effectiveness of Two Rhizobium Sp. Strains in Four Bean (Phaseolus vulgaris L.) Varieties in Peru. Idesia 2019, 37, 73–81. [Google Scholar] [CrossRef]
- Ogilvie, H.A.; Imin, N.; Djordjevic, M.A. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) Gene Family in Angiosperms, and Evolution of Plant-Family Specific CEP Genes. BMC Genom. 2014, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J. Rhizobia and Legume Nodulation Genes. In Brenner’s Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 236–239. [Google Scholar]
- Broughton, W.J.; Dilworth, M.J. Control of Leghaemoglobin Synthesis in Snake Beans. Biochem. J. 1971, 125, 1075. [Google Scholar] [CrossRef]
- De Almeida Leite, R.; Martins, L.C.; Ferreira, L.V.d.S.F.; Barbosa, E.S.; Alves, B.J.R.; Zilli, J.E.; Araújo, A.P.; Jesus, E.D.C. Co-Inoculation of Rhizobium and Bradyrhizobium Promotes Growth and Yield of Common Beans. Appl. Soil. Ecol. 2022, 172, 104356. [Google Scholar] [CrossRef]


| Treatments | Plant Height | Fresh Foliage Weight (g) | Fresh Root Weight (g) | Fresh Nodule Weight (mg) | Dry Nodule Weight (mg) | Dry Foliage Weight (g) | Dry Root Weight (g) | Nodule Number | Number of Pods per Plant | Number of Seeds per Pod | 100-Seed Weight (g) | Pod Length (cm) | Pod Width (cm) | Yield (g/Plant) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 55.45 | a | 47.81 | c | 7.718 | c | 14.45 | d | 2.168 | d | 6.655 | c | 0.932 | c | 5.75 | d | 13.41 | b | 4.24 | c | 43.25 | c | 11.52 | a | 1.105 | bc | 24.58 | c |
| +Rhizobium | 53.58 | a | 59.16 | a | 14.02 | b | 25.94 | cd | 3.892 | cd | 8.576 | a | 1.246 | a | 29.5 | b | 15.54 | ab | 4.348 | bc | 44.27 | bc | 11.29 | a | 1.088 | c | 29.86 | bc |
| ENOD 10−6 M | 53.7 | a | 55.1 | abc | 17.01 | ab | 30.9 | bc | 4.635 | bc | 7.965 | ab | 1.153 | ab | 20.5 | c | 16.34 | a | 4.545 | ab | 46.03 | ab | 11.17 | a | 1.1 | bc | 34.14 | ab |
| ENOD 10−6 M + Rhizobium | 49.75 | a | 58.28 | ab | 18.25 | ab | 49.08 | a | 7.362 | a | 8.589 | a | 1.267 | a | 54.25 | a | 16.49 | a | 4.745 | a | 47.59 | a | 11.35 | a | 1.19 | abc | 37.24 | a |
| CEP 10−6 M | 58.75 | a | 49.11 | bc | 16.1 | ab | 44.53 | ab | 6.679 | ab | 7.273 | bc | 1.075 | b | 16.5 | c | 17.5 | a | 4.398 | bc | 44.6 | bc | 11.73 | a | 1.235 | ab | 34.28 | ab |
| CEP 10−6 M + Rhizobium | 51.35 | a | 55.88 | abc | 19.64 | a | 47.56 | a | 7.133 | a | 7.825 | ab | 1.097 | b | 52.75 | a | 17.82 | a | 4.645 | ab | 47.58 | a | 11.6 | a | 1.265 | a | 39.41 | a |
| p value | 0.1584 | 0.007 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0008 | 0.0004 | <0.0001 | 0.0852 | 0.0017 | <0.0001 | ||||||||||||||
| Significance | ns | ** | **** | **** | **** | **** | **** | **** | *** | *** | **** | ns | ** | **** | ||||||||||||||
| Treatment | Plant Height (cm) | Fresh Foliage Weight (g) | Fresh Root Weight (g) | Fresh Nodule Weight (mg) | Dry Nodule Weight (mg) | Dry Foliage Weight (g) | Dry Root Weight (g) | Nodule Number | Nodule Size (mm) | Number of Pods per Plant | Number of Seeds per Pod | 100-Seed Weight (g) | Pod Length (cm) | Pod Width (cm) | Yield (kg ha−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 51.27 | c | 93.97 | c | 2.951 | c | 82.49 | d | 11.88 | d | 13.84 | b | 0.4247 | d | 15.93 | d | 2.98 | c | 19.06 | b | 3.583 | c | 44.13 | a | 12.05 | a | 0.964 | c | 2341 | d |
| +Rhizobium | 55.5 | ab | 148.3 | abc | 3.363 | bc | 150.3 | cd | 21.67 | cd | 21.8 | ab | 0.4913 | cd | 32.53 | c | 3.15 | bc | 24.44 | ab | 4.542 | a | 44.24 | a | 11.77 | a | 1.051 | bc | 2962 | abc |
| ENOD 10−9 M + Rhizobium + NPK | 52.32 | bc | 114.4 | bc | 3.247 | bc | 152.5 | cd | 22.13 | cd | 16.59 | b | 0.4693 | cd | 31.67 | c | 4.36 | abc | 24.46 | ab | 4.184 | ab | 40.07 | ab | 12.01 | a | 1.164 | a | 2880 | cd |
| ENOD 10−8 M + Rhizobium + NPK | 51.37 | c | 97.49 | c | 3.523 | bc | 177.6 | bc | 25.83 | bc | 14.35 | b | 0.5203 | bc | 37.2 | bc | 4.59 | abc | 26.66 | ab | 3.959 | abc | 44.64 | a | 12.39 | a | 1.151 | ab | 3287 | abc |
| ENOD 10−7 M + Rhizobium + NPK | 57.44 | a | 183.5 | a | 4.357 | a | 263.2 | ab | 38.32 | ab | 26.8 | a | 0.6367 | a | 54.35 | a | 4.923 | ab | 29.33 | a | 3.867 | bc | 38.42 | b | 11.88 | a | 1.087 | ab | 3111 | abc |
| CEP 10−9 M + Rhizobium + NPK | 51.82 | bc | 121.3 | abc | 3.563 | b | 174 | bc | 25.43 | bc | 17.79 | ab | 0.521 | bc | 27.25 | cd | 3.857 | abc | 24.06 | ab | 3.888 | bc | 44.75 | a | 11.28 | a | 1.053 | bc | 2931 | bcd |
| CEP 10−8 M + Rhizobium + NPK | 55.45 | ab | 143.9 | abc | 4.197 | a | 255.5 | ab | 37.69 | ab | 21.37 | ab | 0.6027 | ab | 42.49 | abc | 4.713 | abc | 27.1 | ab | 4.257 | ab | 43.1 | ab | 12.27 | a | 1.146 | ab | 3487 | ab |
| CEP 10−7 M + Rhizobium + NPK | 59.36 | a | 176.1 | ab | 4.263 | a | 286 | a | 41.02 | a | 26.07 | a | 0.623 | a | 49.02 | ab | 5.497 | a | 30.88 | a | 4.039 | abc | 41.04 | ab | 11.28 | a | 1.075 | ab | 3578 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cántaro-Segura, H.; Zúñiga-Dávila, D. Exogenous Application of ENOD40 and CEP1 Peptides Boosts Symbiotic Signaling Gene Expression and Productivity in Common Bean. Plants 2025, 14, 2786. https://doi.org/10.3390/plants14172786
Cántaro-Segura H, Zúñiga-Dávila D. Exogenous Application of ENOD40 and CEP1 Peptides Boosts Symbiotic Signaling Gene Expression and Productivity in Common Bean. Plants. 2025; 14(17):2786. https://doi.org/10.3390/plants14172786
Chicago/Turabian StyleCántaro-Segura, Hector, and Doris Zúñiga-Dávila. 2025. "Exogenous Application of ENOD40 and CEP1 Peptides Boosts Symbiotic Signaling Gene Expression and Productivity in Common Bean" Plants 14, no. 17: 2786. https://doi.org/10.3390/plants14172786
APA StyleCántaro-Segura, H., & Zúñiga-Dávila, D. (2025). Exogenous Application of ENOD40 and CEP1 Peptides Boosts Symbiotic Signaling Gene Expression and Productivity in Common Bean. Plants, 14(17), 2786. https://doi.org/10.3390/plants14172786






