Effects of Roxithromycin Exposure on the Nitrogen Metabolism and Environmental Bacterial Recruitment of Chlorella pyrenoidosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Procedure
2.3. Determination of Microalgal Cell Density and Chlorophyll Content
2.4. Determination of NR Activity
2.5. Determination of Nitrate and Nitrite Concentrations
2.6. Composition Analysis and Function Prediction of Environmental Bacteria
2.7. Statistical Analysis
3. Results and Discussion
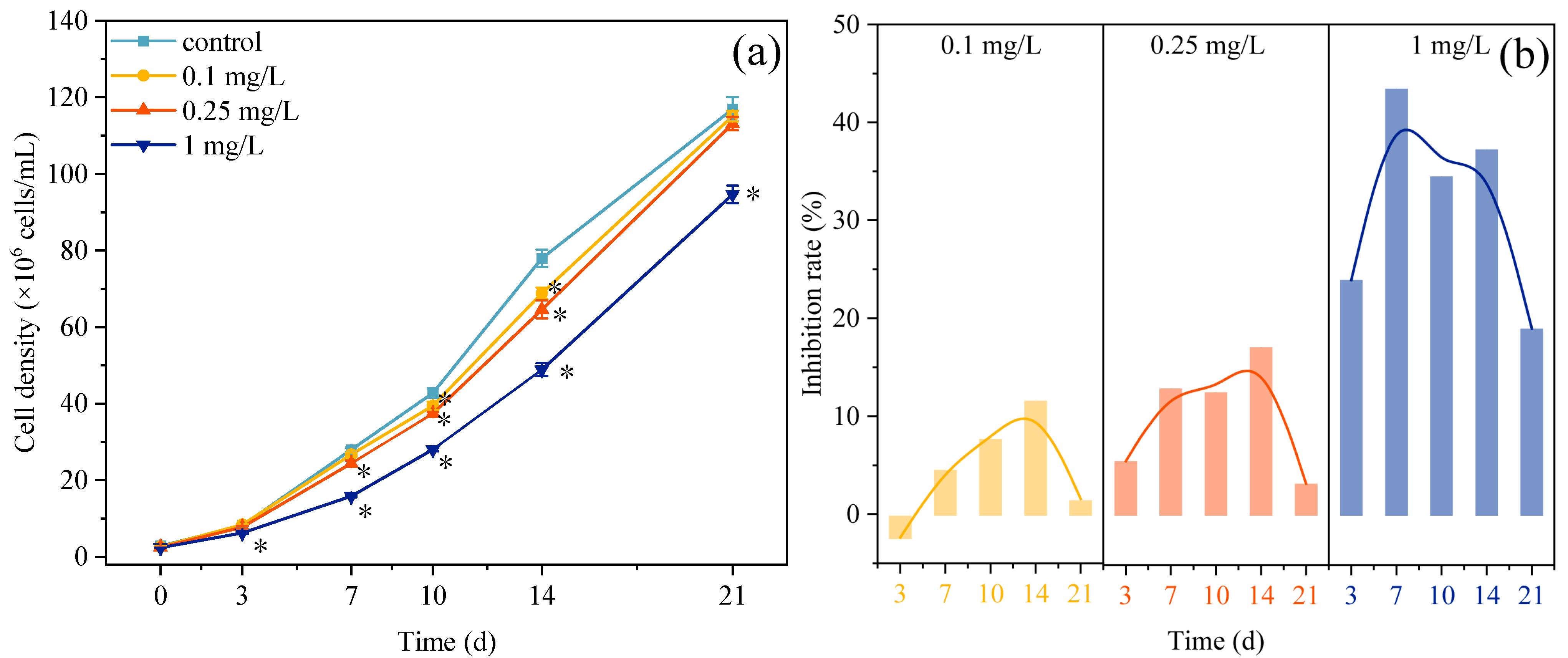
3.1. Growth Changes of Microalgae Under ROX Exposure
3.2. Chlorophyll Contents of Microalgae Under ROX Exposure
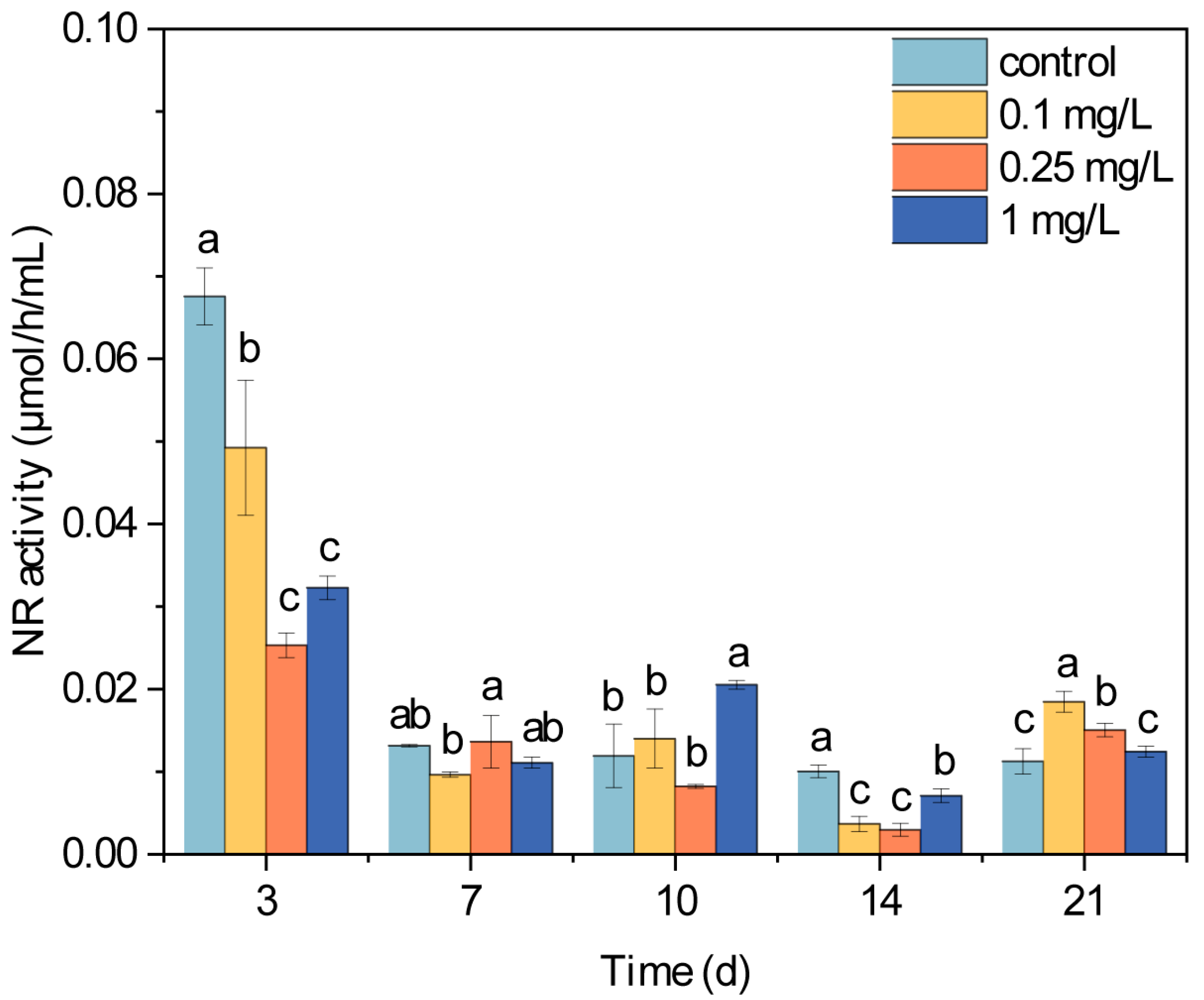
3.3. NR Activity of Microalgae Under ROX Exposure
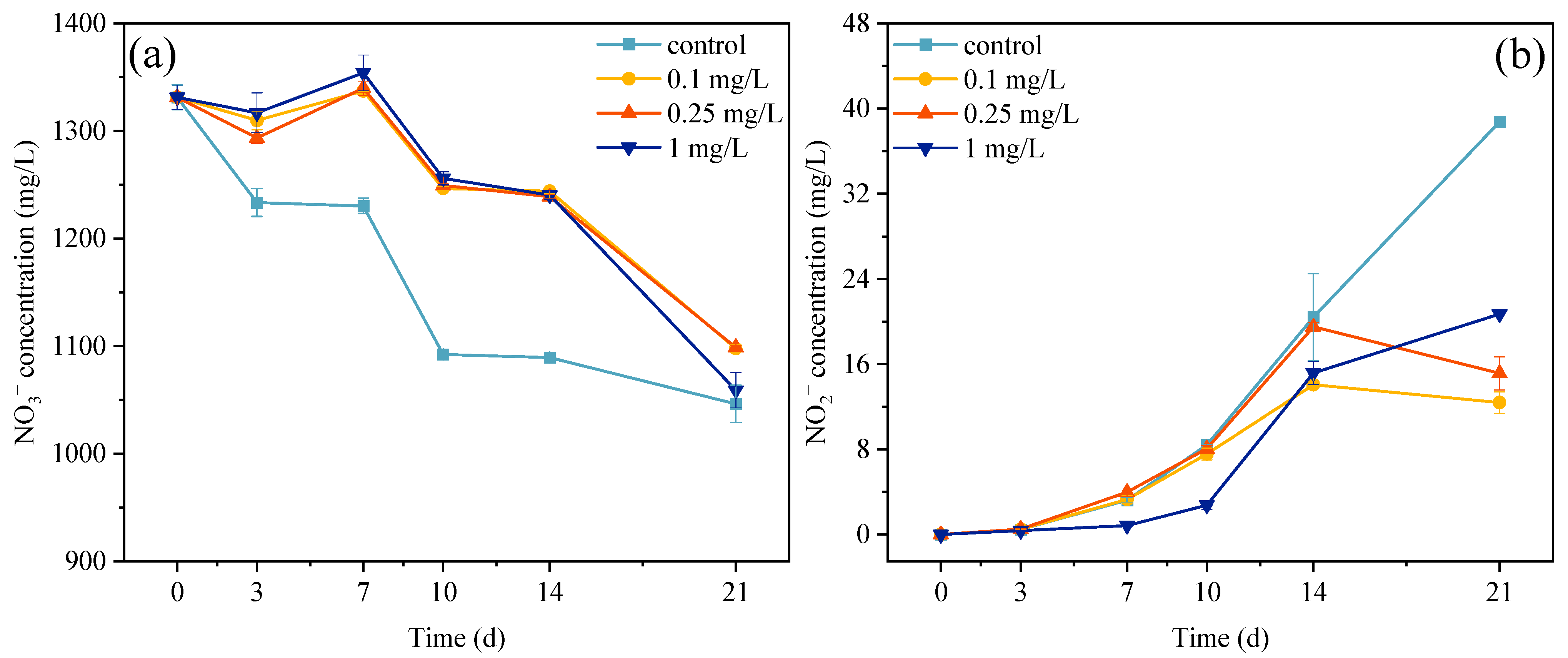
3.4. Concentrations of Nitrate and Nitrite in Exposure System
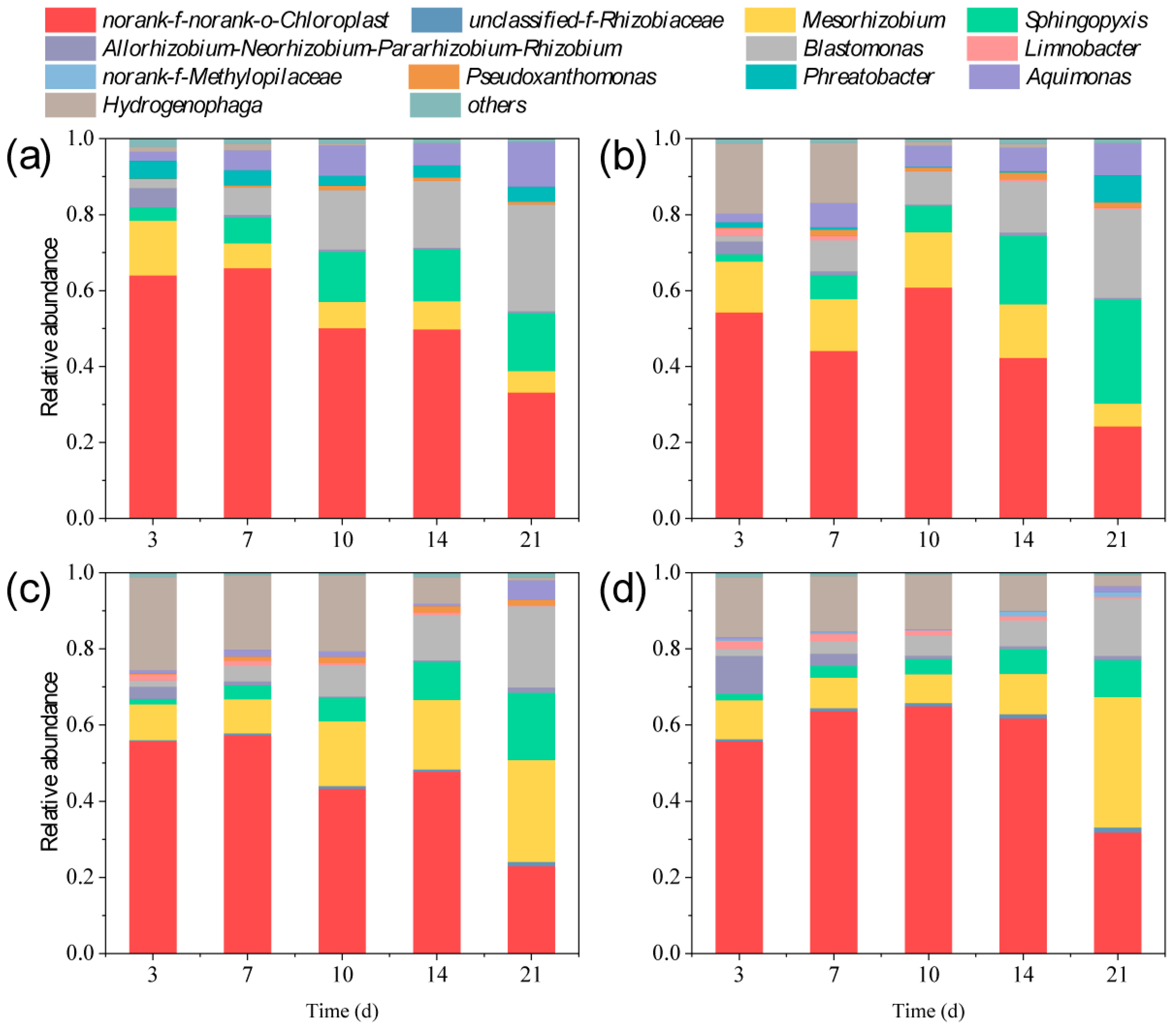
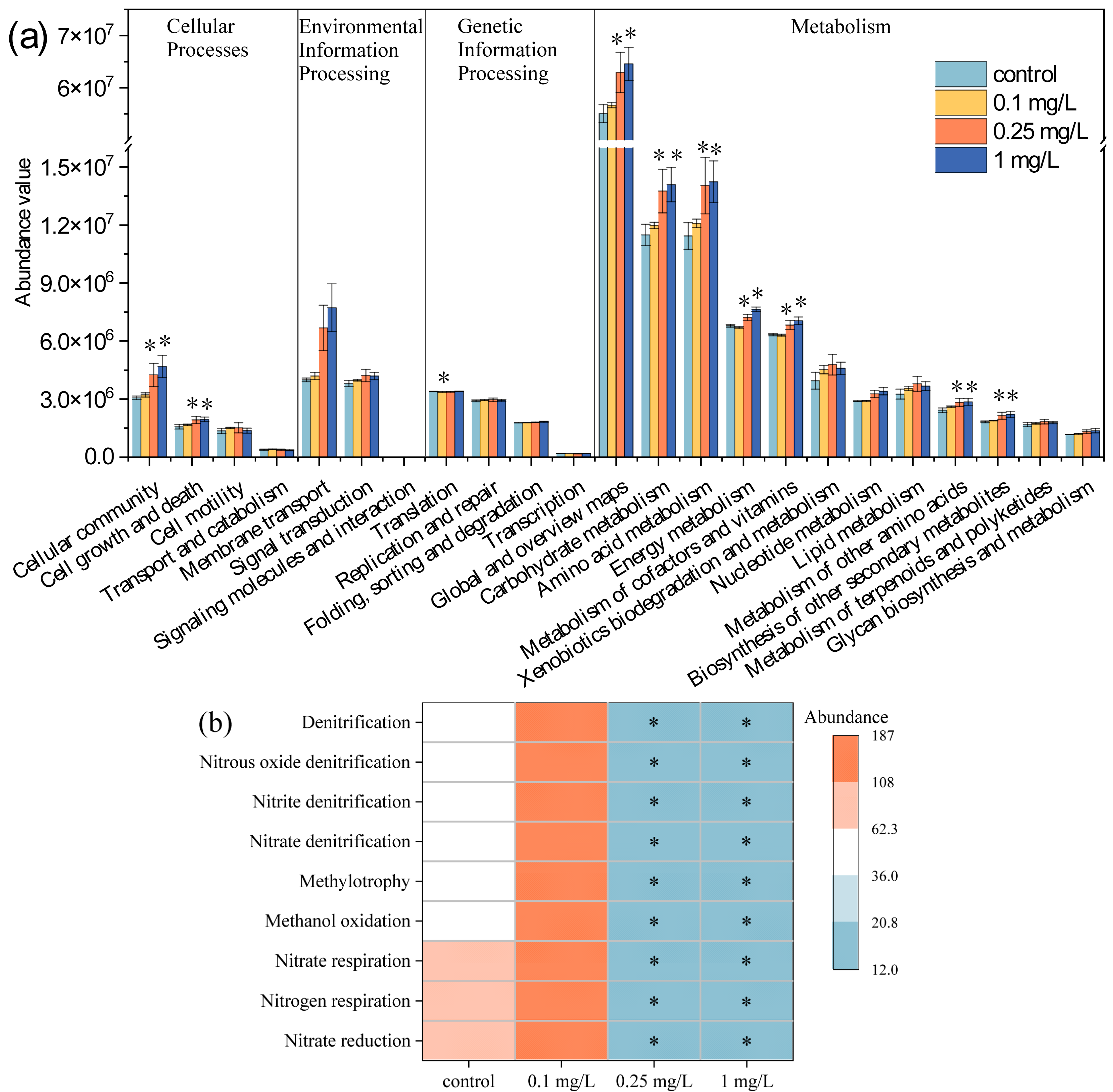
3.5. Changes in the Composition and Diversity of Environmental Bacteria
3.6. Metabolic and Ecological Functions of Environmental Bacteria
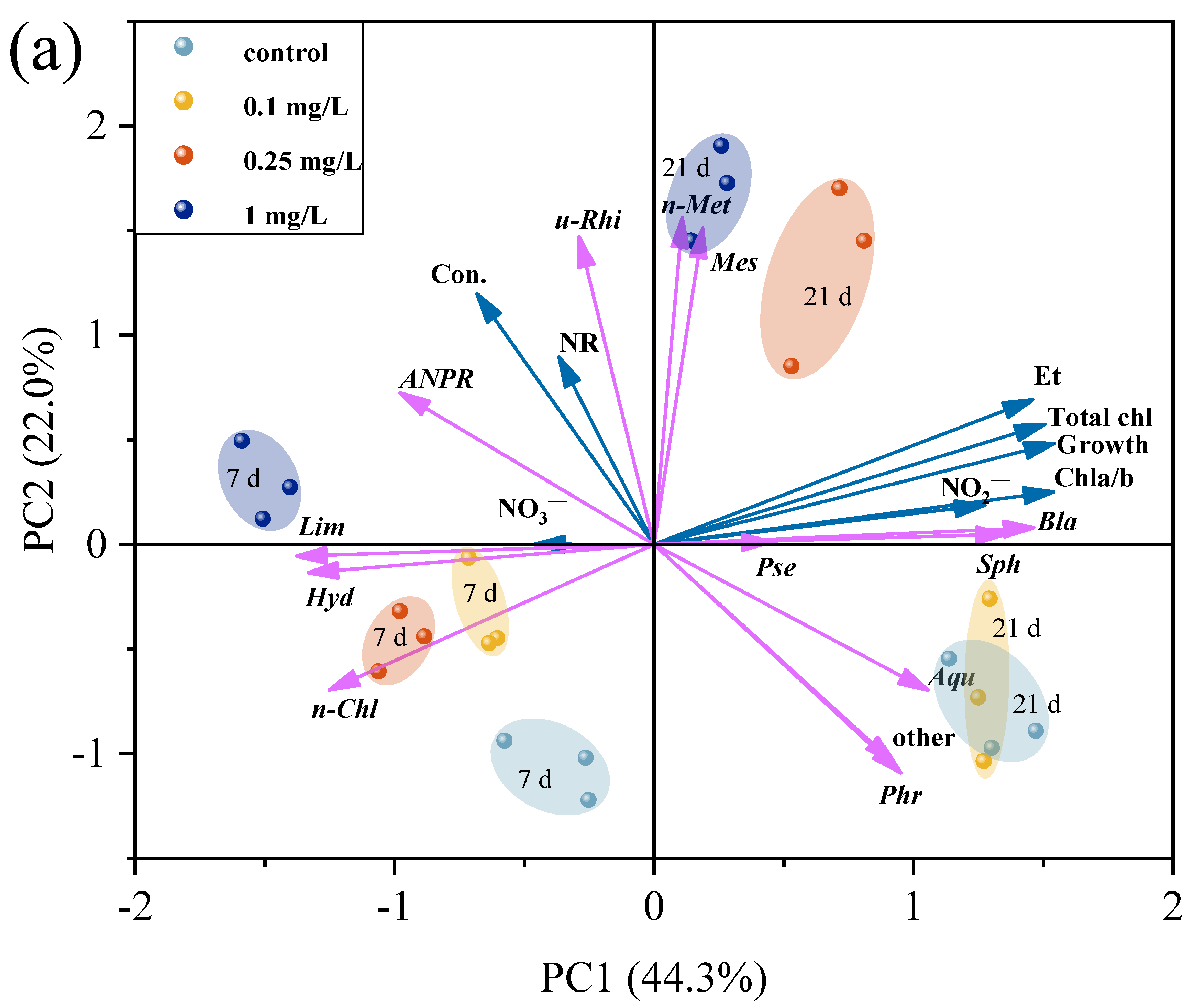
3.7. Correlation Between Microalgae and Environmental Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trott, D.J.; Turnidge, J.; Kovac, J.H.; Simjee, S.; Wilson, D.; Watts, J. Comparative macrolide use in humans and animals: Should macrolides be moved off the World Health Organisation’s critically important antimicrobial list? J. Antimicrob. Chemother. 2021, 76, 1955–1961. [Google Scholar] [CrossRef]
- Kocsmár, É.; Buzás, G.M.; Szirtes, I.; Kocsmár, I.; Kramer, Z.; Szijártó, A.; Fadgyas-Freyler, P.; Szénás, K.; Rugge, M.; Fassan, M. Primary and secondary clarithromycin resistance in Helicobacter pylori and mathematical modeling of the role of macrolides. Nat. Commun. 2021, 12, 2255. [Google Scholar] [CrossRef] [PubMed]
- ResistanceMap. The Center for Disease Dynamics. Economics & Policy. ResistanceMap: Antibiotic Use. 2023. Available online: https://resistancemap.OneHealthTrust.org/AntibioticUse.php (accessed on 30 May 2025).
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Zhang, Y.; Sun, S.; Zhang, B.; Liang, M.; Xu, J.; Wang, L. Occurrence, spatial distribution, source apportionment, and risk assessment of antibiotics in Yangtze river surface water. Emerg. Contam. 2025, 11, 100437. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622, 293–305. [Google Scholar] [CrossRef]
- Gunathilaka, M.L.; Bao, S.; Liu, X.; Li, Y.; Pan, Y. Antibiotic pollution of planktonic ecosystems: A review focused on community analysis and the causal chain linking individual-and community-level responses. Environ. Sci. Technol. 2023, 57, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Wu, Y.; Hu, J.; Zhang, Y.; Sun, Q.; Sun, W.; Geng, J.; Liu, X.; Jia, D. Antibiotics in global rivers. Natl. Sci. Open 2022, 1, 20220029. [Google Scholar] [CrossRef]
- Luisa, A.; Suarez, E.G.P.; Maggio, C.; La Marca, A.; Iovine, R.; Lofrano, G.; Guida, M.; Vaiano, V.; Carotenuto, M.; Libralato, G. Assessment of ecological risks posed by veterinary antibiotics in European aquatic environments: A comprehensive review and analysis. Sci. Total Environ. 2024, 954, 176280. [Google Scholar]
- Zhang, B.; Yu, W.; Liang, J.; Yao, X.; Sun, H.; Iwata, H.; Guo, J. Seasonal variation in structural and functional distribution of periphyton in a macrolide antibiotics-contaminated river. Environ. Pollut. 2024, 345, 123495. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J.; Li, P. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment. J. Hazard. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef]
- Le, V.V.; Tran, Q.-G.; Ko, S.-R.; Lee, S.-A.; Oh, H.-M.; Kim, H.-S.; Ahn, C.-Y. How do freshwater microalgae and cyanobacteria respond to antibiotics? Crit. Rev. Biotechnol. 2023, 43, 191–211. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, Y.; Ma, Z.; Li, H.; Yu, W.; Cao, L.; He, Q. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicol. Environ. Saf. 2021, 222, 112496. [Google Scholar] [CrossRef]
- Mo, J.; Lv, R.; Qin, X.; Wu, X.; Chen, H.; Yan, N.; Shi, J.; Wu, Y.; Liu, W.; Kong, R.Y. Mechanistic insights into hormesis induced by erythromycin in the marine alga Thalassiosira weissflogii. Ecotoxicol. Environ. Saf. 2023, 263, 115242. [Google Scholar] [CrossRef]
- Almeida, A.C.; Gomes, T.; Lomba, J.A.B.; Lillicrap, A. Specific toxicity of azithromycin to the freshwater microalga Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2021, 222, 112553. [Google Scholar] [CrossRef]
- Xin, R.; Yu, X.; Fan, J. Physiological, biochemical and transcriptional responses of cyanobacteria to environmentally relevant concentrations of a typical antibiotic—Roxithromycin. Sci. Total Environ. 2022, 814, 152703. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Min, Z.; Zheng, Q.; Han, J.; Li, P. Physiological, biochemical and transcription effects of roxithromycin before and after phototransformation in Chlorella pyrenoidosa. Aquat. Toxicol. 2021, 238, 105911. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, Z.; Peng, J.; Mo, J.; Li, Q.; Guo, J.; Yang, F. Transcriptomic analysis of Raphidocelis subcapitata exposed to erythromycin: The role of DNA replication in hormesis and growth inhibition. J. Hazard. Mater. 2021, 402, 123512. [Google Scholar] [CrossRef]
- Wang, X.; Dou, X.; Wu, J.; Meng, F. Attenuation pathways of erythromycin and biochemical responses related to algal growth and lipid synthesis in a microalga-effluent system. Environ. Res. 2021, 195, 110873. [Google Scholar] [CrossRef] [PubMed]
- Eheneden, I.; Wang, R.; Zhao, J. Antibiotic removal by microalgae-bacteria consortium: Metabolic pathways and microbial responses. Sci. Total Environ. 2023, 891, 164489. [Google Scholar] [CrossRef]
- Wang, F.; Liu, P.; Li, J.; Xu, S.; Chen, H.; Xie, L. Effects of four antibiotics on the photosynthetic light reactions in the green alga Chlorella pyrenoidosa. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 281, 109927. [Google Scholar] [CrossRef]
- Mao, Y.; Ye, K.; Yang, S.; Salam, M.; Yu, W.; He, Q.; He, R.; Li, H. Repeated Exposure Enhanced Toxicity of Clarithromycin on Microcystis aeruginosa Versus Single Exposure through Photosynthesis, Oxidative Stress, and Energy Metabolism Shift. Environ. Sci. Technol. 2024, 58, 4070–4082. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, L.; Wang, C.; Zhu, X.; Wang, Z.; Xing, B. Photosynthetic response mechanisms in typical C3 and C4 plants upon La2O3 nanoparticle exposure. Environ. Sci. Nano 2020, 7, 81–92. [Google Scholar] [CrossRef]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.; Ghini, V.; Adessi, A.; Fondi, M.; Buchan, A.; Viti, C. Towards a mechanistic understanding of microalgae–bacteria interactions: Integration of metabolomic analysis and computational models. FEMS Microbiol. Rev. 2022, 46, fuac020. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Cao, K.-L.; Lv, S.-B.; Hu, Y.-X.; Lin, J.; Zhou, Q.-Z.; Wang, J.-H. Algal strains, treatment systems and removal mechanisms for treating antibiotic wastewater by microalgae. J. Water Process Eng. 2023, 56, 104266. [Google Scholar] [CrossRef]
- Xue, X.; Su, X.; Zhou, L.; Ji, J.; Qin, Z.; Liu, J.; Li, K.; Wang, H.; Wang, Z. Antibiotic-induced recruitment of specific algae-associated microbiome enhances the adaptability of Chlorella vulgaris to antibiotic stress and incidence of antibiotic resistance. Environ. Sci. Technol. 2023, 57, 13336–13345. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Fang, Y.; Lyu, X.; Zhu, Z.; Wu, C.; Xu, Z.; Li, W.; Liu, N.; Du, C. Phycospheric Bacteria Alleviate the Stress of Erythromycin on Auxenochlorella pyrenoidosa by Regulating Nitrogen Metabolism. Plants 2025, 14, 121. [Google Scholar] [CrossRef]
- Liu, H.; Al-Dhabi, N.A.; Jiang, H.; Liu, B.; Qing, T.; Feng, B.; Ma, T.; Tang, W.; Zhang, P. Toward nitrogen recovery: Co-cultivation of microalgae and bacteria enhances the production of high-value nitrogen-rich cyanophycin. Water Res. 2024, 256, 121624. [Google Scholar] [CrossRef]
- Dao, G.-H.; Wu, G.-X.; Wang, X.-X.; Zhang, T.-Y.; Zhan, X.-M.; Hu, H.-Y. Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res. 2018, 33, 345–351. [Google Scholar] [CrossRef]
- Li, J.; Min, Z.; Li, W.; Xu, L.; Han, J.; Li, P. Interactive effects of roxithromycin and freshwater microalgae, Chlorella pyrenoidosa: Toxicity and removal mechanism. Ecotoxicol. Environ. Saf. 2020, 191, 110156. [Google Scholar] [CrossRef]
- Dai, Y.; Guo, Z.; Guo, X.; Deng, R.; Li, L.; Fan, T.; Cui, K.; Pan, T. Plastic particles and fluorescent brightener co-modify Chlorella pyrenoidosa photosynthesis and a machine learning approach predict algae growth. J. Hazard. Mater. 2024, 477, 135406. [Google Scholar] [CrossRef]
- Wang, T.; Li, D.; Tian, X.; Huang, G.; He, M.; Wang, C.; Kumbhar, A.N.; Woldemicael, A.G. Mitigating salinity stress through interactions between microalgae and different forms (free-living & alginate gel-encapsulated) of bacteria isolated from estuarine environments. Sci. Total Environ. 2024, 926, 171909. [Google Scholar]
- Rezvani, F.; Farazmand, A. Optimizing light/dark cycles and nutrient ratios for continuous microalgae application in nitrate removal and CO2 fixation. Int. J. Environ. Sci. Technol. 2025, 22, 8827–8838. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Zhou, Y.; Li, W.; Cheng, H.; Han, J. Combined toxicity of erythromycin and roxithromycin and their removal by Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2023, 257, 114929. [Google Scholar] [CrossRef]
- Wu, F.; Pan, X.; Zhou, Y.; Zhu, Y.; Liu, K.; Li, W.; Han, J. The key molecular mechanisms of antagonism induced by combined exposure to erythromycin and roxithromycin in Chlorella pyrenoidosa. Aquat. Toxicol. 2025, 280, 107269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, J.; Li, Z.; Wu, Z.; Dang, C.; Fu, J. Enhanced removal efficiency of sulfamethoxazole by acclimated microalgae: Tolerant mechanism, and transformation products and pathways. Bioresour. Technol. 2022, 347, 126461. [Google Scholar] [CrossRef]
- Chu, Y.; Li, S.; Xie, P.; Chen, X.; Li, X.; Ho, S.-H. New insight into the concentration-dependent removal of multiple antibiotics by Chlorella sorokiniana. Bioresour. Technol. 2023, 385, 129409. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Jaén-Gil, A.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Blánquez, P. Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata). Algal Res. 2022, 61, 102560. [Google Scholar] [CrossRef]
- Long, S.; Hamilton, P.B.; Wang, C.; Li, C.; Xue, X.; Zhao, Z.; Wu, P.; Gu, E.; Uddin, M.M.; Li, B. Bioadsorption, bioaccumulation and biodegradation of antibiotics by algae and their association with algal physiological state and antibiotic physicochemical properties. J. Hazard. Mater. 2024, 468, 133787. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Tian, J.; Pan, Z.; Chen, Y.; Ming, F.; Wang, R.; Wang, L.; Zhou, H.; Li, J. Co-cultivation of microalgae-activated sludge for municipal wastewater treatment: Exploring the performance, microbial co-occurrence patterns, microbiota dynamics and function during the startup stage. Bioresour. Technol. 2023, 374, 128733. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Higgins, B.T. Comparison of algae growth and symbiotic mechanisms in the presence of plant growth promoting bacteria and non-plant growth promoting bacteria. Algal Res. 2021, 53, 102156. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Zhang, X.; Ge, S.; Mu, Y.; Shen, J. Unraveling the intracellular and extracellular self-defense of Chlorella sorokiniana toward highly toxic pyridine stress. Bioresour. Technol. 2023, 385, 129366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Zhu, Y.; Pan, X.; Li, W.; Han, J. Mechanisms of hormetic effects of ofloxacin on Chlorella pyrenoidosa under environmental-relevant concentration and long-term exposure. Sci. Total Environ. 2024, 932, 172856. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, Z.; Zhao, D.; Luo, Y.; Lai, C.; Huang, B.; Pan, X. Double-dose responses of Scenedesmus capricornus microalgae exposed to humic acid. Sci. Total Environ. 2022, 806, 150547. [Google Scholar] [CrossRef]
- Cheng, S.; Keang, K.; Cross, J.S. Evidence that microplastics at environmentally relevant concentration and size interfere with energy metabolism of microalgal community. J. Hazard. Mater. 2024, 476, 134995. [Google Scholar] [CrossRef]
- Brück, W.M.; Alfonso, E.; Rienth, M.; Andlauer, W. Heat stress resistance in Chlorella vulgaris enhanced by hydrolyzed whey proteins. Agronomy 2024, 14, 2854. [Google Scholar] [CrossRef]
- VidyaVani, M.; Rajesh, N.; Vinoth, K.; Riazunnisa, K.; Basha, P.O. Generation and characterization of reduced PSII antenna size mutants of Chlorella sorokiniana for improved biomass. J. Appl. Phycol. 2023, 35, 2151–2160. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Chen, J.; Li, W.; Wang, F. Carbon sequestration performance, enzyme and photosynthetic activity, and transcriptome analysis of algae-bacteria symbiotic system after antibiotic exposure. Sci. Total Environ. 2023, 902, 166486. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Na, L.; Bao-Long, W.; Tao, Z.; Peng-Fei, M.; Wei-Xiao, Z.; Sraboni, N.Z.; Zheng, M.; Ying-Qi, Z.; Liu, Y. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J. Environ. Manag. 2022, 320, 115673. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, S.; Guo, T.; Guo, C.; Li, Y.; Hou, Y.; Gao, Y.; Dong, R.; Liu, S. Exploring the influence of sulfadiazine-induced stress on antibiotic removal and transformation pathway using microalgae Chlorella sp. Environ. Res. 2024, 256, 119225. [Google Scholar]
- Yu, C.; Li, C.; Zhang, Y.; Du, X.; Wang, J.-H.; Chi, Z.-Y.; Zhang, Q. Effects of environment-relevant concentrations of antibiotics on seawater Chlorella sp. biofilm in artificial mariculture effluent. Algal Res. 2023, 70, 103008. [Google Scholar] [CrossRef]
- Mao, B.-D.; Li, K.-Y.; Vadiveloo, A.; Gu, J.-J.; Gao, F. Bacterial N-acyl-homoserine lactone enhances the degradation of sulfamethoxazole by microalgae and the associated metabolic regulatory mechanisms. Bioresour. Technol. 2025, 428, 132487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yue, Y.; Chen, X.; Wu, F.; Li, W.; Li, P.; Han, J. Physiological-biochemical responses and transcriptomic analysis reveal the effects and mechanisms of sulfamethoxazole on the carbon fixation function of Chlorella pyrenoidosa. Sci. Total Environ. 2024, 917, 170460. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Chen, M.; Huang, X.; Luo, Z. Effects of nitrogen to phosphorus ratios on algal growth and arsenate metabolism by Microcystis aeruginosa with dissolved organic phosphorus and nitrate as nutrients. Algal Res. 2023, 69, 102922. [Google Scholar] [CrossRef]
- Palacios, O.A.; López, B.R.; de-Bashan, L.E. Microalga Growth-Promoting Bacteria (MGPB): A formal term proposed for beneficial bacteria involved in microalgal–bacterial interactions. Algal Res. 2022, 61, 102585. [Google Scholar] [CrossRef]
- Hao, Y.; Lu, S.; Chu, G.; Gao, M. Effect of salinity on nitrogen removal performance, enzymatic activity and metabolic pathway of Chlorella pyrenoidosa treating aquaculture wastewater. Environ. Res. 2025, 265, 120405. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Romero-Cruz, M.; Leon-Vaz, A.; Vega, J.M.; Vigara, J. Alterations in nitrogen metabolism caused by heavy metals in the acid-tolerant microalga Coccomyxa onubensis. Algal Res. 2024, 84, 103784. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Montero, P.; Aparicio, S.; Ruano, M.; Borrás, L.; Seco, A.; Barat, R. Nitrite inhibition of microalgae induced by the competition between microalgae and nitrifying bacteria. Water Res. 2020, 172, 115499. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Li, X.; Yu, S.; Xu, S.; Qiu, S.; Ge, S. Physiological and genetic responses of Chlorella sp. to nitrite accumulation in microalgal-bacterial consortium with partial nitrification treating municipal wastewater. Water Res. 2025, 280, 123473. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Chen, S.; Liang, C.; Guo, W.; Ngo, H.H.; Peng, L. Inorganic carbon limitation decreases ammonium removal and N2O production in the algae-nitrifying bacteria symbiosis system. Sci. Total Environ. 2024, 928, 172440. [Google Scholar] [CrossRef] [PubMed]
- Qv, M.; Dai, D.; Liu, D.; Wu, Q.; Tang, C.; Li, S.; Zhu, L. Towards advanced nutrient removal by microalgae-bacteria symbiosis system for wastewater treatment. Bioresour. Technol. 2023, 370, 128574. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bai, Y.; Chen, Z.; Mo, J.; Li, Q.; Sun, H.; Zhang, Q. Transcriptomic analysis suggests the inhibition of DNA damage repair in green alga Raphidocelis subcapitata exposed to roxithromycin. Ecotoxicol. Environ. Saf. 2020, 201, 110737. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, Y.; Wang, Y.; Yan, X.; Guo, X.; Lei, Z.; Niu, J.; Liang, J.; Li, Z. Nitrogen-fixing bacteria promote growth and bioactive components accumulation of Astragalus mongholicus by regulating plant metabolism and rhizosphere microbiota. BMC Microbiol. 2024, 24, 261. [Google Scholar] [CrossRef]
- Colombi, E.; Hill, Y.; Lines, R.; Sullivan, J.T.; Kohlmeier, M.G.; Christophersen, C.T.; Ronson, C.W.; Terpolilli, J.J.; Ramsay, J.P. Population genomics of Australian indigenous Mesorhizobium reveals diverse nonsymbiotic genospecies capable of nitrogen-fixing symbioses following horizontal gene transfer. Microb. Genom. 2023, 9, 000918. [Google Scholar] [CrossRef]
- Aburai, N.; Tsukagoshi, T.; Sekiguchi, S.; Arakawa, H.; Imamura, Y.; Abe, K. Mutual supply of carbon and nitrogen sources in the co-culture of aerial microalgae and nitrogen-fixing bacteria. Algal Res. 2023, 70, 103001. [Google Scholar] [CrossRef]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res. 2023, 229, 119397. [Google Scholar] [CrossRef]
- Hung, C.-M.; Chen, C.-W.; Huang, C.-P.; Dong, C.-D. N-doped metal-free biochar activation of peroxymonosulfate for enhancing the degradation of antibiotics sulfadiazine from aquaculture water and its associated bacterial community composition. J. Environ. Chem. Eng. 2022, 10, 107172. [Google Scholar] [CrossRef]
- Lu, T.; Zhu, Y.; Ke, M.; Peijnenburg, W.; Zhang, M.; Wang, T.; Chen, J.; Qian, H. Evaluation of the taxonomic and functional variation of freshwater plankton communities induced by trace amounts of the antibiotic ciprofloxacin. Environ. Int. 2019, 126, 268–278. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, Z.; Chen, Y.; Zhou, Z.; Zhang, J.; Xu, N.; Zhang, Q.; Lu, T.; Peijnenburg, W.; Qian, H. Machine learning predicts the impact of antibiotic properties on the composition and functioning of bacterial community in aquatic habitats. Sci. Total Environ. 2022, 828, 154412. [Google Scholar] [CrossRef]
- Bunbury, F.; Deery, E.; Sayer, A.P.; Bhardwaj, V.; Harrison, E.L.; Warren, M.J.; Smith, A.G. Exploring the onset of B12-based mutualisms using a recently evolved Chlamydomonas auxotroph and B12-producing bacteria. Environ. Microbiol. 2022, 24, 3134–3147. [Google Scholar] [CrossRef]
- Ahmad, N.; Mounsef, J.R.; Lteif, R. Pigment production by Scenedesmus dimorphus using different low-cost and alternative culture media. J. Chem. Technol. Biotechnol. 2022, 97, 287–294. [Google Scholar] [CrossRef]
- Li, R.; Song, M.; Yin, D.; Ye, X.; Yu, J.; Chen, X. Indole-3-acetic acid mediated removal of sludge toxicity by microalgae: Focus on the role of extracellular polymeric substances. Bioresour. Technol. 2023, 387, 129700. [Google Scholar] [CrossRef]
- Gauthier, M.; Senhorinho, G.; Scott, J. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, C.; Cao, H.; Song, J.; Gong, B.; Li, L.; Wang, L.; He, Y.; Liang, M.; Lin, J. Microbial functional assemblages predicted by the FAPROTAX analysis are impacted by physicochemical properties, but C, N and S cycling genes are not in mangrove soil in the Beibu Gulf, China. Ecol. Indic. 2022, 139, 108887. [Google Scholar] [CrossRef]
- Ren, J.; Tang, J.; Min, H.; Tang, D.; Jiang, R.; Liu, Y.; Huang, X. Nitrogen removal characteristics of novel bacterium Klebsiella sp. TSH15 by assimilatory/dissimilatory nitrate reduction and ammonia assimilation. Bioresour. Technol. 2024, 394, 130184. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Xue, Q.; Bu, C.; Wang, Y.; Zhang, B.; Wang, Y.; Qin, Q. Long-term nitrogen and phosphorus removal, shifts of functional bacteria and fate of resistance genes in bioretention systems under sulfamethoxazole stress. J. Environ. Sci. 2023, 126, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tu, W.; Chen, S.; Fan, M.; Jia, L.; Wang, B.; Yang, Y.; Li, S.; Luo, X.; Su, M. Relationships between high-concentration toxic metals in sediment and evolution of microbial community structure and carbon–nitrogen metabolism functions under long-term stress perspective. Environ. Sci. Pollut. Res. 2024, 31, 29763–29776. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fan, X.-Y.; Li, X. Unveiling the characteristics of free-living and particle-associated antibiotic resistance genes associated with bacterial communities along different processes in a full-scale drinking water treatment plant. J. Hazard. Mater. 2024, 476, 135194. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, H.; Lu, X.; Wang, C.; Chen, R.; Cheng, J.; He, T.; Fu, T. Potential of combined reactor and static composting applications for the removal of heavy metals and antibiotic resistance genes from chicken manure. J. Environ. Manag. 2024, 356, 120592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-l.; Li, G.-x.; Ge, Y.-m.; Iqbal, N.M.; Yang, X.; Cui, Z.-d.; Yang, Q. Sphingopyxis microcysteis sp. nov., a novel bioactive exopolysaccharides-bearing Sphingomonadaceae isolated from the Microcystis phycosphere. Antonie Van Leeuwenhoek 2021, 114, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-C.; Zhang, X.; He, Z.-W.; Tian, Y.; Wang, X.C. Role of extracellular polymeric substances on nutrients storage and transfer in algal-bacteria symbiosis sludge system treating wastewater. Bioresour. Technol. 2021, 331, 125010. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, Z.; Yang, Y.; Liu, Z. Growth promotion of Chlorella by symbiotic bacteria under adverse environments. Algal Res. 2022, 66, 102799. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Yu, W.; Tang, S.; Yin, H. Metagenomic insights into the mechanisms of triphenyl phosphate degradation by bioaugmentation with Sphingopyxis sp. GY. Ecotoxicol. Environ. Saf. 2023, 263, 115261. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, S.; Nie, Y.; Liu, R.; Zhu, W.; Zhou, Z.; Ma, Y.; Diao, J. Effect of acetochlor on the symbiotic relationship between microalgae and bacteria. J. Hazard. Mater. 2024, 463, 132848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zeng, T.; Wang, J.; Wang, Z.; Zhao, D.; Wei, J.; Peng, Y.; Miao, L. AHL-mediated quorum sensing drives microbial community succession and metabolic pathway in algal-bacterial biofilm system. Water Res. 2025, 282, 123702. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Cole, N.; Naidu, R.; Megharaj, M. Extracellular polymeric substances drive symbiotic interactions in bacterial–microalgal consortia. Microb. Ecol. 2022, 83, 596–607. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, R.; Li, K.; Sun, J.; Wang, K.; Shao, Y.; Hu, Z.; Zhu, J.; Pan, Z.; Nakhla, G. Microalgae-bacteria symbiosis enhanced nitrogen removal from wastewater in an inversed fluidized bed bioreactor: Performance and microflora. Front. Microbiol. 2025, 16, 1591974. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Xu, Z.; Wu, C.; Zhu, Z.; Lyu, X.; Li, J.; Zhang, X.; Wang, Y.; Luo, Y.; et al. Effects of Roxithromycin Exposure on the Nitrogen Metabolism and Environmental Bacterial Recruitment of Chlorella pyrenoidosa. Plants 2025, 14, 2774. https://doi.org/10.3390/plants14172774
Li J, Wang Y, Xu Z, Wu C, Zhu Z, Lyu X, Li J, Zhang X, Wang Y, Luo Y, et al. Effects of Roxithromycin Exposure on the Nitrogen Metabolism and Environmental Bacterial Recruitment of Chlorella pyrenoidosa. Plants. 2025; 14(17):2774. https://doi.org/10.3390/plants14172774
Chicago/Turabian StyleLi, Jiping, Ying Wang, Zijie Xu, Chenyang Wu, Zixin Zhu, Xingsheng Lyu, Jingjing Li, Xingru Zhang, Yan Wang, Yuming Luo, and et al. 2025. "Effects of Roxithromycin Exposure on the Nitrogen Metabolism and Environmental Bacterial Recruitment of Chlorella pyrenoidosa" Plants 14, no. 17: 2774. https://doi.org/10.3390/plants14172774
APA StyleLi, J., Wang, Y., Xu, Z., Wu, C., Zhu, Z., Lyu, X., Li, J., Zhang, X., Wang, Y., Luo, Y., & Li, W. (2025). Effects of Roxithromycin Exposure on the Nitrogen Metabolism and Environmental Bacterial Recruitment of Chlorella pyrenoidosa. Plants, 14(17), 2774. https://doi.org/10.3390/plants14172774





