Differential Nutrient Contents and Free Amino Acid Levels in Asymptomatic and Symptomatic Leaves of Huanglongbing-Affected Grapefruit Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment Categorization
2.2. Foliage Sampling for CLas Diagnosis, Phloem Extraction, and Nutrient Analysis
2.3. Determination of Amino Acid Content and Foliar Nutrients
2.4. Statistical Analysis
3. Results
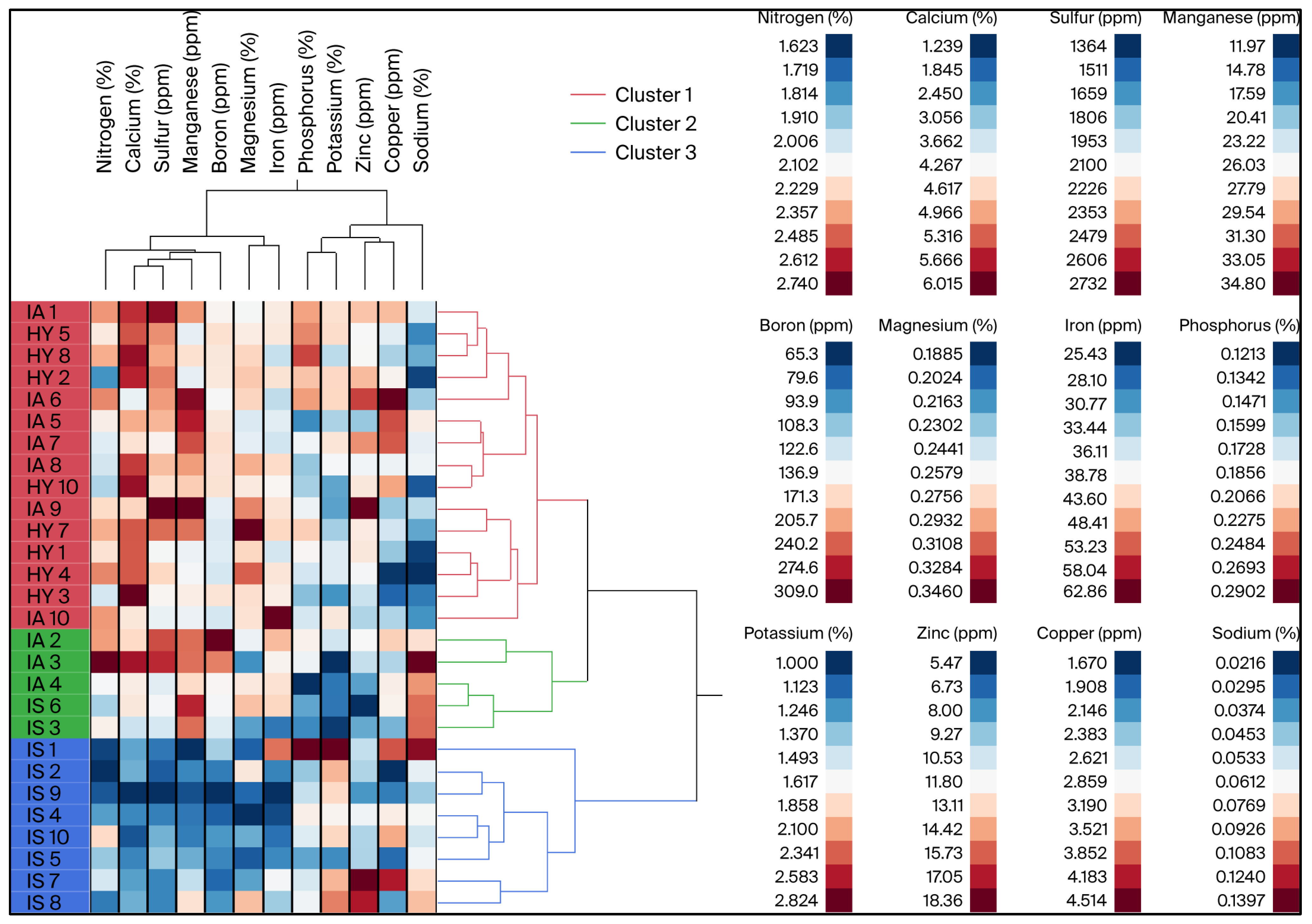
3.1. Leaf Mineral Content of Grapefruit
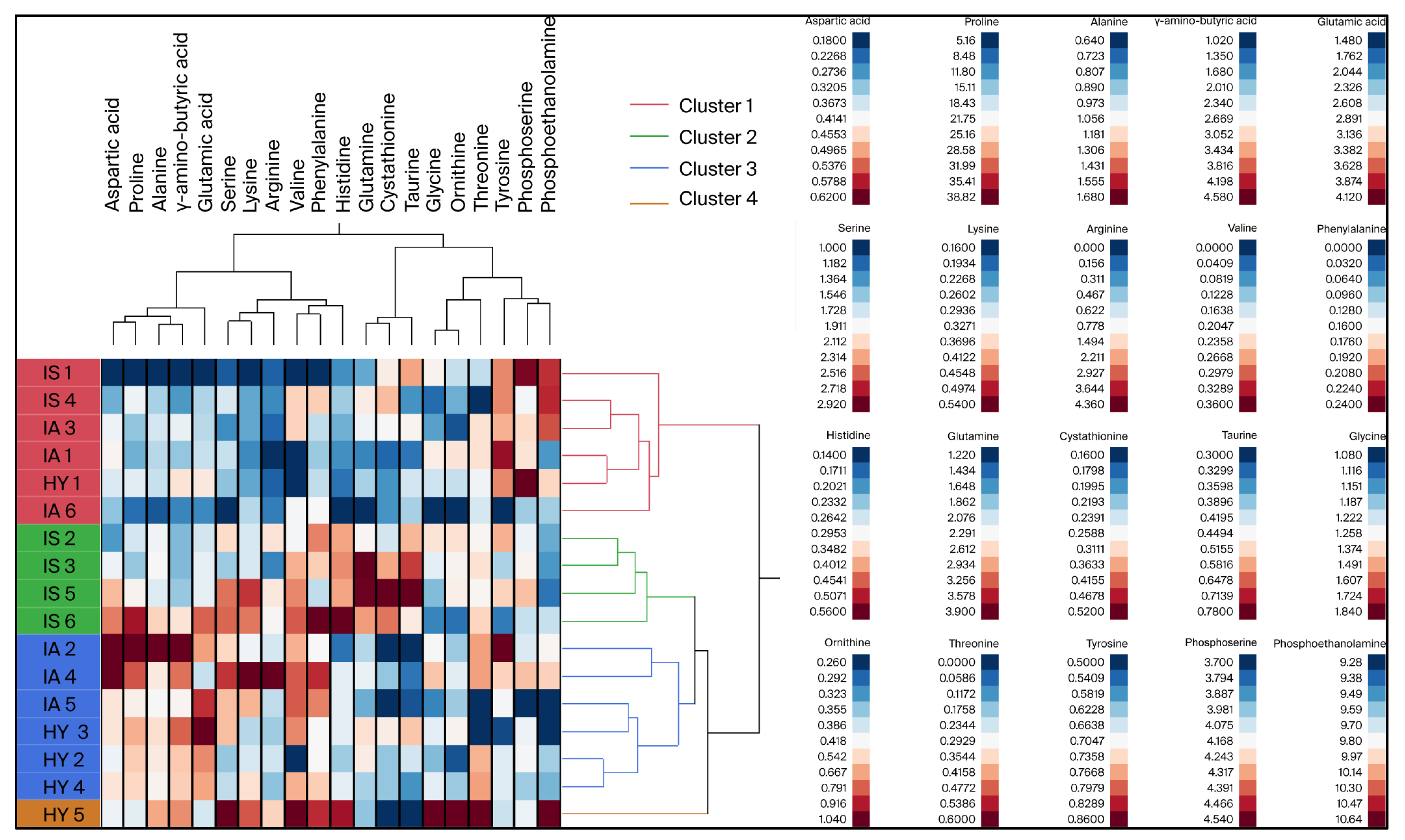
3.2. Phloem Sap Free Amino Acid Levels as Affected by HLB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, J.; Gottwald, T.; Sétamou, M. Status of huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Halbert, S. Establishment of Diaphorina citri and citrus greening in Florida—A case study. J. Insect Sci. 2007, 9, 6. [Google Scholar]
- Sétamou, M.; Alabi, O.J.; Kunta, M.; Dale, J.; da Graça, J.V. Distribution of Candidatus Liberibacter asiaticus in citrus and the Asian citrus psyllid in Texas over a decade. Plant Dis. 2020, 104, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-Term Field Evalua-tion Reveals Huanglongbing Resistance in Citrus Relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Sétamou, M.; Alabi, O.J.; Simpson, C.R.; Jifon, J.L. Contrasting amino acid profiles among permissive and non-permissive hosts of Candidatus Liberibacter asiaticus, putative causal agent of Huanglongbing. PLoS ONE 2017, 12, e0187921. [Google Scholar] [CrossRef]
- Nehela, Y.; Killiny, N. Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of Citrus Metabolites in Symptom Development. Metabolites 2020, 10, 409. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- da Graça, J.V. Citrus greening disease. Annu. Rev. Phytopathol. 1991, 29, 9–136. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Bai, J.; Irey, M.; Cameropn, R.; Luzio, G. Effect of Liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Transcriptional response of susceptible and tolerant citrus to infection with Candidatus Liberibacter asiaticus. Plant Sci. 2012, 185, 118–130. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Yu, X.; Stover, E.; Luo, F.; Duan, Y. Transcriptome Profiling of Huanglongbing (HLB) Tolerant and Susceptible Citrus Plants Reveals the Role of Basal Resistance in HLB Tolerance. Front. Plant Sci. 2016, 7, 933. [Google Scholar] [CrossRef]
- Nwugo, C.C.; Lin, H.; Duan, Y. The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic pro-files and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. BMC Plant Biol. 2013, 13, 59. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; García-Torres, R.; Etxeberria, E.; Reyes-De-Corcuera, J.I. GC-MS analysis of headspace and liquid extracts for metabolomic differentiation of citrus huanglongbing and zinc deficiency in leaves of ‘Valencia’ sweet orange from commercial groves. Phytochem. Anal. 2011, 22, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Achor, D.S.; Etxeberria, E.; Wang, N.; Folimonova, S.Y.; Chung, K.R.; Albrigo, L.G. Sequence of anatomical symptom observations in citrus affected with huanglongbing disease. Plant Pathol. J. 2010, 9, 56–64. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G., Jr.; Li, Z.G. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Dong, Z.; Srivastava, A.K.; Liu, X.; Riaz, M.; Gao, Y.; Liang, X.; Tan, Q.; Sun, X.; Wu, S.; Hu, C. Interactions between nutrient and Huanglongbing pathogen in citrus: An overview and implications. Sci. Hortic. 2021, 290, 110511. [Google Scholar] [CrossRef]
- Masaoka, Y.; Pustika, A.; Subandiyah, S.; Okada, A.; Hanundin, E.; Purwanto, B.; Okuda, M.; Okada, Y.; Saito, A.; Holford, P.; et al. Lower Concentrations of microelements in leaves of citrus infected with ‘Candidatus Liberibacter asiaticus’. Jpn. Agric. Res. Q. JARQ 2011, 45, 269–275. [Google Scholar] [CrossRef]
- Spann, T.M.; Schumann, A.W. The role of plant nutrients in disease development with emphasis on citrus and huanglongbing. Proc. Fla. State Hortic. Soc. 2009, 122, 169–171. [Google Scholar]
- Dutt, M.; Mahmoud, L.M.; Chamusco, K.; Stanton, D.; Chase, C.D.; Nielsen, E.; Quirico, M.; Yu, Q.; Gmitter, F.G., Jr.; Grosser, J.W. Utilization of somatic fusion techniques for the development of HLB tolerant breeding resources employing the Australian finger lime (Citrus australasica). PLoS ONE 2021, 16, e0255842. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M. Novel citrus hybrids incorporating Australian lime genetics: Development of HLB-tolerant citrus rootstocks and physiological changes in ‘Valencia’ sweet orange scions. Front. Plant Sci. 2025, 16, 1614845. [Google Scholar] [CrossRef]
- Liu, J.; Singh, K.; Huff, M.; Gottschalk, C.; Do, M.; Staton, M.; Manjunath, L.K.; Krueger, R.; Ramadugu, C.; Dardick, C. Deep R-gene discovery in HLB resistant wild Australian limes uncovers evolutionary features and potentially important loci for hybrid breeding. Front. Plant Sci. 2025, 15, 1503030. [Google Scholar] [CrossRef]
- Wang, N. The citrus huanglongbing crisis and potential solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Sétamou, M.; Simpson, C.R.; Alabi, O.J.; Nelson, S.D.; Telagamsetty, S.; Jifon, J.L. Quality Matters: Influences of Citrus Flush Physicochemical Characteristics on Population Dynamics of the Asian Citrus Psyllid (Hemiptera: Livider). PLoS ONE 2016, 11, e0168997. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, S.; Kadyampakeni, D.M. Micronutrients Improve Growth and Development of HLB-Affected Citrus Trees in Florida. Plants 2022, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Rouse, R.E.; Ozores-Hampton, M.; Roka, F.M.; Robert, P. Rehabilitation of Huanglongbing-affected Citrus Trees using Severe pruning and Enhanced Foliar Nutritional Treatments. HortScience 2017, 52, 972–978. [Google Scholar] [CrossRef]
- Sétamou, M.; da Graça, J.; Prewett, R. HLB in Texas: Steps and challenges to curb this threat. Citrograph 2012, 3, 32–38. [Google Scholar]
- Kunta, M.; Viloria, Z.; del Rio, H.S.; Louzada, E.S. Diverse DNA extraction methods and PCR primers for detection of Huanglongbing-associated bacteria from roots of ‘Valencia’ sweet orange on sour orange rootstock. Sci. Hortic. 2014, 178, 23–30. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- King, R.W.; Zeevaart, J.A.D. Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974, 53, 96–103. [Google Scholar] [CrossRef]
- Deyl, Z.; Hyanek, J.; Horakova, M. Profiling of amino acids in body fluids and tissues by means of liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1986, 379, 177–250. [Google Scholar] [CrossRef] [PubMed]
- Le Boucher, J.; Charret, C.; Coudray-Lucas, C.; Giboudeau, J.; Cynober, L. Amino acid determination in biological fluids by automated ion-exchange chromatography: Performance of Hitachi L-8500A. Clin. Chem. 1997, 43, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Fekkes, D. State-of-the-art of high-performance liquid chromatographic analysis of amino acids in physiological samples. J. Chromatogr. B Biomed. Sci. Appl. 1996, 682, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.H. Information and entropy in cluster analysis. In Proceedings of the First US/Japan Conference on the Frontiers of Statistical Modeling: An Informational Approach; Springer Nature: Dordrecht, The Netherlands, 1994; Volume 2, pp. 115–147. [Google Scholar]
- JMP Statistical Discovery LLC. JMP® 17 Automation Reference; JMP Statistical Discovery LLC: Cary, NC, USA, 2022. [Google Scholar]
- Ebel, R.C.; Hamido, S.; Morgan, K.T. Interaction of huanglongbing and foliar applications of copper on growth and nutrient acquisition of citrus sinensis cv. Valencia. Hortscience 2019, 54, 297–302. [Google Scholar] [CrossRef]
- Pustika, A.B.; Subandiyah, S.; Holford, P.; Beattie, G.A.C.; Iwanami, T.; Masaoka, Y. Interactions between plant nutrition and symptom expression in mandarin trees infected with the disease Huanglongbing. Australas. Plant Dis. Notes 2008, 3, 112–115. [Google Scholar] [CrossRef]
- Atlio, J.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Aubert, B.; Grisoni, M.; Villemin, M.; Rossolin, G.A. A case study of Huanglongbing (Greening) control in Reunion Island. Proc. Int. Organ. Citrus Virol. Conf. 1996, 13, 276–278. [Google Scholar]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Chapter 6—Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–281. ISBN 9780128197738. [Google Scholar] [CrossRef]
- Tavanti, T.R.; de Melo, A.A.R.; Moreira, L.D.K.; Sanchez, D.E.J.; dos Santos Silva, R.; da Silva, R.M.; Dos Reis, A.R. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Riaz, M.U.; Ayub, M.A.; Khalid, H.; Haq, M.A.; Rasul, A.; Rehman, Z.R.; Ali, S. Fate of Micronutrients in Alkaline Soils. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer Nature: Singapore, 2020; pp. 577–613. [Google Scholar] [CrossRef]
- Boman, B.J.; Zekri, M.; Stover, E. Managing salinity in citrus. HortTechnology 2005, 15, 108–113. [Google Scholar] [CrossRef]
- Ferguson, L.; Grattan, S.R. How salinity damages citrus: Osmotic effects and specific ion toxicities. Tree Physiol. 2005, 12, 195–216. [Google Scholar] [CrossRef]
- Smith, P.F. A case of sodium toxicity in citrus. Proc. Fla. State Hortic. Soc. 1962, 75, 120–124. [Google Scholar]
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil. 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Demidchik, V.; Davenport, R.J.; Tester, M. Nonselective cation channels in plants. Annu. Rev. Plant Biol. 2002, 53, 67–107. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef]
- Yamaya, T.; Oaks, A. Metabolic regulation of ammonium uptake and assimilation. In Nitrogen Acquisition and Assimilation in Higher Plants; Amancio, S., Stulen, I., Eds.; Springer Nature: Dordrecht, The Netherlands, 2004; pp. 35–63. [Google Scholar]
- Albrecht, U.; Tripathi, I.; Bowman, K.D. Rootstock influences the metabolic response to Candidatus Liberibacter asiaticus in grafted sweet orange trees. Trees 2020, 34, 405–431. [Google Scholar] [CrossRef]
- Suh, J.H.; Tang, X.; Zhang, Y.; Gmitter, F.G., Jr.; Wang, Y. Metabolomic analysis provides new insight into tolerance of Huanglongbing in citrus. Front. Plant Sci. 2021, 12, 710598. [Google Scholar] [CrossRef]
- Hung, W.L.; Wang, Y. A targeted mass spectrometry-based metabolomics approach toward the understanding of host responses to Huanglongbing disease. J. Agric. Food. Chem. 2018, 66, 10651–10661. [Google Scholar] [CrossRef]
- Seifi, H.S.; Van Bockhaven, J.; Angenon, G.; Höfte, M. Glutamate metabolism in plant disease and defense: Friend or foe? Mol. Plant-Microbe Interact. 2013, 26, 475–485. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- McCusker, S.; Buff, P.R.; Yu, Z.; Fascetti, A.J. Amino acid content of selected plant, algae and insect species: A search for alternative protein sources for use in pet foods. J. Nutri. Sci. 2014, 3, e39. [Google Scholar] [CrossRef] [PubMed]
- Mejias-Barrera, P. Effect of Reduced Irrigation on Grapevine Physiology, Grape Characteristics and Wine Composition in Three Pinot Noir Vineyards with Contrasting Soils. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2016. [Google Scholar]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Rizwan, M.; Ali, S. Effects of exogenous taurine on growth, photosynthesis, oxidative stress, antioxidant enzymes and nutrient accumulation by Trifolium alexandrinum plants under manganese stress. Chemosphere 2022, 308, 136523. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Rasheed, R.; Rizwan, M.; Hussain, I.; Aslam, R.; Qureshi, F.F.; Ali, S. Effect of exogenous taurine on pea (Pisum sativum L.) plants under salinity & iron deficiency stress. Environ. Res. 2023, 223, 115448. [Google Scholar] [CrossRef]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Trop. Plant. Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Zhang, J.; Corpas, F.J.; Li, J.; Xie, Y. Hydrogen Sulfide and Reactive Oxygen Species, Antioxidant Defense, Abiotic Stress Tolerance Mechanisms in Plants. Int. J. Mol. Sci. 2022, 23, 9463. [Google Scholar] [CrossRef]
- Kaufmann, C.; Sauter, M. Sulfated plant peptide hormones. J. Exp. Bot. 2019, 70, 4267–4277. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, L.; Hall, D.G.; Li, W.; Doddapaneni, H.; Lin, H.; Liu, L.; Vahling, C.M.; Gabriel, D.W.; Williams, K.P.; et al. Complete genome sequence of citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol. Plant Microbe Interact. 2009, 22, 1011–1020. [Google Scholar] [CrossRef]
- Zhang, L.H.; Ren, S.L.; Su, Z.Q.; Xu, P.P.; Ou, D.; Wang, L.J.; Sang, W.; Qiu, B.L. Impact of Huanglongbing Pathogen Infection on the Amino Acid Composition in Both Citrus Plants and the Asian Citrus Psyllid. Front. Physiol. 2021, 12, 777908. [Google Scholar] [CrossRef]
- Nehela, Y.; Killiny, N. Candidatus Liberibacter asiaticus’ and its vector, Diaphorina citri, augment the tricarbox-ylic acid cycle of their host via the γ-aminobutyric acid shunt and polyamines pathway. Mol. Plant-Microbe Interact. 2019, 32, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, S.; Kadyampakeni, D.M.; van Santen, E.; Vashisth, T.; Wright, A. Variable manganese rates influence the performance of Huanglongbing-affected citrus trees in Florida. HortScience 2022, 57, 360–366. [Google Scholar] [CrossRef]
- Kwakye, S.; Kadyampakeni, D.M.; Morgan, K.; Vashisth, T.; Wright, A. Effects of iron rates on growth and development of young Huanglongbing-affected citrus trees in Florida. HortScience 2022, 57, 1092–1098. [Google Scholar] [CrossRef]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar applications of essential nutrients on growth and yield of ‘Valencia’ sweet orange infected with Huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Hasabi, V.; Askari, H.; Alavi, S.M.; Zamanizadeh, H. Effect of amino acid application on induced resistance against citrus canker disease in lime plants. J. Plant Prot. Res. 2014, 54, 144–149. [Google Scholar] [CrossRef]
- Khan, A.S.; Munir, M.; Shaheen, T.; Tassawar, T.; Rafiq, M.A.; Ali, S.; Anwar, R.; Rehman, R.N.U.; Hasan, M.U.; Malik, A.U. Supplemental foliar applied mixture of amino acids and seaweed extract improved vegetative growth, yield and quality of citrus fruit. Sci. Hortic. 2022, 296, 110903. [Google Scholar] [CrossRef]
| Tree ID | Treatment | Description (Tree CLas Status–Foliar Status) | Source Tree HLB Status | Flush–Cq x | Analyses Performed z |
|---|---|---|---|---|---|
| IS 1 | IS | Infected–symptomatic | Positive | 24.2 | AA, MN |
| IS 2 | IS | Infected–symptomatic | Positive | 26.3 | AA, MN |
| IS 3 | IS | Infected–symptomatic | Positive | 23.4 | AA, MN |
| IS 4 | IS | Infected–symptomatic | Positive | 23.9 | AA, MN |
| IS 5 | IS | Infected–symptomatic | Positive | 28.4 | AA, MN |
| IS 6 | IS | Infected–symptomatic | Positive | 29.5 | AA |
| IA 1 | IA | Infected–asymptomatic | Positive | ND y | AA, MN |
| IA 2 | IA | Infected–asymptomatic | Positive | ND | AA, MN |
| IA 3 | IA | Infected–asymptomatic | Positive | ND | AA, MN |
| IA 4 | IA | Infected–asymptomatic | Positive | ND | AA, MN |
| IA 5 | IA | Infected–asymptomatic | Positive | ND | AA, MN |
| IA 6 | IA | Infected–asymptomatic | Positive | ND | AA |
| HY 1 | HY | Healthy | Negative | ND | AA, MN |
| HY 2 | HY | Healthy | Negative | ND | AA, MN |
| HY 3 | HY | Healthy | Negative | ND | AA, MN |
| HY 4 | HY | Healthy | Negative | ND | AA, MN |
| HY 5 | HY | Healthy | Negative | ND | AA, MN |
| Nutrients | Code | HY 1 | IA 1 | IS 1 | F-Value | p-Value | Eta-Squared 4 (η2) | Survey Range 2 |
|---|---|---|---|---|---|---|---|---|
| Nitrogen (%) | N | 2.15 ± 0.06 a | 2.28 ± 0.07 a | 1.88 ± 0.06 b | 9.85 | 0.0006 | 0.4217 | 2.0–2.6 |
| Phosphorus (%) | P | 0.20 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.98 | 0.3896 | 0.0674 | 0.13–0.5 |
| Potassium (%) | K | 1.58 ± 0.09 | 1.52 ± 0.09 | 1.79 ± 0.17 | 1.28 | 0.2955 | 0.0864 | 0.8–2.2 |
| Calcium (%) | Ca | 5.19± 0.27 a | 4.84 ± 0.17 a | 2.64 ± 0.28 b | 30.20 | <0.0001 | 0.6911 | 1.5–5.5 |
| Magnesium (%) | Mg | 0.28 ± 0.01 a | 0.26 ± 0.01 a | 0.23 ± 0.01 b | 7.49 | 0.0022 | 0.3647 | 0.3 |
| Sulfur (ppm) | S | 2228.37 ± 69.6 a | 2365.87 ± 80.3 a | 1687.53 ± 69.7 b | 24.28 | <0.0001 | 0.4248 | 1500–5000 |
| Sodium (%) | Na | 0.03 ± 0.002 b | 0.07 ± 0.01 a | 0.08 ± 0.01 a | 9.97 | 0.0006 | 0.0522 | NA 3 |
| Copper (ppm) | Cu | 2.53 ± 0.18 | 3.21 ± 0.23 | 2.77 ± 0.28 | 2.25 | 0.1248 | 0.2173 | 5.0–20 |
| Manganese (ppm) | Mn | 26.25 ± 0.66 a | 30.78 ± 0.92 a | 20.73 ± 2.32 b | 11.38 | 0.0003 | 0.1428 | 25–200 |
| Zinc (ppm) | Zn | 12.16 ± 0.31 | 12.44 ± 1.03 | 10.90 ± 1.25 | 0.72 | 0.4983 | 0.4574 | 25–150 |
| Iron (ppm) | Fe | 38.35 ± 1.43 ab | 42.65 ± 2.5 a | 33.85 ± 2.69 b | 3.75 | 0.0366 | 0.6513 | 60–200 |
| Boron (ppm) | B | 142.68 ± 5.89 a | 168.84 ± 18 a | 96.61 ± 6.99 b | 9.86 | 0.0006 | 0.4220 | 30–100 |
| Amino Acids | Healthy (HY 1) | Infected–Asymptomatic (IA 1) | Infected–Symptomatic (IS 1) | F Ratio | p Value | Eta-Squared 2 (η2) |
|---|---|---|---|---|---|---|
| Serine | 1.98 ± 0.26 | 1.84 ± 0.25 | 1.92 ± 0.22 | 0.0914 | 0.9132 | 0.013 |
| Glycine | 1.34 ± 0.12 | 1.23 ± 0.05 | 1.22 ± 0.03 | 0.8778 | 0.4374 | 0.111 |
| Alanine | 1.12 ± 0.06 | 1.08 ± 0.13 | 0.98 ± 0.08 | 0.581 | 0.5722 | 0.077 |
| Cysteine | — | — | — | — | — | — |
| Valine | 0.18 ± 0.08 | 0.22 ± 0.05 | 0.21 ± 0.04 | 0.1283 | 0.8806 | 0.018 |
| Leucine | — | — | — | — | — | — |
| Aspartic Acid | 0.41 ± 0.01 | 0.47 ± 0.05 | 0.36 ± 0.05 | 1.5633 | 0.2439 | 0.183 |
| Asparagine | — | 1.15 ± 0.60 | 0.47 ± 0.47 | — | — | |
| Threonine | 0.34 ± 0.10 | 0.29 ± 0.06 | 0.25 ± 0.05 | 0.3492 | 0.7112 | 0.048 |
| Methionine | — | — | — | — | — | — |
| Isoleucine | — | — | — | — | — | — |
| Lysine | 0.33 ± 0.04 | 0.33 ± 0.05 | 0.32 ± 0.05 | 0.0217 | 0.9785 | 0.003 |
| Tyrosine | 0.66 ± 0.04 | 0.73 ± 0.05 | 0.71 ± 0.04 | 0.5884 | 0.5684 | 0.078 |
| Phenylalanine | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.15 ± 0.03 | 0.0799 | 0.9236 | 0.011 |
| Tryptophan | — | — | — | — | — | — |
| Glutamic Acid | 3.33 ± 0.25 | 2.80 ± 0.27 | 2.62 ± 0.28 | 1.7503 | 0.2097 | 0.200 |
| Glutamine | 2.14 ± 0.14 ab | 1.83 ± 0.36 b | 2.87 ± 0.36 a | 4.7168 | 0.0272 | 0.403 |
| Proline | 23.84 ± 1.63 | 22.02 ± 4.75 | 19.74 ± 4.11 | 0.2634 | 0.7721 | 0.036 |
| Arginine | 0.66 ± 0.25 | 1.00 ± 0.68 | 0.65 ± 0.20 | 0.1910 | 0.8282 | 0.027 |
| Ornithine | 0.50 ± 0.08 | 0.36 ± 0.07 | 0.41 ± 0.07 | 0.8517 | 0.4477 | 0.108 |
| Histidine | 0.30 ± 0.06 | 0.22 ± 0.02 | 0.37 ± 0.05 | 2.9292 | 0.0866 | 0.295 |
| Phosphoserine | 4.20 ± 0.09 | 4.09 ± 0.08 | 4.22 ± 0.08 | 0.7753 | 0.4794 | 0.100 |
| Phosphoethanolamine | 9.82 ± 0.20 | 9.76 ± 0.19 | 9.83 ± 0.19 | 0.0419 | 0.9591 | 0.006 |
| Taurine | 0.41 ± 0.05 ab | 0.36 ± 0.04 b | 0.57 ± 0.04 a | 6.5949 | 0.0096 | 0.485 |
| Cystathionine | 0.22 ± 0.03 b | 0.20 ± 0.03 b | 0.36 ± 0.03 a | 9.9158 | 0.0021 | 0.586 |
| γ-amino-butyric Acid | 3.32 ± 0.35 a | 2.86 ± 0.32 ab | 1.93 ± 0.32 b | 4.5925 | 0.0293 | 0.396 |
| Urea | 139.59 ± 24.66 | 126.50 ± 18.22 | 130.60 ± 82.32 | 0.0791 | 0.9243 | 0.011 |
| Total Amino Acids (µg/g) | 195 ± 23.37 | 179 ± 21.33 | 180 ± 21.33 | 0.1040 | 0.8699 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satpute, A.; Simpson, C.; Sétamou, M. Differential Nutrient Contents and Free Amino Acid Levels in Asymptomatic and Symptomatic Leaves of Huanglongbing-Affected Grapefruit Trees. Plants 2025, 14, 2756. https://doi.org/10.3390/plants14172756
Satpute A, Simpson C, Sétamou M. Differential Nutrient Contents and Free Amino Acid Levels in Asymptomatic and Symptomatic Leaves of Huanglongbing-Affected Grapefruit Trees. Plants. 2025; 14(17):2756. https://doi.org/10.3390/plants14172756
Chicago/Turabian StyleSatpute, Aditi, Catherine Simpson, and Mamoudou Sétamou. 2025. "Differential Nutrient Contents and Free Amino Acid Levels in Asymptomatic and Symptomatic Leaves of Huanglongbing-Affected Grapefruit Trees" Plants 14, no. 17: 2756. https://doi.org/10.3390/plants14172756
APA StyleSatpute, A., Simpson, C., & Sétamou, M. (2025). Differential Nutrient Contents and Free Amino Acid Levels in Asymptomatic and Symptomatic Leaves of Huanglongbing-Affected Grapefruit Trees. Plants, 14(17), 2756. https://doi.org/10.3390/plants14172756







