Silicon as a Strategy to Mitigate Abiotic Stresses and Improve Physiological Performance and Grain Yield of Maize Grown Under Tropical Climate Conditions
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Characterization of the Experimental Area
4.2. Climate Assessments
4.3. Experimental Project
4.4. Evaluations
4.5. Economic Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Photosynthetic rate |
| ANOVA | Analysis of variance |
| AS | Sum of clay and silt content |
| Aw | Tropical savanna, megathermic |
| Cd | Differential cost |
| DP | Differential profits |
| EA/UFG | School of Agronomy at the Federal University of Goiás |
| E | Transpiration rate |
| ETc | Crop evapotranspiration |
| ET0 | Reference evapotranspiration |
| es | Saturation vapor pressure |
| ea | Actual vapor pressure |
| γ | Psychrometric constant |
| G | Soil heat flux density |
| gs | Stomatal conductance |
| IGP-DI | General Price Index—Internal Availability |
| Kc | Crop coefficient |
| KCl | Potassium chloride |
| MAP | Monoammonium phosphate |
| R | Rainfall |
| R1 | Onset of the reproductive phase |
| Rd | Differential revenue |
| RH | Relative humidity |
| Rn | Net radiation balance |
| SiBCS | Brazilian Soil Classification System |
| SiKCu | Potassium and copper silicate |
| STO/TAW | Relative water storage in soil |
| STICS | Multidisciplinary Simulator for Standard Cultures |
| Ta | Average temperature |
| TGW | Thousand-grain weight |
| TM | Maximum temperature |
| Tm | Minimum temperature |
| u2 | Wind speed |
| UV | Ultraviolet radiation |
| V4 | First at the four fully expanded leaf stage |
| V8 | Second at the eight-leaf stage |
| Ze | Effective rooting depth |
| θcc | Field capacity |
| θPWP | Permanent wilting point |
| ∆ | Slope of the vapor pressure curve |
References
- Bokor, B.; Santos, C.S.; Kostoláni, D.; Machado, J.; Silva, M.N.; Carvalho, S.M.P.; Vaculík, M.; Vasconcelos, M.W. Mitigation of Climate Change and Environmental Hazards in Plants: Potential Role of the Beneficial Metalloid Silicon. J. Hazard. Mater. 2021, 416, 126193. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; van der Putten, W.H. Climate Change Effects on Plant-Soil Feedbacks and Consequences for Biodiversity and Functioning of Terrestrial Ecosystems. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef]
- Blöschl, G.; Hall, J.; Viglione, A.; Perdigão, R.A.P.; Parajka, J.; Merz, B.; Lun, D.; Arheimer, B.; Aronica, G.T.; Bilibashi, A.; et al. Changing Climate Both Increases and Decreases European River Floods. Nature 2019, 573, 108–111. [Google Scholar] [CrossRef]
- Le Gouis, J.; Oury, F.; Charmet, G. How Changes in Climate and Agricultural Practices Influenced Wheat Production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Nash, K.L.; Halpern, B.S.; Remenyi, T.A.; Corney, S.P.; Fleming, A.; Fulton, E.A.; Hornborg, S.; Johne, A.; Watson, R.A.; et al. Food Production Shocks across Land and Sea. Nat. Sustain. 2019, 2, 130–137. [Google Scholar] [CrossRef]
- Costa, M.G.; Prado, R.M.; Sarah, M.M.S.; Souza, A.E.S.; Souza Júnior, J.P. Silicon Mitigates K Deficiency in Maize by Modifying C, N, and P Stoichiometry and Nutritional Efficiency. Sci. Rep. 2023, 13, 16929. [Google Scholar] [CrossRef]
- Bassu, S.; Brisson, N.; Durand, J.; Boote, K.; Lizaso, J.; Jones, J.W.; Rosenzweig, C.; Ruane, A.C.; Adam, M.; Baron, C.; et al. How Do Various Maize Crop Models Vary in Their Responses to Climate Change Factors? Glob. Change Biol. 2014, 20, 2301–2320. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.P.G.; Andrade, C.L.T.; Magalhães, B.G.; Gontijo Neto, M.M.; Melo, B.F. Produtividade Potencial e Variabilidade da Produtividade de Milho, em Regime de Sequeiro, em Rio Verde, Goiás; Embrapa Milho e Sorgo. Boletim de Pesquisa e Desenvolvimento, 140; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2016. [Google Scholar]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products: Maize. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 2 July 2025).
- FAO. Maize: Water Supply and Crop Yield. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/maize/en/ (accessed on 2 July 2025).
- Conab. Acompanhamento da Safra Brasileira de Grãos; 9; Companhia Nacional de Abastecimento: Brasília, Brazil, 2025. [Google Scholar]
- Battisti, R.; Ferreira, M.D.P.; Tavares, E.B.; Knapp, F.M.; Bender, F.D.; Casaroli, D.; Alves Júnior, J. Rules for Grown Soybean-Maize Cropping System in Midwestern Brazil: Food Production and Economic Profits. Agric. Syst. 2020, 182, 102850. [Google Scholar] [CrossRef]
- Lima, M.A.; Castro, V.F.; Vidal, J.B.; Enéas-Filho, J. Aplicação de silício em milho e feijão-de-corda sob estresse salino. Rev. Ciênc. Agron. 2011, 42, 398–403. [Google Scholar] [CrossRef]
- Shamshiripour, M.; Motesharezadeh, B.; Rahmani, H.A.; Alikhani, H.A.; Etesami, H. Optimal Concentrations of Silicon Enhance the Growth of Soybean (Glycine max L.) Cultivars by Improving Nodulation, Root System Architecture, and Soil Biological Properties. Silicon 2022, 14, 5333–5345. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its Manifold Roles in Plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Thakral, V.; Bhat, J.A.; Kumar, N.; Myaka, B.; Sudhakaran, S.; Patil, G.; Sonah, H.; Shivaraj, S.M.; Deshmukh, R. Role of Silicon under Contrasting Biotic and Abiotic Stress Conditions Provides Benefits for Climate Smart Cropping. Environ. Exp. Bot. 2021, 189, 104545. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Flores, R.A.; Lima, F.S.R.; Xavier, M.F.N.; Bueno, A.M.; Andrade, A.F.; Souza Júnior, J.P.; Campos, C.N.S.; Cunha Júnior, L.C.; Abdala, K.O.; Prado, R.M. Soluble Silicon Source via Foliar Application Improve Plant Physiology and Fruit Quality of Solanum lycopersicum L. Silicon 2024, 16, 1943–1954. Silicon 2024, 16, 1943–1954. [Google Scholar] [CrossRef]
- Xavier, M.F.N.; Flores, R.A.; Carmo, R.T.; Lima, M.L.; Sousa, R.G.; Dapper, F.P.; Abdala, K.O.; Casaroli, D.; Momesso, L.; Santos, G.G.; et al. Influence of Nitrogen Sources and Foliar Silicon Fertilization on Agronomic Characteristics and Differential Profit from Sugarcane Stalk Production. J. Plant Nutr. 2025, 48, 1–24. [Google Scholar] [CrossRef]
- Xavier, M.F.N.; Flores, R.A.; Cruz, D.R.C.; Ferreira, I.V.L.; Castro, J.P.V.; Silva, M.L.; Muniz, M.P.; Silva, V.B.; Milagres, V.A.C.; Abdala, K.O.; et al. Foliar Fertilization with a Soluble Silicon Source Can Alter Pigment Production in Leaves and Increase Fruit Production in Cucumbers (Cucumis sativus L.). J. Plant Nutr. 2025, 48, 2331–2348. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Petrova, A.I.; Lukina, K.A.; Shavarda, A.L. Silicon Ameliorates Iron Deficiency of Cucumber in a pH-Dependent Manner. J. Plant Physiol. 2018, 231, 364–373. [Google Scholar] [CrossRef]
- Araújo, V.S.; Sousa, T.K.R.; Nobre, R.S.; Santos, C.M.; Negreiros, K.K.S.; Carvalho, A.C.C.; Veloso, F.S.; Veloso, R.C.; Rezende, J.S. Influence of Foliar Application of Silicon on the Development and Productivity of Corn under Water Deficit in the Semi-Arid Region of Piauí. Res. Soc. Dev. 2022, 11, e25711528051. [Google Scholar] [CrossRef]
- Flores, R.A.; Arruda, E.M.; Damin, V.; Souza Júnior, J.P.; Maranhão, D.D.C.; Correia, M.A.R.; Prado, R.M. Physiological Quality and Dry Mass Production of Sorghum Bicolor Following Silicon (Si) Foliar Application. Aust. J. Crop Sci. 2018, 12, 631–638. [Google Scholar] [CrossRef]
- Freitas, L.B.; Coelho, E.M.; Maia, S.C.M.; Silva, T.R.B. Adubação foliar com silício na cultura do milho. Rev. Ceres 2011, 58, 262–267. [Google Scholar] [CrossRef]
- Miranda, P.S.; Moraes, T.R.; Santos, J.R.E.; Carvalho, F.D.; Viana, J.P.; Pérez-Maluf, R. Aplicação de silício na cultura do milho. Rev. Ciências Agro-Ambient. 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Munaro, M.F.; Simonetti, A.P.M.M. Aplicação foliar de silício no milho 2a safra: Influência na produtividade. Rev. Cultiv. Saber 2016, 9, 145–154. [Google Scholar]
- Hawerroth, C.; Araujo, L.; Bermúdez-Cardona, M.B.; Silveira, P.R.; Wordell Filho, J.A.; Rodrigues, F.A. Silicon-Mediated Maize Resistance to Macrospora Leaf Spot. Trop. Plant Pathol. 2019, 44, 192–196. [Google Scholar] [CrossRef]
- Mochko, A.C.R.; Silva, B.N.; Oliveira, L.M.; Silva, L.C.; Rodrigues, F.A. Silicon-Mediated Resistance in Maize against Infection by Colletotrichum Graminicola. Plant Soil 2024, 503, 295–312. [Google Scholar] [CrossRef]
- Sarah, M.M.S.; Prado, R.M.; Teixeira, G.C.M.; Souza Júnior, J.P.; Medeiros, R.L.S.; Barreto, R.F. Silicon Supplied Via Roots or Leaves Relieves Potassium Deficiency in Maize Plants. Silicon 2022, 14, 773–782. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Rad, S.N.; Ali, N.; Yvin, J. The Ameliorative Effect of Silicon on Maize Plants Grown in Mg-Deficient Conditions. Int. J. Mol. Sci. 2019, 20, 969. [Google Scholar] [CrossRef]
- Oliveira, K.S.; Prado, R.M.; Guedes, V.H.F. Leaf Spraying of Manganese with Silicon Addition Is Agronomically Viable for Corn and Sorghum Plants. J. Soil Sci. Plant Nutr. 2020, 20, 872–880. [Google Scholar] [CrossRef]
- Campos, C.N.S.; Prado, R.M.; Roque, C.G.; Lima Neto, A.J.; Marques, L.J.P.; Chaves, A.P.; Cruz, C.A. Use of Silicon in Mitigating Ammonium Toxicity in Maize Plants. Am. J. Plant Sci. 2015, 6, 1780–1784. [Google Scholar] [CrossRef]
- Dresler, S.; Wójcik, M.; Bednarek, W.; Hanaka, A.; Tukiendorf, A. The Effect of Silicon on Maize Growth under Cadmium Stress. Russ. J. Plant Physiol. 2015, 62, 86–92. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Sonmez, O.; Ince, F.; Higgs, D. Mitigation Effects of Silicon on Maize Plants Grown at High Zinc. J. Plant Nutr. 2009, 32, 1788–1798. [Google Scholar] [CrossRef]
- Delavar, K.; Ghanati, F.; Behmanesh, M.; Zare-Maivan, H. Physiological Parameters of Silicon-Treated Maize Under Salt Stress Conditions. Silicon 2018, 10, 2585–2592. [Google Scholar] [CrossRef]
- Malčovská, S.M.; Dučaiová, Z.; Bačkor, M. Impact of Silicon on Maize Seedlings Exposed to Short-Term UV-B Irradiation. Biologia 2014, 69, 1349–1355. [Google Scholar] [CrossRef]
- Carvalho, J.S.; Frazão, J.J.; Prado, R.M.; Souza Júnior, J.P.; Costa, M.G. Silicon Modifies C:N:P Stoichiometry and Improves the Physiological Efficiency and Dry Matter Mass Production of Sorghum Grown under Nutritional Sufficiency. Sci. Rep. 2022, 12, 16082. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon Availability Modifies Nutrient Use Efficiency and Content, C:N:P Stoichiometry, and Productivity of Winter Wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef] [PubMed]
- Qamar, R.; Anjum, I.; Atique-ur-Rehman; Safdar, M.E.; Javeed, H.M.R.; Rehman, A.; Ramzan, Y. Mitigating Water Stress on Wheat through Foliar Application of Silicon. Asian J. Agric. Biol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Bellido, L.L. Cultivos Herbaceos; Cereales; Mundi Prensa: Madrid, Spain, 1991; Volume 1, ISBN 84-7114-324-0. [Google Scholar]
- Cruz, J.C.; Pereira Filho, I.A.; Alvarenga, R.C.; Gontijo Neto, M.M.; Viana, J.H.M.; Oliveira, M.F.; Santana, D.P. Manejo da Cultura do Milho; Circular Técnica, 87; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2006. [Google Scholar]
- Fancelli, A.L. Cultivo Racional e Sustentável Requer Maior Conhecimento Sobre Planta Do Milho. Visão Agrícola 2015, 13, 20–23. [Google Scholar]
- Souza, G.M.; Barbosa, A.M. Fatores de Estresse No Milho São Diversos e Exigem Monitoramento Constante. Visão Agrícola 2015, 13, 30–34. [Google Scholar]
- Thornthwaite, C.W.; Mather, J.R. The Water Balance; Drexel Institute of Technology Laboratory of Climatology: Centerton, AR, USA, 1955; Volume 8. [Google Scholar]
- Xavier, M.F.N.; Flores, R.A.; Casaroli, D.; Capuchinho, F.F.; Dapper, F.P.; Carmo, R.T.; Lima, M.L.; Campos, C.N.S.; Santos, G.G.; Damin, V.; et al. CO2 Emission and Physiological Aspects of Sugarcane Ratoon as Interactive Functions of Nitrogen and Silicon Applications. J. Plant Nutr. 2024, 47, 1955–1968. [Google Scholar] [CrossRef]
- Birchall, J.D. The Essentiality of Silicon in Biology. Chem. Soc. Rev. 1995, 24, 351–357. [Google Scholar] [CrossRef]
- Souza Júnior, J.P.; Prado, R.M.; Campos, C.N.S.; Oliveira, D.F.; Cazetta, J.O.; Detoni, J.A. Silicon Foliar Spraying in the Reproductive Stage of Cotton Plays an Equivalent Role to Boron in Increasing Yield, and Combined Boron-Silicon Application, without Polymerization, Increases Fiber Quality. Ind. Crops Prod. 2022, 182, 114888. [Google Scholar] [CrossRef]
- Prado, R.M. Mineral Nutrition of Tropical Plants, 1st ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Wien, H.C. Abiotic Stress Effects on Vegetable Crops. In The Physiology of Vegetable Crops; CABI: Wallingford UK, 2020; pp. 71–93. [Google Scholar]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, S.; Xiong, Y. Silicon Priming Regulates Morpho-Physiological Growth and Oxidative Metabolism in Maize under Drought Stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef]
- Santos, A.F.B.; Teixeira, G.C.M.; Campos, C.N.S.; Baio, F.H.R.; Prado, R.M.; Teodoro, L.P.R.; Vilela, R.G.; Paiva Neto, V.B.; Teodoro, P.E. Silicon Increases Chlorophyll and Photosynthesis and Improves Height and NDVI of Cotton (Gossypium hirsutum L. r. Latifolium hutch). Res. Soc. Dev. 2020, 9, e548973826. [Google Scholar] [CrossRef]
- Souza Júnior, J.P.; Prado, R.M.; Sarah, M.M.S.; Felisberto, G. Silicon Mitigates Boron Deficiency and Toxicity in Cotton Cultivated in Nutrient Solution. J. Plant Nutr. Soil Sci. 2019, 182, 805–814. [Google Scholar] [CrossRef]
- Teixeira, G.C.M.; Prado, R.M.; Oliveira, L.T.; Souza, J.V.C.; Rocha, A.M.S. Silicon Fertigation with Appropriate Source Reduces Water Requirement of Maize under Water Deficit. Plant Soil 2022, 477, 83–97. [Google Scholar] [CrossRef]
- Ahmed, M.; Qadeer, U.; Fayayz-ul-Hassan; Fahad, S.; Naseem, W.; Duangpan, S.; Ahmad, S. Abiotic Stress Tolerance in Wheat and the Role of Silicon: An Experimental Evidence. In Agronomic Crops: Volume 3: Stress Responses and Tolerance; Springer: Singapore, 2020; Volume 3, pp. 443–479. ISBN 978-981-15-0025-1. [Google Scholar]
- Keller, C.; Rizwan, M.; Davidian, J.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J. Effect of Silicon on Wheat Seedlings (Triticum turgidum L.) Grown in Hydroponics and Exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How Does Silicon Mediate Plant Water Uptake and Loss Under Water Deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A Cooperative System of Silicon Transport in Plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. What Effect Does Liming Have on Silicon Availability in Agricultural Soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Idrees, K.; Aziz, A.; Naeem, M.; Azhar, M.F.; Farooq, S.; Hussain, M. Combined Application of Zinc and Silicon Improved Growth, Gas Exchange Traits, and Productivity of Maize (Zea mays L.) Under Water Stress. Silicon 2024, 16, 831–841. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Liu, P.; Wang, W.; Cao, D.; Deng, X.; Zhang, S. Silicon-Mediated Changes in Polyamine and 1-Aminocyclopropane-1-Carboxylic Acid Are Involved in Silicon-Induced Drought Resistance in Sorghum bicolor L. Plant Physiol. Biochem. 2014, 80, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.A.T.; Rena, A.B.; Amaral, J.F.T.D. Crescimento vegetativo sazonal do cafeeiro e sua relação com fotoperíodo, frutificação, resistência estomática e fotossíntese. Pesq. Agropec. Bras. 2006, 41, 377–384. [Google Scholar] [CrossRef]
- Ferreira, S.M. O Efeito do Silício na Cultura do Algodoeiro (Gossypium hirsutum L.): Aspectos Bioquímicos, Qualidade de Fibra e Produtividade. Ph.D. Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2008. [Google Scholar]
- Ober, E.S.; Alahmad, S.; Cockram, J.; Forestan, C.; Hickey, L.T.; Kant, J.; Maccaferri, M.; Marr, E.; Milner, M.; Pinto, F.; et al. Wheat Root Systems as a Breeding Target for Climate Resilience. Theor. Appl. Genet. 2021, 134, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bodner, G.; Rewald, B.; Leitner, D.; Nagel, K.A.; Nakhforoosh, A. Root Architecture Simulation Improves the Inference from Seedling Root Phenotyping towards Mature Root Systems. J. Exp. Bot. 2017, 68, 965–982. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root Traits and Phenotyping Strategies for Plant Improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going Underground: Root Traits as Drivers of Ecosystem Processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Faucon, M.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of Modifying Root Traits in Crops for Agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Costa, A.R. As Relações Hídricas Das Plantas Vasculares; Universidade de Évora: Évora, Portugal, 2001. [Google Scholar]
- French, A.; Ubeda-Tomás, S.; Holman, T.J.; Bennett, M.J.; Pridmore, T. High-Throughput Quantification of Root Growth Using a Novel Image-Analysis Tool. Plant Physiol. 2009, 150, 1784–1795. [Google Scholar] [CrossRef]
- Lobet, G.; Pagès, L.; Draye, X. A Novel Image-Analysis Toolbox Enabling Quantitative Analysis of Root System Architecture. Plant Physiol. 2011, 157, 29–39. [Google Scholar] [CrossRef]
- Medrado, L.C.; Santos, G.G.; Correchel, V.; Silva, G.C.; Flores, R.A.; Severiano, E.C.; Mesquita, M.; Figueiredo, C.C. Evaluation of Sugarcane Root Growth Through Images Obtained via the Minirhizotron Method in a Ferralsol in the Midwest Region of Brazil. Sugar Tech 2023, 25, 638–652. [Google Scholar] [CrossRef]
- Dakora, F.D.; Nelwamondo, A. Silicon Nutrition Promotes Root Growth and Tissue Mechanical Strength in Symbiotic Cowpea. Funct. Plant Biol. 2003, 30, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Signora, L.; De Smet, I.; Foyer, C.H.; Zhang, H. ABA Plays a Central Role in Mediating the Regulatory Effects of Nitrate on Root Branching in Arabidopsis. Plant J. 2001, 28, 655–662. [Google Scholar] [CrossRef]
- Ashfaq, W.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Silicon Improves Root System and Canopy Physiology in Wheat under Drought Stress. Plant Soil 2024, 502, 279–296. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J. Benefits of Plant Silicon for Crops: A Review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Kubicki, J.D.; Heaney, P.J. Molecular Orbital Modeling of Aqueous Organosilicon Complexes: Implications for Silica Biomineralization. Geochim. Cosmochim. Acta 2003, 67, 4113–4121. [Google Scholar] [CrossRef]
- Will, S.; Eichert, T.; Fernández, V.; Möhring, J.; Müller, T.; Römheld, V. Absorption and Mobility of Foliar-Applied Boron in Soybean as Affected by Plant Boron Status and Application as a Polyol Complex. Plant Soil 2011, 344, 283–293. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert. Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef]
- Flores, R.A.; Xavier, M.F.N. Innovative Sources and Ways of Applying Silicon to Plants. In Benefits of Silicon in the Nutrition of Plants; Springer International Publishing: Cham, Switzerland, 2023; pp. 75–85. [Google Scholar]
- Kudryavtsev, P.G.; Figovsky, O.L. Nanocomposite Organomineral Hybrid Materials. Part 2. Nanotehnol. Stroit. 2016, 8, 20–44. [Google Scholar] [CrossRef]
- Souza Júnior, J.P.; Prado, R.M.; Diniz, J.F.; Guedes, V.H.F.; Silva, J.L.F.; Roque, C.G.; Alvarez, R.C.F. Foliar Application of Innovative Sources of Silicon in Soybean, Cotton, and Maize. J. Soil Sci. Plant Nutr. 2022, 22, 3200–3211. [Google Scholar] [CrossRef]
- Flores, R.A.; Arruda, E.M.; Souza Júnior, J.P.; Prado, R.M.; Santos, A.C.A.; Aragão, A.S.; Pedreira, N.G.; Costa, C.F. Nutrition and Production of Helianthus Annuus in a Function of Application of Leaf Silicon. J. Plant Nutr. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Keeping, M.G. Uptake of Silicon by Sugarcane from Applied Sources May Not Reflect Plant-Available Soil Silicon and Total Silicon Content of Sources. Front. Plant Sci. 2017, 8, 760. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Deshmukh, R.; Sonah, H.; Bélanger, R.R. New Evidence Defining the Evolutionary Path of Aquaporins Regulating Silicon Uptake in Land Plants. J. Exp. Bot. 2020, 71, 6775–6788. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Ma, J.F. Linking Transport System of Silicon with Its Accumulation in Different Plant Species. Soil Sci. Plant Nutr. 2021, 67, 10–17. [Google Scholar] [CrossRef]
- Mitani, N.; Yamaji, N.; Ma, J.F. Identification of Maize Silicon Influx Transporters. Plant Cell Physiol. 2009, 50, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani-Ueno, N. Transport of Silicon from Roots to Panicles in Plants. Proc. Jpn. Acad. Ser. B 2011, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ma, J.F. Characterization of Substrate Specificity of a Rice Silicon Transporter, Lsi1. Pflug. Arch. Eur. J. Physiol. 2008, 456, 679–686. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Shivaraj, S.M.; Isenring, P.; Bélanger, R.R. Lsi2: A Black Box in Plant Silicon Transport. Plant Soil 2021, 466, 1–20. [Google Scholar] [CrossRef]
- Snyder, G.H.; Matichenkov, V.V.; Datnoff, L.E. Silicon. In Handbook of Plant Nutrition; Taylor & Francis: Oxfordshire, UK, 2007; pp. 551–562. [Google Scholar]
- Barreto, R.F.; Barão, L. Silicon: Transcellular and Apoplastic Absorption and Transport in the Xylem. In Benefits of Silicon in the Nutrition of Plants; Prado, R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 17–25. ISBN 978-3-031-26673-7. [Google Scholar]
- Mitani, N.; Chiba, Y.; Yamaji, N.; Ma, J.F. Identification and Characterization of Maize and Barley Lsi2-Like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef]
- Oliveira, R.L.L.; Prado, R.M.; Felisberto, G.; Checchio, M.V.; Gratão, P.L. Silicon Mitigates Manganese Deficiency Stress by Regulating the Physiology and Activity of Antioxidant Enzymes in Sorghum Plants. J. Soil Sci. Plant Nutr. 2019, 19, 524–534. [Google Scholar] [CrossRef]
- Moreira, A.R.; Fagan, E.B.; Martins, K.V.; Souza, C.H.E. Resposta da cultura de soja a aplicação de silício foliar. Biosci. J. 2010, 26, 413–423. [Google Scholar]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The Role of Silicon in Plant Biology: A Paradigm Shift in Research Approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef]
- Brunings, A.M.; Datnoff, L.E.; Ma, J.F.; Mitani, N.; Nagamura, Y.; Rathinasabapathi, B.; Kirst, M. Differential Gene Expression of Rice in Response to Silicon and Rice Blast Fungus Magnaporthe Oryzae. Ann. Appl. Biol. 2009, 155, 161–170. [Google Scholar] [CrossRef]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and Plant Disease Resistance against Pathogenic Fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.; Allsopp, P.G.; Gherlenda, A.N.; Johnson, S.N. Increased Root Herbivory under Elevated Atmospheric Carbon Dioxide Concentrations Is Reversed by Silicon-Based Plant Defences. J. Appl. Ecol. 2017, 54, 1310–1319. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; Steppe, K.; Bauweraerts, I.; Kikuchi, S.; Asano, T.; Höfte, M.; De Vleesschauwer, D. Primary Metabolism Plays a Central Role in Moulding Silicon-Inducible Brown Spot Resistance in Rice. Mol. Plant Pathol. 2015, 16, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, M.P.; Gins, V.K. Flavonoids and Silicon in Certain Plant Pollen. Chem. Nat. Compd. 1999, 35, 520–523. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Muneer, S.; Ko, C.H.; Jeong, B.R. Silicon Mitigates Salinity Stress by Regulating the Physiology, Antioxidant Enzyme Activities, and Protein Expression in Capsicum annuum ‘Bugwang’. BioMed Res. Int. 2016, 2016, 3076357. [Google Scholar] [CrossRef]
- Silva, E.S.; Prado, R.M.; Santos, D.M.M.; Cruz, F.J.R.; Júnior de Almeida, H.; Campos, C.N.S. Nitrogen Components, Growth and Gas Exchange in Spring Wheat Plants Grown under Interaction of Silicon (Si) and Nitrogen (N). Aust. J. Crop Sci. 2015, 9, 790–798. [Google Scholar]
- McCree, K.J.; Fernandez, C.J. Simulation Model for Studying Physiological Water Stress Responses of Whole Plants. Crop Sci. 1989, 29, 353–360. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, M.; Warrington, D.N. Growth and Transpiration of Maize and Winter Wheat in Response to Water Deficits in Pots and Plots. Environ. Exp. Bot. 2011, 71, 65–71. [Google Scholar] [CrossRef]
- Herrero, M.P.; Johnson, R.R. Drought Stress and Its Effects on Maize Reproductive Systems. Crop Sci. 1981, 21, 105–110. [Google Scholar] [CrossRef]
- Tombeur, F.; Cooke, J.; Collard, L.; Cisse, D.; Saba, F.; Lefebvre, D.; Burgeon, V.; Nacro, H.B.; Cornelis, J. Biochar Affects Silicification Patterns and Physical Traits of Rice Leaves Cultivated in a Desilicated Soil (Ferric lixisol). Plant Soil 2021, 460, 375–390. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Zhao, Z.G.; Chen, G.C.; Zhou, W.J. Effects of Silicon on Defense of Wheat against Oxidative Stress under Drought at Different Developmental Stages. Biol. Plant. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of Silicon Fertilization on Maize Performance Under Limited Water Supply. Silicon 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 1995; ISBN 978-0-08-057187-4. [Google Scholar]
- Bianchini, H.C.; Marques, D.J. Tolerance to Hydric Stress on Cultivars of Silicon-Fertilized Corn Crops: Absorption and Water-Use Efficiency. Biosci. J. 2019, 35, 527–539. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Snyder, G.H.; Korndörfer, G.H. Silicon in Agriculture, 1st ed.; Studies in Plant Science; Elsevier Science: Amsterdam, The Netherlands, 2001; Volume 8, ISBN 978-0-444-50262-9. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates: Sunderland, UK, 2010. [Google Scholar]
- Walters, J.P.; Archer, D.W.; Sassenrath, G.F.; Hendrickson, J.R.; Hanson, J.D.; Halloran, J.M.; Vadas, P.; Alarcon, V.J. Exploring Agricultural Production Systems and Their Fundamental Components with System Dynamics Modelling. Ecol. Model. 2016, 333, 51–65. [Google Scholar] [CrossRef]
- Flores, R.A.; Souza, M.A.P.; Andrade, A.F.; Bueno, A.M.; Abdala, K.O.; Souza Júnior, J.P.; Prado, R.M.; Santos, G.G.; Mesquita, M. Does Foliar Application of Silicon under Natural Water Stress Conditions Increase Rice Yield in Subtropical Dry Regions? Silicon 2022, 14, 3591–3600. [Google Scholar] [CrossRef]
- Flores, R.A.; Sousa, M.A.P.; Bueno, A.M.; Andrade, A.F.; Souza Júnior, J.P.; Abdala, K.O.; Prado, R.M.; Santos, G.G.; Mesquita, M. Does Foliar Silicon Application Enhance the Biomass Yield of Millet Silage, and Does It Provide Significant Economic Gains? Res. Soc. Dev. 2021, 10, e41610414232. [Google Scholar] [CrossRef]
- Freire, A.H.; Reis, R.P.; Fontes, R.E.; Veiga, R.D. Eficiência econômica da cafeicultura no Sul de Minas Gerais: Uma aplicação da fronteira de produção. Coffee Sci. 2011, 6, 172–183. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Casaroli, D.; Rodrigues, T.R.; Martins, A.P.B.; Evangelista, A.W.E.; Alves Júnior, J. Padrões de Chuva e de Evapotranspiração em Goiânia, GO. Rev. Bras. Meteorol. 2018, 33, 247–256. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Lima, H.N.; Marques, F.A.; et al. Sistema Brasileiro de Classificação de Solos, 6th ed.; Embrapa: Brasília, Brazil, 2025. [Google Scholar]
- WRB World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solos, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Kilmer, V.J. Silicon. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965; pp. 959–962. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Arruda, F.B.; Zullo Júnior, J.; Oliveira, J.B. Soil Parameters for Calculating Available Water Based on Soil Texture. Rev. Bras. Ciência Solo 1987, 11, 11–15. [Google Scholar]
- Pereira, L.S.; Valero, J.A.J.; Buendía, M.R.P.; Martín-Benito, J.M.T. El Riego y Sus Tecnologías; Centro Regional de Estudios del Agua, Universidad de Castilla-La Mancha: Albacete, Spain, 2010. [Google Scholar]
- Camargo, F.A.O.; Battisti, R.; Knapp, F.M.; Dalchiavon, F.C. Maize Yield Gain Using Irrigation in the State of Rio Grande Do Sul, Brazil. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 688–694. [Google Scholar] [CrossRef]
- Lima, M.L. Adubação Foliar Com Silício na Soja e Milho de Segunda Safra. Ph.D. Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2022. [Google Scholar]
- Agranda Sementes Semente Milho Híbrido B2433 PWU. Available online: https://www.agranda.com.br/produto/milho-hibrido-b2433-pwu (accessed on 12 July 2025).
- Brevant Milho B2433PWU. Available online: https://www.brevant.com.br/produtos/milho/b2433pwu.html (accessed on 4 August 2025).
- Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes, 1st ed.; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2009; ISBN 978-85-99851-70-8.
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2004. [Google Scholar]
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009. [Google Scholar]
- Kraska, J.E.; Breitenbeck, G.A. Simple, Robust Method for Quantifying Silicon in Plant Tissue. Commun. Soil Sci. Plant Anal. 2010, 41, 2075–2085. [Google Scholar] [CrossRef]
- Noronha, J.F. Projetos Agropecuários: Administração Financeira, Orçamento e Viabilidade Econômica, 2nd ed.; Atlas: São Paulo, Brazil, 1987. [Google Scholar]
- CEPEA. Indicador do Milho ESALQ/BM&FBOVESPA. Available online: https://www.cepea.esalq.usp.br/br/indicador/milho.aspx (accessed on 12 July 2025).
- FGV. Índice Geral de Preços. Available online: https://portalibre.fgv.br/igp (accessed on 2 July 2025).
- Fundação ABC. Planilha de Custos de Mecanização Agrícola. Available online: https://fundacaoabc.org/wp-content/uploads/2019/11/Custo-de-Mecaniza%C3%A7%C3%A3o-MAIO2019.pdf (accessed on 4 August 2025).
- Barbosa, J.C.; Maldonado Júnior, W. Experimentação Agronômica & AgroEstat—Sistema Para Análises Estatísticas de Ensaios Agronômicos; Universidade Estadual Paulista: Jaboticabal, Brazil, 2015. [Google Scholar]

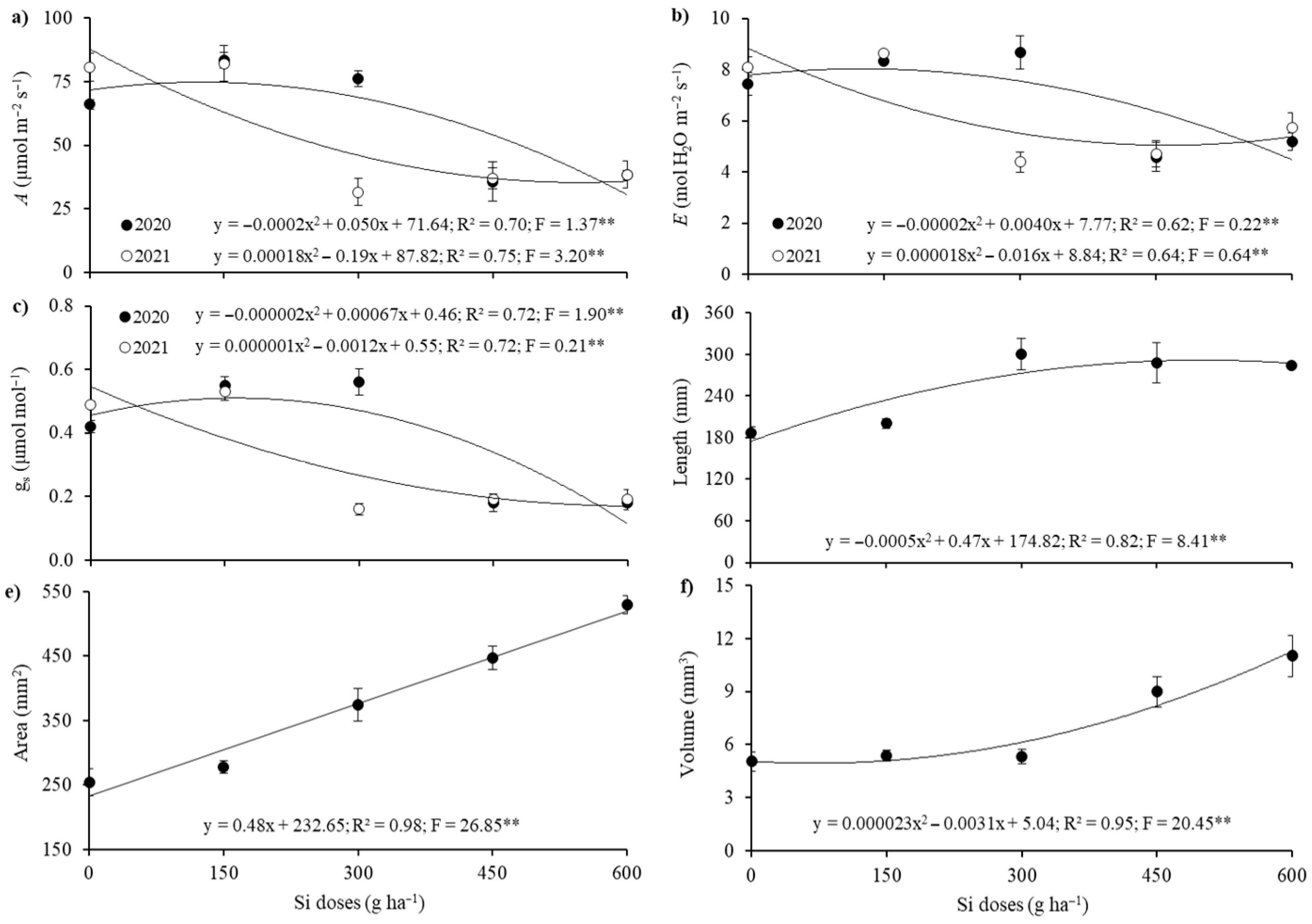
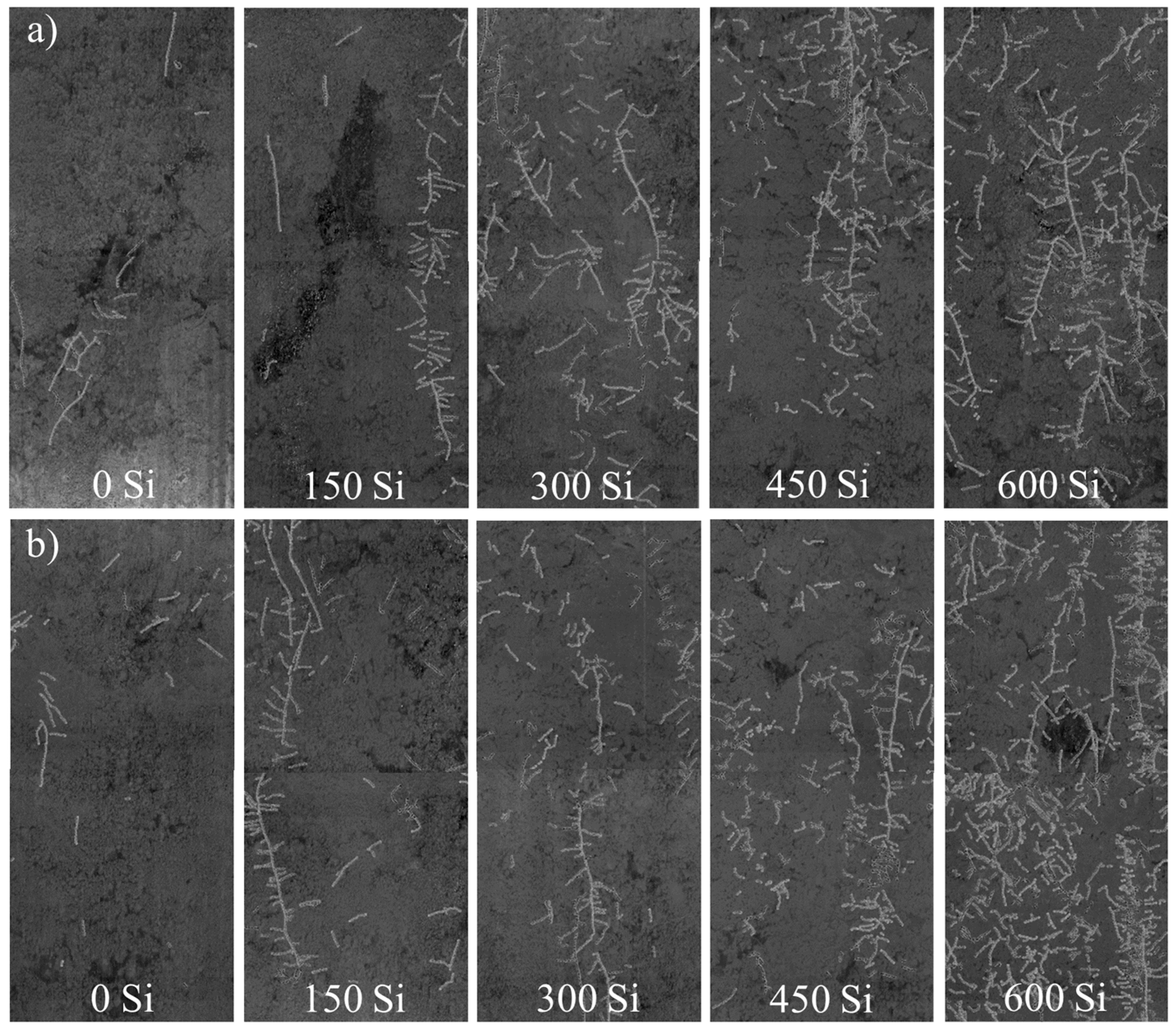
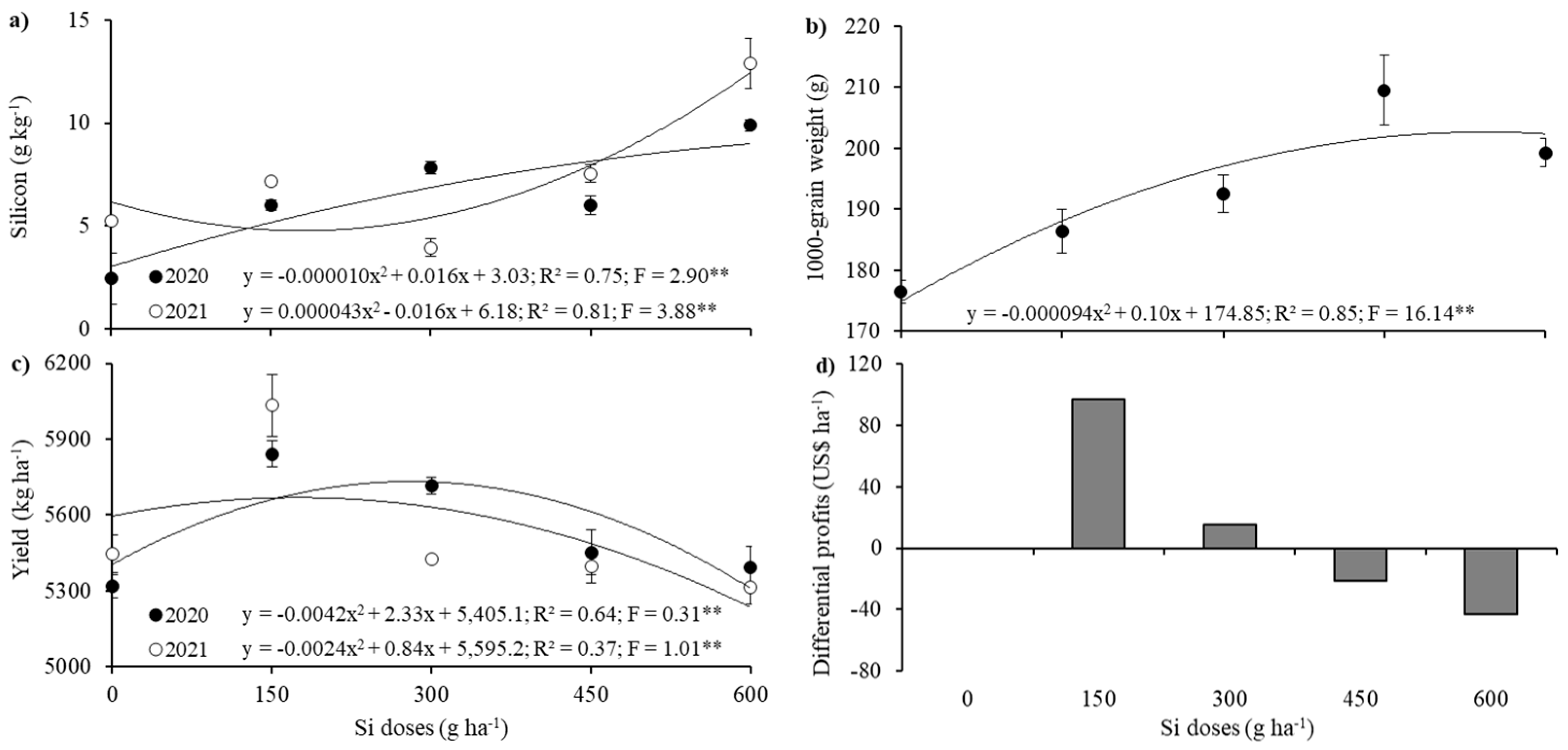
| Treatments | A | E | gs | Length | Area | Volume |
|---|---|---|---|---|---|---|
| Growing Season (GS) | µmol CO2 m−2 s−1 | mmol H2O m−2 s−1 | mol H2O m−2 s−1 | mm | mm2 | mm3 |
| 2020 | 59.94 ± 2.10 a | 6.84 ± 0.42 a | 0.38 ± 0.03 a | 254.45 ± 12.43 a | 387.75 ± 6.64 a | 7.25 ± 0.34 a |
| 2021 | 53.90 ± 2.40 b | 6.31 ± 0.39 a | 0.31 ± 0.02 b | 249.55 ± 15.64 a | 367.15 ± 11.02 a | 7.05 ± 0.33 a |
| F | 13.76 ** | 3.19 ns | 18.36 ** | 0.09 ns | 0.88 ns | 0.14 ns |
| Si dose, g ha−1 (Si) | ||||||
| 0 | 73.36 ± 1.83 b | 7.77 ± 0.42 ab | 0.45 ± 0.01 b | 187.25 ± 8.41 d | 254.25 ± 10.44 e | 5.04 ± 0.27 c |
| 150 | 82.72 ± 2.51 a | 8.46 ± 0.06 a | 0.54 ± 0.03 a | 200.25 ± 6.78 c | 277.62 ± 5.15 d | 5.36 ± 0.16 c |
| 300 | 53.82 ± 2.12 c | 6.53 ± 0.52 bc | 0.36 ± 0.03 c | 300.62 ± 22.42 a | 374.50 ± 12.53 c | 5.32 ± 0.21 c |
| 450 | 36.35 ± 2.93 d | 4.63 ± 0.54 d | 0.19 ± 0.01 d | 287.75 ± 28.58 a | 446.62 ± 8.35 b | 9.00 ± 0.43 b |
| 600 | 38.33 ± 1.84 d | 5.46 ± 0.47 cd | 0.18 ± 0.02 d | 284.12 ± 3.99 b | 529.25 ± 7.07 a | 11.02 ± 0.59 a |
| F | 129.49 ** | 22.73 ** | 81.10 ** | 8.41 ** | 26.85 ** | 20.45 ** |
| Interaction effects (GS × Si) | ||||||
| F | 37.98 ** | 10.12 ** | 27.70 ** | 0.24 ns | 0.20 ns | 0.08 ns |
| Average | 59.92 | 6.57 | 0.34 | 252.00 | 376.45 | 7.15 |
| C.V. | 9.04 | 14.30 | 14.57 | 20.79 | 16.69 | 23.72 |
| Treatments | Si | 1000 G | Yield |
|---|---|---|---|
| Growing Season (GS) | g kg−1 | g | kg ha−1 |
| 2020 | 6.44 ± 0.50 b | 191.80 ± 3.79 a | 5544 ± 65.32 a |
| 2021 | 7.36 ± 0.45 a | 193.86 ± 2.85 a | 5523 ± 62.98 a |
| F | 5.14 * | 0.54 ns | 2.33 ns |
| Si dose, g ha−1 (Si) | |||
| 0 | 3.85 ± 0.70 c | 176.43 ± 1.91 c | 5382 ± 28.96 c |
| 150 | 6.58 ± 0.20 b | 186.42 ± 3.59 bc | 5938 ± 81.40 a |
| 300 | 5.90 ± 0.35 b | 192.50 ± 3.09 b | 5570 ± 57.73 b |
| 450 | 6.78 ± 0.45 b | 209.55 ± 5.67 a | 5424 ± 102.88 bc |
| 600 | 11.40 ± 0.75 a | 199.26 ± 2.34 ab | 5353 ± 49.78 c |
| F | 36.37 ** | 16.14 ** | 18.69 * |
| Interaction effects (GS × Si) | |||
| F | 9.43 ** | 1.68 ns | 8.19 ** |
| Average | 6.90 | 192.83 | 5545 |
| C.V. | 18.83 | 4.58 | 2.68 |
| 2020 Growing Season | |||||||||
| Layer | Clay | Sand | Silt | Cu | Fe2+ | Mn | Zn | OM | pH |
| m | g kg−1 | g kg−1 | g kg−1 | mg dm−3 | mg dm−3 | mg dm−3 | mg dm−3 | g kg−1 | (CaCl2) |
| 0.00–0.20 | 320.00 | 560.00 | 120.00 | 2.00 | 53.00 | 58.00 | 4.00 | 18.00 | 4.80 |
| 0.20–0.40 | 440.00 | 470.00 | 90.00 | 2.00 | 44.00 | 46.00 | 1.80 | 21.00 | 4.80 |
| Layer | P | K | Ca2+ | Mg2+ | H+Al | Al3+ | CEC | m | BS |
| m | mg dm−3 | mg dm−3 | cmolc dm−3 | cmolc dm−3 | cmolc dm−3 | cmolc dm−3 | cmolc dm−3 | % | % |
| 0.00–0.20 | 15.20 | 160.00 | 2.40 | 1.10 | 4.30 | 0.20 | 8.20 | 4.90 | 47.60 |
| 0.20–0.40 | 3.40 | 110.00 | 2.00 | 1.00 | 4.30 | 0.10 | 7.60 | 3.00 | 43.30 |
| 2021 Growing Season | |||||||||
| Layer | Clay | Sand | Silt | Cu | Fe2+ | Mn | Zn | OM | pH |
| m | g kg−1 | g kg−1 | g kg−1 | mg dm−3 | mg dm−3 | mg dm−3 | mg dm−3 | g kg−1 | (CaCl2) |
| 0.00–0.20 | 320.00 | 560.00 | 120.00 | 1.80 | 27.00 | 29.00 | 3.30 | 29.00 | 5.10 |
| 0.20–0.40 | 440.00 | 470.00 | 90.00 | 1.80 | 30.00 | 31.00 | 4.00 | 18.00 | 4.90 |
| Layer | P | K | Ca2+ | Mg2+ | H+Al | Al3+ | CEC | m | BS |
| m | mg dm−3 | mg dm−3 | cmolc dm−3 | cmolc dm−3 | cmolc dm−3 | cmolc dm−3 | cmolc dm−3 | % | % |
| 0.00–0.20 | 25.10 | 115.00 | 2.70 | 1.50 | 2.80 | 0.00 | 7.30 | 0.00 | 61.80 |
| 0.20–0.40 | 8.30 | 64.00 | 1.80 | 1.20 | 3.10 | 0.10 | 6.30 | 3.00 | 50.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.d.L.; Flores, R.A.; Xavier, M.F.N.; Sousa, R.G.d.; Casaroli, D.; Dapper, F.P.; Capuchinho, F.F.; Santos, G.G.; Abdala, K.d.O.; Momesso, L. Silicon as a Strategy to Mitigate Abiotic Stresses and Improve Physiological Performance and Grain Yield of Maize Grown Under Tropical Climate Conditions. Plants 2025, 14, 2755. https://doi.org/10.3390/plants14172755
Lima MdL, Flores RA, Xavier MFN, Sousa RGd, Casaroli D, Dapper FP, Capuchinho FF, Santos GG, Abdala KdO, Momesso L. Silicon as a Strategy to Mitigate Abiotic Stresses and Improve Physiological Performance and Grain Yield of Maize Grown Under Tropical Climate Conditions. Plants. 2025; 14(17):2755. https://doi.org/10.3390/plants14172755
Chicago/Turabian StyleLima, Mateus de Leles, Rilner Alves Flores, Maxuel Fellipe Nunes Xavier, Renato Gomide de Sousa, Derblai Casaroli, Felipe Puff Dapper, Frank Freire Capuchinho, Glenio Guimarães Santos, Klaus de Oliveira Abdala, and Letusa Momesso. 2025. "Silicon as a Strategy to Mitigate Abiotic Stresses and Improve Physiological Performance and Grain Yield of Maize Grown Under Tropical Climate Conditions" Plants 14, no. 17: 2755. https://doi.org/10.3390/plants14172755
APA StyleLima, M. d. L., Flores, R. A., Xavier, M. F. N., Sousa, R. G. d., Casaroli, D., Dapper, F. P., Capuchinho, F. F., Santos, G. G., Abdala, K. d. O., & Momesso, L. (2025). Silicon as a Strategy to Mitigate Abiotic Stresses and Improve Physiological Performance and Grain Yield of Maize Grown Under Tropical Climate Conditions. Plants, 14(17), 2755. https://doi.org/10.3390/plants14172755






