Multidimensional Urbanization Effects on Spontaneous Plant Diversity in a Cold Climate Megacity
Abstract
1. Introduction
2. Results
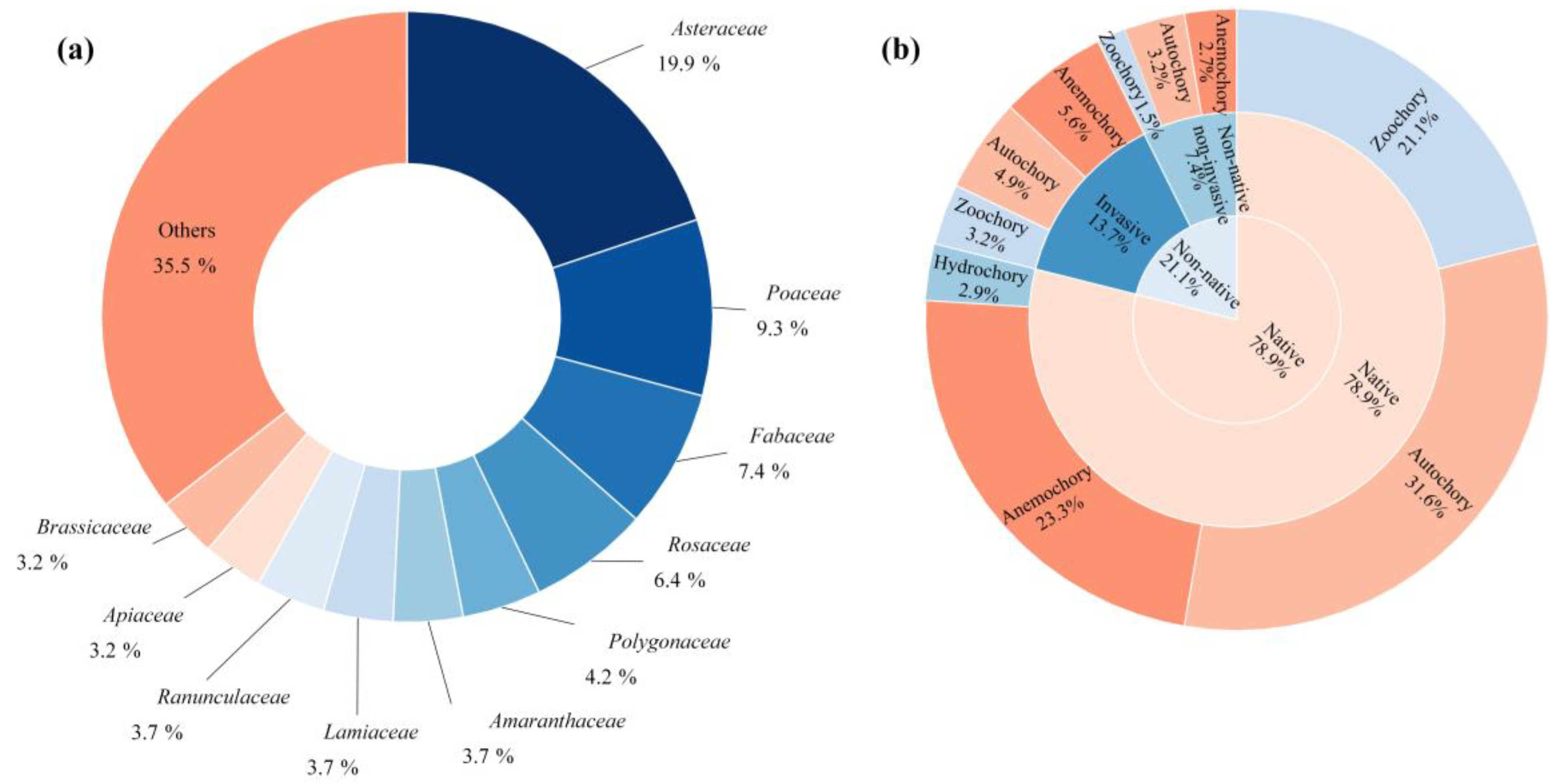
2.1. Species Composition
2.2. Diversity Pattern of Spontaneous Plant Diversity in Changchun
2.3. Environmental Drivers of Spontaneous Plant Diversity
3. Discussion
3.1. Characterizing the Species Composition of Urban Spontaneous Plants
3.2. Distribution Pattern and Causes of Urban Spontaneous Plant Diversity
3.3. Patch Characteristics and Edge Effects
3.4. Joint Effect of Horizontal and Vertical Urbanization on Plant Richness
3.5. Landscape Management Strategies for Urban Biodiversity Enhancement and Maintenance
3.6. Limitations and Future Directions
4. Materials and Methods
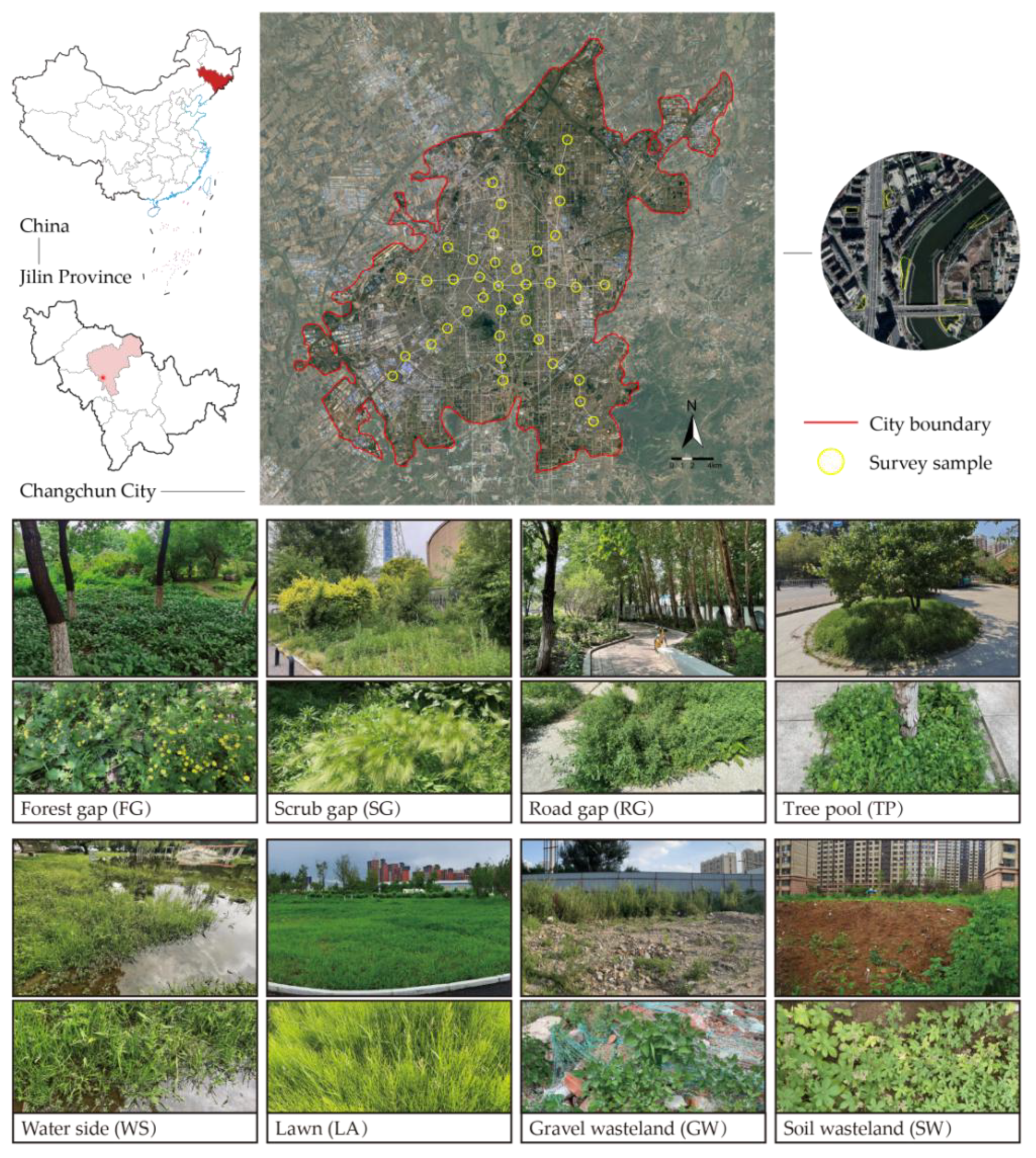
4.1. Study Area
4.2. Field Survey
4.3. Data Analysis
4.3.1. Features of Spontaneous Plant Classification
4.3.2. Species Diversity Analysis
4.3.3. Selection of Drivers for Species Richness
4.3.4. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groffman, P.M.; Cavender-Bares, J.; Bettez, N.D.; Grove, J.M.; Hall, S.J.; Heffernan, J.B.; Hobbie, S.E.; Larson, K.L.; Morse, J.L.; Neill, C.; et al. Ecological homogenization of urban USA. Front. Ecol. Environ. 2014, 12, 74–81. [Google Scholar] [CrossRef]
- Zhao, S.; Da, L.; Tang, Z.; Fang, H.; Song, K.; Fang, J. Ecological consequences of rapid urban expansion: Shanghai, China. Front. Ecol. Environ. 2006, 4, 341–346. [Google Scholar] [CrossRef]
- Norton, B.A.; Evans, K.L.; Warren, P.H. Urban Biodiversity and Landscape Ecology: Patterns, Processes and Planning. Curr. Landsc. Ecol. Rep. 2016, 1, 178–192. [Google Scholar] [CrossRef]
- Yu, D.; Wang, D.; Li, W.; Liu, S.; Zhu, Y.; Wu, W.; Zhou, Y. Decreased Landscape Ecological Security of Peri-Urban Cultivated Land Following Rapid Urbanization: An Impediment to Sustainable Agriculture. Sustainability 2018, 10, 394. [Google Scholar] [CrossRef]
- Bai, L.; Xiu, C.; Feng, X.; Liu, D. Influence of urbanization on regional habitat quality:a case study of Changchun City. Habitat Int. 2019, 93, 102042. [Google Scholar] [CrossRef]
- Marselle, M.R.; Lindley, S.J.; Cook, P.A.; Bonn, A. Biodiversity and Health in the Urban Environment. Curr. Environ. Health Rep. 2021, 8, 146–156. [Google Scholar] [CrossRef]
- Wang, D.; Xu, P.-Y.; An, B.-W.; Guo, Q.-P. Urban green infrastructure: Bridging biodiversity conservation and sustainable urban development through adaptive management approach. Front. Ecol. Evol. 2024, 12, 1440477. [Google Scholar] [CrossRef]
- Gao, Z.; Pan, Y.; Bodegom, P.M.V.; Cieraad, E.; Xing, D.; Yang, Y.; Xia, T.; Luo, X.; Song, K.; Da, L.; et al. Beta diversity of urban spontaneous plants and its drivers in 9 major cities of Yunnan province, China. Landsc. Urban Plan. 2023, 234, 104741. [Google Scholar] [CrossRef]
- Gao, Z.; Pan, Y.; Song, K.; Yang, Y.; Zhuge, M.; Wu, T.; Xia, T.; Hu, Y.; Da, L.; Cieraad, E. Response and sensitivity of urban plants with different seed dispersal modes. Nat. Cities 2024, 2, 28–37. [Google Scholar] [CrossRef]
- Gao, Z.W.; Song, K.; Pan, Y.J.; Malkinson, D.; Zhang, X.J.; Jia, B.; Xia, T.Y.; Guo, X.Y.; Liang, H.; Huang, S.S.; et al. Drivers of spontaneous plant richness patterns in urban green space within a biodiversity hotspot. Urban For. Urban Green. 2021, 61, 127098. [Google Scholar] [CrossRef]
- Prach, K.; Bartha, S.; Joyce, C.B.; Pyšek, P.; Diggelen, R.v.; Wiegleb, G. The Role of Spontaneous Vegetation Succession in Ecosystem Restoration: A Perspective. Appl. Veg. Sci. 2001, 4, 111–114. [Google Scholar] [CrossRef]
- Chen, C.D. Forgotten urban habitats: Analysis of spontaneous vegetation on the urban walls of Chongqing City. Acta Ecol. Sin. 2020, 40, 473–483. [Google Scholar] [CrossRef]
- Huang, L.; Qian, S.H.; Li, T.; Jim, C.Y.; Jin, C.; Zhao, L.; Lin, D.M.; Shang, K.K.; Yang, Y.C. Masonry walls as sieve of urban plant assemblages and refugia of native species in Chongqing, China. Landsc. Urban Plan. 2019, 191, 103620. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Dong, R.R.; Hu, Y.D. Diversity and distribution characteristics of spontaneous plants in urban industrial wasteland in Harbin. Landsc. Archit. 2024, 31, 112–120. [Google Scholar] [CrossRef]
- Liang, X.Y.; You, J.X.; Zhu, S.; Hu, Y.D. Spontaneous vegetation species diversity and distribution in heterogeneous habitats of university campus green spaces of Harbin. Chin. Landsc. Archit. 2023, 39, 138–144. [Google Scholar] [CrossRef]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Oberndorfer, E.; Lundholm, J.; Bass, B.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Koehler, M.; Liu, K.K.Y.; Rowe, B. Green roofs as urban ecosystems: Ecological structures, functions, and services. Bioscience 2007, 57, 823–833. [Google Scholar] [CrossRef]
- Zhang, L.L.; Hao, P.Y.; Dong, L.; Wang, Y.L. Optimization Strategy for Maintenance Management of Herbaceous Layer in Urban Parks Based on Spontaneous Plants: A Case Study of Xicheng District, Beijing. Landsc. Archit. 2024, 31, 46–54. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S. Distribution Patterns of Ruderal Communities and its Responses to Habitat Heterogeneity in Urban Area of Harbin. Ph.D. Thesis, East China Normal University, Shanghai, China, 2014. [Google Scholar]
- Rega-Brodsky, C.C.; Aronson, M.F.J.; Piana, M.R.; Carpenter, E.-S.; Hahs, A.K.; Herrera-Montes, A.; Knapp, S.; Kotze, D.J.; Lepczyk, C.A.; Moretti, M.; et al. Urban biodiversity: State of the science and future directions. Urban Ecosyst. 2022, 25, 1083–1096. [Google Scholar] [CrossRef]
- Liu, R.; Yan, X.; Lin, X.; Sun, Y.; Zhang, T.; Xiao, J. Urban spontaneous plant richness in response to the 2D/3D building and green space patterns in a highly urbanized area. Ecol. Indic. 2023, 154, 110852. [Google Scholar] [CrossRef]
- Malkinson, D.; Kopel, D.; Wittenberg, L. From rural-urban gradients to patch—Matrix frameworks: Plant diversity patterns in urban landscapes. Landsc. Urban Plan. 2018, 169, 260–268. [Google Scholar] [CrossRef]
- Kondratyeva, A.; Knapp, S.; Durka, W.; Kühn, I.; Vallet, J.; Machon, N.; Martin, G.; Motard, E.; Grandcolas, P.; Pavoine, S. Urbanization Effects on Biodiversity Revealed by a Two-Scale Analysis of Species Functional Uniqueness vs. Redundancy. Front. Ecol. Evol. 2020, 8, 73. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, X.; Zhang, C. Quantitative Analysis of the Determinants Influencing Urban Expansion: A Case Study in Beijing, China. Sustainability 2018, 10, 1630. [Google Scholar] [CrossRef]
- Jia, B. Plant Diversity and Community Types of Urban Green Space on Urban-Rural Gradient in Harbin. Master’s Thesis, East China Normal University, Shanghai, China, 2021. [Google Scholar]
- Su, M.; Jie, P.; Li, P.; Yang, F.; Huang, Z.; Shi, X. A review on the mechanisms behind thermal effect of building vertical greenery systems (VGS): Methodology, performance and impact factors. Energy Build. 2024, 303, 113785. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Z.; Zhang, D.; Fu, Y.; Zhai, C.; Wang, T.; Yang, Y.; Wu, J. Analysis of Urban Woody Plant Diversity among Different Administrative Districts and the Enhancement Strategy in Changchun City, China. Sustainability 2022, 14, 7624. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; He, X.; Ren, Z.; Zhai, C.; Yu, X.; Mao, Z.; Wang, P. Effects of forest type and urbanization on species composition and diversity of urban forest in Changchun, Northeast China. Urban Ecosyst. 2015, 19, 455–473. [Google Scholar] [CrossRef]
- Dong, L.D. Changchun Statistical Yearbook; China Statistics Press: Beijing, China, 2024; p. 424. [Google Scholar]
- Chang, S.; Su, K.; Jiang, X.; You, Y.; Li, C.; Wang, L. Impacts and Predictions of Urban Expansion on Habitat Connectivity Networks: A Multi-Scenario Simulation Approach. Forests 2023, 14, 2187. [Google Scholar] [CrossRef]
- Nowak, D.J. Urban Biodiversity and Climate Change. In Urban Biodiversity and Design; Wiley-Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 101–117. [Google Scholar]
- Chen, C.; Mao, L.; Qiu, Y.; Cui, J.; Wang, Y. Walls offer potential to improve urban biodiversity. Sci. Rep. 2020, 10, 9905. [Google Scholar] [CrossRef]
- Prochazka, L.S.; Alcantara, S.; Rando, J.G.; Vasconcelos, T.; Pizzardo, R.C.; Nogueira, A. Resource availability and disturbance frequency shape evolution of plant life forms in Neotropical habitats. New Phytol. 2024, 242, 760–773. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed dynamics in the Mediterranean urban ecosystem: Ecology, biodiversity and management. Weed Res. 2004, 44, 341–354. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, W.B.; Liang, H.; Liu, X.L.; Da, L.J. Dynamics of ruderal species diversity under the rapid urbanization over the past half century in Harbin, Northeast China. Urban Ecosyst. 2014, 17, 455–472. [Google Scholar] [CrossRef]
- Dubois, J.; Cheptou, P.-O. Effects of fragmentation on plant adaptation to urban environments. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2017, 372, 20160038. [Google Scholar] [CrossRef]
- Jiang, H.Q. Distribution of Spontaneous Plants in the Central City of Wuhan and Its Influencing Factor. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar]
- Tian, Z.H. Study on Distribution Patterns of Weed Communities of Terrestrial Ecosystem and Forming Reason in Urban and Rural of Shanghai. Ph.D. Thesis, East China Normal University, Shanghai, China, 2011. [Google Scholar]
- You, Q. Composition and Community Characteristics of Spontaneous Herbaceous Plants in the Main Urban Area of Zhengzhou. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2023. [Google Scholar]
- Herben, T.; Klimešová, J.; Chytrý, M. Effects of disturbance frequency and severity on plant traits: An assessment across a temperate flora. Funct. Ecol. 2018, 32, 799–808. [Google Scholar] [CrossRef]
- Kühn, I.; Klotz, S. Urbanization and homogenization—Comparing the floras of urban and rural areas in Germany. Biol. Conserv. 2006, 127, 292–300. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Handel, S.N.; La Puma, I.P.; Clemants, S.E. Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 2014, 18, 31–45. [Google Scholar] [CrossRef]
- Wang, K. Research on the Spontaneous Herbaceous Plants in Beijing Urban Area. Master’s Thesis, Beijing Forestry University, Beijing, China, 2014. [Google Scholar]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Gaertner, M.; Novoa, A.; Fried, J.; Richardson, D.M. Managing invasive species in cities: A decision support framework applied to Cape Town. Biol. Invasions 2017, 19, 3707–3723. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Li, Z. Seed dispersal modes and landscape application strategies of autogenesis herbs in the parks of Nanjing City. Chin. Landsc. Archit. 2020, 36, 119–123. [Google Scholar] [CrossRef]
- Hope, D.; Gries, C.; Casagrande, D.; Redman, C.L.; Grimm, N.B.; Martin, C. Drivers of Spatial Variation in Plant Diversity Across the Central Arizona-Phoenix Ecosystem. Soc. Nat. Resour. 2006, 19, 101–116. [Google Scholar] [CrossRef]
- Ran, C.; Pan, J.; Lin, Y.; Li, T.; Huang, Y.; Huang, J.; Fan, S.; Fang, W.; Zhao, S.; Liu, Y.; et al. Utilizing spontaneous plants for sustainable development in residential green spaces: Insights from environmental drivers and niche analysis in Fuzhou City, China. J. Environ. Manag. 2024, 364, 122219. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Ossola, A.; Hahs, A.K.; Williams, N.S.G.; Wilson, L.; Livesley, S.J. Variation in Vegetation Structure and Composition across Urban Green Space Types. Front. Ecol. Evol. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, A.; Gacem, A.; Kashyap, S.; Yadav, V.K.; Yadav, K.K.; Hussein, H.S.; Shukla, N.K.; Alsuhaibani, A.M.; Abdellattif, M.H.; et al. Multiple Site Dissimilarities of Herbaceous Species Due to Coal Fly Ash Dumping Based Soil Heavy Metal Toxication. Toxics 2023, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Tredici, P.D. Spontaneous Urban Vegetation: Reflections of Change in a Globalized World. Nat. Cult. 2010, 5, 299–315. [Google Scholar] [CrossRef]
- Xie, C.; Chen, S.; Liu, D.; Jim, C.Y. Unveiling the complex networks of urban tree diversity research: A global perspective. Ecol. Evol. 2024, 14, e11630. [Google Scholar] [CrossRef]
- Heegaard, E.; Økland, R.H.; Bratli, H.; Dramstad, W.E.; Engan, G.; Pedersen, O.; Solstad, H. Regularity of species richness relationships to patch size and shape. Ecography 2007, 30, 589–597. [Google Scholar] [CrossRef]
- Minor, E.S.; Tessel, S.M.; Engelhardt, K.A.M.; Lookingbill, T.R. The role of landscape connectivity in assembling exotic plant communities: A network analysis. Ecology 2009, 90, 1802–1809. [Google Scholar] [CrossRef]
- Connolly, B.M.; Powers, J.; Mack, R.N. Biotic constraints on the establishment and performance of native, naturalized, and invasive plants in Pacific Northwest (USA) steppe and forest. NeoBiota 2017, 34, 21–40. [Google Scholar] [CrossRef][Green Version]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 15, 265–280. [Google Scholar] [CrossRef]
- Jesson, L.; Kelly, D.; Sparrow, A. The importance of dispersal, disturbance, and competition for exotic plant invasions in Arthur’s Pass National Park, New Zealand. N. Z. J. Bot. 2000, 38, 451–468. [Google Scholar] [CrossRef]
- Uroy, L.; Mony, C.; Ernoult, A. Additive effects of connectivity provided by different habitat types drive plant assembly. Sci. Rep. 2019, 9, 13952. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, M.; Yin, F.; Zhang, M.-J.; Wei, J. Tree Cover Improved the Species Diversity of Understory Spontaneous Herbs in a Small City. Forests 2022, 13, 1310. [Google Scholar] [CrossRef]
- Borowy, D.; Swan, C.M. The effects of local filtering processes on the structure and functioning of native plant communities in experimental urban habitats. Ecol. Evol. 2022, 12, e9397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Li, X.L.; Fan, S.X.; Li, K.; Xing, X.Y.; Xu, Y.D.; Hao, P.Y.; Dong, L. Response of spontaneous plant communities to microhabitats in a riparian corridor in Beijing, China. Sci. Rep. 2024, 14, 17642. [Google Scholar] [CrossRef]
- Hu, Y.D.; S, K.K. Create Habitats to Boost Biodiversity in Urban Greening. Science 2023, 75, 6–10+14. [Google Scholar]
- Kühn, N. Intentions for the Unintentional. J. Landsc. Archit. 2006, 1, 46–53. [Google Scholar] [CrossRef]
- Huang, J.L.; Qian, S.H.; Fortin, M.J. Spatiotemporal land use dynamics filter life history strategies to shape urban spontaneous plant assemblages. Ecol. Appl. 2025, 35, e70008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lu, J.; Ma, X.; Wang, D. Constructing a Multivariate Linear Model to Investigate the Wind Propagation Dynamics of Dandelion with Analytic Hierarchy Process. Acad. J. Sci. Technol. 2024, 11, 15–21. [Google Scholar] [CrossRef]
- Gelmi-Candusso, T.A.; Hämäläinen, A.M. Seeds and the City: The Interdependence of Zoochory and Ecosystem Dynamics in Urban Environments. Front. Ecol. Evol. 2019, 7, 41. [Google Scholar] [CrossRef]
- Francis, R.A.; Chadwick, M.A. Urban invasions: Non-native and invasive species in cities. Geography 2015, 100, 144–151. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Humphries, S.E. An Integrated Approach to the Ecology and Management of Plant Invasions. Conserv. Biol. 1995, 9, 761–770. [Google Scholar] [CrossRef]
- Haase, D. Integrating Ecosystem Services, Green Infrastructure and Nature-Based Solutions—New Perspectives in Sustainable Urban Land Management. In Sustainable Land Management in a European Context: A Co-Design Approach; Weith, T., Barkmann, T., Gaasch, N., Rogga, S., Strauß, C., Zscheischler, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 305–318. [Google Scholar]
- Mcgarigal, K. FRAGSTATS Help; University of Massachusetts: Amherst, MA, USA, 2015. [Google Scholar]
- Qin, F.; Xue, T.-T.; Liang, Y.-F.; Zhang, W.-D.; Liu, Q.; Chen, T.-X.; Bussmann, R.W.; Han, B.-C.; Yu, S.-X. Present status, future trends, and control strategies of invasive alien plants in China affected by human activities and climate change. Ecography 2024, 2024, e06919. [Google Scholar] [CrossRef]
- Lin, Q.W.; Xiao, C.; Ma, J.S. A dataset on catalogue of alien plants in China. Biodivers. Sci. 2022, 30, 110–117. [Google Scholar] [CrossRef]
- E.D. Principles of dispersal in higher plants. Biol. Conserv. 1983, 26, 378. [Google Scholar] [CrossRef]
- Sohil, F.; Sohali, M.U.; Shabbir, J. An introduction to statistical learning with applications in R. Stat. Theory Relat. Fields 2021, 6, 87. [Google Scholar] [CrossRef]


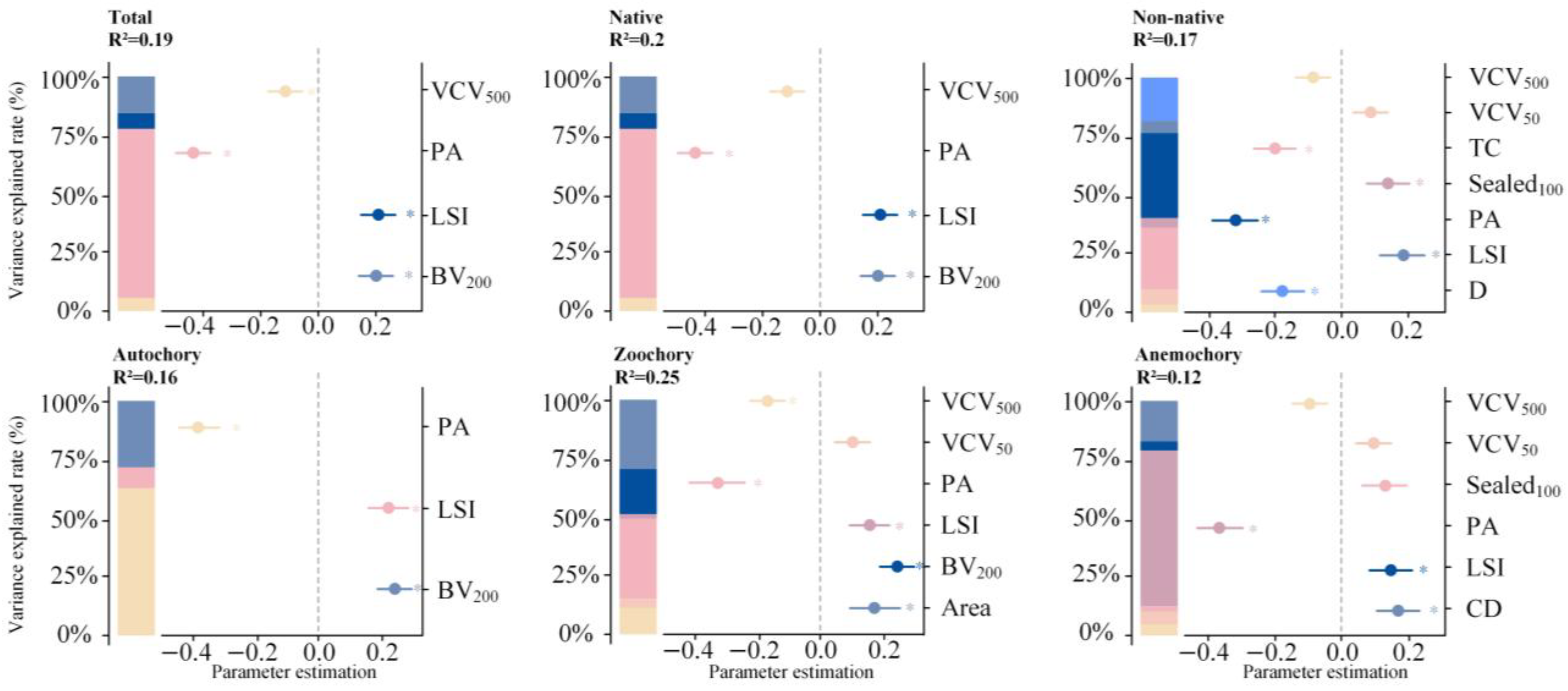
| Driving Factors | Estimate | t Value | SE | R2 | Importance | |
|---|---|---|---|---|---|---|
| Total: R2 = 0.20 adjusted R2 = 0.19 | PA | −0.4378 | −6.9360 | 0.0631 | 0.1385 | 0.4593 |
| LSI | 0.2038 | 3.2099 | 0.0635 | 0.0123 | 0.2138 | |
| BV200 | 0.1981 | 3.4122 | 0.0580 | 0.0296 | 0.2078 | |
| VCV500 | −0.1134 | −1.9693 | 0.0576 | 0.0095 | 0.1190 | |
| Native: R2 = 0.21 adjusted R2 = 0.20 | PA | −0.4509 | −7.1191 | 0.0626 | 0.1492 | 0.4665 |
| LSI | 0.1956 | 3.1055 | 0.0630 | 0.0109 | 0.2024 | |
| BV200 | 0.2086 | 3.6213 | 0.0576 | 0.0336 | 0.2159 | |
| VCV500 | −0.1113 | −1.9477 | 0.0572 | 0.0091 | 0.1152 | |
| Non-native plants: R2 = 0.19 adjusted R2 = 0.17 | PA | −0.3241 | −4.7862 | 0.0677 | 0.0601 | 0.2653 |
| LSI | 0.1772 | 2.6295 | 0.0674 | 0.0091 | 0.1451 | |
| TC | −0.2065 | −3.4588 | 0.0597 | 0.0434 | 0.1691 | |
| D | −0.1798 | −3.0264 | 0.0594 | 0.0302 | 0.1472 | |
| Sealed100 | 0.1352 | 2.0833 | 0.0649 | 0.0080 | 0.1107 | |
| VCV50 | 0.1109 | 1.8208 | 0.0609 | 0.0112 | 0.0908 | |
| VCV500 | −0.0874 | −1.4866 | 0.0588 | 0.0041 | 0.0715 | |
| Autochory: R2 = 0.17 adjusted R2 = 0.16 | PA | −0.3854 | −5.9979 | 0.0642 | 0.1011 | 0.4596 |
| LSI | 0.2158 | 3.3393 | 0.0646 | 0.0147 | 0.2574 | |
| BV200 | 0.2372 | 4.0138 | 0.0591 | 0.0449 | 0.2829 | |
| Zoochory: R2 = 0.27 adjusted R2 = 0.25 | Area | 0.1649 | 1.9795 | 0.0833 | 0.0759 | 0.1461 |
| PA | −0.3223 | −3.5303 | 0.0913 | 0.0870 | 0.2855 | |
| LSI | 0.1468 | 2.2568 | 0.0650 | 0.0058 | 0.1300 | |
| BV200 | 0.2342 | 4.1176 | 0.0568 | 0.0495 | 0.2075 | |
| VCV50 | 0.0952 | 1.6679 | 0.0571 | 0.0093 | 0.0844 | |
| VCV500 | −0.1651 | −2.9799 | 0.0554 | 0.0248 | 0.1463 | |
| Anemochory: R2 = 0.14 adjusted R2 = 0.12 | PA | −0.3592 | −5.1587 | 0.0696 | 0.0775 | 0.3630 |
| LSI | 0.1487 | 2.1789 | 0.0682 | 0.0038 | 0.1502 | |
| CD | 0.1674 | 2.7569 | 0.0607 | 0.0203 | 0.1692 | |
| Sealed100 | 0.1304 | 1.9494 | 0.0669 | 0.0028 | 0.1317 | |
| VCV50 | 0.0918 | 1.4660 | 0.0626 | 0.0065 | 0.0928 | |
| VCV500 | −0.0918 | −1.5241 | 0.0602 | 0.0046 | 0.0928 |
| Types of Green Spaces | Number of Patches | Habitat Types | Number of Plots |
|---|---|---|---|
| Park green space (P-G) | 40 | Forest gap (FG) | 389 |
| Road green space (R-G) | 52 | Scrub gap (SG) | 312 |
| Affiliated green space (I-G) | 30 | Road gap (RG) | 221 |
| Residential green space (RE-G) | 102 | Tree pool (TP) | 13 |
| Square green space (PL-G) | 11 | Water side (WS) | 22 |
| Brownfield site (B-F) | 9 | Lawn (LA) | 146 |
| Protected green space (PR-G) | 1 | Gravel wasteland (GW) | 21 |
| Soil wasteland (SW) | 23 |
| Characterization Factors | Formula | |
|---|---|---|
| Patch characteristic factors | Patch area (Area) | |
| Patch’s perimeter–area ratio (PA) | PA = Perimeter/Area | |
| Landscape shape index (LSI) | ||
| The woody layer cover of the patch (TC) | TC = Area of woody plants/Area of patch, proportion of plants in each patch | |
| Disturbance intensity (D) | ||
| Urbanization characterization factors | Distance from patch to city center (CD) | |
| The proportion of impervious surface within a 100-m buffer zone around the patches (Sealed100) | Sealed.patch = The area sealed/Total area of site, proportion of sealed surface in each patch | |
| Total building volume within a 200-m buffer zone around the patches (BV200) | BV.patch = The product of building height and building area within the patch buffer | |
| Building volume coefficient of variation around the patches within different radii (VCV50, VCV500) | VCV.patch = Standard deviation of building volume/Average value of building volume |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, C.; Yu, M.; Hu, Y.; Gao, Z. Multidimensional Urbanization Effects on Spontaneous Plant Diversity in a Cold Climate Megacity. Plants 2025, 14, 2753. https://doi.org/10.3390/plants14172753
Wang X, Zhao C, Yu M, Hu Y, Gao Z. Multidimensional Urbanization Effects on Spontaneous Plant Diversity in a Cold Climate Megacity. Plants. 2025; 14(17):2753. https://doi.org/10.3390/plants14172753
Chicago/Turabian StyleWang, Xingyuan, Congcong Zhao, Mingyu Yu, Yuandong Hu, and Zhiwen Gao. 2025. "Multidimensional Urbanization Effects on Spontaneous Plant Diversity in a Cold Climate Megacity" Plants 14, no. 17: 2753. https://doi.org/10.3390/plants14172753
APA StyleWang, X., Zhao, C., Yu, M., Hu, Y., & Gao, Z. (2025). Multidimensional Urbanization Effects on Spontaneous Plant Diversity in a Cold Climate Megacity. Plants, 14(17), 2753. https://doi.org/10.3390/plants14172753






