Production and Post-Harvest Quality of Guava Under Saline Water Irrigation Strategies and Foliar Application of Ascorbic Acid
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
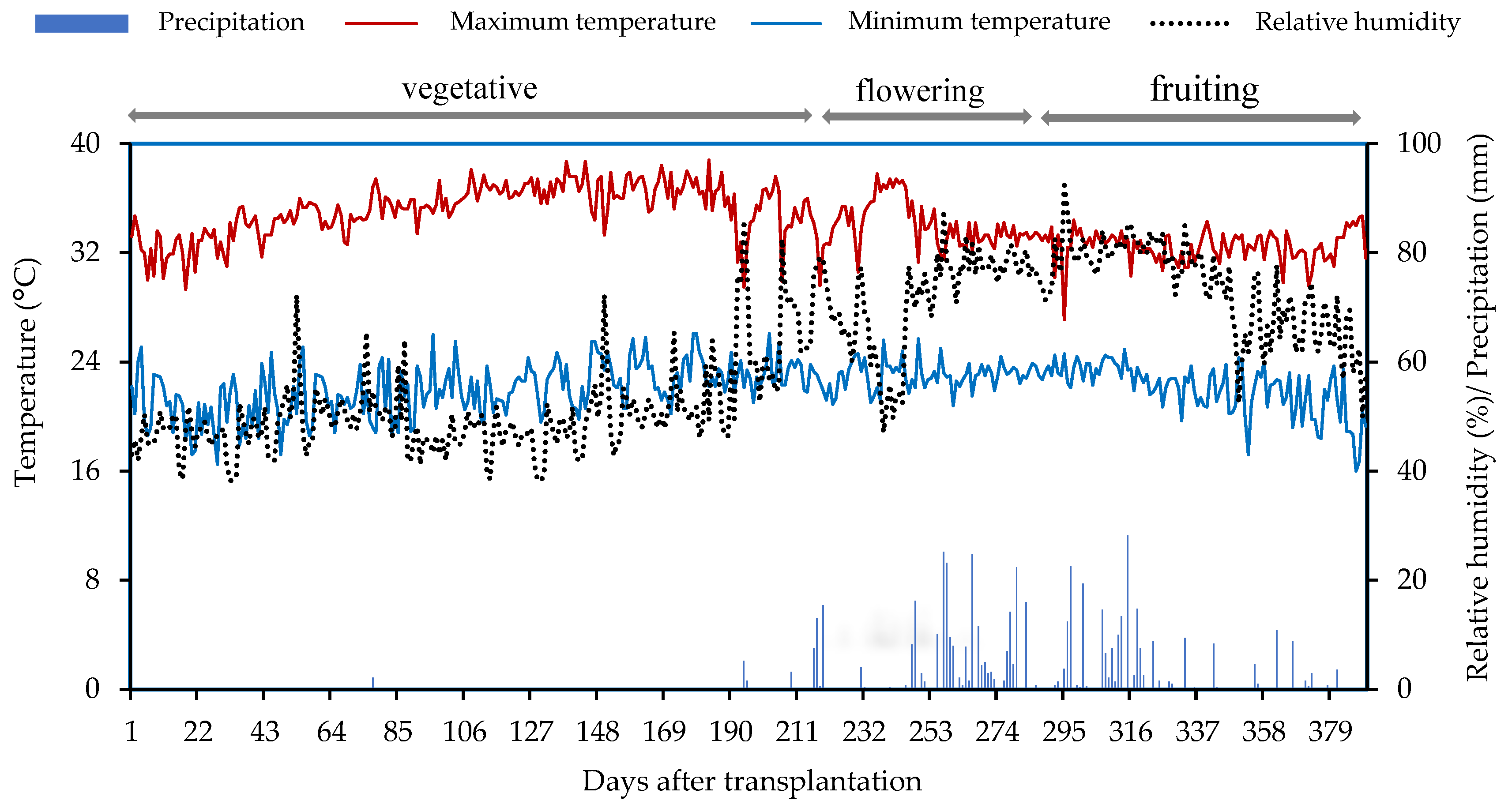
4.1. Location of the Experiment
4.2. Treatments and Experimental Design
4.3. Description of the Experiments
- C = Quantity of salts to be added (mg L−1);
- ECw = Difference between the desired electrical conductivity (3.3 dS m−1) of the water (dS m−1) and the initial value of the water used (2.6 dS m−1).
- Vw = Volume of water to be applied (mL);
- Va = volume applied in the previous irrigation event (mL);
- Vd = Volume of drained water (mL); and
- LF = leaching fraction of 0.10 applied every 20 days.
4.4. Variables Analyzed
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walter, F.H.D.B.; Cavalcante, N.R.; Viana, A.P.; Santos, E.A.; Mendes, D.S.; Oliveira, J.A.V.S.d.; Boechat, M.S.B. Genetic variability and population structure in guava full-sib families via microsatellite markers. Rev. Bras. Frutic. 2024, 46, e-638. [Google Scholar] [CrossRef]
- IBGE-Instituto Brasileiro de Geografia e Estatística. Produção Agrícola Municipal 2023. Available online: https://sidra.ibge.gov.br/pesquisa/pam/tabelas (accessed on 27 January 2024).
- Zaid, A.; Prajapati, P.; Singh, P.; Jaiswal, E.; Yadav, H.; Gaund, M.K.; Mishra, A.; Gautam, H.K.; Kumar, L. Molecular exploration and nutritional composition of guava. Int. J. Adv. Biochem. Res. 2024, 8, 695–702. [Google Scholar] [CrossRef]
- Simões, W.L.; de Andrade, V.P.M.; da Silva, J.S.; Santos, C.A.F.; de Sousa, J.S.C.; Calgaro, M.; Barbosa, K.V.F.; Nascimento, B.R.D. Fruit production and quality of ‘Paluma’ guava with nematode-tolerant rootstock irrigated in the semi-arid region. Rev. Bras. Eng. Agríc. Ambient. 2023, 27, 400–406. [Google Scholar] [CrossRef]
- Magalhães, H.F.; Feitosa, I.S.; de Lima Araújo, E.; Albuquerque, U.P. Perceptions of risks related to climate change in agroecosystems in a semi-arid region of Brazil. Hum. Ecol. 2021, 49, 403–413. [Google Scholar] [CrossRef]
- Nunes, K.G.; Costa, R.N.T.; Cavalcante, I.N.; Gondim, R.S.; Lima, S.C.R.V.; Mateos, L. Ground water resources for agricultural purposes in the Brazilian semi-arid region. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 915–923. [Google Scholar] [CrossRef]
- Santos, G.P.; Cavalcante, L.F.; Nascimento, J.A.M.; Lima Neto, A.J.; Medeiros, S.A.S.; Cavalcante, Í.H.L. Nutritional status of yellow passion fruit fertilized with phosphorus sources and doses. J. Soil. Sci. Plant Nutr. 2018, 18, 388–402. [Google Scholar] [CrossRef]
- Fischer, G.; Melgarejo, L.F. Ecophysiological aspects of guava (Psidium guajava L.): A review. Rev. Colomb. Cienc. Hortíc. 2021, 15, e12355. [Google Scholar] [CrossRef]
- Nóbrega, J.S.; Gomes, V.R.; Soares, L.A.D.A.; de Lima, G.S.; da Silva, A.A.R.; Gheyi, H.R.; Torres, R.A.F.; da Silva, F.J.L.; da Silva, T.I.; da Costa, F.B.; et al. Hydrogen peroxide alleviates salt stress effects on gas exchange, growth, and production of naturally colored cotton. Plants 2024, 13, 390. [Google Scholar] [CrossRef]
- da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Gheyi, H.R.; de Souza, A.R.; Fernandes, P.D. Salicylic acid relieves the effect of saline stress on soursop morphophysiology. Ciênc. Agrotecnol. 2021, 45, e007021. [Google Scholar] [CrossRef]
- de Lacerda, C.N.; de Lima, G.S.; Soares, L.A.D.A.; de Fátima, R.T.; Gheyi, H.R.; de Azevedo, C.A.V. Morphophysiology and production of guava as a function of water salinity and salicylic acid. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 451–458. [Google Scholar] [CrossRef]
- Pinheiro, F.W.A.; de Lima, G.S.; Gheyi, H.R.; Soares, L.A.D.A.; Nobre, R.G.; Fernandes, P.D. Brackish water irrigation strategies and potassium fertilization in the cultivation of yellow passion fruit. Ciênc. Agrotecnol. 2022, 46, e022621. [Google Scholar] [CrossRef]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Alyemeni, M.N. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sharma, A.; Srivastava, A. Ascorbic acid: A metabolite switch for designing stress-smart crops. Crit. Rev. Biotechnol. 2024, 44, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- de Fatima, R.T.; de Lima, G.S.; Soares, L.A.D.A.; Oliveira de Sá, V.K.N.; Guedes, M.A.; Ferreira, J.T.A.; Pereira, W.E. Effect of different timing of water deficit combined with foliar application of ascorbic acid on physiological variables of sour passion fruit. Arid. Land. Res. Manag. 2024, 39, 237–261. [Google Scholar] [CrossRef]
- Torres, R.A.; Nóbrega, J.S.; de Lima, G.S.; Soares, L.A.D.A.; Ferreira, J.T.; Dantas, M.V.; Gheyi, H.R.; Roque, I.A. Ascorbic acid as an elicitor of salt stress on the physiology and growth of guava. Rev. Caatinga 2025, 38, e12425. [Google Scholar] [CrossRef]
- Sahu, S.; Barman, K.; Singh, A. Nitric oxide application for postharvest quality retention of guava fruits. Acta Physiol. Plant. 2020, 42, 156. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Yan, M.; Yu, J.; Zhou, X.; Bao, J.; Zhang, Q.; Wu, C. Effect of saline–alkali stress on sugar metabolism of jujube fruit. Horticulturae 2022, 8, 474. [Google Scholar] [CrossRef]
- de Lacerda, C.N.; de Lima, G.S.; Soares, L.A.D.A.; Arruda, T.F.L.; da Silva, A.A.R.; Gheyi, H.R.; Dantas, M.V. Foliar application of ascorbic acid in guava cultivation under water replacement levels. Rev. Caatinga 2025, 38, e12595. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, M.; Zhang, Q.; Tan, H.; Li, L.; Tang, Y.; Li, Z.; Peng, M.; Deng, W. Metabolism and regulation of ascorbic acid in fruits. Plants 2022, 11, 1602. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef]
- Silva, H.S.S.; Cavalcante, L.F.; Cavalcante, Í.H.L.; de Andrade, R.A.; de Souza, A.P.; Bezerra, F.T.C.; Souto, A.G.D.L.; Dos Santos, R.F. Nutritional status and productivity of guava tree (Psidium guajava L.) irrigated with saline water and treated with biostimulants. Rev. Aracê 2025, 7, 4300–4324. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Thapa, T.; Rawat, V. Impact of post-harvest treatments of plant growth regulators and antioxidant on the quality of guava (Psidium guajava L.). J. Food Chem. Nanotechnol. 2023, 9, 68–75. [Google Scholar] [CrossRef]
- Arabia, A.; Munné-Bosch, S.; Muñoz, P. Ascorbic acid as a master redox regulator of fruit ripening. Postharvest Biol. Technol. 2024, 207, 112614. [Google Scholar] [CrossRef]
- Elsiddig, A.G.; Nour, N.E.A.; Yousif, A.; Ali, A. Effect of exogenous ascorbic acid on two sorghum varieties under different types of salt stress. Chilean J. Agric. Res. 2022, 82, 10–20. [Google Scholar] [CrossRef]
- Silva, W.B.d.; Silva, G.M.C.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Missobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Kalsi, B.S.; Singh, S.; Alam, M.S.; Bhatia, S.K. Application of thermosonication for guava juice processing: Impacts on bioactive, microbial, enzymatic and quality attributes. Ultrason. Sonochem. 2023, 99, 106595. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Cong, Y.; Zhu, P.; Xing, J.; Cui, J.; Xu, W.; Shi, Q.; Diao, M.; Liu, H. Ascorbic acid-induced photosynthetic adaptability of processing tomatoes to salt stress probed by fast OJIP fluorescence rise. Front. Plant Sci. 2021, 12, 594400. [Google Scholar] [CrossRef]
- Feng, C.; Feng, C.; Lin, X.; Liu, S.; Li, Y.; Kang, M. A chromosome-level genome assembly provides insights into ascorbic acid accumulation and fruit softening in guava (Psidium guajava). Plant Biotechnol. J. 2020, 19, 717–730. [Google Scholar] [CrossRef]
- Sonkar, V.; Kumar, S.; Yadav, S.; Kumar, L.; Mitra, D.; Meena, D.; Rawat, S.; Pal, R.K. Effect of foliar spray of micro-nutrients on fruit yield and quality of guava (Psidium guajava L.) cv. Lalit. Int. J. Adv. Biochem. Res. 2024, 8, 2506. [Google Scholar] [CrossRef]
- Nobre, R.G.; Rodrigues Filho, R.A.; de Lima, G.S.; Linhares, E.A.G.; Soares, L.A.D.A.; Silva, L.D.A.; Teixeira, A.J.S.; Macumbi, N.G.M. Gas exchange and photochemical efficiency of guava under saline water irrigation and nitrogen-potassium fertilization. Rev. Bras. Eng. Agríc. Ambient. 2023, 27, 429–437. [Google Scholar] [CrossRef]
- Silva, S.S.D.; Lima, G.S.D.; Lima, V.L.A.D.; Nóbrega, J.S.; Silva, S.T.D.A.; Ferreira, J.T.A.; Dantas, M.V.; Roque, I.A.; Soares, L.A.D.A.; Torres, R.A.F.; et al. Use of proline to induce salt stress tolerance in guava. Plants 2024, 13, 1887. [Google Scholar] [CrossRef]
- Yang, S.; Ding, Y. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, L.; Li, Y.; Li, L.; Wu, C.; Su, M.; Wu, B.; Shi, J.; Zhang, Y.; Zhang, Y.; et al. New insights on the utilitarian balance between fruit flavor development and counteracting saline-alkali stress in ‘Yali’ pear. Sci. Hortic. 2025, 331, 113911. [Google Scholar] [CrossRef]
- Caetano, E.J.M.; da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Veloso, L.L.D.S.A.; Arruda, T.F.D.L.; de Souza, A.R.; Soares, L.A.D.A.; Gheyi, H.R.; Dias, M.D.S.; et al. Application techniques and concentrations of ascorbic acid to reduce saline stress in passion fruit (Passiflora edulis). Plants 2024, 13, 2718. [Google Scholar] [CrossRef] [PubMed]
- Shamili, M.; Homaei, A. Effect of putrescine on biochemical and physiological characteristics of guava (Psidium guajava L.) seedlings under salt stress. Sci. Hortic. 2020, 261, 108961. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, J.; Qin, S.; Yang, W.; Qiao, X.; Yang, B. Effects of genetic background and altitude on sugars, malic acid and ascorbic acid in fruits of wild and cultivated apples (Malus sp.). Foods 2021, 10, 2950. [Google Scholar] [CrossRef]
- Farooq, M.; Ahmad, R.; Shahzad, M.; Sajjad, Y.; Hassan, A.; Shah, M.M.; Siyar, M.; Anjum, S.; Khan, S.A. Differential variations in total flavonoid content and antioxidant enzyme activities in pea under different saline and water stresses. Sci. Hortic. 2021, 287, 110258. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Crizel, R.L.; Reisser, P.L.; Perin, E.C.; da Silva Messias, R.; Rombaldi, C.V.; Galli, V. Changes in the abscisic acid, phenylpropanoids and ascorbic acid metabolism during strawberry fruit growth and ripening. J. Food Compos. Anal. 2022, 108, 104398. [Google Scholar] [CrossRef]
- Wei, L.; Liu, H.; Ni, Y.; Dong, J.; Zhong, C.; Sun, R.; Guo, Y.; Wei, M.; Zhang, H.; Gao, Y. FaAKR23 modulates ascorbic acid and anthocyanin accumulation in strawberry (Fragaria × ananassa) fruits. Antioxidants 2022, 11, 1828. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Chai, W.; Wei, Q.; Yu, Z.; Wang, L. Effect of ascorbic acid on tyrosinase and its anti-browning activity in fresh-cut Fuji apple (Malus domestica). J. Food Biochem. 2021, 46, e13995. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Anthocyanins: Biotechnological targets for enhancing crop tolerance to salinity stress. Sci. Hortic. 2023, 319, 112182. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Wang, Y.; Xu, Y.; Li, J.; Zhao, S.; Liu, C.; Liu, Y. Combined transcriptomic and metabolomic analysis reveals the role of the phenylpropanoid biosynthesis pathway in the salt tolerance process of Sophora alopecuroides. Int. J. Mol. Sci. 2021, 22, 2399. [Google Scholar] [CrossRef]
- Yan, S.; Guo, Y.; Meng, T.; Tian, Y.; Li, J. Comprehensive evaluation of effects of various carbon-rich amendments on tomato production under continuous saline water irrigation: Overall soil quality, plant nutrient uptake, crop yields and fruit quality. Agric. Water Manag. 2021, 255, 106995. [Google Scholar] [CrossRef]
- Saleem, M.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, I.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 187, 234–241. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Bezerra, I.L.; Gheyi, H.R.; Nobre, R.G.; De Lima, G.S.; dos Santos, J.B.; Fernandes, P.D. Interaction between soil salinity and nitrogen on growth and gaseous exchanges in guava. Rev. Ambient. Água 2018, 13, e2130. [Google Scholar] [CrossRef]
- Embrapa. A Cultura da Goiaba, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2010. [Google Scholar]
- Medina, J.C.; Castro, J.C.; Sigrist, J.M.M.; Martin, Z.J.; Kato, K.; Maia, M.L.; Garcia, A.E.B.; Leite, R.S.S.F. Goiaba, 2nd ed.; ITAL: Campinas, Brazil, 1991. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Cavalcanti, F.J.D.A.; Lima Júnior, M.A.; Lima, J.F.W.F. Recomendações de Adubação Para o Estado de Pernambuco: 2ª Aproximação, 3rd ed.; Instituto Agronômico de Pernambuco-IPA: Recife, Brazil, 2008. [Google Scholar]
- Richards, L.A. (Ed.) Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Handbook No. 60; U.S. Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- IAL-Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análises de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- Strohecker, R.; Henning, H.M. Análisis de Vitaminas: Métodos Comprobados; Ediciones Paz Montalvo: Madrid, Spain, 1967. [Google Scholar]
- Benassi, M.T.; Antunes, A.J. A comparison of metaphosphoric and oxalic acids as extractants solutions for the determination of vitamin C in selected vegetables. Arch. Biol. Technol. 1998, 31, 507–513. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of Anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 181–207. [Google Scholar]
- Waterhouse, A.L. Polyphenolics: Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2006, 25, I1.1.1–I1.1.8. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split-plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]


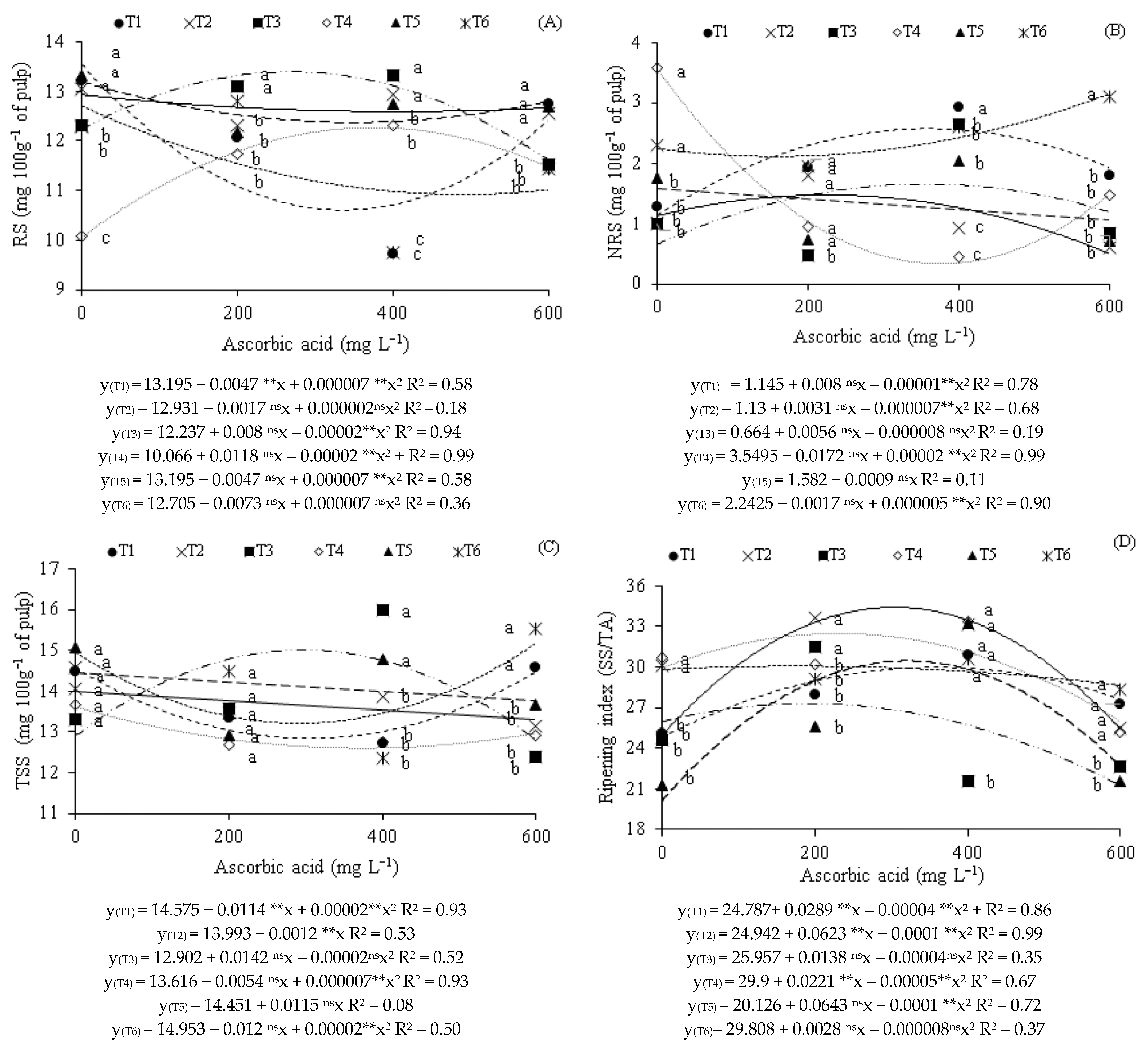
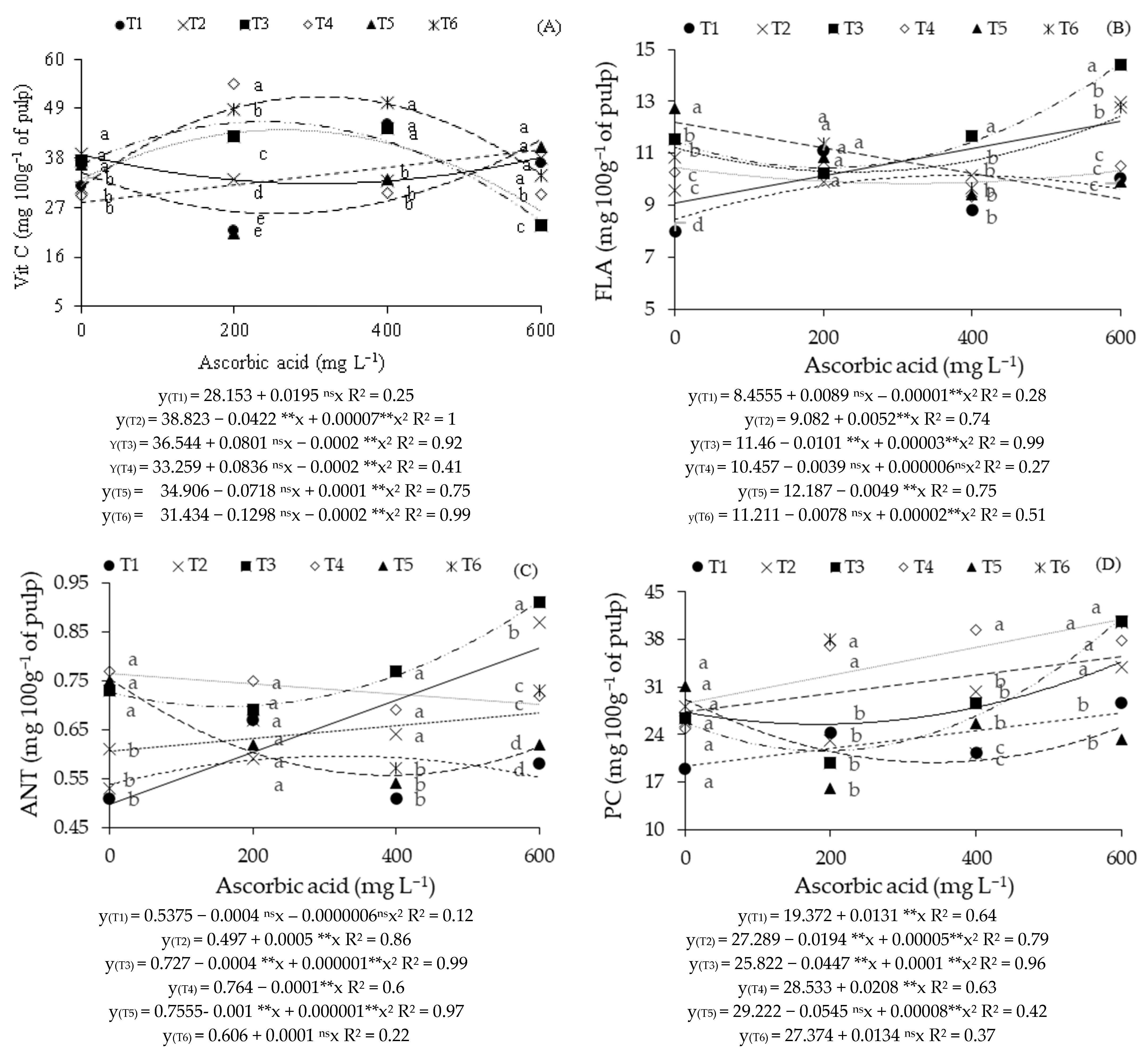


| Source of Variation | DF | Mean Squares | ||||||
|---|---|---|---|---|---|---|---|---|
| AFW | TNF | PD | ED | pH | SS | TA | ||
| Irrigation Strategies (IS) | 5 | 0.001 ns | 193.76 ** | 36.58 ** | 32.26 ** | 0.014 ** | 7.227 ** | 0.002 ns |
| Blocks | 2 | 0.001 ns | 60.29 ns | 11.71 ns | 0.03 ns | 0.002 ns | 0.667 ns | 0.004 ns |
| Residue 1 | 10 | 0.001 | 0.20 | 6.05 | 1.78 | 0.003 * | 0.245 | 0.0013 |
| Ascorbic acid (AsA) | 3 | 0.001 ns | 7.23 ns | 32.40 ** | 54.52 ** | 0.011 ** | 1.097 ** | 0.029 ** |
| Linear regression | 1 | 0.0004 ns | 0.60 ns | 73.19 ** | 53.71 ** | 0.017 ** | 0.46 ns | 0.004 ** |
| Quadratic regression | 1 | 0.0024 ns | 0.33 ns | 9.99 ns | 170.02 ** | 0.037 ** | 0.36 ns | 0.006 ** |
| Interaction (IS × AsA) | 15 | 0.003 * | 9.34 ns | 35.47 ** | 47.05 ** | 0.011 ** | 2.368 ** | 0.002 ns |
| Residue 2 | 36 | 0.001 | 17.37 | 4.364 | 2.23 | 0.011 | 0.2359 | 0.001 |
| CV1 (%) | 19.57 | 27.24 | 3.04 | 2.00 | 0.99 | 3.96 | 8.05 | |
| CV1 (%) | 18.73 | 26.60 | 2.58 | 2.23 | 0.91 | 3.88 | 9.40 | |
| Source of Variation | DF | Mean Squares | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RS | NRS | TSS | RI | Vit C | FLA | ANT | PC | ||
| Irrigation Strategies (IS) | 5 | 8.29 ** | 4.49 ** | 36.58 ** | 55.12 ** | 100.31 ** | 8.51 ** | 0.115 ** | 229.05 ** |
| Blocks | 2 | 0.70 ns | 0.29 ns | 11.71 ns | 55.12 ns | 37.336 ns | 0.01 ns | 0.0001 ns | 3.765 ns |
| Residue 1 | 10 | 0.20 | 0.38 | 6.05 | 6.15 | 9.69 | 1.05 | 0.004 | 28.91 |
| Ascorbic acid (AsA) | 3 | 7.23 ** | 2.42 ** | 32.40 ** | 123.73 ** | 129.53 ** | 11.69 ** | 0.098 ** | 262.62 ** |
| Linear regression | 1 | 3.26 ** | 6.98 ** | 0.69 ns | 26.16 ** | 81.97 ** | 0.02 ns | 0.005 ns | 258.60 ** |
| Quadratic regression | 1 | 4.75 ** | 10.10 ** | 0.99 ** | 44.00 * | 484.25 ** | 0.73 ns | 0.001 ns | 145.28 ** |
| Interaction (IS × AsA) | 15 | 9.34 ** | 1.49 ** | 3.46 ** | 30.46 ** | 285.14 ** | 4.56 ** | 0.040 ** | 118.19 ** |
| Residue 2 | 36 | 0.15 | 0.31 | 1.08 | 6.74 | 12.62 | 0.44 | 0.004 | 16.44 |
| CV1 (%) | 3.77 | 35.38 | 6.11 | 8.92 | 8.61 | 9.61 | 9.98 | 18.84 | |
| CV1 (%) | 3.31 | 32.15 | 7.56 | 9.34 | 9.83 | 6.26 | 9.51 | 14.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.T.A.; Fátima, R.T.d.; Lima, G.S.d.; Soares, L.A.d.A.; Lima, B.d.M.; Lacerda, C.N.d.; Santos, L.F.S.; Oliveira, V.K.N.; Gheyi, H.R.; Almeida, F.d.S.; et al. Production and Post-Harvest Quality of Guava Under Saline Water Irrigation Strategies and Foliar Application of Ascorbic Acid. Plants 2025, 14, 2724. https://doi.org/10.3390/plants14172724
Ferreira JTA, Fátima RTd, Lima GSd, Soares LAdA, Lima BdM, Lacerda CNd, Santos LFS, Oliveira VKN, Gheyi HR, Almeida FdS, et al. Production and Post-Harvest Quality of Guava Under Saline Water Irrigation Strategies and Foliar Application of Ascorbic Acid. Plants. 2025; 14(17):2724. https://doi.org/10.3390/plants14172724
Chicago/Turabian StyleFerreira, Jean Telvio Andrade, Reynaldo Teodoro de Fátima, Geovani Soares de Lima, Lauriane Almeida dos Anjos Soares, Brencarla de Medeiros Lima, Cassiano Nogueira de Lacerda, Larissa Fernanda Souza Santos, Valeska Karolini Nunes Oliveira, Hans Raj Gheyi, Flávia de Sousa Almeida, and et al. 2025. "Production and Post-Harvest Quality of Guava Under Saline Water Irrigation Strategies and Foliar Application of Ascorbic Acid" Plants 14, no. 17: 2724. https://doi.org/10.3390/plants14172724
APA StyleFerreira, J. T. A., Fátima, R. T. d., Lima, G. S. d., Soares, L. A. d. A., Lima, B. d. M., Lacerda, C. N. d., Santos, L. F. S., Oliveira, V. K. N., Gheyi, H. R., Almeida, F. d. S., Silva, S. S. d., Nóbrega, J. S., Silva, L. d. A., Silva, V. M. B. d., & Azevedo, C. A. V. d. (2025). Production and Post-Harvest Quality of Guava Under Saline Water Irrigation Strategies and Foliar Application of Ascorbic Acid. Plants, 14(17), 2724. https://doi.org/10.3390/plants14172724









