Scion, Rootstock and Their Interaction Affect the Photosynthesis of Citrus
Abstract
1. Introduction
2. Results
2.1. Photosynthetic Parameters of the Citrus Scion–Rootstock Combinations
2.2. Chlorophyll Fluorescence of the Citrus Scion–Rootstock Combinations
2.3. Photosynthetic Pigments’ Content of the Citrus Scion–Rootstock Combinations
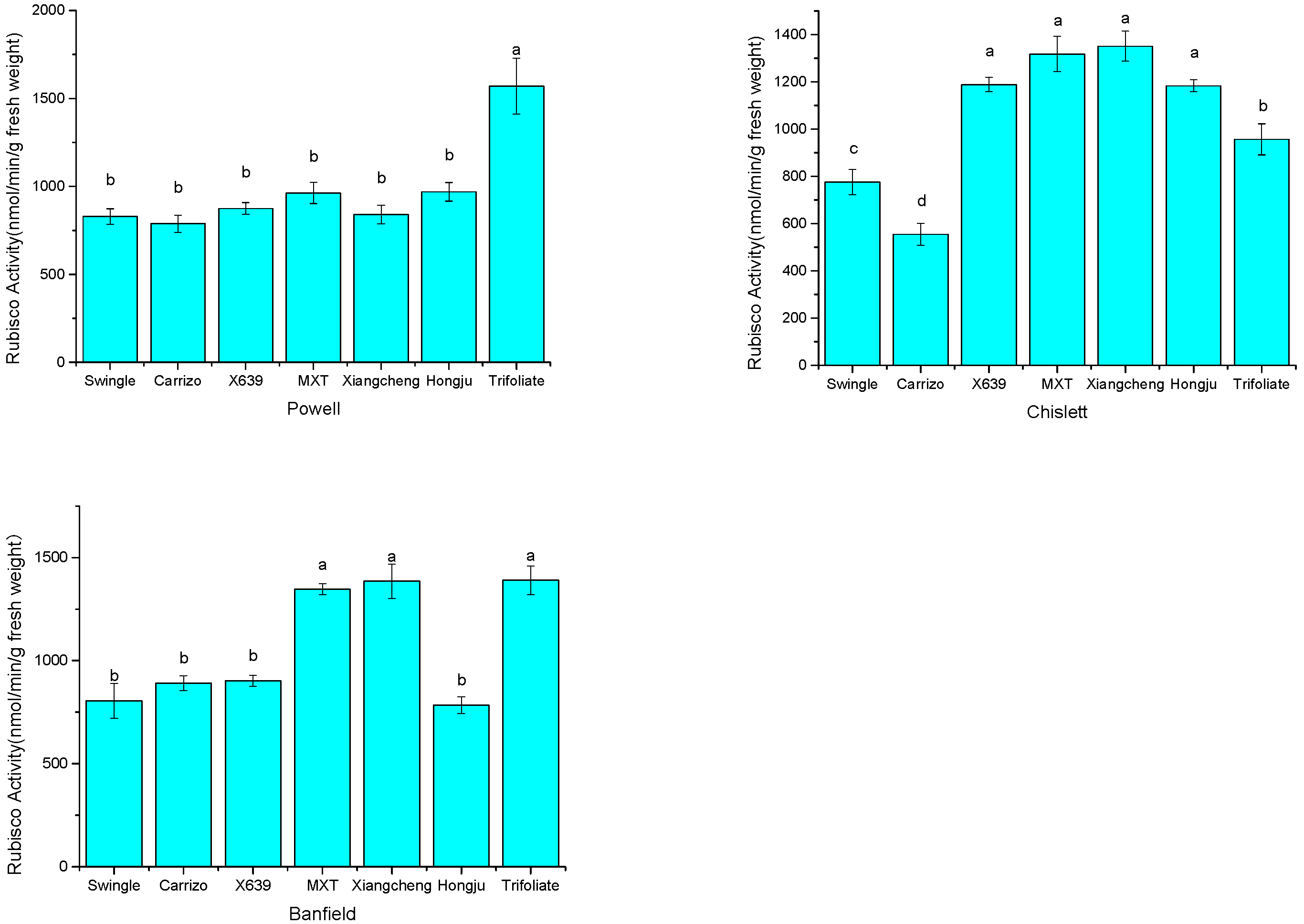
2.4. Rubisco Activity of the Citrus Scion–Rootstock Combinations
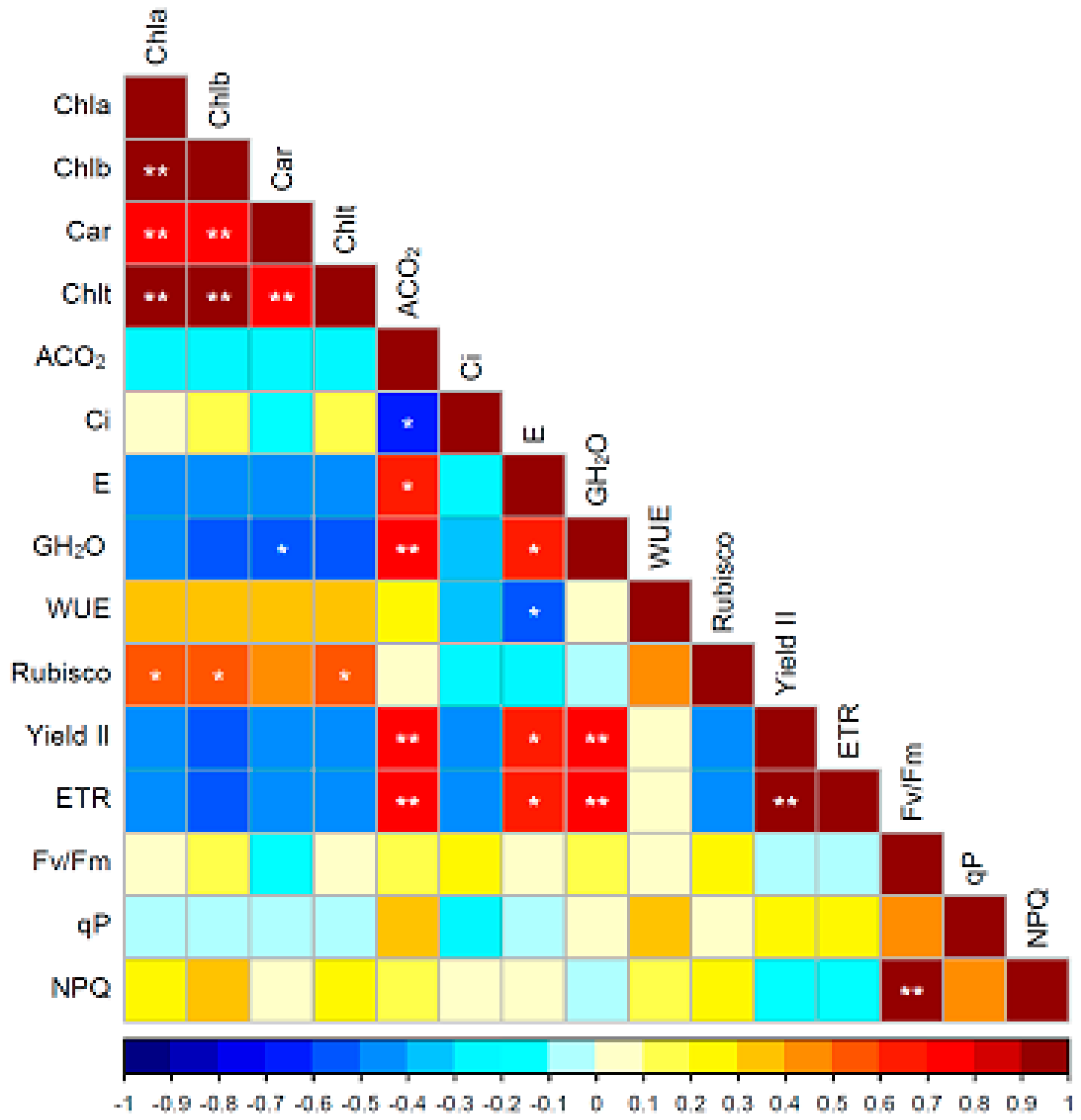
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Leaf Photosynthesis and Chlorophyll Fluorescence
4.3. Photosynthetic Pigment Determination
4.4. Determination of Rubisco Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiang, D.B.; Ma, C.R.; Song, Y.; Wu, Q.; Wu, X.Y.; Sun, Y.X.; Zhao, G.; Wan, Y. Post-anthesis photosynthetic properties provide insights into yield potential of Tartary buckwheat cultivars. Agronomy 2019, 9, 149. [Google Scholar] [CrossRef]
- Yamori, W.; Kondo, E.; Sugiura, D.; Terashima, I.; Suzuki, Y.; Makino, A. Enhanced leaf photosynthesis as a target to increase grain yield: Insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b6/f complex. Plant Cell Environ. 2016, 39, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Sukhova, E.; Sherstneva, O.; Grinberg, M.; Ladeynova, M.; Vodeneev, V.; Sukhov, V. Exogenous abscisic acid can influence photosynthetic processes in peas through a decrease in activity of H(+)-ATP-ase in the plasma membrane. Biology 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Wang, L.X. Photosynthesis. In Biological Chemistry; Wang, J.Y., Zhu, S.G., Xu, C.F., Eds.; Higher Education Press: Beijing, China, 2002; pp. 197–229. (In Chinese) [Google Scholar]
- Scheer, H. Chlorophylls and carotenoids. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 498–505. [Google Scholar]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.L.; Zhang, B.G.; Zhou, R.H.; Hu, Y.F.; Shan, L.L.; Yu, J.H. Effects of different nitrogen forms and ratios on photosynthetic characteristics of Brassica pekinensis. Acta Agri. Zhejiangensis 2015, 27, 761–768. (In Chinese) [Google Scholar]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Wu, Y.W.; Li, Q.; Jin, R.; Chen, W.; Liu, X.L.; Kong, F.L.; Ke, Y.P.; Shi, H.C.; Yuan, J.C. Effect of low-nitrigen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrigen tolerances. J. Interg. Agr. 2019, 18, 1246–1256. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Goltsev, V.N.; Zuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance-Basics and Applications; Taylor and Francis Group, CRC Press: Boca Raton, FL, USA, 2017; pp. 83–180. [Google Scholar]
- Buckley, T.N.; Diaz-Espejo, A. Partitioning changes in photosynthetic rate into contributions from different variables. Plant Cell Environ. 2015, 38, 1200–1211. [Google Scholar] [CrossRef]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.; Vesala, T.; Holtta, T. Leaf carbon and water status control stomatal and nonstomatal limitations of photosynthesis in trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.T.; Zhang, T.Y.; Liu, J.Y.; Sun, X.Z.; Sun, X.; Wang, W.L.; Zheng, C.S. Interactions between rootstock and scion during grafting and their molecular regulation mechanism. Sci. Hortic. 2023, 308, 111554. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; los Santos, M.B.; Melgarego, P.; Martinez-Nicolas, J.J.; Gomez-Perez, R.; Continella, A.; Legua, P. Comparative study of rootstocks on primary and secondary metabolites content in blood orange peel: Potential co-product perspectives. Sci. Hortic. 2025, 342, 114042. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, F.S.; Yu, H.; Zhang, M.M.; Jiang, D.; Huang, T.J.; Xiang, J.S.; Zhu, S.P.; Zhao, X.C. Effects of scion-rootstock interaction on citrus fruit quality related to differentially expressed small RNAs. Sci. Hortic. 2022, 298, 110974. [Google Scholar] [CrossRef]
- Yakushiji, H.; Sugiura, H.; Yamasaki, A.; Azuma, A.; Koshita, Y. Tree growth, productivity, and fruit quality of ‘Fuyu’ persimmon trees onto different dwarfing rootstocks. Sci. Hortic. 2021, 278, 109869. [Google Scholar] [CrossRef]
- Zhu, S.P.; Huang, T.J.; Yu, X.; Hong, Q.B.; Xiang, J.S.; Zeng, A.Z.; Gong, G.Z.; Zhao, X.C. The effects of rootstocks on performances of three late-ripening navel orange varieties. J. Integ. Agr. 2020, 19, 1802–1812. [Google Scholar] [CrossRef]
- Huang, Y.J.; Sun, L.Y.; Wang, J.L.; Chen, Y.H.; He, J.L.; Lyu, D.G. Rootstock–scion interaction affects Malus transcriptome profiles in response to cadmium. Sci. Data 2023, 10, 312. [Google Scholar] [CrossRef]
- Lailheugue, V.; Darriaut, R.; Tran, J.; Morel, M.; Marguerit, E.; Lauvergeat, V. Both the scion and rootstock of grafted grapevines influence the rhizosphere and root endophyte microbiomes, but rootstocks have a greater impact. Environ. Microbiol. 2024, 19, 24. [Google Scholar] [CrossRef]
- Praveenkumar, N.R.; Anjanappa, M.; Shilpashree, N.; Manjunathagowda, D.C. Interaction of scions and rootstocks against bacterial wilt, and grafting effects on plant growth and yield of brinjal (Solanum melongena L.). Plant Physiol. Rep. 2023, 28, 338–344. [Google Scholar] [CrossRef]
- Ranjbar, A.; Imani, A.; Piri, S.; Abdoosi, V. Grafting commercial cultivars of almonds on accurate rootstocks mitigates adverse effects of drought stress. Sci. Hortic. 2022, 293, 110725. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; El-Ezz, S.F.A. Performance of ‘Flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Machado, D.F.S.; Ribeiro, R.V.; da Silveira, J.A.G.; Magalhaes, J.R.; Machado, E.C. Rootstocks induce contrasting photosynthetic responses of orange plants to low night temperature without affecting the antioxidant metabolism. Theor. Exp. Plant Phys. 2013, 25, 26–35. [Google Scholar] [CrossRef][Green Version]
- Ozbahce, A.; Kosker, Y.; Gultekin, R.; Gorgisen, C.; Avag, K.; Demir, Y.; Yucel, S. Impact of different rootstocks and limited water on yield and fruit quality of melon grown in a field naturally infested with Fusarium wilt. Sci. Hortic. 2021, 289, 110482. [Google Scholar] [CrossRef]
- Chen, X.L.; Guo, P.J.; Wang, Z.Y.; Liang, J.Y.; Li, G.H.; He, W.W.; Zhen, A. Grafting improves growth and nitrogen-use efficiency by enhancing NO3− uptake, photosynthesis, and gene expression of nitrate transporters and nitrogen metabolizing enzymes in watermelon under reduced nitrogen application. Plant Soil. 2022, 480, 305–327. [Google Scholar] [CrossRef]
- Freitas, I.S.D.; Roldán, G.Q.; Macedo, A.C.; Mello, S.D.C. The responses of photosynthesis, fruit yield and quality of mini-cucumber to LED-interlighting and grafting. Hortic. Bras. 2021, 39, 86–93. [Google Scholar] [CrossRef]
- Hu, W.; Di, Q.; Wei, J.Y.; Zhang, J.; Liu, J. Grafting tobacco onto nutrient-efficient rootstocks improves photosynthesis. J. Am. Soc. Hortic. Sci. 2021, 146, 286–293. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, P.; Jin, H.J.; Ding, X.T.; Yu, J.Z. Effects of high temperature on the growth, photosnthesis and flurescence characteristics of grafted cucumber with different rootstocks. Acta Agri. Shanghai 2016, 32, 40–45. (In Chinese) [Google Scholar]
- De Carvalho, D.U.; Neves, C.S.V.J.; da Cruz, M.A.; Colombo, R.C.; Yada, I.F.U.; Junior, R.P.L.; Tazima, Z.H. Performance of ‘Salustiana’ sweet orange on different rootstocks under Brazilian subtropical conditions. Sci. Hortic. 2021, 287, 1–11. [Google Scholar] [CrossRef]
- Ferrer, V.; Paymal, N.; Quinton, C.; Costantino, G.; Paoli, M.; Froelicher, Y.; Ollitrault, P.; Tomi, F.; Luro, F. Influence of the rootstock and the ploidy level of the scion and the rootstock on sweet orange (Citrus sinensis) peel essential oil yield, composition and aromatic properties. Agriculture 2022, 12, 214. [Google Scholar] [CrossRef]
- Kunwar, S.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field performance of ‘Hamlin’ orange trees grown on various rootstocks in Huanglongbing-endemic conditions. HortScience 2021, 56, 244–253. [Google Scholar] [CrossRef]
- Maciá-Vázquez, A.A.; Martínez-Nicolás, J.J.; Núñez-Gómez, D.; Melgarejo, P.; Legua, P. Influence of rootstock on yield, morphological, biochemical and sensory characteristics of ‘Afourer’ variety mandarins. Sci. Hortic. 2024, 325, 112644. [Google Scholar] [CrossRef]
- Tirado-Corbalá, R.; Segarra-Carmona, A.; Matos-Rodríguez, M.; Rivera-Ocasio, D.; Estévez de Jensen, C.; Pagán, J. Assessment of two sweet orange cultivars grafted on selected rootstocks grown on an inceptisol in Puerto Rico. Horticulturae 2020, 6, 30. [Google Scholar] [CrossRef]
- Miranda, M.T.; Da Silva, S.F.; Silveira, N.M.; Pereira, L.; Machado, E.C.; Ribeiro, R.V. Root osmotic adjustment and stomatal control of leaf gas exchange are dependent on citrus rootstocks under water deficit. J. Plant Growth Regul. 2021, 40, 11–19. [Google Scholar] [CrossRef]
- Sampaio, A.H.R.; Silva, R.O.; Brito, R.B.F.; Filho, W.D.S.S.; Gesteira, A.D.S.; Souza, L.D.; Filho, M.A.C. Sweet orange acclimatisation to water stress: A rootstock dependency. Sci. Hortic. 2021, 276, 109727. [Google Scholar] [CrossRef]
- Silva, S.F.; Miranda, M.T.; Cunha, C.P.; Domingues, A.P., Jr.; Aricetti, J.A.; Caldana, C.; Machado, E.C.; Ribeiro, R.V. Metabolic profiling of drought tolerance: Revealing how citrus rootstocks modulate plant metabolism under varying water availability. Environ. Exp. Bot. 2023, 206, 105169. [Google Scholar] [CrossRef]
- Jover, S.; Martínez-Alcántara, B.; Rodríguez-Gamir, J.; Legaz, F.; Primo-Millo, E.; Forner, J.; Forner-Giner, M.A. Influence of rootstocks on photosynthesis in navel orange leaves: Effects on growth, yield, and carbohydrate distribution. Crop Sci. 2012, 52, 836–848. [Google Scholar] [CrossRef]
- Liao, L.; Cao, S.Y.; Rong, Y.; Gu, X.J.; Li, Q.N.; Ye, S.; Qiu, X.; Wang, Z.H. Effects of diferent rootstocks on photosynthetic characteristics, activities and gene expression of key enzymes of photosynthesis in Huangguogan. Acta Agri. Zhejiang 2016, 28, 769–775. [Google Scholar]
- Choi, H.G. Correlation among phenotypic parameters related to the growth and photosynthesis of strawberry (Fragaria x ananassa Duch.) grown under various light intensity conditions. Front. Plant Sci. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Gonza’lez-Mas, M.C.; Llosa, M.J.; Quijano, A.; Forner-Giner, M.A. Rootstock effects on leaf photosynthesis in ‘Navelina’ trees grown in calcareous soil. Hortscience 2009, 44, 280–283. [Google Scholar] [CrossRef]
- Slattery, R.A.; Walker, B.J.; Weber, A.P.M.; Ort, D.R. The impacts of fluctuating light on crop peformance. Plant Physiol. 2018, 176, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Ma, G.H.; Zheng, W.Y.; Song, C.F.; Sun, J.; Qin, R.J. Effects of nutrigen fertilizer level on chlorophyll fluorescence characteristics in flag leaf of super hybrid rice at late growth stage. Rice Sci. 2013, 20, 220–228. [Google Scholar] [CrossRef]
- He, Z.L.; Liu, C.X.; Wang, X.N.; Wang, R.; Tian, Y.; Chen, Y.Z. Leaf transcriptome and weight gene co-expression network analysis uncovers genes associated with photosynthetic efficiency in Camellia oleifera. Biochem. Genet. 2020, 59, 398–421. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, Y.T.; Jiang, X.L.; Jia, H.H.; Tan, C.M.; Hu, G.; Hu, Y.B.; Rao, M.J.; Deng, X.X.; Xu, Q. Genome of a citrus rootstock and global DNA demethylation caused by heterografting. Hortic. Res. 2021, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Bjorkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dabrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Kooten, O.V.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Li, H.S. Modern Plant Physiological Biochemical Experiment Principle and Technology; Higher Education Press: Beijing, China, 2000; pp. 134–137. [Google Scholar]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef]
- Tietel, Z.; Srivastava, S.; Fait, A.; Tel-Zur, N.; Carmi, N.; Raveh, E. Impact of scion/rootstock reciprocal effects on metabolomics of fruit juice and phloem sap in grafted Citrus reticulata. PLoS ONE 2020, 15, e0227192. [Google Scholar] [CrossRef]
| Scions | Rootstocks | ACO2 (µmol m−2 s−1) | Ci (μmol mol−1) | E (mmol m−2 s−1) | GH2O (mmol m−2 s−1) |
|---|---|---|---|---|---|
| BF | CA | 7.82 ± 0.24 d | 349.04 ± 3.00 a | 2.43 ± 0.13 c | 132.56 ± 0.77 bc |
| HJ | 6.67 ± 0.03 e | 325.48 ± 2.80 bc | 1.74 ± 0.03 e | 96.73 ± 2.68 f | |
| MXT | 8.93 ± 0.11 c | 335.68 ± 3.76 b | 2.84 ± 0.03 b | 122.45 ± 1.89 de | |
| TO | 9.75 ± 0.19 b | 310.34 ± 4.86 d | 2.74 ± 0.07 b | 136.43 ± 3.79 b | |
| SW | 11.22 ± 0.23 a | 327.50 ± 3.17 bc | 4.30 ± 0.08 a | 163.92 ± 3.82 a | |
| X639 | 7.14 ± 0.25 e | 353.97 ± 3.54 a | 2.18 ± 0.03 d | 117.69 ± 2.31 e | |
| ZY | 8.82 ± 0.12 c | 322.61 ± 4.20 c | 2.21 ± 0.02 d | 127.63 ± 2.66 cd | |
| CH | CA | 7.32 ± 0.16 d | 357.05 ± 0.76 a | 2.85 ± 0.10 b | 110.82 ± 3.05 cd |
| HJ | 10.19 ± 0.21 a | 304.88 ± 1.63 d | 2.54 ± 0.08 c | 120.24 ± 3.18 c | |
| MXT | 8.34 ± 0.22 c | 306.62 ± 2.53 d | 1.68 ± 0.03 e | 113.00 ± 1.23 cd | |
| TO | 5.58 ± 0.25 e | 388.59 ± 0.24 a | 2.32 ± 0.08 cd | 94.40 ± 6.34 d | |
| SW | 10.77 ± 0.18 a | 307.75 ± 3.92 d | 2.38 ± 0.01 cd | 159.31 ± 6.34 a | |
| X639 | 9.47 ± 0.09 b | 322.22 ± 5.71 c | 3.16 ± 0.11 a | 146.67 ± 4.65 b | |
| ZY | 8.47 ± 0.25 c | 349.32 ± 2.35 b | 2.28 ± 0.09 d | 90.34 ± 7.51 d | |
| PW | CA | 7.62 ± 0.08 e | 304.63 ± 3.21 d | 3.00 ± 0.02 c | 103.82 ± 1.63 c |
| HJ | 7.97 ± 0.04 d | 344.45 ± 1.67 b | 2.43 ± 0.05 d | 138.04 ± 3.09 b | |
| MXT | 12.27 ± 0.14 a | 329.06 ± 5.87 c | 3.91 ± 0.09 a | 139.23 ± 5.02 b | |
| TO | 10.12 ± 0.17 c | 329.39 ± 0.98 c | 3.27 ± 0.08 b | 153.97 ± 1.88 a | |
| SW | 11.69 ± 0.17 b | 312.94 ± 2.23 d | 3.36 ± 0.05 b | 139.73 ± 1.44 b | |
| X639 | 8.11 ± 0.03 d | 355.35 ± 1.84 a | 2.38 ± 0.03 d | 134.03 ± 2.65 b | |
| ZY | 7.07 ± 0.04 f | 336.30 ± 4.22 bc | 1.78 ± 0.04 d | 97.38 ± 3.60 c | |
| p | Scion | 1.29 × 10−9 *** | 0.7012 | 4.19 × 10−15 *** | 6.05 × 10−9 *** |
| Rootstock | 1.74 × 10−27 *** | 0.00015 ** | 1.00 × 10−25 *** | 1.68 × 10−23 *** | |
| Interaction | 8.91 × 10−26 *** | 8.47 × 10−8 *** | 3.25 × 10−28 *** | 1.10 × 10−21 *** |
| Scions | Rootstocks | Yield II | ETR (µmol Electrons m−2 s−1) | Fv/Fm | qP | NPQ |
|---|---|---|---|---|---|---|
| BF | CA | 0.28 ± 0.03 bc | 116.31 ± 2.23 b | 0.83 ± 0.00 a | 0.22 ± 0.00 a | 3.06 ± 0.02 a |
| HJ | 0.20 ± 0.02 b | 81.88 ± 1.20 d | 0.80 ± 0.01 ab | 0.21 ± 0.05 a | 2.62 ± 0.09 a | |
| MXT | 0.31 ± 0.03 a | 125.69 ± 3.26 a | 0.84 ± 0.00 a | 0.17 ± 0.04 b | 2.90 ± 0.09 a | |
| TO | 0.28 ± 0.03 ab | 114.71 ± 1.68 b | 0.74 ± 0.04 b | 0.26 ± 0.17 a | 1.50 ± 0.56 b | |
| SW | 0.32 ± 0.03 a | 132.24 ± 4.15 a | 0.83 ± 0.00 a | 0.17 ± 0.04 b | 2.70 ± 0.07 a | |
| X639 | 0.28 ± 0.04 ab | 112.33 ± 3.53 b | 0.82 ± 0.01 a | 0.17 ± 0.01 b | 2.29 ± 0.21 ab | |
| ZY | 0.23 ± 0.02 ab | 94.42 ± 0.94 c | 0.80 ± 0.01 ab | 0.19 ± 0.02 ab | 2.71 ± 0.41 a | |
| CH | CA | 0.27 ± 0.03 bc | 110.82 ± 2.14 d | 0.68 ± 0.02 b | 0.11 ± 0.00 b | 1.13 ± 0.22 d |
| HJ | 0.29 ± 0.03 abc | 120.24 ± 2.83 d | 0.81 ± 0.01 a | 0.31 ± 0.10 a | 2.98 ± 0.18 ab | |
| MXT | 0.27 ± 0.02 bc | 113.00 ± 3.20 d | 0.79 ± 0.02 a | 0.17 ± 0.02 ab | 2.03 ± 0.07 d | |
| TO | 0.23 ± 0.02 c | 94.09 ± 0.64 e | 0.82 ± 0.01 a | 0.22 ± 0.03 ab | 2.57 ± 0.04 abc | |
| SW | 0.38 ± 0.04 a | 158.39 ± 2.08 a | 0.77 ± 0.02 ab | 0.20 ± 0.03 ab | 1.89 ± 0.14 bcd | |
| X639 | 0.36 ± 0.04 ab | 146.67 ± 2.22 b | 0.80 ± 0.03 a | 0.29 ± 0.01 a | 2.68 ± 0.58 abc | |
| ZY | 0.22 ± 0.02 c | 90.22 ± 0.26 e | 0.81 ± 0.01 a | 0.24 ± 0.02 ab | 3.09 ± 0.39 a | |
| PW | CA | 0.28 ± 0.02 bc | 116.75 ± 3.60 c | 0.77 ± 0.04 b | 0.21 ± 0.02 ab | 2.56 ± 0.30 ab |
| HJ | 0.24 ± 0.04 c | 100.31 ± 1.28 d | 0.82 ± 0.01 a | 0.24 ± 0.03 ab | 2.84 ± 0.23 ab | |
| MXT | 0.36 ± 0.04 ab | 145.48 ± 0.97 b | 0.82 ± 0.00 a | 0.31 ± 0.02 a | 3.13 ± 0.05 a | |
| TO | 0.27 ± 0.03 bc | 110.92 ± 2.15 c | 0.82 ± 0.00 a | 0.22 ± 0.03 ab | 3.08 ± 0.04 a | |
| SW | 0.38 ± 0.04 a | 154.59 ± 3.33 a | 0.82 ± 0.00 a | 0.30 ± 0.07 a | 3.22 ± 0.10 a | |
| X639 | 0.22 ± 0.02 c | 90.85 ± 1.03 e | 0.83 ± 0.01 a | 0.14 ± 0.00 b | 2.40 ± 0.07 b | |
| ZY | 0.20 ± 0.02 c | 83.00 ± 0.57 f | 0.81 ± 0.01 a | 0.17 ± 0.02 b | 2.77 ± 0.28 ab | |
| p | Scion | 0.5235 | 0.00275 * | 0.0068 * | 0.2838 | 0.0033 * |
| Rootstock | 7.68 × 10−6 *** | 1.95 × 10−26 *** | 0.0114 | 0.2214 | 0.0606 | |
| Interaction | 0.0169 | 7.85 × 10−18 *** | 0.0014 * | 0.0071 * | 0.0004 ** |
| Scions | Rootstocks | Chla (μmol s−1 Kg−1 FW) | Chlb (μmol s−1 Kg−1 FW) | Car (μmol s−1 Kg−1 FW) | Chlt (μmol s−1 Kg−1 FW) |
|---|---|---|---|---|---|
| BF | CA | 12.69 ± 0.18 a | 5.52 ± 0.16 ab | 2.43 ± 0.25 ab | 18.21 ± 0.31 a |
| HJ | 12.43 ± 0.50 a | 5.41 ± 0.28 ab | 2.69 ± 0.01 a | 17.84 ± 0.75 ab | |
| MXT | 12.32 ± 0.15 a | 5.44 ± 0.18 ab | 2.52 ± 0.09 ab | 17.75 ± 0.33 ab | |
| TO | 12.05 ± 0.38 b | 5.12 ± 0.15 b | 2.49 ± 0.13 ab | 17.17 ± 0.50 b | |
| SW | 11.10 ± 0.12 c | 4.64 ± 0.14 c | 2.25 ± 0.03 b | 15.74 ± 0.24 c | |
| X639 | 12.91 ± 0.08 a | 5.53 ± 0.02 ab | 2.56 ± 0.09 ab | 18.44 ± 0.09 a | |
| ZY | 13.68 ± 0.23 a | 6.15 ± 0.20 a | 2.42 ± 0.05 ab | 19.83 ± 0.40 a | |
| CH | CA | 12.06 ± 0.03 c | 5.18 ± 0.08 b | 2.33 ± 0.12 bc | 17.24 ± 0.10 c |
| HJ | 11.19 ± 0.50 c | 4.62 ± 0.19 c | 2.31 ± 0.11 bc | 15.81 ± 0.69 c | |
| MXT | 11.15 ± 0.36 c | 4.98.0 ± 0.20 bc | 2.09 ± 0.05 c | 16.13 ± 0.55 c | |
| TO | 11.43 ± 0.08 c | 4.74 ± 0.06 c | 2.11 ± 0.16 c | 16.17 ± 0.15 c | |
| SW | 11.21 ± 0.53 c | 4.53 ± 0.22 c | 2.24 ± 0.08 bc | 15.74 ± 1.18 c | |
| X639 | 12.72 ± 0.81 b | 5.42 ± 0.37 b | 2.42 ± 0.21 ab | 18.14 ± 1.15 b | |
| ZY | 14.33 ± 0.34 a | 6.64 ± 0.25 a | 2.81 ± 0.06 a | 20.97 ± 0.69 a | |
| PW | CA | 11.83 ± 0.44 cd | 5.10 ± 0.28 cd | 2.44 ± 0.19 ab | 16.93 ± 0.68 de |
| HJ | 11.52 ± 0.21 e | 5.22 ± 0.20 cd | 2.21 ± 0.08 b | 16.74 ± 0.41 e | |
| MXT | 11.79 ± 0.23 de | 5.24 ± 0.03 cd | 2.24 ± 0.05 b | 17.03 ± 0.25 cd | |
| TO | 11.37 ± 0.33 e | 4.82 ± 0.17 d | 2.19 ± 0.13 b | 16.19 ± 0.48 e | |
| SW | 12.89 ± 0.66 bc | 5.62 ± 0.35 bc | 2.34 ± 0.15 ab | 18.51 ± 1.01 bc | |
| X639 | 13.14 ± 0.15 ab | 5.80 ± 0.07 b | 2.52 ± 0.15 ab | 18.94 ± 0.19 b | |
| ZY | 13.98 ± 0.49 a | 6.41 ± 0.13 a | 2.63 ± 0.21 a | 20.39 ± 0.61 a | |
| p | Scion | 0.3020 | 0.1502 | 0.0213 | 0.2589 |
| Rootstock | 7.21 × 10−6 *** | 5.04 × 10−8 *** | 0.0157 | 1.32 × 10−6 *** | |
| Interaction | 0.5623 | 0.3350 | 0.0719 | 0.4847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Liu, M.; Luo, G.; Hu, Z.; Zhang, X.; Xiang, J.; Yang, R.; Hu, S.; Cai, X.; Yu, X. Scion, Rootstock and Their Interaction Affect the Photosynthesis of Citrus. Plants 2025, 14, 2718. https://doi.org/10.3390/plants14172718
Zhu S, Liu M, Luo G, Hu Z, Zhang X, Xiang J, Yang R, Hu S, Cai X, Yu X. Scion, Rootstock and Their Interaction Affect the Photosynthesis of Citrus. Plants. 2025; 14(17):2718. https://doi.org/10.3390/plants14172718
Chicago/Turabian StyleZhu, Shiping, Mengyu Liu, Guotao Luo, Zhou Hu, Xiaonan Zhang, Jinsong Xiang, Rong Yang, Shixue Hu, Xiaodong Cai, and Xin Yu. 2025. "Scion, Rootstock and Their Interaction Affect the Photosynthesis of Citrus" Plants 14, no. 17: 2718. https://doi.org/10.3390/plants14172718
APA StyleZhu, S., Liu, M., Luo, G., Hu, Z., Zhang, X., Xiang, J., Yang, R., Hu, S., Cai, X., & Yu, X. (2025). Scion, Rootstock and Their Interaction Affect the Photosynthesis of Citrus. Plants, 14(17), 2718. https://doi.org/10.3390/plants14172718





