The Age-Dependent Response of Carbon Coordination in the Organs of Pinus yunnanensis Seedlings Under Shade Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Material and Experimental Design
2.3. Indicator Measurements
2.4. Statistical Analysis
3. Results and Analysis
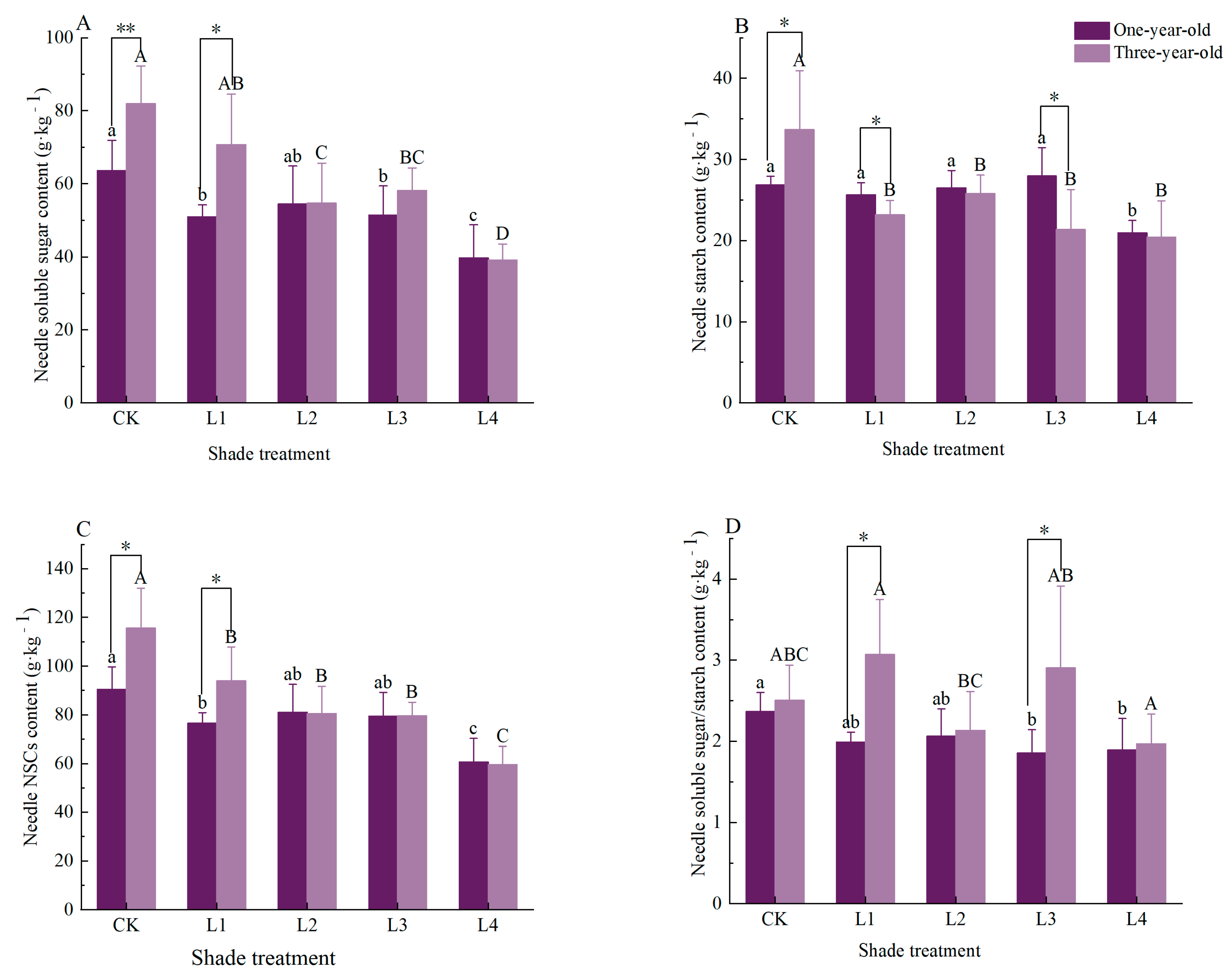
3.1. Effects of Shading on Needle Non-Structural Carbohydrates and Their Components in Pinus yunnanensis Seedlings of Different Ages
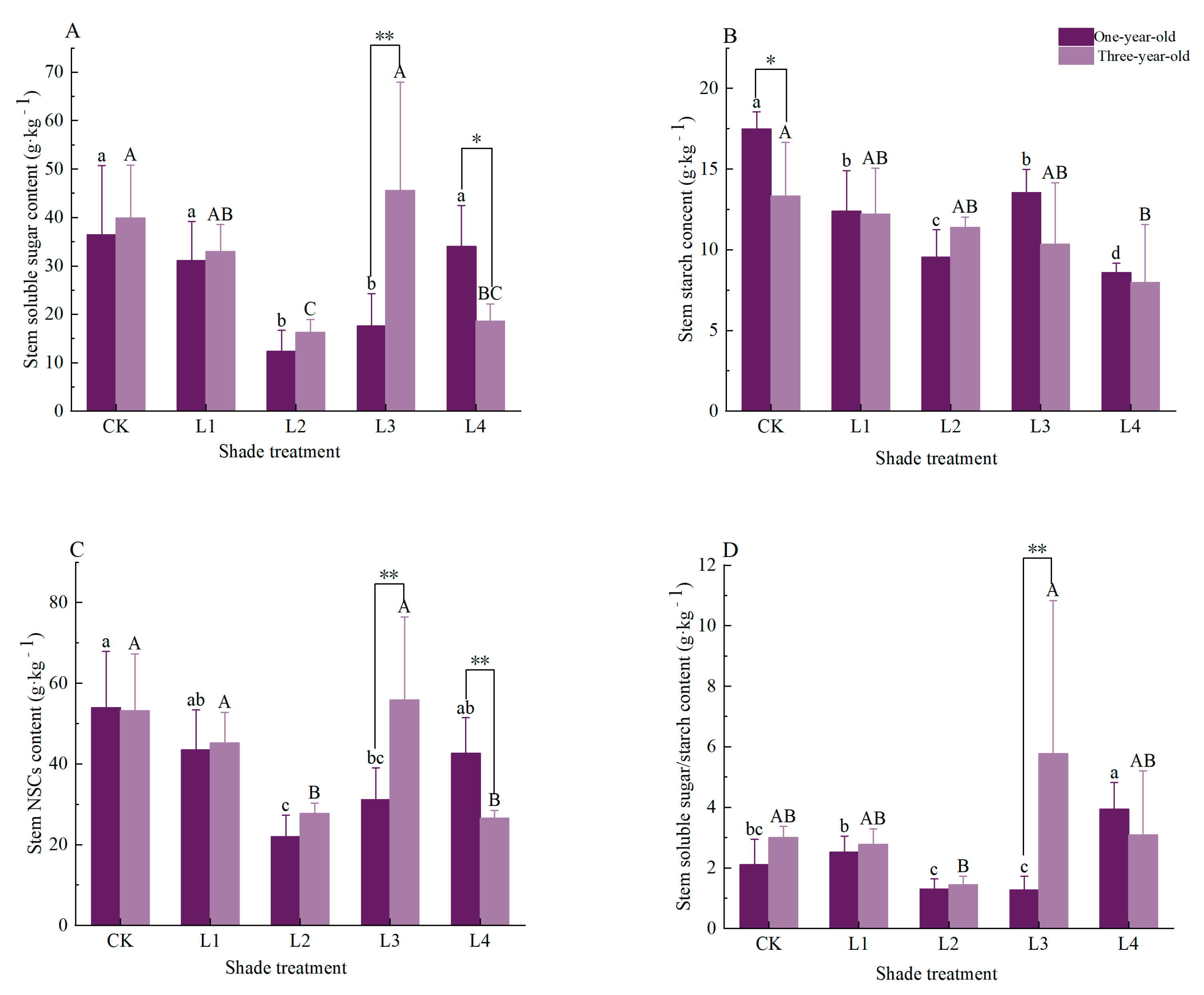
3.2. Effects of Shading on Stem Non-Structural Carbohydrates and Their Components in Pinus yunnanensis Seedlings of Different Ages
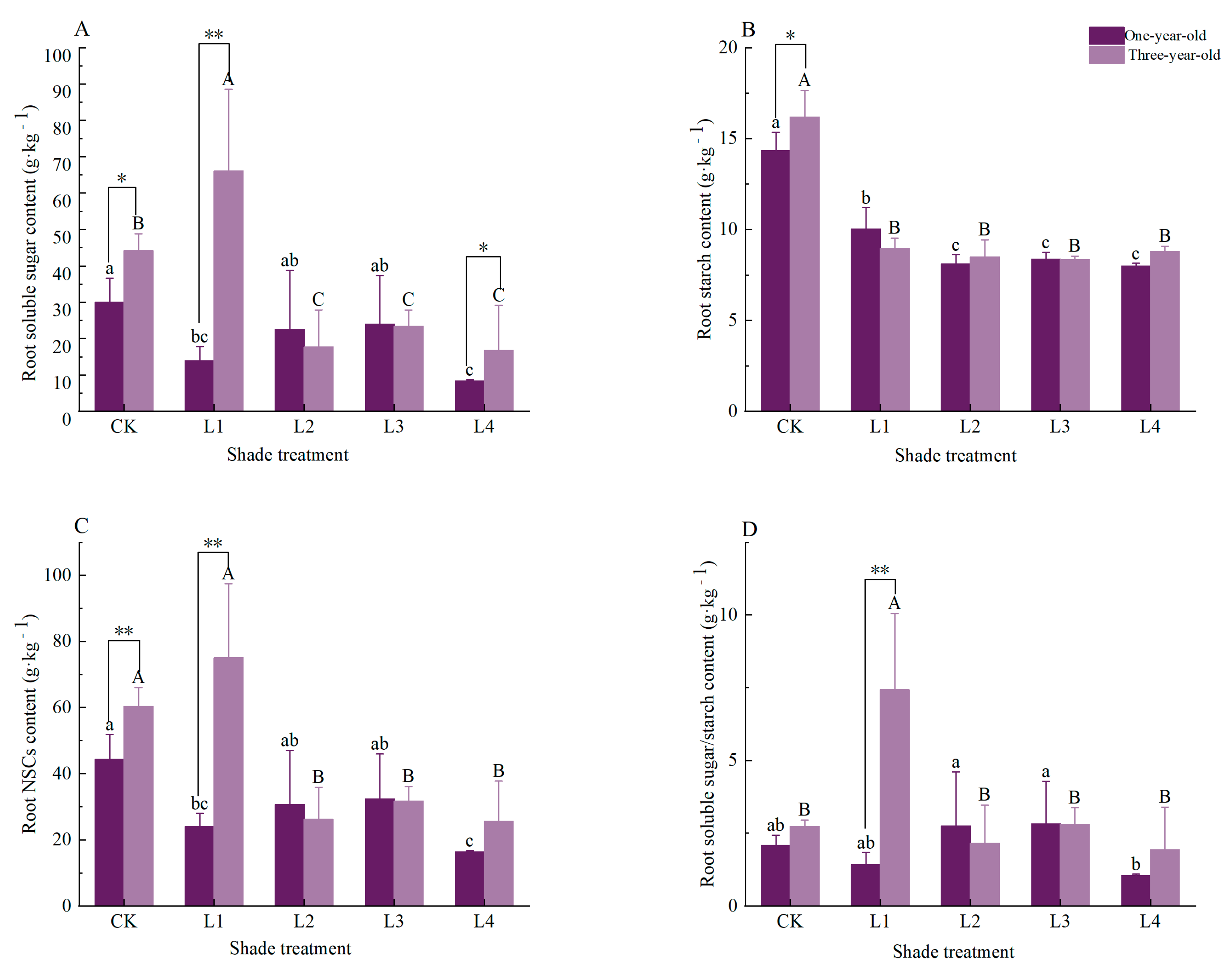
3.3. Effects of Shading on Root Non-Structural Carbohydrates and Their Components in Pinus yunnanensis Seedlings of Different Ages
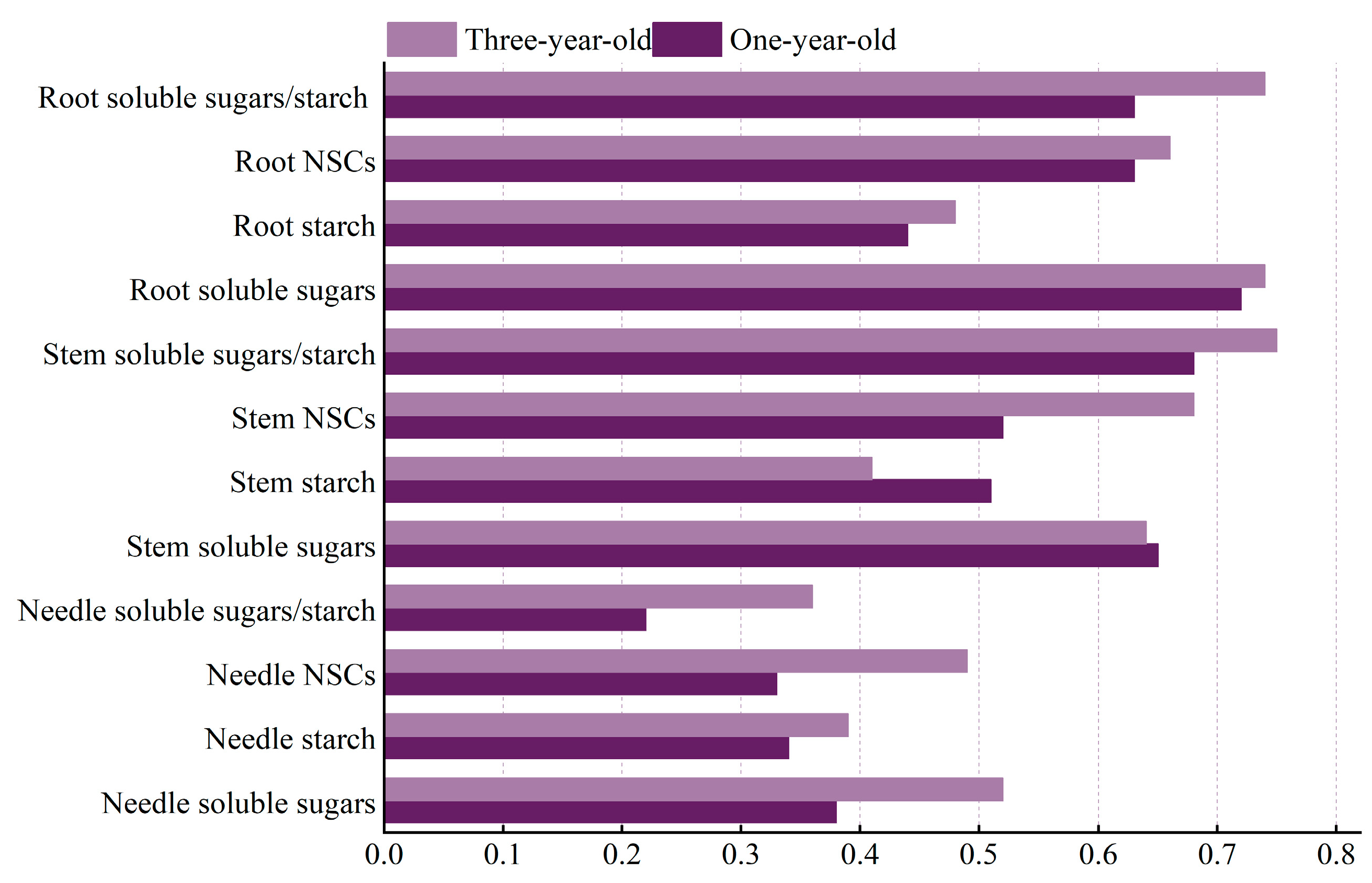
3.4. Analysis of Phenotypic Plasticity in Non-Structural Carbohydrate Response to Shading in Pinus yunnanensis Seedlings of Different Ages
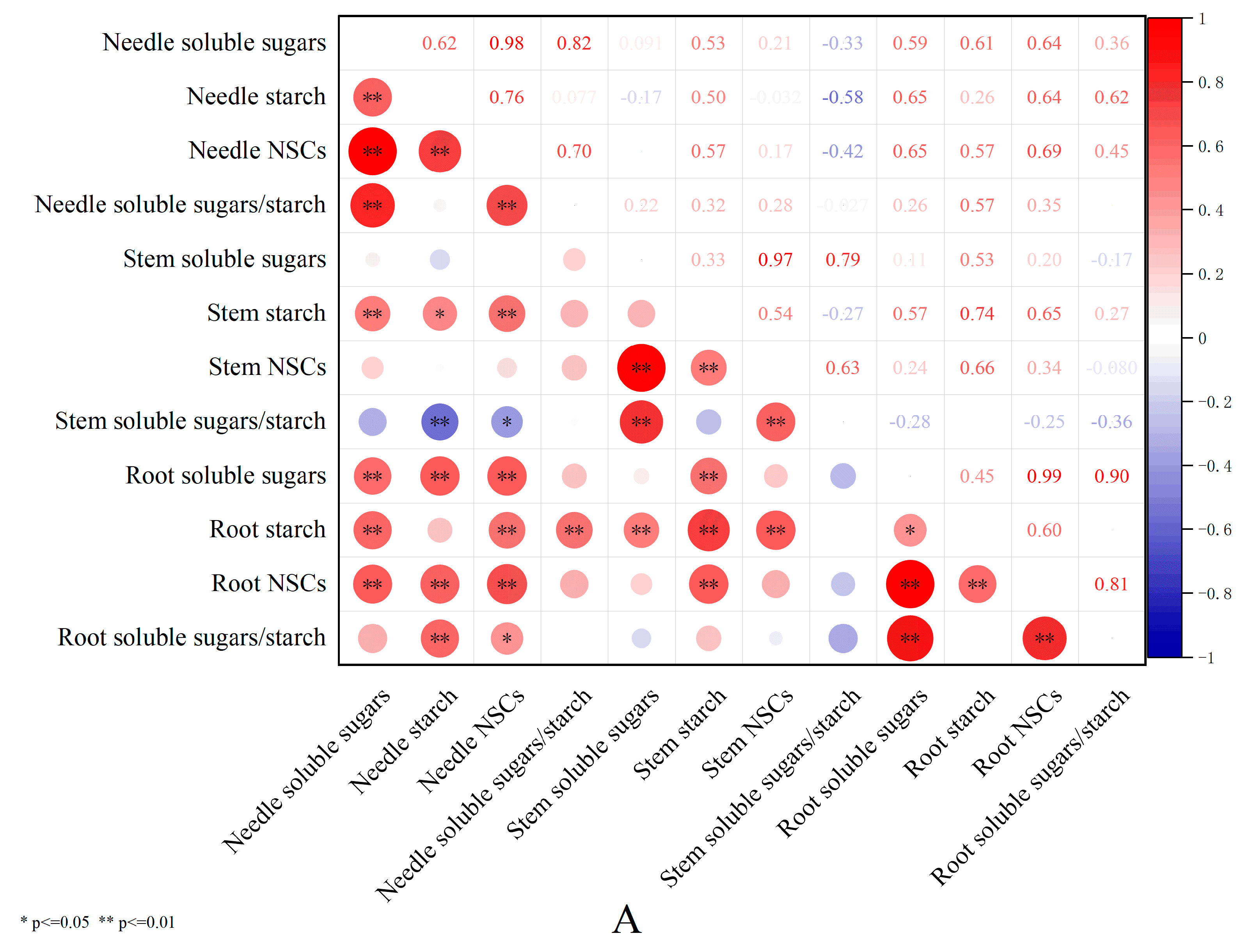
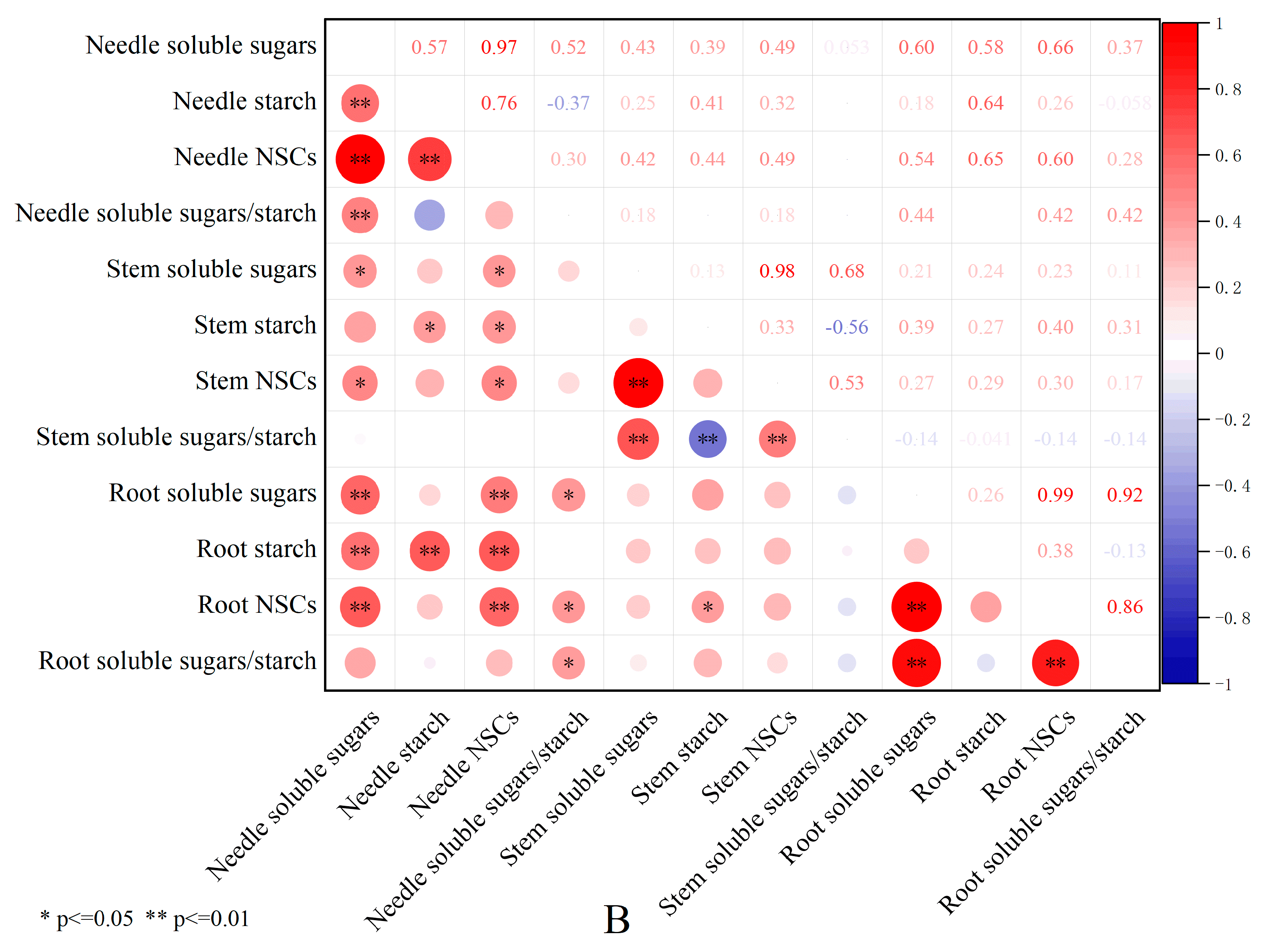
3.5. Correlation Analysis of Shading Effects on Non-Structural Carbohydrates in Pinus yunnanensis Seedlings of Different Ages
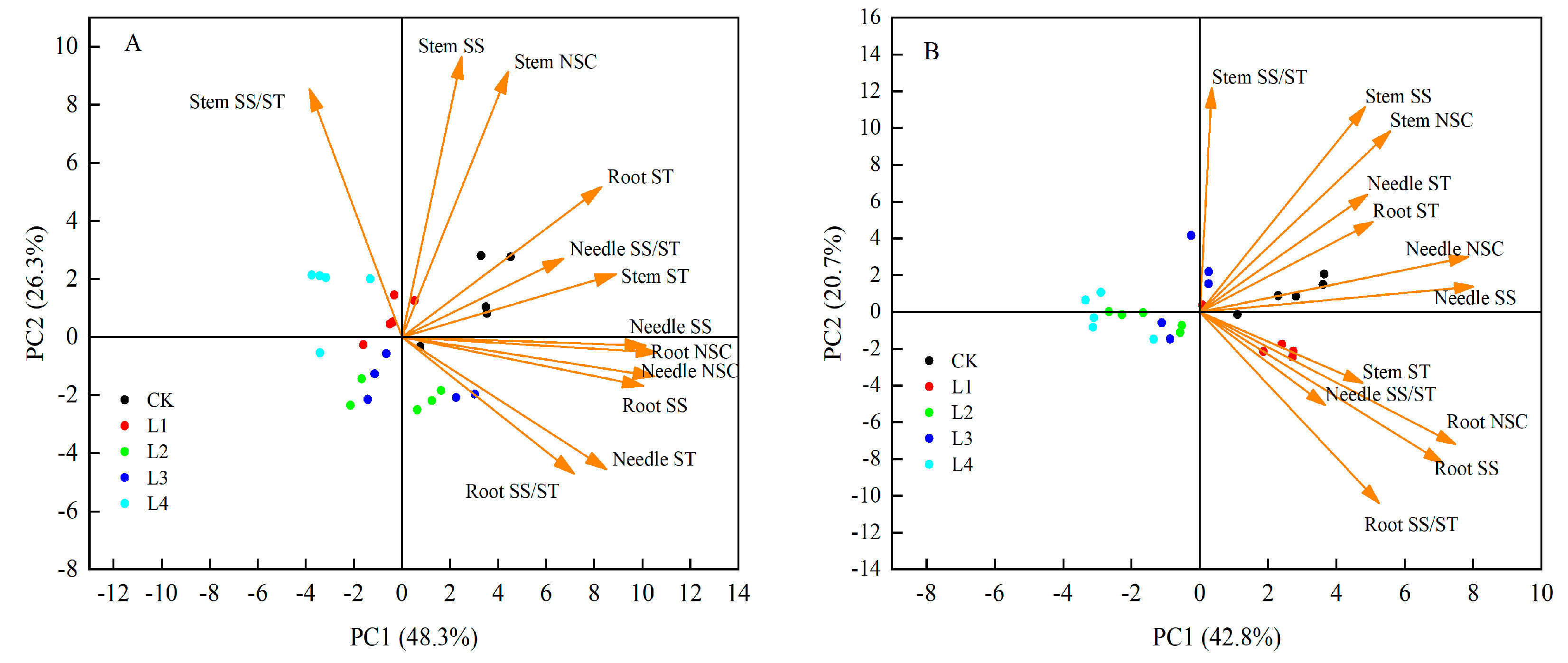
3.6. Principal Component Analysis (PCA) of Shading Effects on Non-Structural Carbohydrates in Pinus yunnanensis Seedlings of Different Ages
4. Discussion
4.1. Organ-Specific Non-Structural Carbohydrate Responses to Shading Stress in Pinus yunnanensis Seedlings of Different Ages
4.2. Organ-Specific Coordinated Resource Allocation Strategies in Pinus yunnanensis Seedlings of Different Ages Under Shading Stress
4.3. Ontogenetic Shade Thresholds Informing Regeneration Management for Pinus yunnanensis Plantations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Ji, M.F.; Deng, Y.; Su, X. Research progress on asymmetric light competition in plants. Pratacultural Sci. 2020, 37, 156–167. [Google Scholar] [CrossRef]
- Bartolini, S.; Viti, R.; Andreini, L. The effect of summer shading on flower bud morphogenesis in apricot (Prunus armeniaca L.). Open Life Sci. 2013, 8, 54–63. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.Y.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. var youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. The effect of low irradiance on leaf nitrogen allocation and mesophyll conductance to CO2 in seedlings of four tree species in subtropical China. Plants 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R.; Vega, D.; Poorter, H. Interactions of drought and shade effects on seedlings of four Quercus species: Physiological and structural leaf responses. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Zheng, X.W.; Liu, Y.J.; Fang, L.; Pan, Z.F.; Bao, M.H.; Huang, P. Effect of oridonin on cytochrome P450 expression and activities in HepaRG cell. Pharmacology 2018, 101, 246–254. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; St Onge, K.R.; Voesenek, L.A.C.J.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- He, L.; Shan, Y.Q.; Liu, C.; Cao, H.; Liu, X.N.; Guo, Y. Prediction of bedload transport inside vegetation canopies with natural morphology. J. Hydrodyn. 2024, 36, 556–569. [Google Scholar] [CrossRef]
- Binkley, D.; Campoe, O.C.; Gspaltl, M.; Forrester, D.I. Light absorption and use efficiency in forests: Why patterns differ for trees and stands. For. Ecol. Manag. 2013, 288, 5–13. [Google Scholar] [CrossRef]
- Nelson, A.S.; Landhäusser, S.M.; Lieffers, V.J. Light absorption and light-use efficiency of juvenile white spruce trees in natural stands and plantations. For. Ecol. Manag. 2016, 376, 158–165. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Cheng, Y.; Tan, N.D.; Jiang, F.; Liu, S.Z.; Chu, G.-W.; Meng, Z.; Liu, J.X. Comparative eco-physiological adaptability of four tree species to experimental warming in a lower-subtropical evergreen broad-leaved forest. Chin. J. Plant Ecol. 2020, 44, 1203–1214. [Google Scholar] [CrossRef]
- He, Q.; Zhan, J.; Liu, X.; Dong, C.; Tian, D.; Fu, Q. Multispectral polarimetric bidirectional reflectance research of plant canopy. Opt. Lasers Eng. 2025, 184, 108688. [Google Scholar] [CrossRef]
- Yu, Q.; Shen, Y.; Wang, Q.; Wang, X.; Fan, L.; Wang, Y.; Zhang, M. Light deficiency and waterlogging affect chlorophyll metabolism and photosynthesis in Magnolia sinostellata. Trees 2019, 33, 11–22. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015; ISBN 978-1-60535-255-8. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Pan, Q.M.; Han, X.G.; Bai, Y.-F.; Yang, J.C. Advances in the ecophysiology of non-structural carbohydrate storage in plants. Chin. Bull. Bot. 2002, 19, 30–38. [Google Scholar] [CrossRef]
- Hao, X.Y.; Li, D.D.; Yao, Y.T.; Wang, L. Effects of shading-induced carbon uptake limitation on non-structural carbohydrates, cold tolerance and subsequent growth of Platycladus orientalis seedlings. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2017, 37, 360–365. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Shen, Z.G.; Luo, Y.; Cheng, J.M.; Ding, X. Effects of shading on photosynthetic characteristics of Paeonia ostii‘Fengdanbai’. J. Northwest For. Univ. 2017, 32, 46–51. [Google Scholar] [CrossRef]
- Chen, Z.C.; Liu, X.J.; Liu, C.; Wan, X.C. Responses of Quercus aliena var acuteserrata seedling growth, photosynthesis and non-structural carbohydrates to shading and simulated sunflecks. Chin. J. Ecol. 2017, 36, 935–943. [Google Scholar] [CrossRef]
- Shan, C.; Dong, K.; Wen, D.; Cui, Z.; Cao, J. A comprehensive review of m6A modification in plant development and potential quality improvement. Int. J. Biol. Macromol. 2025, 308, 142597. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Schulze, E.D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Deng, X.Q.; Huang, B.L.; Wen, Q.Z.; Hua, C.L.; Tao, J. Study on the distribution of Pinus yunnanensis forest in Yunnan Province. J. Yunnan Univ. (Nat. Sci.) 2013, 35, 843–848. [Google Scholar] [CrossRef]
- Cao, Z.L.; Wang, X.L.; Zhang, Q.X.; Li, G.Q. Characteristics of population structure and soil seed bank of Pinus yunnanensis forests invaded by Ageratina adenophora. J. Yunnan Univ. (Nat. Sci.) 2016, 38, 958–964. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, G.Q.; Li, L.F.; Zhao, M.C.; Liu, D.Y. Growth characteristics and accelerated cultivation of Pinus yunnanensis. J. Shaanxi For. Sci. Technol. 2008, 36, 4–7. [Google Scholar] [CrossRef]
- Liang, J.; Chen, X.; Yang, Z.; Liu, J.; Wang, J.; Chen, H. Photosynthetic rate in response to light intensity and CO2 concentration in needles of a mixed Pinus yunnanensis and Pinus armandii plantation. For. Res. 2009, 22, 21–25. [Google Scholar] [CrossRef]
- Shangguan, H.L.; Liu, H.Y.; Hu, G.Z.; Guo, W.C. Seasonal patterns and driving factors of non-structural carbohydrates in different tree species at a xeric timberline. Acta Sci. Nat. Univ. Pekin. 2019, 55, 553–560. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Ma, X.Q.; Huang, Z.J.; Guo, S.; Wang, D.Y.; Wang, C.H.; Liu, B. Effects of light intensity on morphological characteristics and leaf non-structural carbohydrate content of Chinese fir seedlings. Acta Ecol. Sin. 2019, 39, 4455–4462. [Google Scholar]
- Tang, G.; Zhang, H.; Xing, H.; Yuan, T.; Gontcharov, A.A.; Yang, L. Light adaptation strategies of Quercus mongolica at different ages in four plantations. For. Res. 2024, 4, e005. [Google Scholar] [CrossRef]
- Jin, S.; Peng, Z. Responses of Robinia pseudoacacia seedlings’ carbohydrate physiological parameters to long-term drought and rewatering. J. Beijing For. Univ. 2023, 45, 43–56. [Google Scholar]
- Ma, Y.; Su, B.-L.; Han, Y.-G.; Wu, X.-H.; Zhou, W.-M.; Wang, Q.-W.; Zhou, L.; Yu, D.P. Responses of photosynthetic characteristics and non-structural carbohydrate accumulation of Betula platyphylla seedlings to drought stress. J. Appl. Ecol. 2021, 32, 513–520. [Google Scholar] [CrossRef]
- Wang, X.L.; Shi, L.; Sun, J.X.; Zhang, J.Z.; Liu, L.A.; Lu, R.Q.; Jiang, C.D. Effect of shading on growth characteristics and biomass distribution of Liriope spicata (Thunb.) Lour. Bull. Bot. Res. 2006, 26, 225–228. [Google Scholar] [CrossRef]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiological and Biochemical Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2015; ISBN 978-7-04-039646-1. [Google Scholar]
- Board, J.E.; Harville, B.G. Explanations for greater light interception in narrow- vs. wide-row. Crop Sci. 1992, 32, 198–202. [Google Scholar] [CrossRef]
- Dai, Y.X.; Wang, L.; Wan, X.C. Effects of shading and girdling on carbon allocation and hydraulic traits in seedlings of Robinia pseudoacacia and Platycladus orientalis. Sci. Silvae Sin. 2017, 53, 37–44. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Yan, L.F.; Yang, Q.P.; Zheng, W.H.; Huang, K.; Zhao, F.X. Responses of non-structural carbohydrates in Cunninghamia lanceolata seedlings to shading and subsequent light restoration. Acta Bot. Boreal-Occident. Sin. 2020, 40, 311–318. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Adams, H.D. Getting to the root of carbon reserve dynamics in woody plants: Progress, challenges and goals. Tree Physiol. 2024, 44, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Chen, J.; Yang, L.; Zeng, S.; Su, Y.; Li, J.; He, Q.; Qiu, Q. Global bibliometric analysis of research on the application of biochar in forest soils. Forests 2023, 14, 2238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Liu, Y.; Zhang, W.; Duan, G.; Chen, J.; Zhu, W.; Wu, J. The Age-Dependent Response of Carbon Coordination in the Organs of Pinus yunnanensis Seedlings Under Shade Stress. Plants 2025, 14, 2679. https://doi.org/10.3390/plants14172679
Han J, Liu Y, Zhang W, Duan G, Chen J, Zhu W, Wu J. The Age-Dependent Response of Carbon Coordination in the Organs of Pinus yunnanensis Seedlings Under Shade Stress. Plants. 2025; 14(17):2679. https://doi.org/10.3390/plants14172679
Chicago/Turabian StyleHan, Juncheng, Yuanxi Liu, Wenhao Zhang, Guihe Duan, Jialan Chen, Weisong Zhu, and Junwen Wu. 2025. "The Age-Dependent Response of Carbon Coordination in the Organs of Pinus yunnanensis Seedlings Under Shade Stress" Plants 14, no. 17: 2679. https://doi.org/10.3390/plants14172679
APA StyleHan, J., Liu, Y., Zhang, W., Duan, G., Chen, J., Zhu, W., & Wu, J. (2025). The Age-Dependent Response of Carbon Coordination in the Organs of Pinus yunnanensis Seedlings Under Shade Stress. Plants, 14(17), 2679. https://doi.org/10.3390/plants14172679


.png)

