Supercritical CO2 Antisolvent Fractionation of Citrus aurantium Flower Extracts: Enrichment and Characterization of Bioactive Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Precipitate Yield Optimization
2.2. Recovery and Quantification of Target Bioactives
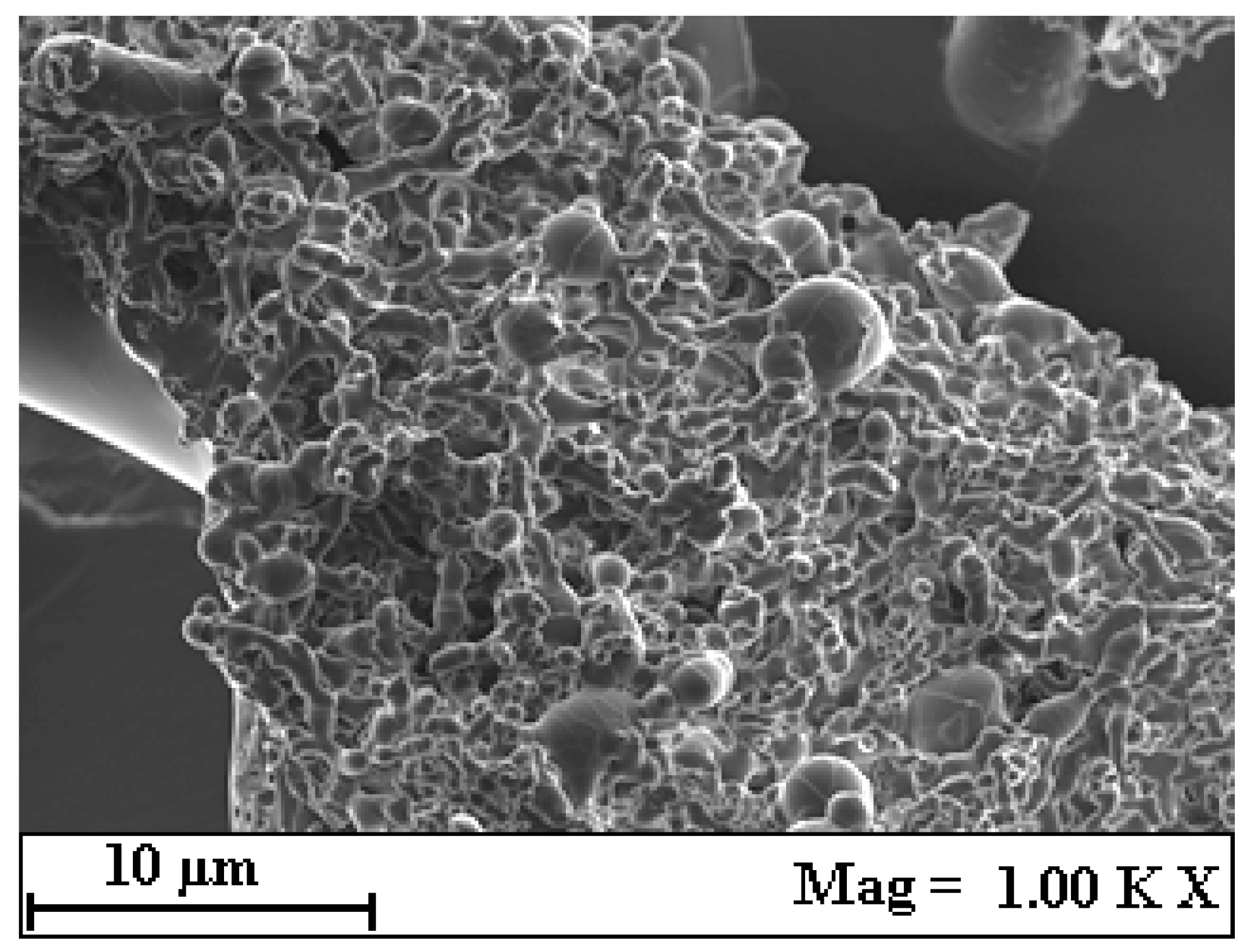
2.3. SEM Image Analysis and Morphological Characterization
2.4. In Silico ADMET and Skin Permeability Predictions
2.4.1. ADME-Tox Profile of Target Compounds
2.4.2. In Silico Skin Penetration Modelling Results (COSMOperm)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Feedstock and Sample Preparation
3.3. Feed Solution Preparation for SAF Experiments
3.4. Supercritical Antisolvent Fractionation (SAF) Process
3.5. Experimental Design and Statistical Analysis
3.6. Microscopy Observations
3.7. Analysis of the Extracts
3.8. Computational Methodology: ADMET and Skin Permeation Modelling
3.8.1. In Silico ADME and Toxicity Prediction
3.8.2. Skin Permeation Modelling: COSMOperm Method
- stratum corneum (“SC”; outer horny layer);
- stratum granulosum (“SG”; granular layer, the outermost viable layer);
- stratum spinosum (“SS”; viable prickle layer, releasing neutral barrier lipids);
- stratum basale (“SB”; basal layer, metabolically active;
- appendageal compartment (“shunt”; through the shunts provided by the hair follicles, sweat glands, and sebaceous glands).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ABPR | Automated Backpressure Regulator |
| ADME | Absorption, Distribution, Metabolism and Excretion |
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| ANOVA | Analysis of Variance |
| BBB | Blood–Brain Barrier |
| BPR | Backpressure Regulator |
| CCD | Central Composite Design |
| CO2 | Carbon Dioxide |
| COSMOperm | Conductor-like Screening Model for Real Solvents—Permeability module |
| COSMOtherm | Conductor-like Screening Model for Real Solvents—Thermodynamic software suite |
| DFT | Density Functional Theory |
| DERMWIN | Dermal Permeability Estimation Program (US EPA) |
| DV | Downstream Vessel |
| EPA | Environmental Protection Agency (USA) |
| FESEM | Field Emission Scanning Electron Microscope |
| FS | Feed Solution |
| HIA | Human Intestinal Absorption |
| HPLC | High-Performance Liquid Chromatography |
| LD50 | Lethal Dose for 50% of the population |
| PV | Precipitation Vessel |
| QSAR | Quantitative Structure–Activity Relationship |
| RSM | Response Surface Methodology |
| SAF | Supercritical Antisolvent Fractionation |
| SAS | Supercritical Antisolvent |
| SC | Stratum Corneum |
| SEM | Scanning Electron Microscopy |
| SFE | Supercritical Fluid Extraction |
| TMPRSS2 | Transmembrane Protease, Serine 2 |
| TZVP | Triple-Zeta Valence Polarized (basis set in DFT) |
| UV | Ultraviolet-Visible |
| VEGA | Virtual models for property evaluation of chemicals |
| VLE | Vapor–Liquid Equilibrium |
References
- Herraiz, T.; Galisteo, J. Tetrahydro-β-carboline alkaloids occur in fruits and fruit juices: Activity as antioxidants and radical scavengers. J. Agric. Food Chem. 2003, 51, 7156–7161. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Murakami, A.; Ohigashi, H. Nobiletin, a citrus flavonoid, downregulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci. Biotechnol. Biochem. 2005, 69, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Tavakoli, H.; Pourheidari, G.; Sobhani, A.; Shafiee, A. Radioprotective effects of citrus extract against γ irradiation in mouse bone marrow cells. J. Radiat. Res. 2003, 44, 237–241. [Google Scholar] [CrossRef]
- Suryawanshi, J.A.S. An overview of Citrus aurantium used in treatment of various diseases. Afr. J. Plant Sci. 2011, 5, 390–395. [Google Scholar]
- Arctander, S. Perfume and Flavor Materials of Natural Origin; Orchard Innovations: Montclair, NJ, USA, 1960; p. 436. [Google Scholar]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef]
- Riolo, M.; Moreno Villena, A.; Calpe, J.; Luz, C.; Meca, G.; Tuccitto, N.; Cacciola, S.O. A Circular Economy Approach: A New Formulation Based on a Lemon Peel Medium Activated with Lactobacilli for Sustainable Control of Post-Harvest Fungal Rots in Fresh Citrus Fruit. Biol. Control 2024, 189, 105443. [Google Scholar] [CrossRef]
- Fernández-Cabal, J.; Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Recent Developments in Citrus aurantium L.: An Overview of Bioactive Compounds, Extraction Techniques, and Technological Applications. Processes 2025, 13, 120. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, H.J.; Lee, J.S.; Lee, M.K.; Kim, H.O.; Park, E.J.; Kim, H.K.; Jeong, T.S.; Choi, M.S. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003, 22, 561–568. [Google Scholar] [CrossRef]
- Seo, H.J.; Jeong, K.S.; Lee, M.K.; Park, Y.B.; Jung, U.J.; Kim, H.J.; Choi, M.S. Role of naringin supplement in regulation of lipid and ethanol metabolism in rats. Life Sci. 2003, 73, 933–946. [Google Scholar] [CrossRef]
- Suarez, J.; Herrera, M.D.; Marhuenda, E. Effect of hesperidin and neohesperidin dihydrochalcone on different experimentally induced gastric ulcers. Phytother. Res. 1996, 10, 616–618. [Google Scholar] [CrossRef]
- Park, J.H.; Keeley, L.L. The effect of biogenic amines and their analogs on carbohydrate metabolism in the fat body of the cockroach Blaberus discoidalis. Gen. Comp. Endocrinol. 1998, 110, 88–95. [Google Scholar] [CrossRef]
- Carpéné, C.; Galitzky, J.; Fontana, E.; Atgié, C.; Lafontan, M.; Berlan, M. Selective activation of β3-adrenoceptors by octopamine: Comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 359, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Shekelle, P.G.; Hardy, M.L.; Morton, S.C.; Maglione, M.; Mojica, W.A.; Suttorp, M.J.; Rhodes, S.L.; Jungvig, L.; Gagné, J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: A meta-analysis. Clin. J. Sport Med. 2004, 14, 188–189. [Google Scholar]
- Kandeel, M.; Kitade, Y.; Almubarak, A. Repurposing FDA-approved phytomedicines, natural products, antivirals and cell protectives against SARS-CoV-2 (COVID-19) RNA-dependent RNA polymerase. PeerJ 2020, 8, e10480. [Google Scholar] [CrossRef]
- Chikhale, R.V.; Gupta, V.K.; Eldesoky, G.E.; Wabaidur, S.M.; Patil, S.A.; Islam, M.A. Identification of potential anti-TMPRSS2 natural products through homology modelling, virtual screening and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2021, 39, 6660–6675. [Google Scholar] [CrossRef]
- Liu, L.Z.; Song, Z.Q.; Zhang, L.; Li, L.F.; Wang, Y.S. Determination of three chemical components in Fructus aurantii immaturus. Zhongguo Zhong Yao Za Zhi 2006, 31, 1425–1427. [Google Scholar] [PubMed]
- Reverchon, E.; Della Porta, G.; Lamberti, G. Modelling of orange flower concrete fractionation by supercritical CO2. J. Supercrit. Fluids 1999, 14, 115–121. [Google Scholar] [CrossRef]
- González, A.; Martín, L.; Mainar, A.M.; Urieta, J.S.; Fraga, B.M.; Rodriguez, V.; Díaz, C.E. Supercritical extraction and supercritical antisolvent fractionation of natural products from plant material: Comparative results on Persea indica. Phytochem. Rev. 2012, 11, 433–446. [Google Scholar] [CrossRef]
- Marqués, J.L.; Della Porta, G.; Reverchon, E.; Renuncio, J.A.R.; Mainar, A.M. Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. J. Supercrit. Fluids 2013, 82, 238–243. [Google Scholar] [CrossRef]
- Langa, E.; Pardo, J.I.; Giménez-Rota, C.; González-Coloma, A.; Hernáiz, M.J.; Mainar, A.M. Supercritical anti-solvent fractionation of Artemisia absinthium L. conventional extracts: Tracking artemetin and casticin. J. Supercrit. Fluids 2019, 151, 15–23. [Google Scholar] [CrossRef]
- Cardoso, M.A.T.; Antunes, S.; Van Keulen, F.; Ferreira, B.S.; Geraldes, A.; Cabral, J.M.S.; Palavra, A.M.F. Supercritical antisolvent micronization of synthetic all-trans β-carotene with tetrahydrofuran as solvent and carbon dioxide as antisolvent. J. Chem. Technol. Biotechnol. 2009, 84, 215–222. [Google Scholar] [CrossRef]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Langa, E.; Urieta, J.S.; Hernáiz, M.J.; Mainar, A.M. Supercritical antisolvent fractionation of antioxidant compounds from Lavandula luisieri (Rozeira) Riv.-Mart. J. Supercrit. Fluids 2020, 161, 104821. [Google Scholar] [CrossRef]
- Chen, K.X.; Zhang, X.Y.; Pan, J.; Zhang, W.C.; Yin, W.H. Gas antisolvent precipitation of Ginkgo ginkgolides with supercritical CO2. Powder Technol. 2005, 152, 127–132. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem. Eng. J. 2011, 169, 358–370. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Schwöbel, J.A.H.; Klamt, A. Mechanistic skin penetration model by the COSMOperm method: Routes of permeation, vehicle effects and skin variations in the healthy and compromised skin. Comput. Toxicol. 2019, 11, 50–64. [Google Scholar] [CrossRef]
- Schwöbel, J.A.H.; Ebert, A.; Bittermann, K.; Huniar, U.; Goss, K.; Klamt, A. COSMOperm: Mechanistic prediction of passive membrane permeability for neutral compounds and ions and its pH dependence. J. Phys. Chem. B 2020, 124, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Mur, R.; Pardo, J.I.; Pino-Otín, M.R.; Urieta, J.S.; Mainar, A.M. Supercritical Antisolvent Fractionation of Antioxidant Compounds from Salvia officinalis. Int. J. Mol. Sci. 2021, 22, 9351. [Google Scholar] [CrossRef]
- Mur, R.; Langa, E.; Pino-Otín, M.R.; Urieta, J.S.; Mainar, A.M. Concentration of Antioxidant Compounds from Calendula officinalis through Sustainable Supercritical Technologies, and Computational Study of Their Permeability in Skin for Cosmetic Use. Antioxidants 2022, 11, 96. [Google Scholar] [CrossRef]
- Sulejmanović, M.; Jerković, I.; Zloh, M.; Nastić, N.; Milić, N.; Drljača, J.; Jokić, S.; Aladić, K.; Vidović, S. Supercritical Fluid Extraction of Ginger Herbal Dust Bioactives with an Estimation of Pharmacological Potential Using In Silico and In Vitro Analysis. Food Biosci. 2024, 59, 104074. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef]
- Joung, S.N.; Yoo, C.W.; Shin, H.Y.; Kim, S.Y.; Yoo, K.P.; Lee, C.S.; Wan, S.H. Measurements and correlation of high-pressure VLE of binary CO2–alcohol systems (methanol, ethanol, 2-methoxyethanol and 2-ethoxyethanol). Fluid Phase Equilib. 2001, 185, 219–230. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Martín, Á.; Cocero, M.J. Solubility Parameter Estimation by Group Contribution Methods: Application to Supercritical Systems. J. Supercrit. Fluids 2012, 66, 107–115. [Google Scholar] [CrossRef]
- Wu, J.J.; Shen, C.T.; Jong, T.T.; Young, C.C.; Yang, H.L.; Hsue, S.L.; Chang, C.M.J.; Jen Shieh, C. Supercritical carbon dioxide anti-solvent process for purification of micronized propolis particulates and associated anti-cancer activity. Sep. Purif. Technol. 2009, 70, 190–198. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Lee, Y.H.; Charles, A.L.; Kung, H.F.; Tang Ho, C.; Huang, T.C. Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind. Crops Prod. 2010, 31, 59–64. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcuspluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Avula, B.; Upparapalli, S.K.; Navarrete, A.; Khan, I.A. Simultaneous quantification of adrenergic Amines and flavonoids in C. aurantium, various Citrus species, and dietary supplements by liquid chromatography. J. AOAC Int. 2005, 88, 1593–1606. [Google Scholar] [CrossRef]
- Giannuzzo, A.N.; Boggetti, H.J.; Nazareno, M.A.; Mishima, H.T. Supercritical fluid extraction of naringin from the peel of Citrus paradise. Phytochem. Anal. 2003, 14, 221–223. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2017, 18, 8615–8627. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibáñez, E. Sub- and Supercritical Fluid Extraction of Functional Ingredients from Different Natural Sources: Plants, Food-by-Products, Algae and Microalgae. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Sugino, M.; Todo, H.; Suzuki, T.; Nakada, K.; Tsuji, K.; Tokunaga, H.; Jinno, H.; Sugibayashi, K. Safety Prediction of Topically Exposed Biocides Using Permeability Coefficients and the Desquamation Rate at the Stratum Corneum. J. Toxicol. Sci. 2014, 39, 475–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- VEGA HUB—Virtual Models for Property Evaluation of Chemicals Within a Global Architecture. Available online: https://www.vegahub.eu (accessed on 3 January 2025).
- EPA (EPI/DERMWIN 2.0). Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 22 February 2025).
- ANSI/ASAE S319.3 (2001); Method of Determining and Expressing Fineness of Feed Materials by Sieving. Standard of the American Society of Agricultural Engineers: St. Joseph, MI, USA.
- De Marco, I.; Reverchon, E. Influence of Pressure, Temperature and Concentration on the Mechanisms of Particle Precipitation in Supercritical Antisolvent Micronization. J. Supercrit. Fluids 2011, 58, 295–302. [Google Scholar] [CrossRef]
- Martin, L.; Gonzalez-Coloma, A.; Adami, R.; Scognamiglio, M.; Reverchon, E.; Della Porta, G.; Urieta, J.S.; Mainar, A.M. Supercritical Antisolvent Fractionation of Ryanodol from Persea indica. J. Supercrit. Fluids 2011, 60, 16–20. [Google Scholar] [CrossRef]
- Chang, C.J.; Day, C.; Ko, C.; Chiu, K. Densities and P-x-y diagrams for carbon dioxide dissolution in methanol, ethanol, and acetone mixtures. Fluid Phase Equilib. 1997, 131, 243–258. [Google Scholar] [CrossRef]
- Galicia-Luna, L.A.; Ortega-Rodríguez, A.; Richon, D. New Apparatus for the fast determination of high-pressure vapor–liquid equilibria of mixtures and of accurate critical pressures. J. Chem. Eng. Data 2000, 45, 265–271. [Google Scholar] [CrossRef]
- He, X.G.; Lian, L.Z.; Lin, L.Z.; Bernart, M.W. High-performance liquid chromatography-electrospray mass spectrometry analysis of sour orange (Citrus aurantium L.). J. Chromatogr. A 1997, 791, 127–134. [Google Scholar] [CrossRef]
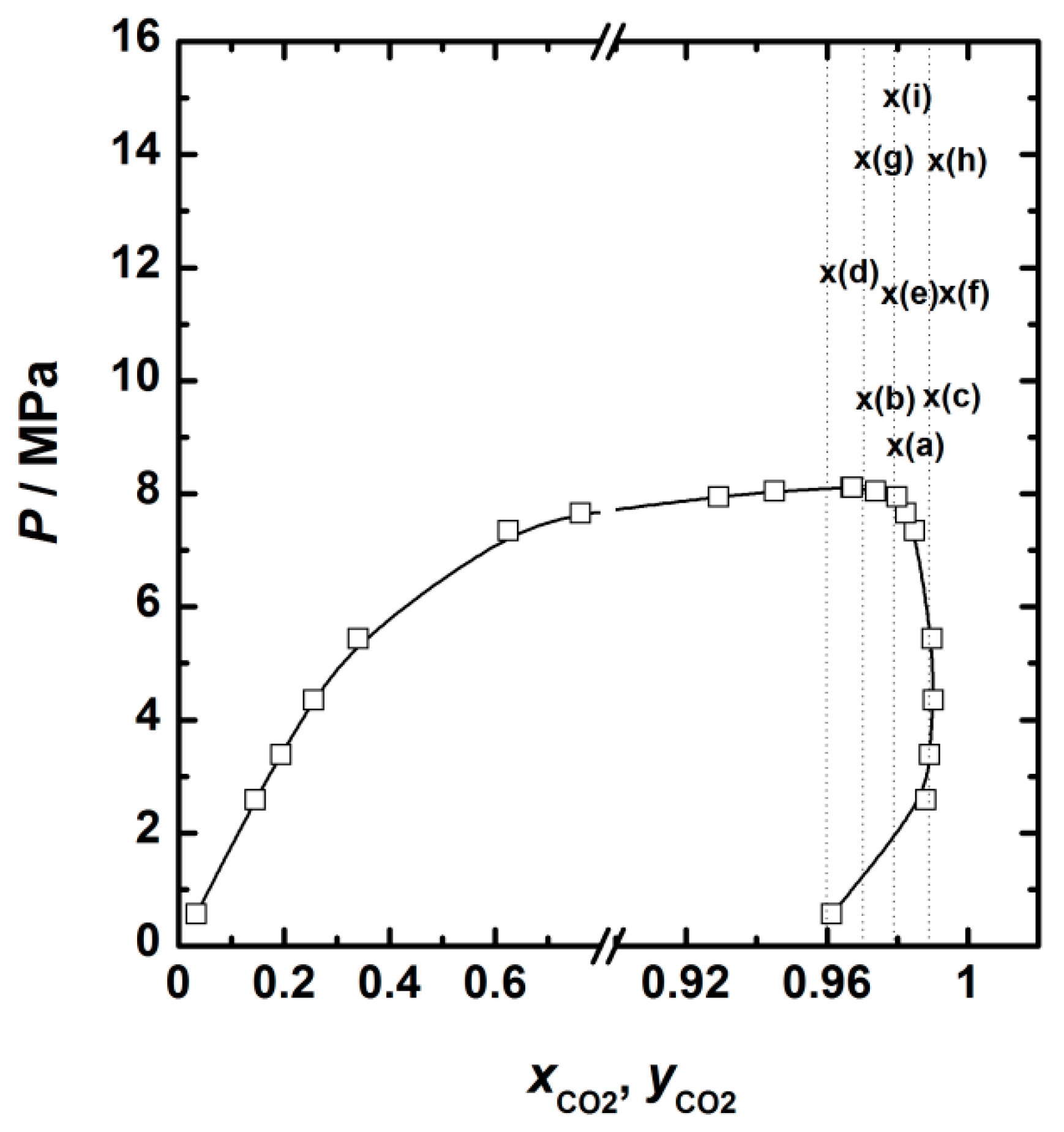
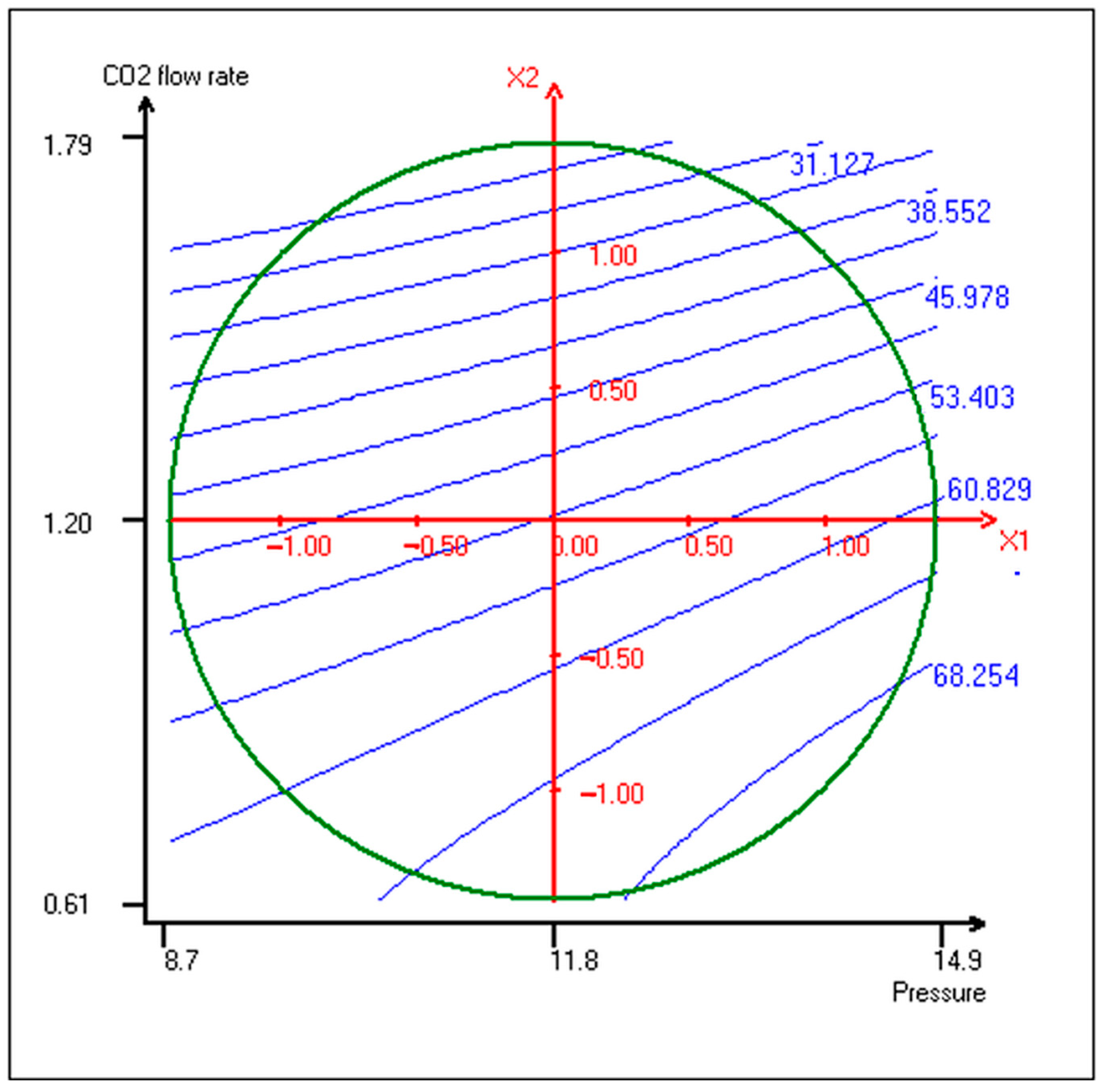

| Run | Variables | CO2 Molar Fraction | Yexp. (%) 1 | Ycalc. (%) 2 | Deviation (%) 3 | |
|---|---|---|---|---|---|---|
| x1 | x2 | |||||
| CO2 Pressure (MPa) | CO2 Flowrate (kg/h) | |||||
| 1 | 9.6 | 0.8 | 0.97 | 62.0 | 59.7 | 1.9 |
| 2 | 14.1 | 0.8 | 0.97 | 68.2 | 69.8 | 2.3 |
| 3 | 9.6 | 1.6 | 0.99 | 28.7 | 29.6 | 2.9 |
| 4 | 14.1 | 1.6 | 0.99 | 37.3 | 41.0 | 9.9 |
| 5 | 8.7 | 1.2 | 0.98 | 46.7 | 47.4 | 1.6 |
| 6 | 15.0 | 1.2 | 0.98 | 65.3 | 62.0 | 4.9 |
| 7 | 11.8 | 0.6 | 0.96 | 66.9 | 67.1 | 0.3 |
| 8 | 11.8 | 1.8 | 0.99 | 27.4 | 24.7 | 9.8 |
| 9 | 11.8 | 1.2 | 0.98 | 51.7 | 53.7 | 3.8 |
| 10 | 11.8 | 1.2 | 0.98 | 55.3 | 53.7 | 2.9 |
| 11 | 11.8 | 1.2 | 0.98 | 54.1 | 53.7 | 0.7 |
| Symbol | Coefficient | Inflation Factor | Standard Deviation | t. exp. | Signif. % 1 |
|---|---|---|---|---|---|
| b0 | 53.75 | 1.71 | 31.44 | *** | |
| b1 | 5.14 | 1.00 | 1.04 | 4.91 | ** |
| b2 | −15.00 | 1.00 | 1.04 | −14.33 | *** |
| b11 | 0.49 | 1.09 | 1.24 | 0.39 | 70.8% |
| b22 | −3.92 | 1.09 | 1.24 | −3.15 | * |
| b12 | 0.59 | 1.00 | 1.48 | 0.40 | 70.5% |
| Components | Percentage (%) 1 |
|---|---|
| Synephrine | 11.7 |
| Naringin | 25.0 |
| Neohesperidine | 14.9 |
| Substance | LD50 Rat Model (mol/kg) | HIA | BBB | Carcinogens | Cytochrome p450 Inhibition/Substrate | Oral Acute Toxicity | AMES Toxicity |
|---|---|---|---|---|---|---|---|
| Naringin | 2.2619 | HIA+ 0.8645 | BBB− 0.8414 | No | Non-Substrate Non-Inhibitor | Category III | Yes |
| Neohesperidin | 2.4045 | HIA+ 0.7271 | BBB− 0.9396 | No | Non-Substrate Only inhibited CYP450-3A4 | Category III | No |
| Synephrine | 2.6480 | HIA+ 0.9943 | BBB− 0.9115 | No | Non-Substrate Non-Inhibitor | Category II | No |
| Parameter | Naringin | Neohesperidin | Synephrine |
|---|---|---|---|
| Vehicle | water | water | water |
| Skin membrane | epidermis | epidermis | epidermis |
| Rate limiting step | SC via polar transcorneoite pathway | SC via polar transcorneoite pathway | SC via polar transcorneoite pathway |
| RSC (s/m) | 3.72 × 1010 | 9.20 × 109 | 3.07 × 107 |
| RSC,inter (s/m) | 8.82 × 1023 | 2.99 × 1020 | 4.10 × 1010 |
| RSC,trans (s/m) | 3.72 × 1010 | 9.20 × 109 | 3.07 × 107 |
| log Kp, SC (cm/s) | −8.57 | −7.96 | −5.49 |
| RSG (s/m) | 1.94 × 109 | 4.32 × 108 | 2.92 × 105 |
| log Kp, SG (cm/s) | −7.29 | −6.64 | −3.46 |
| RSS (s/m) | 4.39 × 109 | 9.80 × 108 | 6.64 × 105 |
| log Kp, SS (cm/s) | −7.64 | −6.99 | −3.82 |
| RSB (s/m) | 3.04 × 108 | 6.78 × 107 | 1.97 × 105 |
| log Kp, SB (cm/s) | −6.48 | −5.83 | −3.29 |
| Rcells (s/m) | 4.38 × 1010 | 1.07 × 1010 | 3.18 × 107 |
| Rshunt (s/m) | 5.00 × 1010 | 5.00 × 1010 | 5.00 × 1010 |
| Rskin (s/m) | 2.33 × 1010 | 8.80 × 109 | 4.20 × 108 |
| log Kp (pred.) (cm/s) | −8.37 | −7.94 | −5.50 |
| log Kp (pred.) + offset (cm/s) | −9.49 | −9.06 | −6.62 |
| log Kp (VEGA) 1 (cm/s) | −8.64 | −8.66 | −6.86 |
| Percent relative deviation 2 | 8.96 | 4.42 | 3.62 |
| log Kp (DERMWIN) 3 (cm/s) | −9.91 | −9.15 | −7.29 |
| Percent relative deviation 2 | 4.43 | 0.99 | 10.1 |
| Run | P (MPa) | QCO2 (kg/h) | SAF Extracts (%) 1 | |||
|---|---|---|---|---|---|---|
| Synephrine | Naringin | Neohesperidin | Total 2 | |||
| 1 | 9.6 | 0.8 | 5.3 | 15.9 | 12.6 | 33.7 |
| 2 | 14.1 | 0.8 | 8.5 | 27.8 | 18.2 | 54.5 |
| 3 | 9.6 | 1.6 | 7.2 | 24.8 | 11.9 | 43.9 |
| 4 | 14.1 | 1.6 | 8.7 | 27.5 | 18.0 | 54.1 |
| 5 | 8.7 | 1.2 | 9.0 | 33.7 | 20.0 | 62.7 |
| 6 | 15.0 | 1.2 | 8.0 | 28.9 | 21.6 | 58.5 |
| 7 | 11.8 | 0.6 | 6.9 | 24.4 | 15.6 | 46.9 |
| 8 | 11.8 | 1.8 | 7.6 | 26.3 | 17.9 | 51.7 |
| 9 | 11.8 | 1.2 | 6.4 | 31.1 | 18.4 | 55.9 |
| 10 | 11.8 | 1.2 | 8.6 | 26.4 | 19.5 | 54.5 |
| 11 | 11.8 | 1.2 | 5,1 | 27.2 | 18.1 | 50.4 |
| Run | P (MPa) | QCO2 (kg/h) | Compound Yield (g/kg of Dry Flowers) 1 | ||
|---|---|---|---|---|---|
| Synephrine | Naringin | Neohesperidin | |||
| 1 | 9.6 | 0.8 | 3.8 | 11.5 | 9.2 |
| 2 | 14.1 | 0.8 | 6.8 | 22.2 | 14.5 |
| 3 | 9.6 | 1.6 | 2.4 | 8.3 | 4.0 |
| 4 | 14.1 | 1.6 | 3.8 | 12.1 | 7.9 |
| 5 | 8.7 | 1.2 | 4.9 | 18.5 | 11.0 |
| 6 | 15.0 | 1.2 | 6.1 | 22.1 | 16.6 |
| 7 | 11.8 | 0.6 | 5.4 | 19.3 | 12.2 |
| 8 | 11.8 | 1.8 | 2.4 | 8.4 | 5.7 |
| 9 | 11.8 | 1.2 | 4.0 | 19.4 | 11.5 |
| 10 | 11.8 | 1.2 | 5.6 | 17.1 | 12.6 |
| 11 | 11.8 | 1.2 | 3.2 | 17.2 | 11.5 |
| Factors | Symbol | Range and Levels of Independent Factors | ||||
|---|---|---|---|---|---|---|
| −1.41 | −1 | 0 | 1 | 1.41 | ||
| (P) CO2 (MPa) | (x1) | 8.7 | 9.6 | 11.8 | 14.1 | 15.0 |
| Q CO2 (kg/h) | (x2) | 0.6 | 0.8 | 1.2 | 1.6 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trabelsi, D.; Martínez-López, J.F.; Abderrabba, M.; Urieta, J.S.; Mainar, A.M. Supercritical CO2 Antisolvent Fractionation of Citrus aurantium Flower Extracts: Enrichment and Characterization of Bioactive Compounds. Plants 2025, 14, 2678. https://doi.org/10.3390/plants14172678
Trabelsi D, Martínez-López JF, Abderrabba M, Urieta JS, Mainar AM. Supercritical CO2 Antisolvent Fractionation of Citrus aurantium Flower Extracts: Enrichment and Characterization of Bioactive Compounds. Plants. 2025; 14(17):2678. https://doi.org/10.3390/plants14172678
Chicago/Turabian StyleTrabelsi, Dhekra, José F. Martínez-López, Manef Abderrabba, José S. Urieta, and Ana M. Mainar. 2025. "Supercritical CO2 Antisolvent Fractionation of Citrus aurantium Flower Extracts: Enrichment and Characterization of Bioactive Compounds" Plants 14, no. 17: 2678. https://doi.org/10.3390/plants14172678
APA StyleTrabelsi, D., Martínez-López, J. F., Abderrabba, M., Urieta, J. S., & Mainar, A. M. (2025). Supercritical CO2 Antisolvent Fractionation of Citrus aurantium Flower Extracts: Enrichment and Characterization of Bioactive Compounds. Plants, 14(17), 2678. https://doi.org/10.3390/plants14172678








