Potentially Toxic Elements in Terrestrial Mosses in the Vicinity of a Stibnite Mine in Pinal de Amoles, Mexico
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of the Moss Species Collected
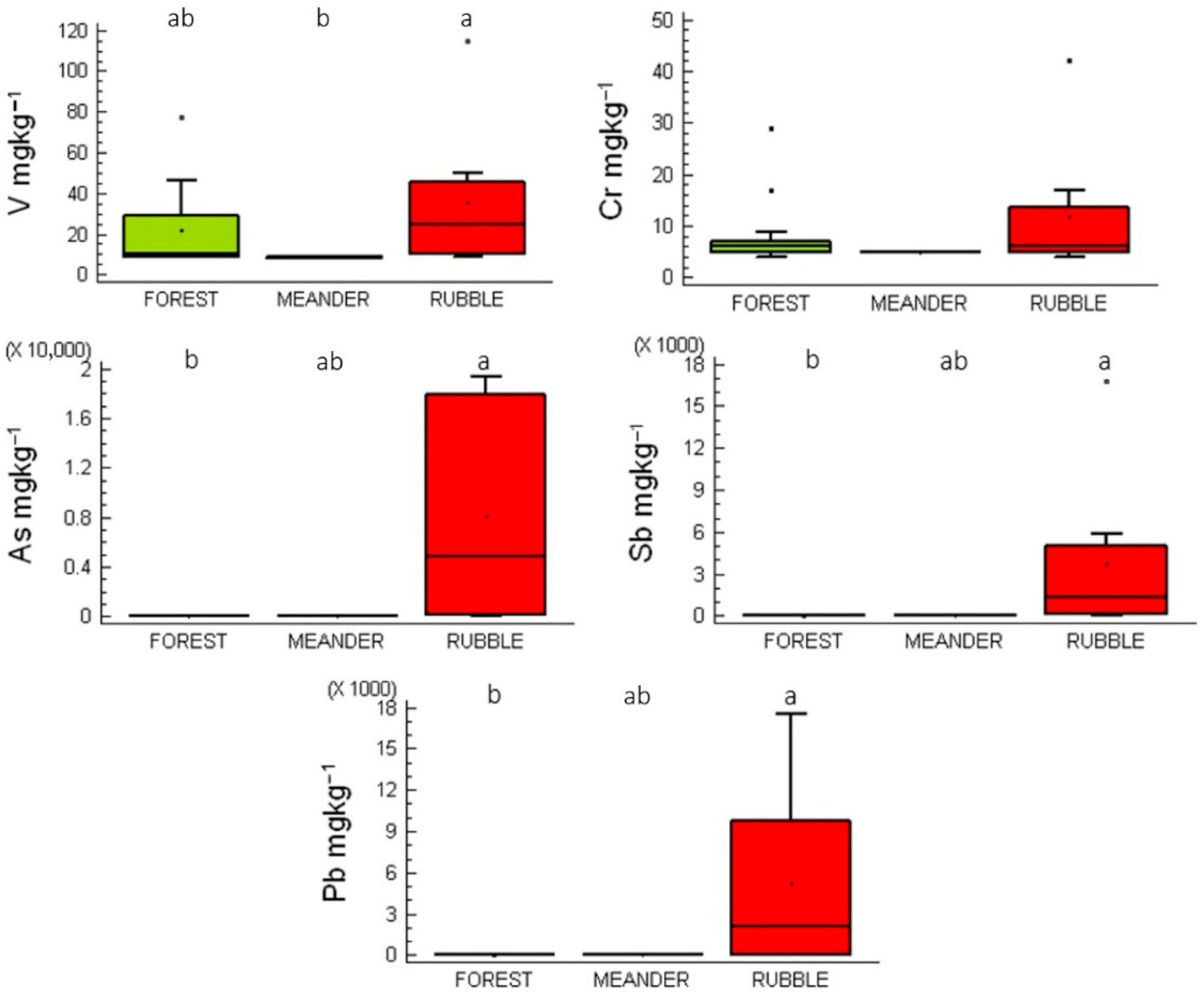
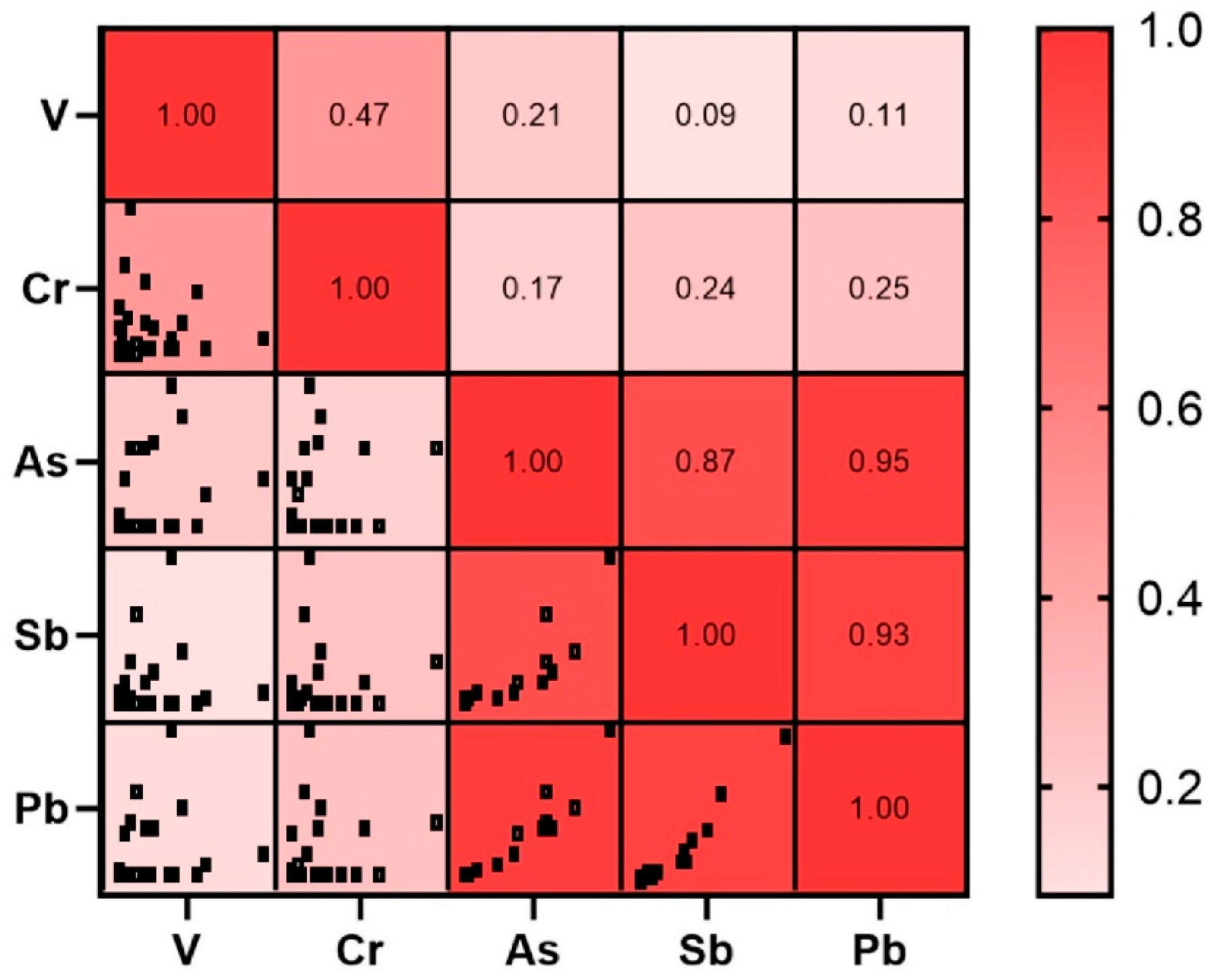
2.2. PTEs Content in Reference Soils and Mosses
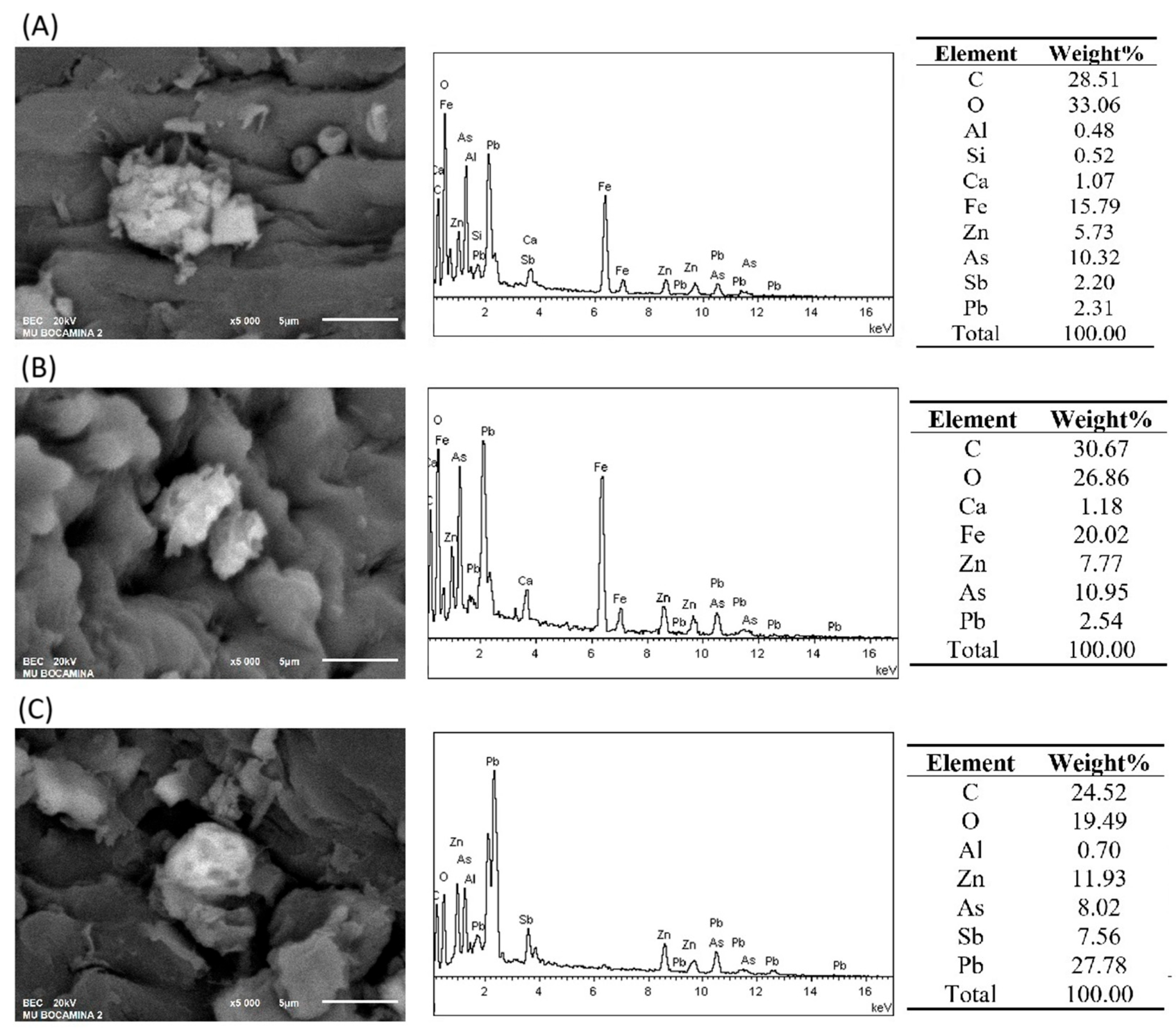
2.3. Analysis of Stems/Phyllidia Samples by SEM-EDS
3. Materials and Methods
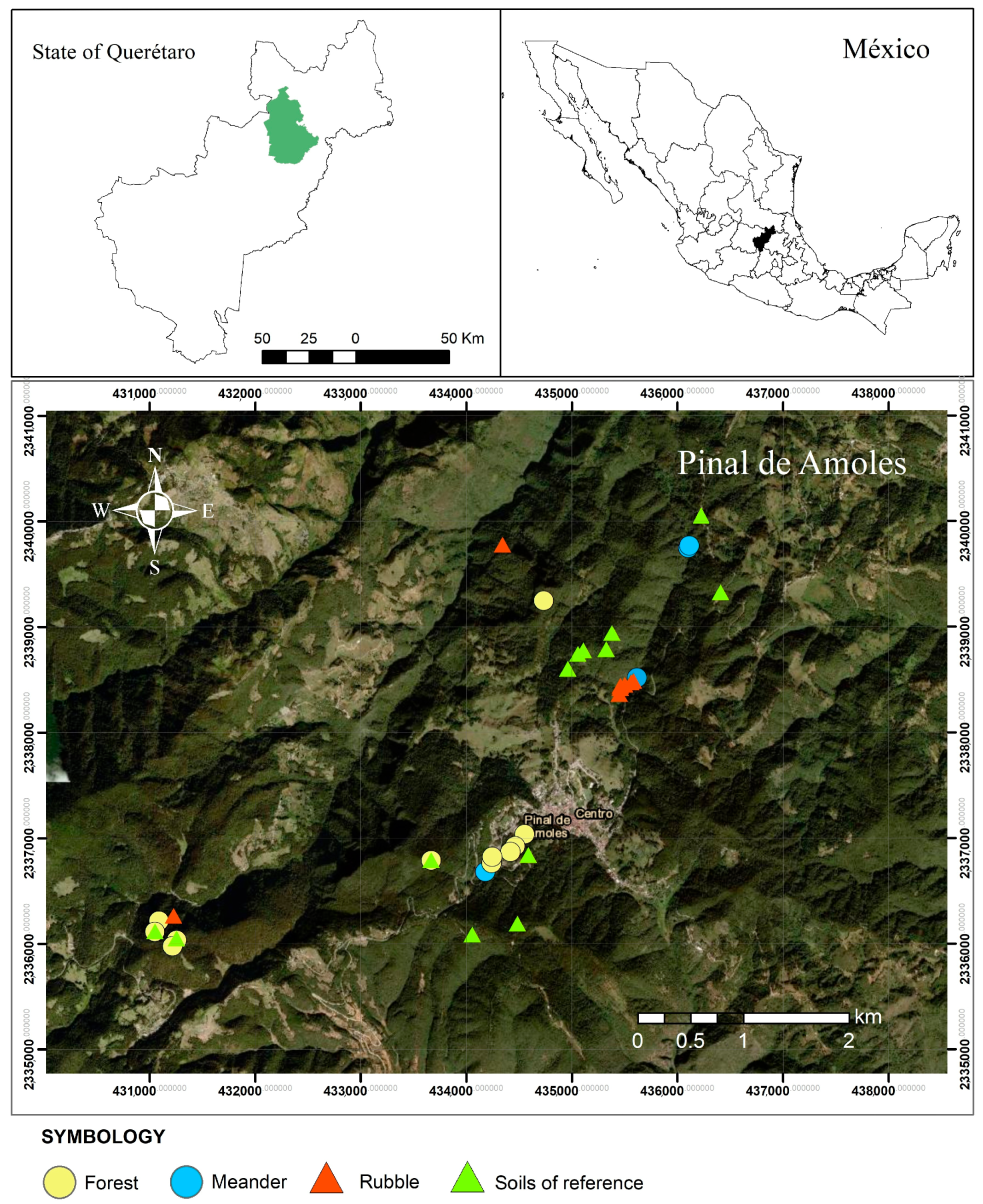
3.1. Study Area
3.2. Sampling and Sample Preparation
3.3. Taxonomic Identification
3.4. Sample Preparation
3.5. Sample Analysis
3.6. Enrichment Factor
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTE | Potentially toxic elements |
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Caritat, P.; Reimann, C.; Bogatyrev, I.; Chekushin, V.; Finne, T.E.; Halleraker, J.H.; Äyräs, M. Regional distribution of Al, B, Ba, Ca, K, La, Mg, Mn, Na, P, Rb, Si, Sr, Th, U and Y in terrestrial moss within a 188,000 km2 area of the central Barents region: Influence of geology, seaspray and human activity. Appl. Geochem. 2001, 16, 137–159. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Yang, J.C.; Zhang, Z.W.; Yuan, M.; Song, C.; Yuan, S. Biomonitoring heavy metal contaminations by moss visible parameters. J. Hazard. Mater. 2015, 296, 201–209. [Google Scholar] [CrossRef]
- Covarrubias, S.A.; Peña-Cabriales, J.J.P. Contaminación ambiental por elementos tóxicos en México: Problemática y estrategias de fitorremediación. Rev. Int. Contam. Ambient. 2017, 33, 7–21. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Rühling, A.; Tyler, G. Ecological approach to the lead problem. Bot. Not. 1968, 121, 321–342. [Google Scholar]
- Fernández, J.A.; Ederra, A.; Nunez, E.; Martinez-Abaigar, J.; Infante, M.; Heras, P.; Elias, M.J.; Maximpaka, V.; Carballeira, A. Biomonitoring of metal deposition in northern Spain by moss analysis. Sci. Total Environ. 2002, 300, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Macedo-Miranda, G.; Avila-Pérez, P.; Gil-Vargas, P.; Zarazúa, G.; Sánchez-Meza, J.C.; Zepeda-Gómez, C.; Tejeda, S. Accumulation of heavy metals in mosses: A biomonitoring study. SpringerPlus 2016, 5, 715. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Z.; Qiu, G.; Wu, P.; Zhang, R. Arsenic accumulation and speciation in epilithic moss collected from an abandoned mercury mining area, south-western China. Atmos. Pollut. Res. 2019, 10, 455–461. [Google Scholar] [CrossRef]
- Ávila-Pérez, P.; Ortiz-Oliveros, H.B.; Zarazúa-Ortega, G.; Tejeda-Vega, S.; Villalva, A.; Sánchez-Muñoz, R. Determining of risk areas due to exposure to heavy metals in the Toluca Valley using epiphytic mosses as a biomonitor. J. Environ. Manag. 2019, 241, 138–148. [Google Scholar] [CrossRef]
- Lara-Almazán, N.; Zarazúa-Ortega, G.; García-Chávez, M.D.L.Á.; Gómez-Hinojos, A.M.; Barrera-Díaz, C.E.; Ávila-Pérez, P. Biomonitoreo activo con Leskea angustata en la zona metropolitana del valle de Toluca. Rev. Int. Contam. Ambient. 2021, 37, 293–306. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Roy, M. Global ambient air quality monitoring: Can mosses help? A systematic meta-analysis of literature about passive moss biomonitoring. Environ. Dev. Sustain. 2024, 26, 5735–5773. [Google Scholar] [CrossRef]
- Díaz, S.; Villares, R.; Vázquez, M.D.; Carballeria, A. Physiological Effects of Exposure to Arsenic, Mercury, Antimony and Selenium in the Aquatic Moss Fontinalis antipyretica Hedw. Water Air Soil Pollut. 2013, 224, 1659. [Google Scholar] [CrossRef]
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Asadi Karam, E.; Lentini, M.; et al. In-field and in vitro study of the moss Leptodictyum riparium as bioindicator of toxic metal pollution in the aquatic environment: Ultrastructural damage, oxidative stress and HSP70 induction. PLoS ONE 2018, 13, e0195717. [Google Scholar] [CrossRef]
- Klos, A.; Czora, M.; Rajfur, M.; Wacławek, M. Mechanisms for translocation of heavy metals from soil to epigeal mosses. Water Air Soil Pollut. 2012, 223, 1829–1836. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Wang, Z. Effects of heavy metals on moss diversity and analysis of moss indicator species in Nancha manganese mining area, Southwestern China. Glob. Ecol. Conserv. 2021, 28, e01665. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, R.; Zhang, J.; Gao, L.; Ni, X. Distribution and dispersión of heavy metals in the rock-soil-moss system of the black sale áreas in the southeast of Guizhou Province, China. Environ. Sci. Pollut. Res. 2022, 29, 854–867. [Google Scholar] [CrossRef]
- Adamo, P.; Giordano, S.; Naimo, D.; Bargagli, R. Geochemical properties of airborne particulate matter (PM10) collected by automatic device and biomonitors in a Mediterranean urban environment. Atmos. Environ. 2008, 42, 346–357. [Google Scholar] [CrossRef]
- Gallego-Cartagena, E.; Morillas, H.; Carrero, J.; Madariaga, J.; Maguregui, M. Naturally growing grimmiaceae family mosses as passive biomonitos of heavy metals pollution in urban-industrial atmospheres from the Bilbao Metropolitan área. Chemosphere 2021, 263, 128190. [Google Scholar] [CrossRef]
- Delgadillo-Moya, C. Biodiversidad de Bryophyta (musgos) en México. Rev. Mex. Biodivers. 2014, 85, 100–105. [Google Scholar] [CrossRef]
- Herrera-Paniagua, P.; Martínez, M. Musgos de bosques húmedos de montaña en la Sierra Madre Oriental: Nuevos registros regionales. Bot. Sci. 2014, 92, 81–88. [Google Scholar] [CrossRef]
- Fernández-Nava, R.; Robles, J.A.C. Notas sobre la vegetación y flora del municipio de San Joaquín, Querétaro, México. Polibotánica 1997, 4, 10–36. [Google Scholar]
- INE. Programa de manejo de la Reserva de la Biosfera Sierra Gorda, México. In Secretaría de Medio Ambiente, Recursos Naturales y Pesca; INE: Mexico City, Mexico, 1999. [Google Scholar]
- Abad-Cuevas, N.C. Modelación Hidrológica de las Cuencas El Chuveje y Arroyo Real Como Herramienta en la Implementación de Pago Por Servicios Ecológicos en la Reserva de La Biosfera Sierra Gorda, Querétaro, México (Tesis de Maestría) Repositorio del Tecnológico de Monterrey. 2006. Available online: https://repositorio.tec.mx/items/23a7da51-a290-4d2d-ae1a-85e583f1b8aa (accessed on 14 May 2025).
- Klos, A.; Rajfur, M.; Šrámek, I.; Waclawek, M. Mercury concentration in lichen, moss and soil samples collected from the forest áreas of Praded and Glacensis Euroregions (Poland and Czech Republic). Environ. Monit. Assess. 2012, 184, 6765–6774. [Google Scholar] [CrossRef]
- Wojtun, B.; Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. Decreasing concentrations of metals in Sphagnum mosses in ombrotrophic mires of the Sudety mountains (SW Poland) since late 1980s. Chemosphere 2013, 91, 1456–1461. [Google Scholar] [CrossRef]
- Kosior, G.; Klanova, J.; Vankova, L.; Kukucka, P.; Chropenova, M.; Brudzinska-Kosior, A.; Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. Pleurozium schreberi as an ecological indicator of polybrominated diphenyl ethers (PBDEs) in a heavily industrialized urban área. Ecol. Indic. 2015, 48, 492–497. [Google Scholar] [CrossRef]
- Zarazúa-Ortega, G.; Poblano-Bata, J.; Tejeda-Vega, S.; Ávila-Pérez, P.; Zepeda-Gómez, C.; Ortiz-Oliveros, H.; Macedo-Miranda, G. Assessment of spatial variability of heavy metals in metropolitan zone of Toluca valley, Mexico, using the biomonitoring technique in mosses and TXRF analysis. Sci. World J. 2013, 2013, 426492. [Google Scholar] [CrossRef]
- Macedo-Miranda, M.G.; Barrera-Díaz, C.E.; Ávila-Pérez, P.; López-Solórzano, E.; Ortiz-Oliveros, H.B.; Zavala-Arce, R.E. Bioconcentration Capacity of Moss Leskea angustata Tayl. for Heavy Metals and Its Application in the Atmospheric Biomonitoring of a Metropolitan Area. Atmos. Environ. 2024, 331, 120579. [Google Scholar] [CrossRef]
- Gómez-Ensastegui, C.; Avila-Pérez, P.; García-Rivas, J.L.; Barrera-Díaz, C.E.; Ortiz-Oliveros, H.B.; Martínez-Gallegos, S. Evaluation of an aquatic liverwort and terrestrial moss as biomonitors of heavy metals associated with particulate matter. Sci. Rep. 2025, 15, 4127. [Google Scholar] [CrossRef]
- Xiao, J.; Han, X.; Sun, S.; Wang, L.; Rinklebe, J. Heavy metals in different moss species in alpine ecosystems of Mountain Gongga, China: Geochemical characteristics and controlling factors. Environ. Pollut. 2020, 272, 115991. [Google Scholar] [CrossRef]
- Fernández, J.A.; Boquete, M.T.; Carballeira, A.; Aboal, J.R. A critical review of protocols for moss biomonitoring of atmospheric deposition: Sampling and sample preparation. Sci. Total Environ. 2015, 517, 132–150. [Google Scholar] [CrossRef]
- Schröder, W.; Nickel, S. Moss species-specific accumulation of atmospheric deposition? Environ. Sci. Eur. 2019, 31, 78. [Google Scholar] [CrossRef]
- Świsłowski, P.; Nowak, A.; Wacławek, S.; Silvestri, D.; Rajfur, M. Bioaccumulation of Trace Elements from Aqueous Solutions by Selected Terrestrial Moss Species. Biology 2022, 11, 1692. [Google Scholar] [CrossRef]
- Trejo-Reséndiz, P. Metales Pesados Presentes en Los Cauces de la Cuenca Presa Jalpan. Master’s Thesis, Ciencias en Hidrología Ambiental, Facultad de Ingeniería, Universidad Autónoma de Querétaro, Querétaro, Mexico, 2025. [Google Scholar]
- SGM; Casto-Díaz, J. Evaluación de los suministros de mercurio primario y secundario en México; Comisión para la Cooperación Ambiental: Montreal, QC, Canada, 2013. [Google Scholar]
- Zhao, S.; Shi, T.; Terada, A.; Riya, S. Evaluation of Pollution Level, Spatial Distribution, and Ecological Effects of Antimony in Soils of Mining Areas: A Review. Int. J. Environ. Res. Public Health 2023, 20, 242. [Google Scholar] [CrossRef]
- Navarro, R. Estudio de Los Suelos Contaminados Con Mercurio del Estado de Querétaro, Sus Especies y Ensayos de Biodisponibilidad. Bachelor’s Thesis, Repositorio Institucional de la Universidad Nacional Autónoma de México, Mexico City, Mexico, 2009. [Google Scholar]
- Hernández-Silva, G.; García-Martínez, R.; Solís-Valdez, S.; Martínez-Trinidad, S.; Mercado-Sotelo, I.; Ramírez-Islas, M.; Scharek, P.; Solorio-Munguía, G. Presencia del Hg total en una relación suelo-planta-atmósfera al sur de la Sierra Gorda de Querétaro, México. TIP Rev. Espec. En Cienc. Químico-Biológicas 2012, 15, 5–15. [Google Scholar]
- INECC. Evaluación de Las Fuentes Principales en el Sector Minería Primaria en Querétaro y Desarrollo de Inventario de Sitios; INECC: Mexico City, Mexico, 2020. [Google Scholar]
- Balcázar-Regalado, R. Estudio Genotóxico en Renacuajos (Ecnomiohyla miotympanum) del Río Escanela Expuestos a Residuos Mineros de Pinal de Amoles, Querétaro. Ph.D. Thesis, Facultad de Química, Universidad Autónoma de Querétaro, Repositorio Institucional DGBSDI-UAQ, Querétaro, Mexico, 2011. [Google Scholar]
- Sun, X.; Li, B.; Han, F.; Xiao, E.; Xiao, T.; Sun, W. Impacts of arsenic and antimony co-contamination on sedimentary microbial communities in rivers with different pollution gradients. Microb. Ecol. 2019, 78, 589–602. [Google Scholar] [CrossRef]
- Rebolloso-Hernández, C.A.; Vallejo-Pérez, M.R.; Carrizales-Yáñez, L.; Garrigos-Lomelí, G.J.; Razo-Soto, I.; Diaz-Barriga, F. Arsenic and mercury exposure in different insect trophic guilds from mercury mining areas in Mexico. Environ. Monit. Assess. 2024, 196, 422. [Google Scholar] [CrossRef]
- Delgadillo-Moya, C. Manual de Briofitas; UNAM: Mexico City, Mexico, 1990. [Google Scholar]
- Sharp, A.J.; Crum, H.A.; Eckel, P. The Moss flora of Mexico. Pt. 1. Sphagnales to Bryales—Pt. 2. Orthotrichales to Polytrichales. In Memoirs of the New York Botanical Garden (USA); Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- De Vos, W.; Tarvainen, T.; Salminen, R.; Reeder, S.; De Vivo, B.; Demetriades, A.; Pirc, S.; Batista, M.J.; Marsina, K.; Ottesen, R.T.; et al. Geochemical Atlas of Europe. Part 2. Interpretation of Geochemical Maps, Additional Tables, Figures, Maps, and Related Publications; Geological Survey of Finland: Espoo, Finland, 2006; ISBN 951–690-956-6. Available online: http://weppi.gtk.fi/publ/foregsatlas/ (accessed on 14 May 2025).
- Preston, W.; Clistenes, W.; Miranda-Biondi, C.; de Souza-Junior, V.; Ramos, W.; Alves-Ferreira, H. Valores de referência de qualidade para metais pesados em solos do rio Grande do Norte. Rev. Bras. Cienc. Solo 2014, 38, 1028–1037. [Google Scholar] [CrossRef]
- Vassallo-Morales, L.F.; Flores, L.; Lazcano, L.M.; Hernández, G.; Solorio, G.; Maples, M.; Girón, P.; Garduño, C. El gabro de Arperos y su aportación de Cr-Ni a la subcuenca del río Silao, Guanajuato, México: Ing. Hidrául. Méx. 2001, 16, 63–71. [Google Scholar]
- Gutiérrez-Ruiz, M.; Romero, F.M.; González-Hernández, G. Suelos y sedimentos afectados por la dispersión de jales inactivos de sulfuros metálicos en la zona minera de Santa Bárbara, Chihuahua, México. Rev. Mex. Cienc. Geológicas 2007, 24, 170–184. [Google Scholar]
- Álvarez-Ayuso, E.; Otones, V.; Murciego, A.; García-Sánchez, A.; Santa Regina, I. Antimony, arsenic and lead distribution in soils and plants of an agricultural area impacted by former mining activities. Sci. Total Environ. 2012, 439, 35–43. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils; Blackie Academic & Professional: Glasgow, UK, 1995. [Google Scholar]
- Bellini, E.; Bandoni, E.; Giardini, S.; Sorce, C.; Spanó, C.; Bottega, S.; Fontanini, D.; Kola, A.; Valensin, D.; Bertolini, A.; et al. Glutathione and phytochelatins jointly allow intracellular and extracellular detoxification of cadmium in the liverwort Marchantia polymorpha. Environ. Exp. Bot. 2023, 209, 105303. [Google Scholar] [CrossRef]
- Świsłowski, P.; Nowak, A.; Rajfurc, M. Comparison of exposure techniques and vitality assessment of mosses in active biomonitoring for their suitability in assessing heavy metal pollution in atmospheric aerosol. Environ. Toxicol. Chem. 2022, 41, 1429–1438. [Google Scholar] [CrossRef]
- Bagherifam, S.; Brown, T.C.; Fellows, C.M.; Naidu, R. Bioavailability of arsenic and antimony in terrestrial ecosystems: A Review. Pedosphere 2019, 29, 681–720. [Google Scholar] [CrossRef]
- Huang, J.L.; Li, Z.Y.; Mao, J.Y.; Chen, Z.M.; Liu, H.L.; Liang, G.Y.; Zhang, D.B.; Wen, P.J.; Mo, Z.Y.; Jiang, Y.M. Contamination and health risks brought by arsenic, lead and cadmium in a water-soil-plant system nearby a non-ferrous metal mining area. Ecotoxicol. Environ. Saf. 2024, 270, 115873. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Koz, B.; Çevik, U.; Akbulut, S. Heavy metal analysis around Murgul (Artvin) copper mining area of Turkey using moss and soil. Ecol. Indic. 2012, 20, 17–23. [Google Scholar] [CrossRef]
- Bargagli, R.; Monaci, F.; Borghini, F.; Bravi, F.; Agnorelli, C. Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 2002, 116, 279–287. [Google Scholar] [CrossRef]
- Oishi, Y. Biomonitoring of transboundary pollutants using moss in Japan’s mountains. Environ. Sci. Pollut. Res. 2022, 29, 15018–15025. [Google Scholar] [CrossRef] [PubMed]
- Veress, M. Rubble Mines in the Environs of Veszprém (Bakony Region, Hungary). Mining 2023, 3, 579–604. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.F.; Olvera-Balderas, D. Elementos potencialmente tóxicos (Cd, Hg, Pb Y Zn) en suelos impactados por planta recicladora de plomo (Zacatecas, México), a una década de parar operaciones. Rev. Int. Contam. Ambient. 2019, 35, 651–669. [Google Scholar] [CrossRef]
- ICP Vegetation. Heavy Metals, Nitrogen and POPs in European Mosses: Monitoring Manual, 2020 Survey. United Nations Economic Commission for Europe Convention on Long-Range Transboundary Air Pollution. 2020. Available online: https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf (accessed on 14 May 2025).
- Herrera-Paniagua, P.; Delgadillo-Moya, C.; Ríos, J.L.V.; Luna-Vega, I. Floristics and biogeography of the mosses of the state of Querétaro, Mexico. Bryologist 2008, 111, 41–56. [Google Scholar] [CrossRef]
- Garcias, Y.; Torres, R.; Ojeda, G.; de los Santos-Villalobos, S.; Tejeda, S.; Velasco, H. Using Geochemical Fingerprints for Assessing Sediment Source Apportionment in an Agricultural Catchment in Central Argentina. Water 2021, 13, 3632. [Google Scholar] [CrossRef]
- Tejeda, S.; Zarazúa-Ortega, G.; Ávila-Pérez, P.; García-Mejía, A.; Carapia-Morales, L.; Díaz-Delgado, C. Major and trace elements in sediments of the upper course of Lerma river. J. Radioanal. Nucl. Chem. 2006, 270, 9–14. [Google Scholar] [CrossRef]
- Torres-Astorga, R.; Garcias, Y.; Borgatello, G.; Velasco, H.; Padilla, R.; Dercon, G.; Mabit, L. Use of geochemical fingerprints to trace sediment sources in an agricultural catchment of Argentina. Int. Soil Water Conserv. Res. 2020, 8, 410–417. [Google Scholar] [CrossRef]
- Boquete, M.T.; Lang, I.; Weidinger, M.; Richards, C.L.; Alonso, C. Patterns and mechanisms of heavy metal accumulation and tolerance in two terrestrial moss species with contrasting habitat specialization. Environ. Exp. Bot. 2021, 182, 104336. [Google Scholar] [CrossRef]
- Søndergaard, J.; Jørgensen, C.J. Field Portable X-Ray Fluorescence (pXRF) Spectrometry for Chemical Dust Source Characterization: Investigations of Natural and Mining-Related Dust Sources in Greenland (Kangerlussuaq Area). Water Air Soil Pollut. 2021, 232, 144. [Google Scholar] [CrossRef]
- Schropp, S.; Lewis, F.; Windom, H.; Ryan, J.; Calder, F.; Burney, L. Interpretation of metal concentrations in estuarine sediments of Florida using Aluminum as a reference element. Estuaries 1990, 13, 227–235. [Google Scholar] [CrossRef]
- Machado, A.; García, N.; Colina, G.; García, C.; Granadillo, V. Estudio de cinc, cromo, níquel y plomo mediante factores de enriquecimiento como indicadores de contaminación en suelo y sedimentos viales en la ciudad de Maracaibo. Ciencia 2010, 16, 448–456. [Google Scholar]
- Shah, M.H.; Shaheen, N.; Nazir, R. Assessment of the trace elements level in urban atmospheric particulate matter and source apportionment in Islamabad, Pakistan. Atmos. Pollut. Res. 2012, 3, 39–45. [Google Scholar] [CrossRef]
- Dubey, B.; Pal, A.K.; Singh, G. Trace metal composition of airborne particulate matter in the coal mining and non–mining areas of Dhanbad Region, Jharkhand, India. Atmos. Pollut. Res. 2012, 3, 238–246. [Google Scholar] [CrossRef]



| PTE | Forest | Meander | Mine Rubble | Gongga Mountain, China 1 | Murgul Mine, Turkey 2 | Colline Metallifere Italy 3 | Wanshan District China 4 | Spain 5 |
|---|---|---|---|---|---|---|---|---|
| V | 10 | 2 | 25 | --- | 70.9 | 1.4 | --- | --- |
| Cr | 6 | 1 | 6 | 8.3 | 43.4 | 5.1 | --- | 2.6 |
| As | 10 | 42 | 4847 | --- | 18.1 | 1.4 | 3.5 | 0.4 |
| Sb | 0.1 | 37 | 1291 | --- | --- | --- | --- | --- |
| Pb | 10 | 50 | 2082 | 6.6 | 19.0 | 2.7 | --- | 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejeda, S.; Zarazúa, G.; Juárez-Acosta, E.A.; Barrera-Díaz, C.E.; León, L.R.; Avila-Pérez, P.; Zepeda-Gómez, C. Potentially Toxic Elements in Terrestrial Mosses in the Vicinity of a Stibnite Mine in Pinal de Amoles, Mexico. Plants 2025, 14, 2657. https://doi.org/10.3390/plants14172657
Tejeda S, Zarazúa G, Juárez-Acosta EA, Barrera-Díaz CE, León LR, Avila-Pérez P, Zepeda-Gómez C. Potentially Toxic Elements in Terrestrial Mosses in the Vicinity of a Stibnite Mine in Pinal de Amoles, Mexico. Plants. 2025; 14(17):2657. https://doi.org/10.3390/plants14172657
Chicago/Turabian StyleTejeda, Samuel, Graciela Zarazúa, Emma A. Juárez-Acosta, Carlos E. Barrera-Díaz, Luis R. León, Pedro Avila-Pérez, and Carmen Zepeda-Gómez. 2025. "Potentially Toxic Elements in Terrestrial Mosses in the Vicinity of a Stibnite Mine in Pinal de Amoles, Mexico" Plants 14, no. 17: 2657. https://doi.org/10.3390/plants14172657
APA StyleTejeda, S., Zarazúa, G., Juárez-Acosta, E. A., Barrera-Díaz, C. E., León, L. R., Avila-Pérez, P., & Zepeda-Gómez, C. (2025). Potentially Toxic Elements in Terrestrial Mosses in the Vicinity of a Stibnite Mine in Pinal de Amoles, Mexico. Plants, 14(17), 2657. https://doi.org/10.3390/plants14172657







