Biodiversity Conservation, a Crucial Step Towards Food and Nutritional Security, Food Justice and Climate Change Resilience in Africa
Abstract
1. Introduction
1.1. Significance of the Study
- The methods of biodiversity conservation adopted in Africa and impacts on human survival;
- Highly utilised African wild indigenous vegetables, climbers and trailers, and fruit trees and their roles in food and nutritional security, especially during drought and food scarcity;
- The impact of community-inclusive biodiversity conservation methods on conservation outcomes and their various challenges;
- The role of African indigenous wild fruit trees in carbon sequestration and climate resilience; and
- The overall objective was to identify gaps in African biodiversity conservation and develop a conceptual framework to highlight the integral principles crucial for effective biodiversity conservation in Africa.
1.2. Methodology
2. Results and Discussions
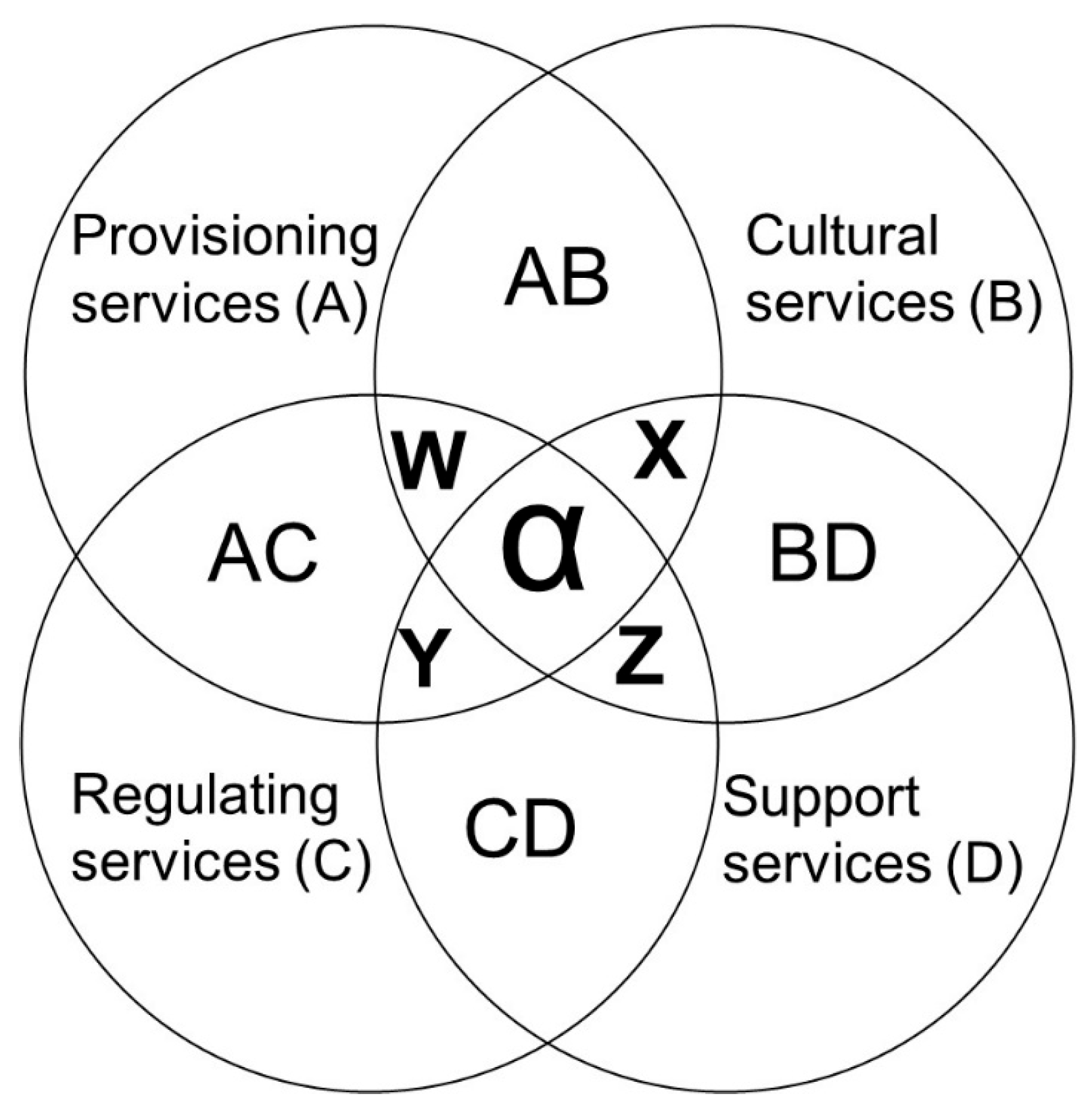
2.1. Ecosystem Services
2.2. Biodiversity Conservation Policies/Methods and Outcomes
2.3. Wild Indigenous Fruit and Vegetable Species with Provisioning Services (Food and Natural Medicine)
2.3.1. African Wild Indigenous Fruit Trees with Various Ecosystem Services
| Plant Specie | Provisional Services (Food and Medicinal Uses) |
|---|---|
| Sclerocarya birrea subsp. caffra (Sond.) Kokwaro | Fruit pulp and edible nuts are consumed [34] and used in the production of beverages and cosmetics [42]. Fruits are used to produce sweets, wine and flavourings [34]. Oil is extracted from the kernels for domestic use [43]. The oil is used to preserve meat and meat products [44]. Wood is used as construction material and leaves serve as fodder [45]. Root is used to treat an internal ailment (kati), while the bark is used to treat stomach disorders [46]. The bark decoction is used to treat dysentery, diarrhoea, and rheumatism and has a prophylactic effect against malaria [47]; and bark is used to treat haemorrhoids; roots and bark are used as laxatives, and a drink made from the leaves is used to treat gonorrhoea [47]. |
| Dovyalis afra (Hook.f. & Harv.) Warb. (often referred to as Dovyalis caffra (Hook.f. & Harv.) Hook.f.) | The fruit is eaten raw by people and wild animals, or cooked, utilised as jelly, pickles and jam [48] and often used to produce juices and wine [49]. Its juice is added to boiled millet or sorghum porridge among the Bapedi people of South Africa [50]. The boiled fruit is used to flavour meat and fish and a brief simmering of fruits is performed, with products added to fillings used in pies, puddings and cakes [51]. |
| Parinari curatellifolia Planch. ex Benth. | The plant is used for the treatment of cancer, pneumonia, fever, malaria, typhoid, hypertension, microbial infections, pain, anti-inflammation, and toothache [52,53]. The tree is a valuable source of food and ethnomedicines across Africa as a result of its rich nutritional content and phytochemicals in various parts of the plant [54]. The fruit is utilised as food and in the production of traditional alcoholic drinks [55,56]. |
| Strychnos madagascariensis Poir. | Fruit pulp is consumed raw as a snack and processed into value-added food products (such as fruit rolls, powders, jams, or juices) [57,58]. |
| Strychnos spinosa Lam. | Fruit is used to produce alcohol, fruit juice, and jam [59]. Fermented combinations of maize meal and S. spinosa pulp [59] and sorghum porridge mixed with S. spinosa pulp [60] are consumed in rural KwaZulu-Natal and Zimbabwe, respectively. Plant parts are used to treat snakebites, ulcers, wounds, headaches, gastric and intestinal problems, venereal diseases, leprosy, diarrhoea, and fever [61]. Leaf decoction combined with bark powder is used to treat wounds, while the dried powdered leaves are added to food to treat liver damage [61]. The green fruits are used as an antidote for snakebite [62,63]. Bark decoction is used to treat stomachache [59,64]. |
| Amaranthus (various species) | Several studies have shown that oil extracted from Amaranthus seed or leaves can benefit those with cardiovascular disease and hypertension [65]. Vegetable Amaranthus has been a good source of medicine for young children, lactating mothers, and other patients with constipation and in addition, it is used to treat fever, anaemia, or kidney complaints [66]. Amaranth may present a potential source of cancer treatment as the seeds are a natural source of squalene [67], a valuable antioxidant known for anticancer activity [68]. Amaranth oil contains 6 to 8% squalene [69]. |
| Corchorus olitorus L. | It is used to treat heart failure, diarrhoea, typhoid fever and colic [70]. Leaves are used to cure gonorrhoea, chronic cystitis, pain, fever, and tumours [71]. |
| Cleome gynandra L. (also referred to as Gynandropsis gynandra (L.) Briq.) | Spider plant is used as a traditional medicine all over Uganda to hasten childbirth as a result of its uterotonic activity [72]. The entire plant has been utilised traditionally to treat a variety of diseases and conditions such as anaemia, arthritis, diabetes, cancer, piles, rheumatism, scurvy, tumours, cardiovascular diseases, chest pains, constipation, malaria, a relieving eyewash [73,74] migraine headaches, epilepsy [75] and stomach ache [76]. The extract is used to treat snake bites, food poisoning and severe pain caused by scorpion stings [77]. The sap of the leaves is used to manage severe threadworm infections and relieve cerebral pain [78]. Also, the sap from pounded young leaves is squeezed into the ears, nose, and eyes to control epileptic seizures and relieve earache [78]. The decoction of leaves and roots relieves fever and headaches and alleviates sexual weakness [79]. |
| Momordica balsamina L. | Fresh leaves are consumed as vegetables [80]. Leaves are used to treat diabetes, jaundice, fever, gonorrhoea, tuberculosis, and viral infections [81]. |
| Momordica charantia L. | The young fruits and shoots are consumed in some parts of West Africa, and they are used as an emmenagogue to facilitate childbirth in Ivory Coast [82]. It is used to treat diabetes, measles and chicken pox [83], tumours, wound, rheumatism, malaria, vaginal discharge and to expel intestinal gas, while the seeds are used to induce abortion [84,85]. In Nigeria and Ghana, the root of the plant is used as an abortifacient together with the fruit as well as an ingredient in aphrodisiac preparation [84]. |
| Citrullus colocynthis (L.) Schrad. | In Northeastern Morocco it is used to treat various cardiovascular system diseases [86]. In East Africa, seed tar is applied to the skin by nomads. But, the digestion of this fruit results in acute toxic colitis, bloody diarrhoea, and changes in the colon [87]. In southern Tunisia, C. colocynthis is a useful medicine for gout, arthritis, and inflammatory disorders and its kernels are used in food preparation in many African countries [88]. |
| Wild Forest Species | Nutritional, Mineral and Bioactive Contents |
|---|---|
| Sclerocarya birrea | Marula pulp and nuts are rich in various healthy saturated fatty acids, such as tetradecanoic and hexadecanoic acid, and unsaturated fatty acids, such as oleic acid, linoleic acid, α-linolenic acid, and eicosanoid acid with cardioprotective activity [40,89,90]. |
| Dovyalis caffra (Hook.f. & Harv) Hook.f. | The fruit is a valuable source of ascorbates [35,91]. Chlorogenic acid, catechin, and gallic acid are the main constituents of the fruit while hesperidin, rutin, ellagic acid, quercetin, kaempferol and apigenin were detected in lower quantities [92]. Fruits contain p-coumaric acid, p-hydroxyphenylacetic acid, 3-methoxy-4-hydroxyphenylacetic acid, m-hydroxybenzoic acid, vanillic acid [93], chlorogenic acid, procatechic acid [94], Pyrogallol and Catechin [95]. Kei–apple fruit juice also contains a high concentration of ascorbic acid [93]. |
| Parinari curatellifolia Planch. ex Benth. | The fruit is rich in vitamin C, protein and calcium [55,56]. The leaves contain compounds such as alkaloids, flavonoids, and saponins [52], while the stem contains saponins, alkaloids, tannins, cardiac glycosides, flavonoids, digitalis glycosides, phenols, terpenes, and steroids [96]. |
| Strychnos madagascariensis Poir. | Fruits have high sugar, fibre, potassium and iron content [97,98]. |
| Strychnos spinosa Lam. | Fruit pulp contains fibre, carbohydrates, and vitamin C [99]. |
| Amaranthus species | Amaranth seeds contain approximately 7% squalene [69]. Amino acid in Amaranth grain includes arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine [100]. Phytonutrients in Amaranth grain include flavonoids, quercetin, nicotiflorin, rutin, ferulic acid, gallic acid, caffeic acid, p-coumaric acid, isoquercitrin, anthocyanins, syringic acid and vanillic acid [101]. Leaves contain high protein [102]. |
| Corchorus olitorus L. | The leaves are rich in beta-carotene, iron, calcium, fibre, vitamins C, A, and E, proteins, sodium and folic acid [103], amino acids, and essential minerals [104]. The plant parts, such as the roots, bark, leaves and seeds, contain flavonoids, cardiac glycosides, fatty acids, triterpenoids, polysaccharides and phenolics [105,106,107,108]. Chlorogenic acid is present in leaves [109]. Other phenolic compounds present are 3,5-dicaffeoylquinic acid, quinic acid, gallic acid, protocatechuic acid, 4-O-caffeoylquinic acid, caffeic acid, 1,3-di-O-caffeoylquinic acid, feruloyl-quinic acids, and 4,5-di-O-caffeoylquinic acid, which were identified from the leaves of C. olitorius [110]. Trans-ferulic acid, p-coumaric acid, and rosmarinic acid were detected from C. olitorius [107,111]. The leaves contain flavones (cirsilineol and cirsiliol), flavones glycosides (apigenin, apegenin-7-O-glucoside), flavanones (naringenin, naringin), astragalin (kaempferol-3-O-β-D-glucopyranoside), tolifolin (kaempferol-3-O-β-D-galactopyranoside), and jugulanin (kaempferol-3-O-β-L-arabinopyranoside) [111]. |
| Cleome gynandra L. | It has high α-carotene, α-tocopherol, β-tocopherol and γ-tocopherol, ascorbic acid, β-carotene, lutein, violaxanthin, and β-cryptoxanthin content [74]. Leaves contain magnesium, calcium, iron, and zinc [112]. It is rich in flavonoids and phenolics [113]. |
| Momordica balsamina L. | The leaves, fruits, seeds, and bark of the M. balsamina contain alkaloids, flavonoids, glycosides, steroids, terpenes, cardiac glycoside, saponins, tannins and lectins [114]. Balsamin found in leaves, fruit, stem of M. balsamina has anticancer activity [115]. |
| Momordica charantia L. | The leaves and flowers of M. charantia contain triterpenoids (momordicine and charantin), carotenoids (antheraxanthin, lutein, violaxanthin, α-carotene, and β-carotene), and phenylpropanoids (caffeic acid, chlorogenic acid, epicatechin, gallic acid, p-coumaric acid, rutin, and trans-cinnamic acid) [116]. The leaves of contain Momordicin I, Momordicin IV, aglycone of Momordicoside, aglycone of Momordicoside L and Karavilagenin D [117]. |
| Citrullus colocynthis (L.) Schrad. | The major minerals in the seeds are calcium, magnesium and potassium [118]. Twelve alkaloids, including quinoline, nicotinamide, uracil, 2-hydroxyquinoline, 2-methylquinoline, 4-hydroxyquinoline, 4-methylquinoline, 6-hydroxyquinoline, 6-methylquinoline, 7, 8-benzoquinoline, 8-hydroxyquinoline, and 8-methylquinoline, were detected in C. colocynthis fruits [119]. Citrullus colocynthis contain ketones, epoxy compounds, hydrocarbons [120], and fatty acids [121]. |
2.3.2. Wild Edible Vegetables in African Forests
2.4. Contribution of Indigenous People to Conservation Engagements
2.5. Biodiversity Conservation as a Climate Change Resilience Tool
2.5.1. Role of Drought-Tolerant African Wild Leafy Vegetables and Fruits in Sustaining Livelihoods During Drought
2.5.2. African Indigenous Wild Fruit Trees as a Tool for Carbon Sequestration and Climate Resilience
2.6. Challenges Associated with Community-Based Biodiversity Conservation Projects
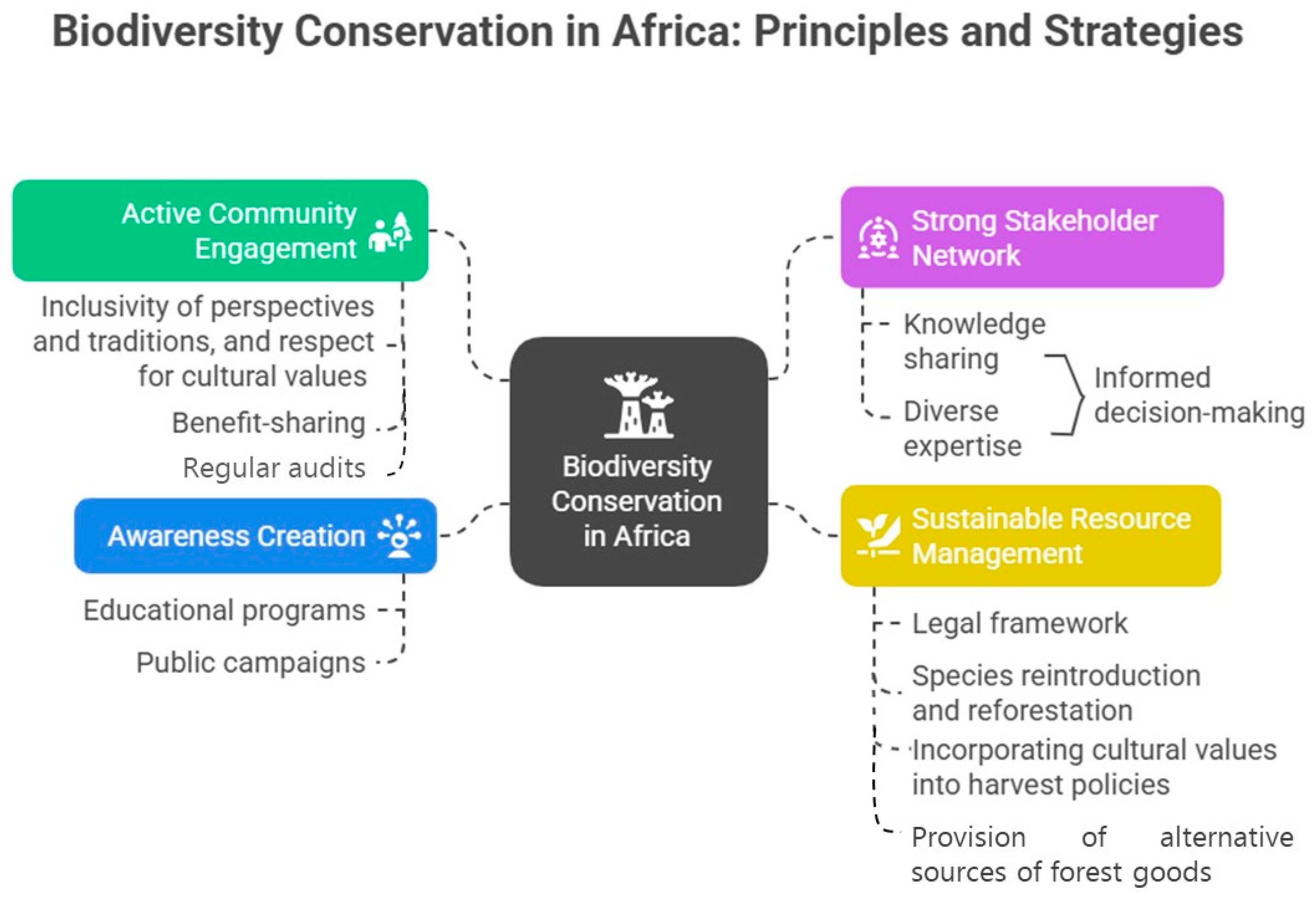
2.7. Conceptual Framework for Biodiversity Conservation in Africa
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment 2015: How Are the World’s Forests Changing? 2nd ed.; FAO: Rome, Italy, 2016. [Google Scholar]
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar]
- Ajanaku, B.A.; Collins, A.R. Economic Growth and Deforestation in African Countries: Is the Environmental Kuznets Curve Hypothesis Applicable? For. Policy Econ. 2021, 129, 102488. [Google Scholar] [CrossRef]
- Kissinger, G.; Herold, M.; De Sy, V. Drivers of Deforestation and Forest Degradation: A Synthesis Report for REDD+ Policymakers; REDD+ Policymakers Report; Lexeme Consulting: Vancouver, BC, Canada, 2012; Volume 48. [Google Scholar]
- Rudel, T.K. The National Determinants of Deforestation in Sub-Saharan Africa. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120405. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020: Key Findings; FAO: Rome, Italy, 2020. [Google Scholar]
- Baptista, D.M.S.; Farid, M.M.; Fayad, D.; Kemoe, L.; Lanci, L.S.; Mitra, M.P.; Muehlschlegel, T.S.; Okou, C.; Spray, J.A.; Tuitoek, K.; et al. Climate Change and Chronic Food Insecurity in Sub-Saharan Africa; International Monetary Fund: Washington, DC, USA, 2022. [Google Scholar]
- Aryee, G.A.; Sardinha, I.D.; Branquinho, C. Linking drivers of food insecurity and ecosystem services in Africa. Front. Sustain. Food Syst. 2024, 8, 1272332. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.; Chan, K.M.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Dodo, M.K. Understanding Africa’s food security challenges. In Food Security in Africa; IntechOpen: London, UK, 2020; pp. 1–17. [Google Scholar]
- Ayanlade, A.; Radeny, M. COVID-19 and food security in Sub-Saharan Africa: Implications of lockdown during agricultural planting seasons. npj Sci. Food 2020, 4, 13. [Google Scholar] [CrossRef]
- Bouwens, M. Towards More Foundational Humanitarian Self-Sovereign Identity Systems: Exploring Strategies for Humanitarian Organizations to Nurture Support for SSI Systems in Kenya; Delft University of Technology: Delft, The Netherlands, 2020. [Google Scholar]
- Abdul Mumin, Y.; Abdulai, A. Informing food security and nutrition strategies in sub-Saharan African countries: An overview and empirical analysis. Appl. Econ. Perspect. Policy 2022, 44, 364–393. [Google Scholar] [CrossRef]
- Govender, L.; Pillay, K.; Siwela, M.; Modi, A.; Mabhaudhi, T. Food and nutrition insecurity in selected rural communities of KwaZulu-Natal, South Africa—Linking human nutrition and agriculture. Int. J. Environ. Res. Public Health 2017, 14, 17. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of the World’s Forests 2018: Forest Pathways to Sustainable Development; FAO: Rome, Italy, 2018. [Google Scholar]
- Lunku, H.S.; Li, Z.; Yang, S.; Shayo, A.; Ngoma, J.B. The dynamics of deforestation in sub-Saharan Africa: The impact of environmental policy and foreign direct investment on forest conversion. For. Policy Econ. 2024, 169, 103342. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Wallace, K.J. Classification of ecosystem services: Problems and solutions. Biol. Conserv. 2007, 139, 235–246. [Google Scholar] [CrossRef]
- Domínguez, R.L.; Luoma, C. Decolonising conservation policy: How colonial land and conservation ideologies persist and perpetuate indigenous injustices at the expense of the environment. Land 2020, 9, 65. [Google Scholar] [CrossRef]
- Dowie, M. Conservation Refugees: The Hundred-Year Conflict Between Global Conservation and Native Peoples; MIT Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Colchester, M. Conservation policy and indigenous peoples. Environ. Sci. Policy 2004, 7, 145–153. [Google Scholar] [CrossRef]
- Hummel, C.; Poursanidis, D.; Orenstein, D.; Elliott, M.; Adamescu, M.C.; Cazacu, C.; Ziv, G.; Chrysoulakis, N.; van der Meer, J.; Hummel, H. Protected area management: Fusion and confusion with the ecosystem services approach. Sci. Total Environ. 2019, 651, 2432–2443. [Google Scholar] [CrossRef]
- Plumwood, V. Decolonizing relationships with nature. In Decolonizing Nature; Routledge: Abingdon, UK, 2012; pp. 51–78. [Google Scholar]
- Doolittle, A.A. Fortress conservation. In Encyclopedia of Environment and Society; Sage Publications: Thousand Oaks, CA, USA, 2007; Volume 1, p. 5. [Google Scholar]
- Piccolo, J.J.; Taylor, B.; Washington, H.; Kopnina, H.; Gray, J.; Alberro, H.; Orlikowska, E. “Nature’s contributions to people” and peoples’ moral obligations to nature. Biol. Conserv. 2022, 270, 109572. [Google Scholar] [CrossRef]
- Jonas, H.D.; Ahmadia, G.N.; Bingham, H.C.; Briggs, J.; Butchart, S.H.; Cariño, J.; Chassot, O.; Chaudhary, S.; Darling, E.; Degemmis, A.; et al. Equitable and effective area-based conservation: Towards the conserved areas paradigm. Parks 2021, 27, 71–84. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Kabra, A.; Kepe, T.; Garnett, S.; Merino, R. Re-centering social justice in conservation science: Progressive policies, methods, and practices. Biol. Conserv. 2024, 294, 110600. [Google Scholar] [CrossRef]
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective—A review article. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- Medicinal Plant Names Services Portal. Royal Botanic Gardens, Kew. 2024. Available online: https://mpns.science.kew.org (accessed on 28 July 2025).
- Shackleton, S.E.; Shackleton, C.M.; Cunningham, T.; Lombard, C.; Sullivan, C.A.; Netshiluvhi, T.R. Knowledge on Sclerocarya birrea subsp. caffra with emphasis on its importance as a non-timber forest product in South and southern Africa: A summary: Part 1: Taxonomy, ecology and role in rural livelihoods. South Afr. For. J. 2002, 196, 67–77. [Google Scholar]
- Kokwaro, J.O.; Gillett, J.B. Notes on the Anacardiaceae of eastern Africa. Kew Bull. 1980, 34, 745–760. [Google Scholar] [CrossRef]
- Hyde, M.A.; Wursten, B. Flora of Zimbabwe: Species information: Sclerocarya birrea subsp. caffra. Available online: http://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=136510 (accessed on 16 February 2025).
- Wilken, L.E.; Kleinert, A.; Schmeisser, M.; Dzikiti, S. Leaf gas exchange, water use, and yield of marula (S. birrea) and Kei apple (D. caffra): South African indigenous fruit trees with domestication potential. S. Afr. J. Bot. 2024, 173, 266–278. [Google Scholar] [CrossRef]
- Olas, B. Marula [Sclerocarya birrea (A. Rich.) Hochst.] products as a food and medicine. Front. Pharmacol. 2025, 16, 1552355. [Google Scholar] [CrossRef]
- Ngemakwe, P.N.; Remize, F.; Thaoge, M.L.; Sivakumar, D. Phytochemical and nutritional properties of underutilised fruits in the southern African region. S. Afr. J. Bot. 2017, 113, 137–149. [Google Scholar] [CrossRef]
- Dlamini, N.R.; Dube, S. Studies on the physico-chemical, nutritional and microbiological changes during the traditional preparation of marula wine in Gwanda, Zimbabwe. Nutr. Food Sci. 2008, 38, 61–69. [Google Scholar] [CrossRef]
- Rose, A.J. Amino acid nutrition and metabolism in health and disease. Nutrients 2019, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Hlangwani, E.; van Hal, P.H.; Moganedi, K.L.; Dlamini, B.C. The Future of African Wild Fruits—A Drive towards Responsible Production and Consumption of the Marula Fruit. Front. Sustain. Food Syst. 2023, 7, 1294437. [Google Scholar] [CrossRef]
- Nkosi, N.N.; Mostert, T.H.C.; Dzikiti, S.; Ntuli, N.R. Prioritization of indigenous fruit tree species with domestication and commercialization potential in KwaZulu-Natal, South Africa. Genet. Resour. Crop Evol. 2020, 67, 1567–1575. [Google Scholar] [CrossRef]
- Mashau, M.E.; Kgatla, T.E.; Makhado, M.V.; Mikasi, M.S.; Ramashia, S.E. Nutritional composition, polyphenolic compounds and biological activities of marula fruit (Sclerocarya birrea) with its potential food applications: A review. Int. J. Food Prop. 2022, 25, 1549–1575. [Google Scholar] [CrossRef]
- Tautsagae, A. Storage Temperature and Atmosphere Influenced Postharvest Quality of Marula (Sclerocarya birrea subsp. caffra) Fruits. Master’s Thesis, Botswana University of Agriculture and Natural Resources, Gaborone, Botswana, 2020. [Google Scholar]
- Akinnifesi, F.K.; Kwesiga, F.; Mhango, J.; Chilanga, T.; Mkonda, A.; Kadu, C.A.C.; Kadzere, I.; Mithofer, D.; Saka, J.D.K.; Sileshi, G.; et al. Towards the development of miombo fruit trees as commercial tree crops in southern Africa. For. Trees Livelihoods 2006, 16, 103–121. [Google Scholar] [CrossRef]
- Mojeremane, W.; Tshwenyane, S.O. The resource role of morula (Sclerocarya birrea): A multipurpose indigenous fruit tree of Botswana. J. Biol. Sci. 2004, 4, 771–775. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J. Ethnobiol. Ethnomed. 2013, 9, 31. [Google Scholar] [CrossRef]
- Daldoum, D.M.A.; Massaud, M.M.; Adam, Y.O. Distribution, fruit production and natural regeneration of Sclerocarya birrea (A. Rich.) Hochst. subsp. birrea in the Nuba Mountains, Sudan. World J. Agric. Sci. 2012, 8, 234–239. [Google Scholar]
- Kokwaro, J.O. Medicinal Plants of East Africa; East African Literature Bureau: Nairobi, Kenya, 1976. [Google Scholar]
- Mutshinyalo, T.; Tshisevhe, J. Sclerocarya birrea (A. Rich.) Hochst. subsp. caffra (Sond.) Kokwaro; South African National Biodiversity Institute: Pretoria, South Africa, 2003. [Google Scholar]
- Augustyn, W.A.; Regnier, T.; De Jager, K.; Hajari, E.; Du Preez, R.; Nonyane, D. A preliminary study on the chemical characteristics of Kei apple (Dovyalis caffra), an undervalued South African fruit. S. Afr. J. Bot. 2018, 117, 268–275. [Google Scholar] [CrossRef]
- Waweru, D.M.; Arimi, J.M.; Marete, E.; Jacquier, J.C.; Harbourne, N. Current status of utilization and potential of Dovyalis caffra fruit: Major focus on Kenya—A review. Sci. Afr. 2022, 16, e01097. [Google Scholar] [CrossRef]
- Aremu, A.O.; Ncama, K.; Omotayo, A.O. Ethnobotanical uses, biological activities and chemical properties of Kei-apple [Dovyalis caffra (Hook.f. & Harv.) Sim]: An indigenous fruit tree of southern Africa. J. Ethnopharmacol. 2019, 241, 111963. [Google Scholar]
- National Research Council. Lost Crops of Africa: Volume III: Fruits; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Kundishora, A.; Sithole, S.; Mukanganyama, S. Determination of the cytotoxic effect of different leaf extracts from Parinari curatellifolia (Chrysobalanaceae). J. Toxicol. 2020, 2020, 8831545. [Google Scholar] [CrossRef] [PubMed]
- Omoniwa, B.P.; Okaiyeto, K.; Omoniwa, D.O.; Olorunyomi, O.A. In vitro antiplasmodial evaluation of ethanoic and n-hexane extracts of Parinari curatellifolia stem bark. J. Pharm. Bioresour. 2021, 18, 103–112. [Google Scholar] [CrossRef]
- Kaseke, T.; Pfukwa, T.M.; Nxumalo, K.A.; Shinga, M.H.; Opara, U.L.; Fawole, O.A. Parinari curatellifolia: A treasure trove of phytochemicals, nutritional benefits, and biological activities. Heliyon 2025, 11, e41647. [Google Scholar] [CrossRef] [PubMed]
- Benhura, M.A.N.; Muchuweti, M.; Gombiro, P.E.; Benhura, C. Properties of Parinari curatellifolia (Hacha or Chakata) fruit from different parts of Harare, Zimbabwe. Afr. J. Food Agric. Nutr. Dev. 2013, 13, 8004–8018. [Google Scholar]
- Shoko, T.; Saka, J.K.; Apostolides, Z. Headspace volatiles of the edible fruit pulp of Parinari curatellifolia growing in Malawi using solid phase microextraction. S. Afr. J. Bot. 2014, 90, 128–130. [Google Scholar] [CrossRef]
- Ngadze, R.T.; Linnemann, A.R.; Fogliano, V.; Verkerk, R. Monkey orange fruit juice improves the nutritional quality of a maize-based diet. Food Res. Int. 2019, 116, 870–877. [Google Scholar] [CrossRef]
- Kunene, E.N.; Nxumalo, K.A.; Ngwenya, M.P.; Masarirambi, M.T. Domesticating and commercialisation of indigenous fruit and nut tree crops for food security and income generation in the Kingdom of Eswatini. Curr. J. Appl. Sci. Technol. 2020, 39, 37–52. [Google Scholar] [CrossRef]
- Mbhele, Z.; Zharare, G.E.; Zimudzi, C.; Ntuli, N.R. Indigenous knowledge on the uses and morphological variation among Strychnos spinosa Lam. at Oyemeni area, KwaZulu-Natal, South Africa. Sustainability 2022, 14, 6623. [Google Scholar] [CrossRef]
- Ngadze, R.T.; Verkerk, R.; Nyanga, L.K.; Fogliano, V.; Linnemann, A.R. Improvement of traditional processing of local monkey orange (Strychnos spp.) fruits to enhance nutrition security in Zimbabwe. Food Secur. 2017, 9, 621–633. [Google Scholar] [CrossRef]
- Neuwinger, H.D. African Ethnobotany Poisons and Drugs: Chemistry, Pharmacology, Toxicology; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Hedberg, I.; Hedberg, O.; Madat, P.J.; Mshigeni, K.E.; Mshiu, E.N.; Samuelsson, G. Inventory of plants used in traditional medicine in Tanzania. II. Plants of the families Dilleniaceae—Opiliaceae. J. Ethnopharmacol. 1983, 9, 105–127. [Google Scholar] [CrossRef]
- Mors, W.B.; do Nascimento, M.C.; Pereira, B.M.R.; Pereira, N.A. Plant natural products active against snake bite—The molecular approach. Phytochemistry 2000, 55, 627–642. [Google Scholar] [CrossRef]
- Avakoudjo, H.G.G.; Hounkpèvi, A.; Idohou, R.; Koné, M.W.; Assogbadjo, A.E. Local knowledge, uses, and factors determining the use of Strychnos spinosa organs in Benin (West Africa). Econ. Bot. 2020, 74, 15–31. [Google Scholar] [CrossRef]
- Ruth, O.N.; Unathi, K.; Nomali, N.; Chinsamy, M. Underutilization versus nutritional-nutraceutical potential of the Amaranthus food plant: A mini-review. Appl. Sci. 2021, 11, 6879. [Google Scholar] [CrossRef]
- Achigan-Dako, E.G.; Sogbohossou, O.E.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Gouws, C. The interactive effects of drought and heat stress on photosynthetic efficiency and biochemical defense mechanisms of Amaranthus species. Plant-Environ. Interact. 2022, 3, 212–225. [Google Scholar] [CrossRef]
- Ronco, A.L.; De Stéfani, E. Squalene: A multi-task link in the crossroads of cancer and aging. Funct. Foods Health Dis. 2013, 3, 462–476. [Google Scholar] [CrossRef]
- He, H.P.; Cai, Y.; Sun, M.; Corke, H. Extraction and purification of squalene from Amaranthus grain. J. Agric. Food Chem. 2002, 50, 368–372. [Google Scholar] [CrossRef]
- Ta Bi, I.H.; Koffi, N.G.; Lezin, B.E.; Assa, R.R.; Séverin, A. Etude ethnobotanique de quelques espèces du genre Corchorus rencontrées en Côte d’Ivoire. Eur. Sci. J. 2016, 12, 24. [Google Scholar]
- Ndlovu, J.; Afolayan, A.J. Nutritional analysis of the South African wild vegetable Corchorus olitorius L. Asian J. Plant Sci. 2008, 7, 615–618. [Google Scholar]
- Monima, L.A.; Buhari, M.; Lawal, S.; Isaac, E.; Fred, S.; Elna, O.; Edmund, B.; Diaz, M.E.F.; Bassey, A.V.; Ikwap, K. Effect of Cleome gynandra leaf extract on the estrous cycle and histology of the ovary and uterus of Wistar albino rats. Anat. J. Afr. 2019, 8, 1385–1394. [Google Scholar] [CrossRef]
- Mishra, S.S.; Moharana, S.K.; Dash, M.R. Review on Cleome gynandra. Int. J. Res. Pharm. Chem. 2011, 1, 681–689. [Google Scholar]
- Moyo, M.; Aremu, A.O. Nutritional, phytochemical and diverse health-promoting qualities of Cleome gynandra. Crit. Rev. Food Sci. Nutr. 2022, 62, 3535–3552. [Google Scholar] [CrossRef] [PubMed]
- Hamill, F.A.; Apio, S.; Mubiru, N.K.; Bukenya-Ziraba, R.; Mosango, M.; Maganyi, O.W.; Soejarto, D.D. Traditional herbal drugs of Southern Uganda, II: Literature analysis and antimicrobial assays. J. Ethnopharmacol. 2003, 84, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Okello, J.; Ssegawa, P. Medicinal plants used by communities of Ngai Subcounty, Apac District, northern Uganda. Afr. J. Ecol. 2007, 45, 76–83. [Google Scholar] [CrossRef]
- Al-Asmari, A.; Manthiri, R.A.; Abdo, N.; Al-Duaiji, F.A.; Khan, H.A. Saudi medicinal plants for the treatment of scorpion sting envenomation. Saudi J. Biol. Sci. 2017, 24, 1204–1211. [Google Scholar] [CrossRef]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef]
- Bala, A.; Haldar, P.K.; Kar, B.; Naskar, S.; Saha, P.; KunduSen, S.; Gupta, M.; Mazumder, U.K. Antioxidant activity of the fractions of Cleome gynandra promotes antitumor activity in Ehrlich Ascites Carcinoma. Asian J. Chem. 2011, 23, 5055. [Google Scholar]
- Ramalhete, C.; Gonçalves, B.M.; Barbosa, F.; Duarte, N.; Ferreira, M.J.U. Momordica balsamina: Phytochemistry and pharmacological potential of a gifted species. Phytochem. Rev. 2022, 21, 617–646. [Google Scholar] [CrossRef] [PubMed]
- Mushaphi, L.F.; Magoro, M.; Tshiambara, P. Nutritious edible indigenous vegetables. In Working with Indigenous Knowledge: Strategies for Health Professionals; AOSIS Books: Cape Town, South Africa, 2022. [Google Scholar]
- Burkil, H.M. Useful Plants of West Tropical Africa, 2nd ed.; Kew Royal Botanical Garden: Richmond, UK, 1985; pp. 597–599. [Google Scholar]
- Dossou-Yovo, H.O.; Kindomihou, V.; Vodouhè, F.G.; Sinsin, B. Assessment of the diversity of medico-magic knowledge on four herbaceous species in Benin. Sci. World J. 2021, 2021, 6650704. [Google Scholar] [CrossRef]
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa, 2nd ed.; Spectrum Books Ltd.: Ibadan, Nigeria, 2006; pp. 151–153. [Google Scholar]
- Taylor, L. Bitter melon: Herbal properties and actions. In The Healing Power of Rainforest Herbs; Taylor, L., Ed.; Square One Publication Inc.: New York, NY, USA, 2005; pp. 1–5. [Google Scholar]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of hypertension and diabetes in oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef]
- Abdel-Hassan, I.A.; Abdel-Barry, J.A.; Mohammeda, S.T. The hypoglycaemic and antihyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J. Ethnopharmacol. 2000, 71, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Poonia, A. Citrullus colocynthis (bitter apple): Bioactive compounds, nutritional profile, nutraceutical properties and potential food applications—A review. Food Prod. Process. Nutr. 2023, 5, 4. [Google Scholar] [CrossRef]
- Mariod, A.A.; Abdelwahab, S.I. Sclerocarya birrea (marula), an African tree of nutritional and medicinal uses: A review. Food Rev. Int. 2012, 28, 375–388. [Google Scholar] [CrossRef]
- Sybille, B.; Suarez, C.; Beckett, K. Marula fruit: The next beverage innovation. Nutraceutical Bus. Technol. 2012, 8, 34–37. [Google Scholar]
- Sibiya, N.P.; Kayitesi, E.; Moteetee, A.N. Proximate analyses and amino acid composition of selected wild indigenous fruits of Southern Africa. Plants 2021, 10, 721. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; TM, A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Loots, D.T.; van der Westhuizen, F.H.; Jerling, J. Polyphenol composition and antioxidant activity of Kei-apple (Dovyalis caffra) juice. J. Agric. Food Chem. 2006, 54, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Minnaar, P.P.; Jolly, N.P.; Paulsen, V.; Du Plessis, H.W.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: Effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237. [Google Scholar] [CrossRef]
- Mpai, S.; Du Preez, R.; Sultanbawa, Y.; Sivakumar, D. Phytochemicals and nutritional composition in accessions of Kei-apple (Dovyalis caffra): Southern African indigenous fruit. Food Chem. 2018, 253, 37–45. [Google Scholar] [CrossRef]
- Halilu, E.M. Characterization of crude saponins from stem bark extract of Parinari curatellifolia and evaluation of its antioxidant and antibacterial activities. Phys. Sci. Rev. 2024, 9, 2077–2095. [Google Scholar] [CrossRef]
- Van Rayne, K.K.; Adebo, O.A.; Ngobese, N.Z. Nutritional and physicochemical characterization of Strychnos madagascariensis Poir (black monkey orange) seeds as a potential food source. Foods 2020, 9, 1060. [Google Scholar] [CrossRef]
- Shaffer, L.J. A Landscape of Possibilities: Seeking Food Security in Matutúine District, Mozambique. Ecol. Environ. Anthropol. 2008, 4, 37–53. [Google Scholar]
- Van Rayne, K.K.; Adebo, O.A.; Wokadala, O.C.; Ngobese, N.Z. The potential of Strychnos spp. L. utilization in food insecurity alleviation: A review. Food Rev. Int. 2023, 39, 3400–3414. [Google Scholar] [CrossRef]
- USDA. Subset, Food, and All Foods. In National Nutrient Database for Standard Reference: Amaranth Grain; USDA: Washington, DC, USA, 2016. [Google Scholar]
- Soriano-García, M.; Aguirre-Díaz, I.S. Nutritional functional value and therapeutic utilization of amaranth. In Nutritional Value of Amaranth; IntechOpen: London, UK, 2019. [Google Scholar]
- Shukla, S.; Pandey, V.; Pachauri, G.; Dixit, B.S.; Banerji, R.; Singh, S.P. Nutritional contents of different foliage cuttings of vegetable amaranth. Plant Foods Hum. Nutr. 2003, 58, 1–8. [Google Scholar] [CrossRef]
- Maseko, I.; Ncube, B.; Mabhaudhi, T.; Tesfay, S.; Chimonyo, V.G.P.; Araya, H.T.; Fessehazion, M.; Du Plooy, C.P. Moisture stress on physiology and yield of some indigenous leafy vegetables under field conditions. S. Afr. J. Bot. 2019, 126, 85–91. [Google Scholar] [CrossRef]
- Idris, S.; Yisa, J.; Ndamitso, M.M. Nutritional composition of Corchorus olitorius leaves. Anim. Prod. Res. Adv. 2009, 5, 83–87. [Google Scholar] [CrossRef]
- Ashafa, A.O.; Abass, A.A.; Osinaike, T.; Lewu, F.B. Morphological characters and ascorbic acid content of an elite genotype of Corchorus olitorius: The influence of moisture stress. S. Afr. J. Plant Soil 2013, 30, 113–117. [Google Scholar] [CrossRef]
- Ewetola, E.A.; Fasanmi, T.F. Growth responses of okra (Abelmoschus esculentus) and jute mallow (Corchorus olitorius) to water stress and non-water stress conditions. Int. Lett. Chem. Phys. Astron. 2015, 59, 11. [Google Scholar] [CrossRef]
- Yakoub, A.R.B.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Bkhairia, I.; Nasri, M.; Ferchichi, A. Bioactive polysaccharides and their soluble fraction from Tossa jute (Corchorus olitorius L.) leaves. Food Biosci. 2020, 37, 100741. [Google Scholar] [CrossRef]
- Biswas, A.; Dey, S.; Li, D.; Liu, Y.; Zhang, J.; Huang, S.; Pan, G.; Deng, Y. Comparison of phytochemical profile, mineral content, and in vitro antioxidant activities of Corchorus capsularis and Corchorus olitorius leaf extracts from different populations. J. Food Qual. 2020, 2020, 2931097. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, H.H.L.; Kim, Y.; Lee, H.B.; Lee, E.; Park, J.H.; Park, H.Y. Corchorus olitorius L. ameliorates alcoholic liver disease by regulating gut-liver axis. J. Funct. Foods 2021, 85, 104648. [Google Scholar] [CrossRef]
- Guzzetti, L.; Panzeri, D.; Ulaszewska, M.; Sacco, G.; Forcella, M.; Fusi, P.; Tommasi, N.; Fiorini, A.; Campone, L.; Labra, M. Assessment of dietary bioactive phenolic compounds and agricultural sustainability of an African leafy vegetable Corchorus olitorius L. Front. Nutr. 2021, 8, 667812. [Google Scholar] [CrossRef]
- Yakoub, A.R.B.; Abdehedi, O.; Jridi, M.; Elfalleh, W.; Nasri, M.; Ferchichi, A. Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leaves (Corchorus olitorius L.). Ind. Crops Prod. 2018, 118, 206–213. [Google Scholar] [CrossRef]
- Jinazali, H.; Mtimuni, B.; Chilembwe, E. Nutrient composition of cats whiskers (Cleome gynandra L.) from different agroecological zones in Malawi. Afr. J. Food Sci. 2017, 11, 24–29. [Google Scholar]
- Thovhogi, F.; Ntushelo, N.; Gwata, E.T. A comparative study of the presence of minerals, flavonoids and total phenolic compounds in the leaves of common traditional vegetables. Appl. Sci. 2023, 13, 8503. [Google Scholar] [CrossRef]
- Madala, N.E.; Tugizimana, F.; Steenkamp, P.A. Development and optimization of an UPLC-QTOF-MS/MS method based on an in-source collision induced dissociation approach for comprehensive discrimination of chlorogenic acids isomers from Momordica plant species. J. Anal. Methods Chem. 2014, 2014, 650879. [Google Scholar] [CrossRef] [PubMed]
- Ajji, P.K.; Binder, M.J.; Walder, K.; Puri, M. Balsamin Induces Apoptosis in Breast Cancer Cells via DNA Fragmentation and Cell Cycle Arrest. Mol. Cell. Biochem. 2017, 432, 189–198. [Google Scholar] [CrossRef]
- Sathasivam, R.; Park, C.H.; Yeo, H.J.; Park, Y.E.; Kim, J.K.; Park, S.U. Analysis of triterpenoids, carotenoids, and phenylpropanoids in the flowers, leaves, roots, and stems of white bitter melon (Cucurbitaceae, Momordica charantia). Trop. J. Pharm. Res. 2021, 20, 155–160. [Google Scholar] [CrossRef]
- Li, W.; Lin, Z.; Yang, C.; Wang, Y.; Qiao, Y. Study on the chemical constituents of Momordica charantia L. leaves and method for their quantitative determination. Biomed. Res. 2015, 26, 415–419. [Google Scholar]
- Sadou, H.; Sabo, H.; Alma, M.M.; Saadou, M.; Leger, C.L. Chemical content of the seeds and physico-chemical characteristic of the seed oils from Citrullus colocynthis, Coccinia grandis, Cucumis metuliferus and Cucumis prophetarum of Niger. Bull. Chem. Soc. Ethiop. 2007, 21, 323–330. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, H.S. Biofunctional constituent isolated from Citrullus colocynthis fruits and structure–activity relationships of its analogues show acaricidal and insecticidal efficacy. J. Agric. Food Chem. 2014, 62, 8663–8667. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Ramanathan, T.; Satyavani, K. Characterization of volatile compounds from bitter apple (Citrullus colocynthis) using GC-MS. Int. J. Chem. Anal. Sci. 2011, 2, 108–110. [Google Scholar]
- Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Bitter apple (Citrullus colocynthis): An overview of chemical composition and biomedical potentials. Asian J. Plant Sci. 2010, 9, 394–401. [Google Scholar] [CrossRef]
- Schmidt, E.; Lotter, M.; McCleland, W. Trees and Shrubs of Mpumalanga and Kruger National Park; Jacana Media: Johannesburg, South Africa, 2002. [Google Scholar]
- Malengue, A.S.; Miranda, I.; Simões, R.; Lourenço, A.; Gominho, J.; Pereira, H. Cork cellular and chemical features underlying bark environmental protection in the miombo species Parinari curatellifolia. Heliyon 2023, 9, e21135. [Google Scholar] [CrossRef]
- Onipe, O.O.; Matshisevhe, M.M.; Ramashia, S.E.; Mashau, M.E. Physicochemical and functional properties of finger millet (Eleusine coracana) flour supplemented with Parinari curatellifolia flour. Sci. Afr. 2024, 23, e02092. [Google Scholar] [CrossRef]
- Akweni, A.L.; Sibanda, S.; Zharare, G.E.; Zimudzi, C. Fruit-based allometry of Strychnos madagascariensis and S. spinosa (Loganiaceae) in the savannah woodlands of the Umhlabuyalingana municipality, KwaZulu-Natal, South Africa. Trees For. People 2020, 2, 100025. [Google Scholar] [CrossRef]
- Hyde, M.A.; Wursten, B.T.; Ballings, P.; Coates Palgrave, M. Flora of Zimbabwe: Species Information: Strychnos innocua. 2025. Available online: https://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=144350 (accessed on 9 August 2025).
- Thothela, S.N.S.; Kola, E.; Dalu, M.T.; Ndhlovu, P.T. Indigenous knowledge and utilisation of Strychnos spinosa Lam. in Sub-Saharan Africa: A systematic review of its medicinal, nutritional, and cultural significance. Diversity 2025, 17, 228. [Google Scholar] [CrossRef]
- Madzimure, J.; Nyahangare, E.T.; Hamudikuwanda, H.; Hove, T.; Belmain, S.R.; Stevenson, P.C.; Mvumi, B.M. Efficacy of Strychnos spinosa (Lam.) and Solanum incanum L. aqueous fruit extracts against cattle ticks. Trop. Anim. Health Prod. 2013, 45, 1341–1347. [Google Scholar] [CrossRef]
- Thiombiano, D.N.E.; Lamien, N.; Castro-Euler, A.M.; Vinceti, B.; Agundez, D.; Boussim, I.J. Local communities demand for food tree species and the potentialities of their landscapes in two ecological zones of Burkina Faso. Open J. For. 2013, 3, 79–87. [Google Scholar] [CrossRef]
- Bruschi, P.; Mancini, M.; Mattioli, E.; Morganti, M.; Signorini, M.A. Traditional uses of plants in a rural community of Mozambique and possible links with Miombo degradation and harvesting sustainability. J. Ethnobiol. Ethnomed. 2014, 10, 59. [Google Scholar] [CrossRef]
- Djidohokpin, D.; Dassou, G.H.; Hounkpèvi, A.; Ouachinou, J.M.A.S.; Favi, G.A.; Houessou, G.L.; Yédomonhan, H.; Adomou, A.C. Factors affecting population structure and fruit production of Strychnos innocua Delile and Strychnos spinosa Lam. in Benin, West Africa. All Life 2024, 17, 2401896. [Google Scholar] [CrossRef]
- Akoègninou, A.; Van der Burg, W.J.; Van der Maesen, L.J.G. Flore Analytique du Bénin; Backhuys Publishers: Leiden, The Netherlands, 2006. [Google Scholar]
- Dassou, G.H. Diversité, Ethnobotanique, Écologie et Statut de Conservation des Plantes Utilisées en Médecine Vétérinaire Traditionnelle au Bénin. Doctoral Thesis, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, Cotonou, Bénin, 2016. [Google Scholar]
- Ouachinou, J.M.A.S. Evaluation et Valorisation de la Flore Fourragère et Médicinale à Usages Gastro-Intestinaux Chez les Bovins au Bénin; Université d’Abomey-Calavi: Cotonou, Bénin, 2020. [Google Scholar]
- Djidohokpin, D.; Dassou, G.H.; Favi, G.A.; Zountangni, O.M.; Ouachinou, J.M.A.S.; Makponsè, J.; Kpetikou, G.; Tossou, G.M.; Yédomonhan, H.; Adomou, A.C. Traditional uses and importance of Strychnos L. (Loganiaceae): A cross-cultural and spatial variation study in Benin. Trees For. People 2025, 20, 100865. [Google Scholar] [CrossRef]
- Tabuti, J.R.S.; Dhillion, S.S.; Lye, K.A. The status of wild food plants in Bulamogi County, Uganda. Int. J. Food Sci. Nutr. 2004, 55, 485–498. [Google Scholar] [CrossRef]
- Onyango, M.A. Seed production and support systems for African leafy vegetables in three communities in western Kenya. Afr. J. Food Agric. Nutr. Dev. 2016, 7, 82. [Google Scholar]
- Maundu, P.; Achigan-Dako, E.; Morimoto, Y. Biodiversity of African vegetables. In African Indigenous Vegetables in Urban Agriculture; Routledge: London, UK, 2009; pp. 65–104. [Google Scholar]
- Kyambo, O.M. Determinants of Adoption of Improved Amaranth Among Small Scale Farmers in Buuri Sub-County, Meru County, Kenya. Doctoral Dissertation, Egerton University, Nakuru, Kenya, 2014. [Google Scholar]
- N’Danikou, S.; Assogba, T.M.; Avohou, E.S.; Achigan-Dako, E.G. Selected species of traditional vegetable of Benin by family. In Traditional Vegetables in Benin; Institut National des Recherches Agricoles du Bénin: Cotonou, Bénin, 2010; pp. 93–197. [Google Scholar]
- Sogbohossou, O.E.; Achigan-Dako, E.G.; Assogba Komlan, F.; Ahanchede, A. Diversity and differential utilization of Amaranthus spp. along the urban-rural continuum of southern Benin. Econ. Bot. 2015, 69, 9–25. [Google Scholar] [CrossRef]
- Ngone Abwe Mercy, N.; Monah, N.L.; Mathias, M.A. Survey of wild vegetables in the Lebialem Highlands of South Western Cameroon. J. Plant Sci. 2016, 4, 172–184. [Google Scholar]
- Ogwu, M.C. Value of Amaranthus [L.] Species in Nigeria; IntechOpen: London, UK, 2020; pp. 1–21. [Google Scholar]
- Emmanuel, O.C.; Babalola, O.O. Amaranth production and consumption in South Africa: The challenges of sustainability for food and nutrition security. Int. J. Agric. Sustain. 2022, 20, 449–460. [Google Scholar] [CrossRef]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Okaiyeto, K.; Oguntibeju, O.O.; Laubscher, C.P. A systematic review on Amaranthus-related research. Horticulturae 2022, 8, 239. [Google Scholar] [CrossRef]
- Antwi, G.; Essilfie, M.K. Patterns of phytochemical variation in populations of Amaranthus viridis in two ecological zones of Ghana. Int. J. Contemp. Microbiol. 2024, 10, 1. [Google Scholar] [CrossRef]
- Rabiou, H.; Maazou, R.; Mahamane, M.; Issaharou-Matchi, I.; Mahamane, A.; Lykke, A.M. Diversity and socio-cultural importance of wild food herbs and cyanobacteria in the Lake Chad Basin (Niger). Ethnobot. Res. Appl. 2023, 25, 1–14. [Google Scholar] [CrossRef]
- Emire, S.A.; Arega, M. Value added product development and quality characterization of amaranth (Amaranthus caudatus L.) grown in East Africa. Afr. J. Food Sci. Technol. 2012, 3, 129–141. [Google Scholar]
- Maroyi, A. Traditional uses of wild and tended plants in maintaining ecosystem services in agricultural landscapes of the Eastern Cape Province in South Africa. J. Ethnobiol. Ethnomed. 2022, 18, 17. [Google Scholar] [CrossRef]
- Mukuwapasi, B.; Mavengahama, S.; Gerrano, A.S. Grain amaranth: A versatile untapped climate-smart crop for enhancing food and nutritional security. Discov. Agric. 2024, 2, 44. [Google Scholar] [CrossRef]
- Andini, R.; Yoshida, S.; Ohsawa, R. Variation in protein content and amino acids in the leaves of grain, vegetable and weedy types of amaranths. Agronomy 2013, 3, 391–403. [Google Scholar] [CrossRef]
- Ndungu, Z.W.; Kuria, E.N.; Gikonyo, N.K.; Mbithe, D.K. Efficacy of amaranth grain consumption on CD4 count and morbidity patterns among adults living with HIV in Nyeri, Kenya. J. AIDS HIV Res. 2017, 9, 81–88. [Google Scholar]
- Pholoma, S.B.; Haki, G.; Malambane, G.; Tshwenyane, S.; Adjetey, J. Corchorus olitorius: A promising medicinal plant in Southern Africa and effects of growing conditions on its bioactive compounds—A review. J. Biosci. Med. 2024, 12, 255–274. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.; Abdelwahab, M.F.; Abdelmohsen, U.R.; Hamed, A.N. Pharmacological and Phytochemical Biodiversity of Corchorus olitorius. RSC Adv. 2022, 12, 35103–35114. [Google Scholar] [CrossRef]
- Chigurupati, S.; Aladhadh, H.S.; Alhowail, A.; Selvarajan, K.K.; Bhatia, S. Phytochemical composition, antioxidant and antidiabetic potential of methanolic extract from Corchorus olitorius Linn. grown in Saudi Arabia. Med. Plants-Int. J. Phytomed. Relat. Ind. 2020, 12, 71–76. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G.; Aragbaiye, F.P.; Oyeleye, S.I.; Ogunsuyi, O.B. Antioxidant properties and in vitro α-amylase and α-glucosidase inhibitory properties of phenolics constituents from different varieties of Corchorus spp. J. Taibah Univ. Med. Sci. 2015, 10, 278–287. [Google Scholar] [CrossRef]
- Tropical Plants Database, Ken Fern. tropical.theferns.info. 20 August 2025. Available online: https://tropical.theferns.info/viewtropical.php?id=Cleome+gynandra (accessed on 9 May 2025).
- Yimer, A.; Forsido, S.F.; Addis, G.; Ayelign, A. Phytochemical profile and antioxidant capacity of some wild edible plants consumed in Southwest Ethiopia. Heliyon 2023, 9, e15331. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Manyevere, A.; Chakauya, E. Cleome gynandra: A wonder climate-smart plant for nutritional security for millions in semi-arid areas. Front. Plant Sci. 2022, 13, 1003080. [Google Scholar] [CrossRef]
- Omondi, E.O.; Engels, C.; Nambafu, G.; Schreiner, M.; Neugart, S.; Abukutsa-Onyango, M.; Winkelmann, T. Nutritional compound analysis and morphological characterization of spider plant (Cleome gynandra)—An African indigenous leafy vegetable. Food Res. Int. 2017, 100, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Olarewaju, O.O.; Fajinmi, O.O.; Arthur, G.D.; Coopoosamy, R.M.; Naidoo, K.K. Food and medicinal relevance of Cucurbitaceae species in Eastern and Southern Africa. Bull. Natl. Res. Cent. 2021, 45, 208. [Google Scholar] [CrossRef]
- Fajinmi, O.O.; Olarewaju, O.O.; Arthur, G.D.; Coopoosamy, R.M.; Naidoo, K. Sociocultural relevance of the bottle gourd and selected species of Cucurbitaceae family in West Africa. J. Med. Plants Econ. Dev. 2022, 6, 139. [Google Scholar] [CrossRef]
- Olarewaju, O.O.; Fajinmi, O.O.; Arthur, G.D.; Coopoosamy, R.M.; Naidoo, K. Effect of climate change on the production of Cucurbitaceae species in North African countries. J. Agric. Food Res. 2023, 14, 100742. [Google Scholar] [CrossRef]
- Shahid, M.; Rao, N.K. Diversity of Citrullus colocynthis (L.) Schrad. (Cucurbitaceae) in the United Arab Emirates. J. New Biol. Rep. 2014, 3, 145–150. [Google Scholar]
- Dalziel, J.M. The Useful Plants of West Tropical Africa; Appendix to the Flora of West Tropical Africa; Crown Agents: London, UK, 1937. [Google Scholar]
- Marzouk, B.; Marzouk, Z.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef]
- Fajinmi, O.O.; Olarewaju, O.O.; Arthur, G.D.; Naidoo, K.; Coopoosamy, R. Cucurbitaceae species used as traditional medicine in West Africa. J. Med. Plants Econ. Dev. 2022, 6, 163. [Google Scholar] [CrossRef]
- Assogbadjo, C.S.; Avocèvou-Ayisso, C.; Idohou, R.; Wouyou, H.G.; Houndonougbo, J.S.H.; Hounsou-Dindin, G.; Assogbadjo, A.E. A review of Momordica species in Africa (Cucurbitaceae): Current knowledge and perspectives for sustainable management. Ethnobot. Res. Appl. 2025, 30, 1–22. [Google Scholar] [CrossRef]
- Kgosana, M.R.; Mayimele, N.N. The use and benefits of Momordica balsamina L. (Nkaka) amongst Bantu people in southern Africa: From traditional food source to modern medicine. J. Appl. Pharm. Sci. 2025, 15, 1–7. [Google Scholar] [CrossRef]
- Beloin, N.; Gbeassor, M.; Akpagana, K.; Hudson, J.; de Soussa, K.; Koumaglo, K.; Arnason, J.T. Ethnomedicinal uses of Momordica charantia (Cucurbitaceae) in Togo and relation to its phytochemistry and biological activity. J. Ethnopharmacol. 2005, 96, 49–55. [Google Scholar] [CrossRef]
- Cornevin, R. Histoire du Togo; Berger-Levrault: Paris, France, 1969; 554p. [Google Scholar]
- Clapp, J.; Moseley, W.G.; Burlingame, B.; Termine, P. The case for a six-dimensional food security framework. Food Policy 2022, 106, 102164. [Google Scholar] [CrossRef]
- Golay, C. The Right to Food and Access to Justice: Examples at the National, Regional and International Levels; FAO: Rome, 2009; p. 9. [Google Scholar]
- Story, M.; Hamm, M.W.; Wallinga, D. Food systems and public health: Linkages to achieve healthier diets and healthier communities. J. Hunger Environ. Nutr. 2009, 4, 219–224. [Google Scholar] [CrossRef]
- Murray, S.; Gale, F.; Adams, D.; Dalton, L. A scoping review of the conceptualisations of food justice. Public Health Nutr. 2023, 26, 725–737. [Google Scholar] [CrossRef]
- Fajinmi, O.O.; Olarewaju, O.O.; Van Staden, J. Propagation of medicinal plants for sustainable livelihoods, economic development, and biodiversity conservation in South Africa. Plants 2023, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Murye, A.F.; Pelser, A.J. Commercial harvesting of marula (Sclerocarya birrea) in Swaziland: A quest for sustainability. Sel. Stud. Biodivers. 2018, 14, 303–317. [Google Scholar]
- Bezeng, B.S.; Ameka, G.; Angui, C.M.V.; Atuah, L.; Azihou, F.; Bouchenak-Khelladi, Y.; Carlisle, F.; Doubi, B.T.S.; Gaoue, O.G.; Gatarabirwa, W.; et al. An African perspective to biodiversity conservation in the twenty-first century. Philos. Trans. R. Soc. B 2025, 380, 20230443. [Google Scholar] [CrossRef]
- Chapman, C.A.; Abernathy, K.; Chapman, L.J.; Downs, C.; Effiom, E.O.; Gogarten, J.F.; Golooba, M.; Kalbitzer, U.; Lawes, M.J.; Mekonnen, A.; et al. The future of sub-Saharan Africa’s biodiversity in the face of climate and societal change. Front. Ecol. Evol. 2022, 10, 790552. [Google Scholar] [CrossRef]
- Archer, E.; Dziba, L.; Mulongoy, K.J.; Maoela, M.A.; Walters, M.; Biggs, R.O.; Salem, M.C.C.; DeClerck, F.; Diaw, M.C.; Dunham, A.E.; et al. The Regional Assessment Report on Biodiversity and Ecosystem Services for Africa: Summary for Policymakers; IPBES Secretariat: Bonn, Germany, 2018; p. 49. [Google Scholar]
- Dawson, N.M.; Coolsaet, B.; Sterling, E.J.; Loveridge, R.; Gross-Camp, N.D.; Wongbusarakum, S.; Sangha, K.K.; Scherl, L.M.; Phan, H.P.; Zafra-Calvo, N.; et al. The role of Indigenous peoples and local communities in effective and equitable conservation. Ecol. Soc. 2021, 26, 19. [Google Scholar] [CrossRef]
- Brosius, J.P. Indigenous peoples and protected areas at the World Parks Congress. Conserv. Biol. 2004, 18, 609–612. [Google Scholar] [CrossRef]
- Diaz, S.; Settele, J.; Brondizio, E.S.; Ngo, H.T.; Gueze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. IPBES: Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Garnett, S.T.; Burgess, N.D.; Fa, J.E.; Fernández-Llamazares, Á.; Molnár, Z.; Robinson, C.J.; Watson, J.E.; Zander, K.K.; Austin, B.; Brondizio, E.S.; et al. A spatial overview of the global importance of Indigenous lands for conservation. Nat. Sustain. 2018, 1, 369–374. [Google Scholar] [CrossRef]
- Reyes-García, V.; Fernández-Llamazares, Á.; McElwee, P.; Molnár, Z.; Öllerer, K.; Wilson, S.J.; Brondizio, E.S. The contributions of Indigenous Peoples and local communities to ecological restoration. Restor. Ecol. 2019, 27, 3–8. [Google Scholar] [CrossRef]
- Fa, J.E.; Watson, J.E.; Leiper, I.; Potapov, P.; Evans, T.D.; Burgess, N.D.; Molnár, Z.; Fernández-Llamazares, Á.; Duncan, T.; Wang, S.; et al. Importance of Indigenous Peoples’ lands for the conservation of Intact Forest Landscapes. Front. Ecol. Environ. 2020, 18, 135–140. [Google Scholar] [CrossRef]
- Campion, O.B.; West, S.; Degnian, K.; Djarrbal, M.; Ignjic, E.; Ramandjarri, C.; Malibirr, G.W.; Guwankil, M.; Djigirr, P.; Biridjala, F.; et al. Balpara: A practical approach to working with ontological difference in indigenous land & sea management. Soc. Nat. Resour. 2023, 37, 695–715. [Google Scholar] [CrossRef]
- Troeger, U.; Wittmer, H. South Africa’s Biodiversity Stewardship Programme: Case Study for the GIZ Project BioFrame and Partners. 2023. Available online: https://www.international-climate-initiative.com/fileadmin/iki/Dokumente/Publikationen/Projekte/19_IV_101/IKI_Bioframe_Case_Study_South_Africa_barrierefrei.pdf (accessed on 12 May 2025).
- South African National Biodiversity Institute (SANBI). The Business Case for Biodiversity Stewardship; SANBI Fact-Sheet Series; South African National Biodiversity Institute: Pretoria, South Africa, 2017. [Google Scholar]
- Sundberg, J.O. Conserving Biodiversity on South Africa’s Privately-Owned Grasslands: Farmer Experiences with Protected Areas; Lincoln Institute of Land Policy: Cambridge, MA, USA, 2022. [Google Scholar]
- Department of Environmental Affairs (DEA). Factsheet: Biodiversity Stewardship Achievements; South African National Biodiversity Institute: Pretoria, South Africa, 2017. [Google Scholar]
- Climate Impact Partners. Gola Rainforest REDD+, Sierra Leone. 2025. Available online: https://www.climateimpact.com/global-projects/gola-rainforest-redd-sierra-leone/ (accessed on 16 May 2025).
- Barrow, E. 300,000 Hectares Restored in Shinyanga, Tanzania—But what did it really take to achieve this restoration? SAPIENS 2014, 7, 2. [Google Scholar]
- Makindi, S.M. Local Communities, Biodiversity Conservation and Ecotourism: A Case Study of the Kimana Community Wildlife Sanctuary, Kenya. Afr. J. Hosp. Tour. Leis. 2016, 5, 3. [Google Scholar]
- Sghaier, M.; Fetoui, M.; Frija, A.; Ben Salem, F.; Ayadi, N.; Robinson, L.W. Community-Based Rangeland Management in Tataouine, South-East Tunisia: Institutional Settings to Revive Traditional Land Restoration “Gdel”; International Livestock Research Institute: Nairobi, Kenya, 2020. [Google Scholar]
- Ferraro, P.J.; Lawlor, K.; Mullan, K.L.; Pattanayak, S.K. Forest figures: Ecosystem services valuation and policy evaluation in developing countries. Rev. Environ. Econ. Policy 2012, 6, 20–44. [Google Scholar] [CrossRef]
- FAO. Forests for Resilience to Natural, Climate and Human-Induced Disasters and Crises; Forestry Department: Rome, Italy, 2019. [Google Scholar]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; De Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Jourdan, M.; Kunstler, G.; Morin, X. How neighbourhood interactions control the temporal stability and resilience to drought of trees in mountain forests. J. Ecol. 2020, 108, 666–677. [Google Scholar] [CrossRef]
- Kiplimo, I.T. Ecosystem-based Adaptation and Climate Change Mitigation. 2021. Available online: https://www.linkedin.com/pulse/ecosystem-based-adaptation-climate-change-mitigation-kiplimo/ (accessed on 26 April 2025).
- IPCC. Summary for Policymakers; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Baccini, A.G.S.J.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Change 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Jury, M.R. Climate trends in southern Africa. S. Afr. J. Sci. 2013, 109, 111. [Google Scholar] [CrossRef]
- Serdeczny, O.; Adams, S.; Baarsch, F.; Coumou, D.; Robinson, A.; Hare, W.; Schaeffer, M.; Perrette, M.; Reinhardt, J. Climate change impacts in Sub-Saharan Africa: From physical changes to their social repercussions. Reg. Environ. Change 2017, 17, 1585–1600. [Google Scholar] [CrossRef]
- Prudhomme, C.; Giuntoli, I.; Robinson, E.L.; Clark, D.B.; Arnell, N.W.; Dankers, R.; Fekete, B.M.; Franssen, W.; Gerten, D.; Gosling, S.N.; et al. Hydrological droughts in the 21st century, hotspots and uncertainties from a global multimodel ensemble experiment. Proc. Natl. Acad. Sci. USA 2014, 111, 3262–3267. [Google Scholar] [CrossRef]
- Vogel, C.; van Zyl, K. Drought: In search of sustainable solutions to a persistent, ‘wicked’ problem in South Africa. In Climate Change Adaptation Strategies—An Upstream-Downstream Perspective; Springer: Cham, Switzerland, 2016; pp. 195–211. [Google Scholar]
- Grossiord, C. Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytol. 2020, 228, 42–49. [Google Scholar] [CrossRef]
- Schnabel, F.; Liu, X.; Kunz, M.; Barry, K.E.; Bongers, F.J.; Bruelheide, H.; Fichtner, A.; Härdtle, W.; Li, S.; Pfaff, C.T.; et al. Species richness stabilizes productivity via asynchrony and drought-tolerance diversity in a large-scale tree biodiversity experiment. Sci. Adv. 2021, 7, eabk1643. [Google Scholar] [CrossRef] [PubMed]
- Noack, F.; Riekhof, M.C.; Di Falco, S. Droughts, biodiversity, and rural incomes in the tropics. J. Assoc. Environ. Resour. Econ. 2019, 6, 823–852. [Google Scholar] [CrossRef]
- Jones, K. Zero Hunger, Zero Emissions: Land-Based Climate Change Mitigation, Food Security, and Equity; Oxfam: Washington, DC, USA, 2020. [Google Scholar]
- Fanzo, J.; Davis, C.; McLaren, R.; Choufani, J. The effect of climate change across food systems: Implications for nutrition outcomes. Glob. Food Secur. 2018, 18, 12–19. [Google Scholar] [CrossRef]
- Muriuki, E.N.; Sila, D.N.; Onyango, A. Nutritional diversity of leafy amaranth species grown in Kenya. J. Appl. Biosci. 2014, 79, 6818–6825. [Google Scholar] [CrossRef]
- Jamalluddin, N.; Massawe, F.J.; Mayes, S.; Ho, W.K.; Singh, A.; Symonds, R.C. Physiological screening for drought tolerance traits in vegetable amaranth (Amaranthus tricolor) germplasm. Agriculture 2021, 11, 994. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Van Der Kooy, F.; Oladimeji, O.; Gouws, C. Influence of drought and heat stress on mineral content, antioxidant activity and bioactive compound accumulation in four African Amaranthus species. Plants 2023, 12, 953. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Cernansky, R. Super vegetables. Nature 2015, 522, 146. [Google Scholar] [CrossRef]
- Raju, A.J.; Rani, D.S. Reproductive ecology of Cleome gynandra and Cleome viscosa (Capparaceae). Phytol. Balcanica 2016, 22, 15–28. [Google Scholar]
- Ventosa-Febles, E. Cleome gynandra (Wild Spider Flower). In CABI Compendium; CABI International: Wallingford, UK, 2022. [Google Scholar]
- Giang Doan, H.; Tokhtar, V.K. Ecological and biological features of tropical species of the genus Momordica (Cucurbitaceae) introduced under the conditions of Belgorod region (Russia). Ukr. Bot. J. 2014, 71, 41–44. [Google Scholar] [CrossRef]
- Rao, P.G. Recent advances in breeding of bitter gourd (Momordica charantia L.). In Advances in Plant Breeding Strategies: Vegetable Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Rathod, V.; Behera, T.K.; Munshi, A.D.; Gaikwad, A.B.; Singh, S.; Vinay, N.D.; Boopalakrishnan, G.; Jat, G.S. Developing partial interspecific hybrids of Momordica charantia × Momordica balsamina and their advance generations. Sci. Hortic. 2021, 281, 109985. [Google Scholar] [CrossRef]
- Dane, F.; Liu, J.; Zhang, C. Phylogeography of the bitter apple, Citrullus colocynthis. Genet. Resour. Crop Evol. 2007, 54, 327–336. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Goertzen, L.R.; McElroy, J.S.; Dane, F. Analysis of the Citrullus colocynthis transcriptome during water deficit stress. PLoS ONE 2014, 9, e104657. [Google Scholar] [CrossRef]
- Jabeen, S.; Al Mahruqi, Z.M.H.; Nadeem, F.; Khalid, T. Bitter Apple (Citrullus colocynthis)—A Review of a Wild Plant Growing from Asia to Africa with High Medicinal Potentials. Int. J. Chem. Biochem. Sci. 2017, 11, 65–70. [Google Scholar]
- Nyoka, B.I.; Chanyenga, T.; Mng’omba, S.A.; Akinnifesi, F.K.; Sagona, W. Variation in growth and fruit yield of populations of Sclerocarya birrea (A. Rich.) Hochst. Agrofor. Syst. 2015, 89, 397–407. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa; Briza Publications: Pretoria, South Africa, 1997; p. 304. [Google Scholar]
- Chirwa, P.W.; Bwanali, R.J.; Meke, G.; Sagona, W.; Munthali, C.R.Y.; Mwabumba, L. Growth and phenology of a three- to four-year-old Sclerocarya birrea international provenance trial in Malawi. South. Hemisph. For. J. 2007, 69, 49–54. [Google Scholar] [CrossRef]
- Sinthumule, N.I.; Mzamani, L.C.M. Communities and conservation: Marula trees (Sclerocarya birrea subsp. caffra) under communal management at Matiyane Village, Limpopo Province, South Africa. Trop. Conserv. Sci. 2019, 12, 194. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional Uses, Phytochemical and Pharmacological Properties of Dovyalis caffra. J. Pharm. Nutr. Sci. 2018, 8, 230–238. [Google Scholar] [CrossRef]
- Dzikiti, S.; Ntuli, N.R.; Nkosi, N.N.; Ntshidi, Z.; Ncapai, L.; Gush, M.B.; Mostert, T.H.C.; Du Preez, R.; Mpandeli, N.M.S.; Pienaar, H.H. Contrasting water use patterns of two drought adapted native fruit tree species growing on nutrient poor sandy soils in northern KwaZulu-Natal. S. Afr. J. Bot. 2022, 147, 197–207. [Google Scholar] [CrossRef]
- Mwamba, C.K. Monkey orange: Strychnos cocculoides (No. 8). In Crops Future; Southampton Centre for Underutilised Crops: Southampton, UK, 2006. [Google Scholar]
- Uchechukwu, A.C.; Obiageli, N.M. Studies of the wood of some Nigerian alkaloid-rich Strychnos species. J. Hortic. For. 2020, 12, 57–62. [Google Scholar] [CrossRef]
- Di Matteo, G.; Luzzi, G.; Basile, A.; Sposato, A.; Bertini, G.; Neri, U.; Pennelli, B.; Napoli, R.; Nardi, P. Carbon concentrations and carbon storage capacity of three old-growth forests in the Sila National Park, Southern Italy. J. For. Res. 2023, 34, 233–242. [Google Scholar] [CrossRef]
- Abbas, S. Climate change and major crop production: Evidence from Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 5406–5414. [Google Scholar] [CrossRef]
- Nzabarinda, V.; Bao, A.; Tie, L.; Uwamahoro, S.; Kayiranga, A.; Ochege, F.U.; Muhirwa, F.; Bao, J. Expanding forest carbon sinks to mitigate climate change in Africa. Renew. Sustain. Energy Rev. 2025, 207, 114849. [Google Scholar] [CrossRef]
- Song, R.; Zhu, Z.; Zhang, L.; Li, H.; Wang, H. A simple method using an allometric model to quantify the carbon sequestration capacity in vineyards. Plants 2023, 12, 997. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. CO2 and Greenhouse Gas Emissions; Our World Data: Oxford, UK, 2023. [Google Scholar]
- Ball, J. State of the World’s Forests 2011; FAO: Rome, Italy, 2011. [Google Scholar]
- Mayaux, P.; Pekel, J.F.; Desclée, B.; Donnay, F.; Lupi, A.; Achard, F.; Clerici, M.; Bodart, C.; Brink, A.; Nasi, R.; et al. State and evolution of the African rainforests between 1990 and 2010. Philos. Trans. R. Soc. B 2013, 368, 20120300. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.A.; Consequences of Deforestation. Mongabay. 2019. Available online: https://rainforests.mongabay.com/0901.htm (accessed on 25 July 2025).
- Walters, G.; Baruah, M.; Karambiri, M.; Adjei, P.O.W.; Samb, C.; Barrow, E. The power of choice: How institutional selection influences restoration success in Africa. Land Use Policy 2021, 104, 104090. [Google Scholar] [CrossRef]
- Gelaye, Y.; Getahun, S. A review of the carbon sequestration potential of fruit trees and their implications for climate change mitigation: The case of Ethiopia. Cogent Food Agric. 2024, 10, 2294544. [Google Scholar] [CrossRef]
- Hurd, C.L.; Law, C.S.; Bach, L.T.; Britton, D.; Hovenden, M.; Paine, E.R.; Raven, J.A.; Tamsitt, V.; Boyd, P.W. Forensic carbon accounting: Assessing the role of seaweeds for carbon sequestration. J. Phycol. 2022, 58, 347–363. [Google Scholar] [CrossRef]
- Dibaba, A.; Soromessa, T.; Workineh, B. Carbon stock of the various carbon pools in Gerba-Dima moist Afromontane Forest, South-western Ethiopia. Carbon Balance Manag. 2019, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, J.; Wang, G.; Dang, Q.L. Combined effects of elevated CO2 and warmer temperature on limitations to photosynthesis and carbon sequestration in yellow birch. Tree Physiol. 2023, 43, 379–389. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Cukor, J. European forests under global climate change: Review of tree growth processes, crises and management strategies. J. Environ. Manag. 2023, 332, 117353. [Google Scholar] [CrossRef]
- Harris, S.H.; Betts, M.G. Selecting among land sparing, sharing and Triad in a temperate rainforest depends on biodiversity and timber production targets. J. Appl. Ecol. 2023, 60, 737–750. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, 7005. [Google Scholar] [CrossRef]
- Pimbert, M.P.; Pretty, J.N. Diversity and sustainability in community-based conservation. In Proceedings of the UNESCO-IIPA Regional Workshop on Community-Based Conservation, India, 9–12 February 1997. [Google Scholar]
- Mountjoy, N.J.; Whiles, M.R.; Spyreas, G.; Lovvorn, J.R.; Seekamp, E. Assessing the efficacy of community-based natural resource management planning with a multi-watershed approach. Biol. Conserv. 2016, 201, 120–128. [Google Scholar] [CrossRef]
- Chiutsi, S.; Saarinen, J. Local participation in transfrontier tourism: Case of Sengwe community in Great Limpopo Transfrontier Conservation Area, Zimbabwe. Dev. South. Afr. 2017, 34, 260–275. [Google Scholar] [CrossRef]
- Hoon, P. Elephants are like our diamonds: Recentralizing community-based natural resource management in Botswana, 1996–2012. Afr. Stud. Q. 2014, 15, 55–70. [Google Scholar]
- Chigonda, T. More than just story telling: A review of biodiversity conservation and utilisation from precolonial to postcolonial Zimbabwe. Scientifica 2018, 2018, 6214318. [Google Scholar] [CrossRef] [PubMed]
- Chiutsi, S.; Saarinen, J. The limits of inclusivity and sustainability in Transfrontier peace parks: Case of Sengwe community in Great Limpopo Transfrontier Conservation Area, Zimbabwe. Crit. Afr. Stud. 2019, 11, 348–360. [Google Scholar] [CrossRef]
- Logah, V.; Abubakari, F.; Issifu, H.; Adjei-Gyapong, T.; Baidoo, E.; Abubakari, A.; Okonkwo, G.; Hamelink, J.; Pyck, M.; Ocansey, C.M.; et al. Soil carbon, nutrient, and vegetation dynamics of an old Anogeissus grove in Mole National Park, Ghana. Biotropica 2024, 56, e13299. [Google Scholar] [CrossRef]
- Constant, N.L.; Tshisikhawe, M.P. Hierarchies of knowledge: Ethnobotanical knowledge, practices and beliefs of the Vhavenda in South Africa for biodiversity conservation. J. Ethnobiol. Ethnomed. 2018, 14, 28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajinmi, O.O.; Mabhaudhi, T.; Van Staden, J. Biodiversity Conservation, a Crucial Step Towards Food and Nutritional Security, Food Justice and Climate Change Resilience in Africa. Plants 2025, 14, 2649. https://doi.org/10.3390/plants14172649
Fajinmi OO, Mabhaudhi T, Van Staden J. Biodiversity Conservation, a Crucial Step Towards Food and Nutritional Security, Food Justice and Climate Change Resilience in Africa. Plants. 2025; 14(17):2649. https://doi.org/10.3390/plants14172649
Chicago/Turabian StyleFajinmi, Olufunke Omowumi, Tafadzwanashe Mabhaudhi, and Johannes Van Staden. 2025. "Biodiversity Conservation, a Crucial Step Towards Food and Nutritional Security, Food Justice and Climate Change Resilience in Africa" Plants 14, no. 17: 2649. https://doi.org/10.3390/plants14172649
APA StyleFajinmi, O. O., Mabhaudhi, T., & Van Staden, J. (2025). Biodiversity Conservation, a Crucial Step Towards Food and Nutritional Security, Food Justice and Climate Change Resilience in Africa. Plants, 14(17), 2649. https://doi.org/10.3390/plants14172649







