Native Plant Responses and Elemental Accumulation in Mining and Metallurgical Mediterranean Ecosystems
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization and PTE Concentration in Soil
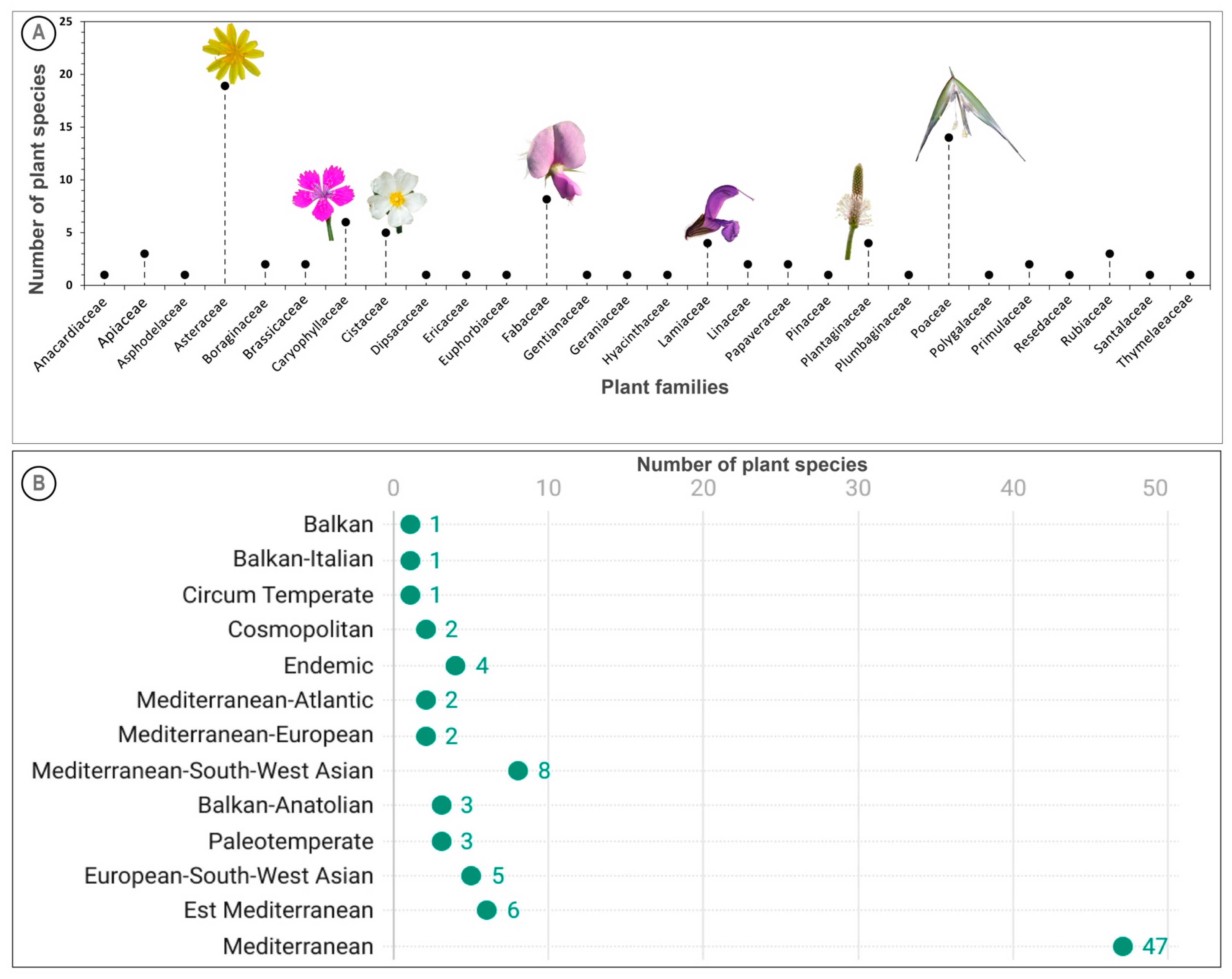
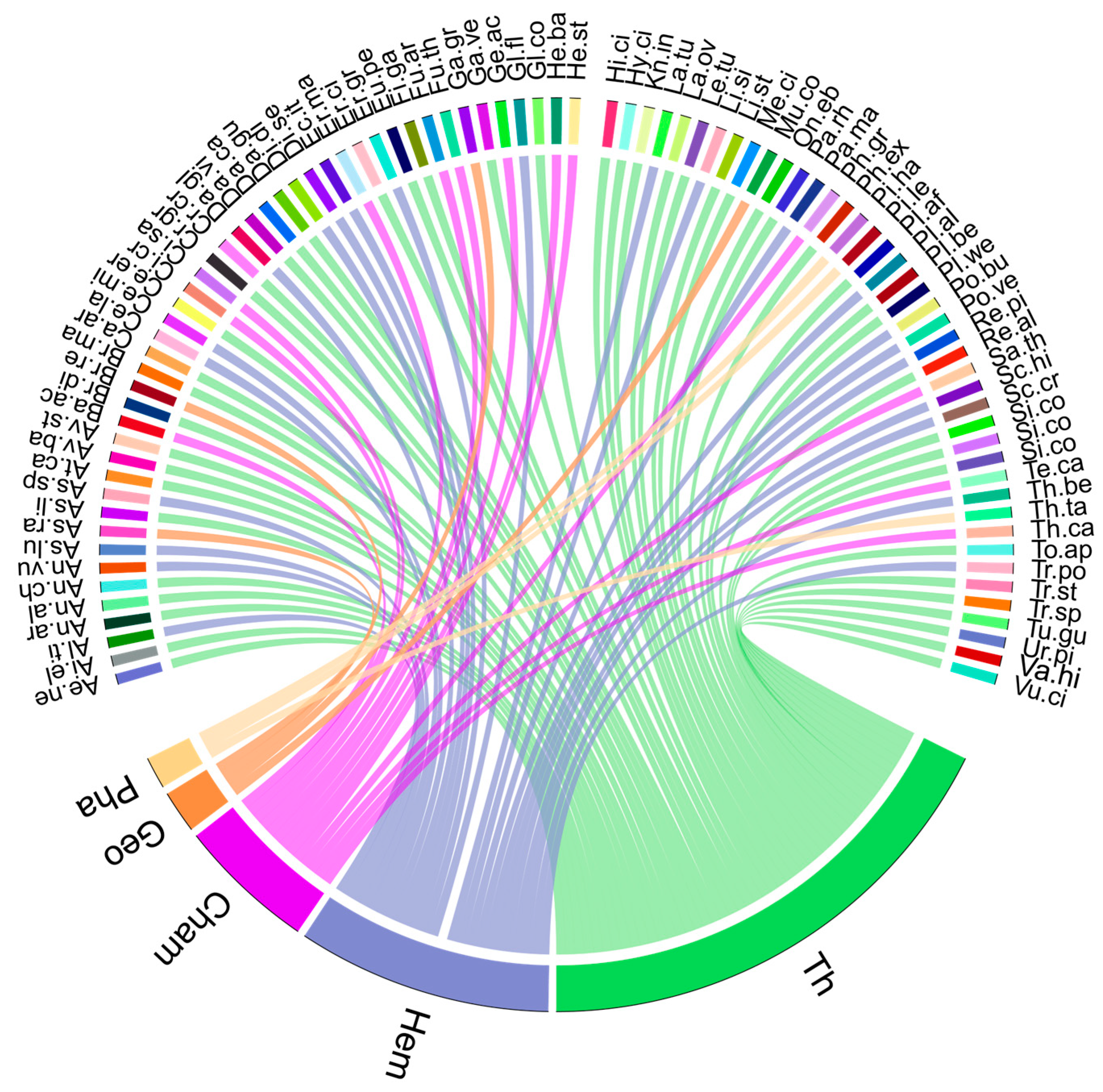
2.2. Plant Diversity and Floristic Analysis
2.3. PTE Concentrations in Plant Aerial Parts
2.4. Bioconcentration Factor
2.5. Candidate Plant Species for Phytostabilization of Polluted Areas
3. Materials and Methods
3.1. Study Sites Description
3.2. Soil Sampling and Analyses
3.3. Plant Collection and Identification
3.4. Potential Toxic Elements Concentration and Bioconcentration Assessment
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive analysis of heavy metal soil contamination in mining environments: Impacts, monitoring techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Upadhyay, V.; Kumari, A.; Kumar, S. From soil to health hazards: Heavy metals contamination in northern India and health risk assessment. Chemosphere 2024, 354, 141697. [Google Scholar] [CrossRef] [PubMed]
- Hudson-Edwards, K.A. Sources, mineralogy, chemistry and fate of heavy metal-bearing particles in mining-affected environments. Minerals 2016, 7, 116. [Google Scholar]
- Pacetti, T.; Lompi, M.; Petri, C.; Caporali, E. Mining activity impacts on soil erodibility and reservoirs silting: Evaluation of mining decommissioning strategies. J. Hydrol. 2020, 589, 125107. [Google Scholar] [CrossRef]
- Liang, Y.; Yi, X.; Dang, Z.; Wang, Q.; Luo, H.; Tang, J. Heavy metal contamination and health risk assessment in the vicinity of a tailing pond in Guangdong, China. Int. J. Environ. Res. Public Health 2017, 14, 1557. [Google Scholar] [CrossRef]
- Gul, I.; Manzoor, M.; Kallerhoff, J.; Arshad, M. Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere 2020, 258, 127405. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Bashir, O.; Haq, S.A.U.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Ernst, W.H.O.; Van der Ent, A.; Malaisse, F.; Ginocchio, R. Metallophytes: The unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America. In Ecology of Industrial Pollution; Batty, L.C., Hallberg, K.H., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 7–40. [Google Scholar]
- Wójcik, M.; Gonnellix, C.; Selvix, F.; Dresler, S.; Rostanski, A.; Vangronsveld, J. Metallophytes of serpentine and calamine soils—Their unique ecophysiology and potential for phytoremediation. Adv. Bot. Res. 2017, 83, 1–42. [Google Scholar]
- Vangronsveld, J.; Clijsters, H. Toxic effects of metals. In Biology of Metal-Accumulating Plants; Van der Lelie, H., Van der Lelie, D., Vangronsveld, J., Eds.; VCH Press: Weinheim, Germany, 1994; pp. 149–177. [Google Scholar]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators—An analysis: Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Chen, L.; Beiyuan, J.; Hu, W.; Zhang, Z.; Duan, C.; Cui, Q.; Zhu, X.; He, H.; Huang, X.; Fang, L. Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): A comprehensive review. Chemosphere 2022, 293, 133577. [Google Scholar] [CrossRef]
- Zine, H.; Hakkou, R.; Papazoglou, E.G.; Elmansour, A.; Abrar, F.; Benzaazoua, M. Revegetation and ecosystem reclamation of post-mined land: Toward sustainable mining. Int. J. Environ. Sci. Technol. 2024, 21, 9775–9798. [Google Scholar] [CrossRef]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laffont-Schwob, I. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a non-destructive and integrative approach. J. Environ. Manag. 2016, 183, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A state-of-the-art of phytoremediation approach for sustainable management of heavy metals recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Thijs, S.; Vasilev, A.; Wójcik, M.; Vangronsveld, J. Phytomanagement of Pollutants in Soil and Groundwater; Ok, Y.S., Rinklebe, J., Hou, D., Tsang, D.C.W., Tack, F.M.G., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 131–151. [Google Scholar]
- Lavanya, M.B.; Viswanath, D.S.; Sivapullaiah, P.V. Phytoremediation: An eco-friendly approach for remediation of heavy metal-contaminated soils—A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100975. [Google Scholar] [CrossRef]
- Papazoglou, E.G.; Karantounias, G.A.; Vemmos, S.N.; Bouranis, D.L. Photosynthesis and growth responses of giant reed (Arundo donax L.) to the heavy metals Cd and Ni. Environ. Int. 2005, 31, 243–249. [Google Scholar] [CrossRef]
- Vasilatos, C.; Economou-Eliopoulos, M. Fossilized bacteria in Fe-Mn-mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece). Minerals 2018, 8, 107. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Mavrogonatos, C.; Photiades, A.; Moraiti, E.; Rieck, B.; Kolitsch, U.; Tarantola, A.; Scheffer, C.; Morin, D.; et al. The Lavrion mines: A unique site of geological and mineralogical heritage. Minerals 2021, 11, 76. [Google Scholar] [CrossRef]
- Makropoulos, W.; Konteye, C.; Eikmann, T.; Einbrodt, H.J.; Hatzakis, A.; Papanagiotou, G. Cross-sectional epidemiological study on the lead burden of children and workers in Greece. Toxicol. Environ. Chem. 1991, 31, 467–477. [Google Scholar] [CrossRef]
- Makropoulos, W.; Stilianakis, N.; Eikmann, T.J.; Einbrodt, H.J.; Hatzakis, A.; Nikolau-Papanagiotou, A. Cross-sectional epidemiological study of the effect of various pollutants on the health of children in Greece. Fresenius Environ. Bull. 1992, 1, 117–122. [Google Scholar]
- Kafourou, A.; Touloumi, C.; Makropoulos, V.; Loutradi, A.; Papanagiotou, A.; Hatzakis, A. Effects of lead on the somatic growth of children. Arch. Environ. Health 1997, 52, 377–383. [Google Scholar] [CrossRef]
- Kalyvas, G.; Gasparatos, D.; Papassiopi, N.; Massas, I. Topsoil pollution as ecological footprint of historical mining activities in Greece. Land Degrad. Dev. 2017, 29, 2025–2035. [Google Scholar] [CrossRef]
- Ross, J.; Voudouris, P.; Melfos, V.; Vaxevanopoulos, M.; Soukis, K.; Merigot, K. The Lavrion silver district: Reassessing its ancient mining history. Geoarchaeology 2021, 36, 617–642. [Google Scholar] [CrossRef]
- Antoniadis, V.; Thalassinos, G.; Levizou, E.; Wang, J.; Wang, S.L.; Shaheen, S.M.; Rinklebe, J. Hazardous enrichment of toxic elements in soils and olives in the urban zone of Lavrio, Greece, a legacy, millennia-old silver/lead mining area and related health risk assessment. J. Hazard. Mater. 2022, 434, 128906. [Google Scholar] [CrossRef]
- Pavlopoulos, K.; Chrisanthaki, I.; Economou–Eliopoulos, M.; Lekkas, S. Hydrochemical study of metals in the groundwater of the wider area of Koropi. Environ. Earth Sci. 2011, 20, 169–176. [Google Scholar]
- Triantafyllidis, S.S.; Anastasakis, G.; Papanastasiou, A.; Stylianou, C.; Kavros, N.; Pappa, F.K.; Tombros, S.F.; Fitros, M.; Skliros, V. Provenance of coastal and seabed sediments relative to mining and processing wastes: The case of Lavrion, Attiki Peninsula, Greece. Minerals 2024, 14, 33. [Google Scholar] [CrossRef]
- Kontopoulos, A.; Komnitsas, K.; Xenidis, A.; Papassiopi, N. Environmental characterisation of the sulphidic tailings in Lavrion. Miner. Eng. 1995, 8, 1209–1219. [Google Scholar] [CrossRef]
- The Council of the European Communities. European Soil Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. Off. J. Eur. Communities 1986, L181, 6–12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:31986L0278 (accessed on 12 December 2024).
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Taylor and Francis Group: Boca Raton, FL, USA, 2011; p. 505. [Google Scholar]
- Kafkala, I.G.; Parpodis, K.; Serelis, K.G.; Papazoglou, E.G. Soil pollution as a tool for the detection of ancient metallurgy workshops and associated native flora. In Proceedings of the Third Conference on Environmental Management, Engineering, Planning and Economics (CEMEPE 2011) & SECOTOX Conference, Skiathos, Greece, 19–24 June 2011. [Google Scholar]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece. An Annotated Checklist; Englera, 31; Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany, 2013; pp. 1–372. [Google Scholar]
- Tshwene-Mauchaza, B.; Aguirre-Gutiérrez, J. Climatic drivers of plant species distributions across spatial grains in Southern Africa tropical forests. Front. For. Glob. Change 2019, 2, 69. [Google Scholar] [CrossRef]
- Médail, F. Plant biogeography and vegetation patterns of the Mediterranean islands. Bot. Rev. 2022, 88, 63–129. [Google Scholar] [CrossRef]
- Zine, H.; Hakkou, R.; Elmansour, A.; Elgadi, S.; Ouhammou, A.; Benzaazoua, M. Native plant diversity for ecological reclamation in Moroccan open-pit phosphate mines. Biodivers. Data J. 2023, 11, e104592. [Google Scholar] [CrossRef] [PubMed]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Rundel, P.W.; Dillon, M.O. The effects of atmospheric nitrogen deposition on Mediterranean-type ecosystems. Biogeochemistry 1998, 43, 77–113. [Google Scholar]
- Nardini, A.; Lo Gullo, M.A.; Trifilò, P.; Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: Responses and adaptations. Environ. Exp. Bot. 2014, 103, 68–79. [Google Scholar] [CrossRef]
- Gutterman, Y. Seed Germination in Desert Plants; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Sádlo, J.; Chytrý, M.; Pergl, J.; Pyšek, P. Plant dispersal strategies: A new classification based on the multiple dispersal modes of individual species. Biol. Conserv. 2018, 218, 217–219. [Google Scholar] [CrossRef]
- Herrera, C.M. Plant-vertebrate seed dispersal systems in the Mediterranean: Ecological, evolutionary, and historical determinants. Annu. Rev. Ecol. Syst. 1995, 26, 705–727. [Google Scholar] [CrossRef]
- Sjögren, P.; Van der Knaap, W.O.; Van Leeuwen, J.F.N. Pollen dispersal properties of Poaceae and Cyperaceae: First estimates of their absolute pollen productivities. Rev. Palaeobot. Palynol. 2015, 216, 123–131. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Kumar, A.; Tripti; Raj, D.; Maiti, S.K.; Maleva, M.; Borisova, G. Soil pollution and plant efficiency indices for phytoremediation of heavy metal(loid)s: Two-decade study (2002–2021). Metals 2022, 12, 1330. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Peco, J.D.; Higueras, P.; Campos, J.A.; Esbrí, J.M.; Moreno, M.M.; Battaglia-Brunet, F.; Sandalio, L.M. Abandoned mine lands reclamation by plant remediation technologies. Sustainability 2021, 13, 6555. [Google Scholar] [CrossRef]
- Mankė, J.; Praspaliauskas, M.; Pedišius, N.; Sujetovienė, G. Evaluation of phytoremediation efficiency of shooting range soil using the bioaccumulation potential and sensitivity of different plant species. Ecol. Eng. 2024, 198, 107134. [Google Scholar] [CrossRef]
- Wójcik, M.; Sugier, P.; Siebielec, G. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef]
- Abreu, M.M.; Tavares, M.T.; Batista, M.J. Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugal. J. Geochem. Explor. 2008, 96, 210–222. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Peñalosa, J.M.; Manzano, R.; Carpena-Ruiz, R.O.; Gamarra, R.; Esteban, E. Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wild flora. J. Hazard. Mater. 2009, 162, 854–859. [Google Scholar] [CrossRef] [PubMed]
- El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Assessment of the transfer of trace metals to spontaneous plants on abandoned pyrrhotite mine: Potential application for phytostabilization of phosphate wastes. Plants 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological potential of plants for phytoremediation and ecorestoration of fly ash deposits and mine wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhou, Y.; Gong, T.; Wang, J.; Ge, Y. Enhanced phytoremediation of mixed heavy metal (mercury)-organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S-transferase and human P450 2E1. J. Hazard. Mater. 2013, 260, 1100–1107. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Rostański, A. Microevolutionary changes in ecotypes of calamine waste heap vegetation near Olkusz, Poland: A review. Acta Biol. Cracov. Ser. Bot. 2002, 44, 7–19. [Google Scholar]
- Shuihong, Y.; Jiangtao, Q.; Xinhua, P.; Bin, Z. The effects of vegetation on restoration of physical stability of a severely degraded soil in China. Ecol. Eng. 2009, 35, 723–734. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef]
- Kapás, R.E.; Plue, J.; Kimberley, A.; Cousins, S.A. Grazing livestock increases both vegetation and seed bank diversity in remnant and restored grasslands. J. Veg. Sci. 2020, 31, 1053–1065. [Google Scholar] [CrossRef]
- Terschanski, J.; Nunes, M.H.; Aalto, I.; Pellikka, P.; Wekesa, C.; Maeda, E.E. The role of vegetation structural diversity in regulating the microclimate of human-modified tropical ecosystems. J. Environ. Manag. 2024, 360, 121128. [Google Scholar] [CrossRef]
- Torbati, S.; Esmailbegi Kermani, S.; Abedini, A. Remediation of heavy metals by native plant species grown in Iran’s richest gold mine and study of plants’ pollution tolerance strategies. Front. Earth Sci. 2024, 12, 1304497. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Droulia, F.; Tsiros, I.X. Projecting bioclimatic change over the South-Eastern European agricultural and natural areas via ultrahigh-resolution analysis of the de Martonne Index. Atmosphere 2023, 14, 858. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Droulia, F.; Kokkoris, I.P.; Dimopoulos, P. Projections on the spatiotemporal bioclimatic change over the phytogeographical regions of Greece by the Emberger Index. Water 2024, 16, 2070. [Google Scholar] [CrossRef]
- Day, P.R. Particle formation and particle-size analysis. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Properties; Black, C.A., Ed.; Including Statistics of Measurements and Sampling; American Society of Agronomy and the Soil Science Society of America: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Meers, E.; Samson, R.; Tack, F.M.G.; Ruttens, A.; Vandegehuchte, M.; Vangronsveld, J.; Verloo, M.G. Phytoavailability assessment of heavy metals in soils by single extractions and accumulation by Phaseolus vulgaris. Environ. Exp. Bot. 2007, 60, 385–396. [Google Scholar] [CrossRef]
- Van Ranst, E.; Verloo, M.; Demeyer, A.; Pauwels, J.M. Manual for the Soil Chemistry and Fertility Laboratory; Ghent University, Faculty of Agricultural and Applied Biological Sciences: Ghent, Belgium, 1999; p. 243. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DPTA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Strid, A.; Tan, K. Flora Hellenica 1; Koeltz Scientific Books: Königstein, Germany, 1997. [Google Scholar]
- Strid, A.; Tan, K. Flora Hellenica 2; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2002. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora. Part 1: Text & Plates. Englera 2016, 33, 1–700. [Google Scholar]
- Rotkittikhun, P.; Kruatrachue, M.; Chaiyarat, R.; Ngernsansaruay, C.; Pokethitiyook, P.; Paijitprapaporn, A.; Baker, A.J.M. Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ. Pollut. 2006, 144, 681–688. [Google Scholar] [CrossRef] [PubMed]
- NIST SRM 1570a; Spinach Leaves. NIST: National Institute of Standards and Technology (NIST), U.S. Department of Commerce: Gaithersburg, MD, USA, 2003.



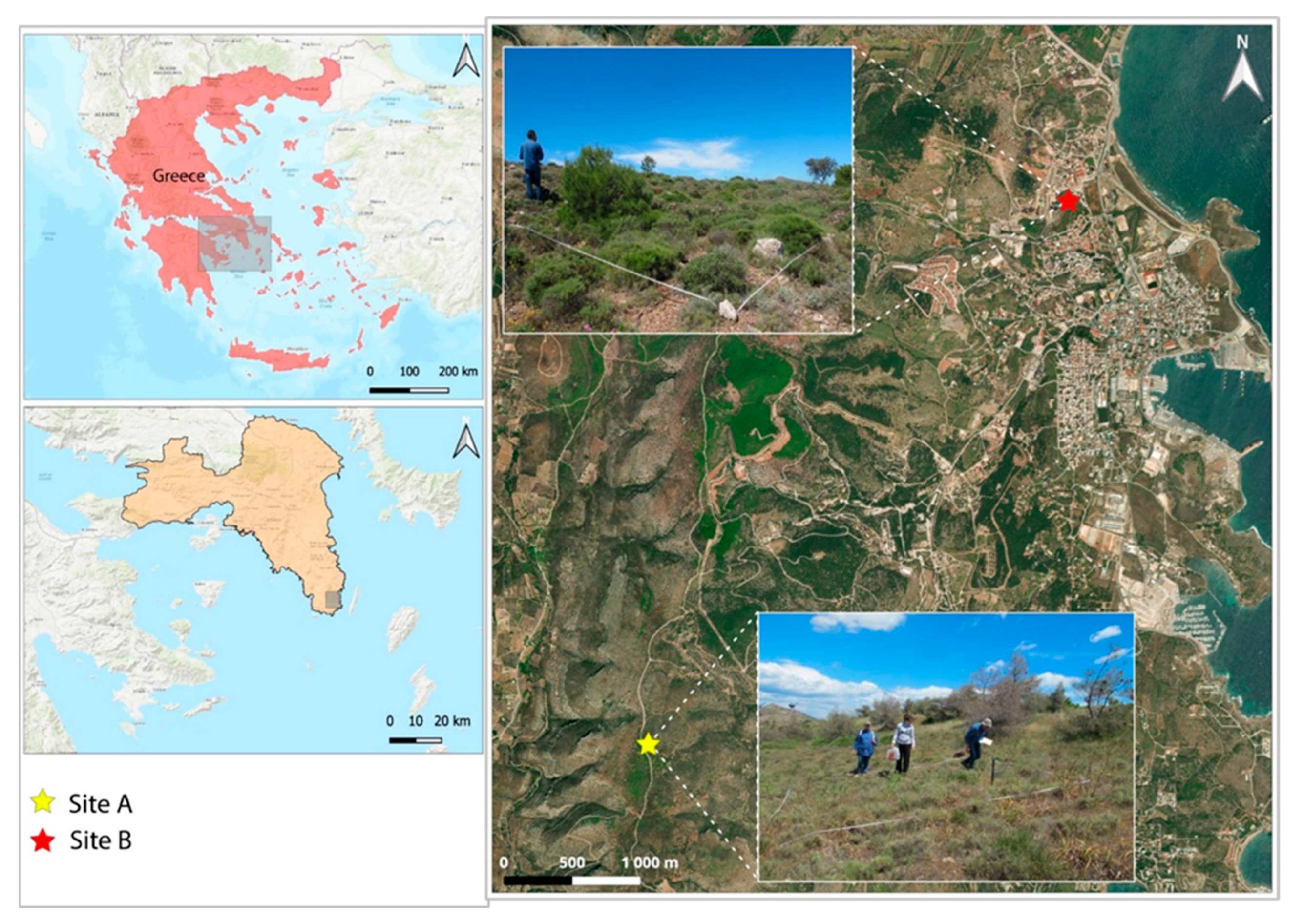
| Site A | Site B | |
|---|---|---|
| Clay (%) | 8.0 | 17.0 |
| Silt (%) | 21.0 | 18.0 |
| Sand (%) | 71.0 | 65.0 |
| Texture | Sandy loam | Sandy loam |
| pH | 7.62 | 7.58 |
| Organic matter (g 100−1 g DW) | 4.4 | 9.6 |
| Conductivity (μS/cm) | 225 | 300 |
| CEC (cmol(+)kg−1, DW) | 18 | 18 |
| PTE | Site A (Mining Area) | Site B (Metallurgical Area) | Threshold Values (♦) & Natural Background Levels (‡) in Soil (mg kg−1) | ||
|---|---|---|---|---|---|
| Total Concentrations | DTPA Extractable Concentrations | Total Concentrations | DTPA Extractable Concentrations | ||
| As | 387.67–497.08 a | 0.1–0.3 A | 3184.87–3430.68 b | 0.27–0.68 B | 5.0–6.0 ‡ |
| Cd | 132.05–138.05 a | 27.3–37.8 A | 128.86–132.45 a | 17.80–27.78 Β | 1.0–3.0 ♦ |
| Co | 8.32–13.11 a | 0.08–0.23 A | 5.23–7.56 b | 0.02–0.04 B | 20.0–50.0) ‡ |
| Cr | 198.92–356.80 a | <D.L. | 127.36–242.44 a | <D.L. | 10.0–100.0 ‡ |
| Cu | 144.00–168.45 a | 1.0–1.21 A | 601.67–724.91 b | 16.19–26.53 B | 50.0–140.0 ♦ |
| Fe | 28,597.99–39,175.34 a | 0.41–0.97 A | 61,058.76–62,947.18 b | 0.56–0.88 B | 10,000.0–100,000.0 ‡ |
| Mn | 1545.68–1797.30 a | 0.18–0.25 A | 2653.39–2948.21 b | 0.22–0.39 B | 350.0–2000.0 ‡ |
| Ni | 179.76–200.84 a | 1.15–2.10 A | 124.52–146.83 b | 1.45–2.47 B | 30.0–75.0 ♦ |
| Pb | 23,260.27–27,046.81 a | 323.23–425.15 A | 22,770.64–25,875.72 a | 119.14–132.31 B | 50.0–300.0 ♦ |
| Sb | 192.1–279.40 a | 0.07–0.29 A | 253.49–369.59 a | 0.30–0.62 B | 0.1–10.0 ‡ |
| Zn | 17,472.22–19,200.24 a | 665.81–751.55 A | 14,805.01–16,821.60 b | 741.50–788.72 B | 150.0–300.0 ♦ |
| Plant Species | Family | Chorology | Life Form | Abbreviation | Location Site |
|---|---|---|---|---|---|
| Aegilops neglecta | Poaceae | Mediterranean-SW Asian | Therophyte | Ae.ne | B |
| Aira elegantissima * | Poaceae | Mediterranean-SW Asian | Therophyte | Ai.el | A, B |
| Alkanna tinctoria * | Boraginaceae | Mediterranean | Hemicryptophyte | Al.ti | B |
| Anagallis arvensis | Primulaceae | Cosmopolitan | Therophyte | An.ar | A |
| Anthemis altissima | Asteraceae | European-SW Asian | Therophyte | An.al | B |
| Anthemis chia | Asteraceae | Mediterranean | Therophyte | An.ch | B |
| Anthyllis vulneraria * | Fabaceae | European | Hemicryptophyte | An.vu | Β |
| Asperula lutea subsp. rigidula * | Rubiaceae | Greek endemic | Chamaephyte | As.lu | A |
| Asphodelus ramosus | Asphodelaceae | Mediterranean | Geophyte | As.ra | A, B |
| Asterolinon linum-stellatum | Primulaceae | Mediterranean | Therophyte | As.li | A |
| Astragalus spruneri | Fabaceae | Balkan | Hemicryptophyte | As.sp | A |
| Atractylis cancellata | Asteraceae | Mediterranean | Therophyte | At.ca | Β |
| Avena barbata | Poaceae | Mediterranean | Therophyte | Av.ba | Β |
| Avena sterilis | Poaceae | Mediterranean-SW Asian | Therophyte | Av.st | Β |
| Ballota acetabulosa * | Lamiaceae | Balkan-Anatolian | Chamaephyte | Ba.ac | Β |
| Brachypodium distachyon | Poaceae | Mediterranean-SW Asian | Therophyte | Br.di | A, B |
| Brachypodium retusum * | Poaceae | Mediterranean | Hemicryptophyte | Br.re | A, B |
| Bromus madritensis | Poaceae | Mediterranean-SW Asian | Therophyte | Br.ma | A, B |
| Calendula arvensis | Asteraceae | Mediterranean | Therophyte | Ca.ar | Β |
| Centaurea laureotica * | Asteraceae | Greek endemic | Hemicryptophyte | Ce.la | A |
| Centaurea raphanina subsp. mixta | Asteraceae | Greek endemic | Hemicryptophyte | Ce.mi | A, B |
| Centaurium erythraea | Gentianaceae | European-SW Asian | Therophyte | Ce.er | A, B |
| Cistus creticus * | Cistaceae | Mediterranean | Chamaephyte | Ci.cr | A |
| Cistus salviifolius * | Cistaceae | Mediterranean | Chamaephyte | Ci.sa | A |
| Crepis neglecta subsp. graeca | Asteraceae | Greek endemic | Therophyte | Cr.gr | A |
| Crupina crupinastrum | Asteraceae | European-SW Asian | Therophyte | Cr.cr | Β |
| Dactylis glomerata | Poaceae | Paleotemperate | Hemicryptophyte | Da.gl | A, Β |
| Dasypyrum villosum * | Poaceae | Mediterranean-SW Asian | Therophyte | Da.vi | Β |
| Daucus carota | Apiaceae | Paleotemperate | Therophyte | Da.ca | Β |
| Daucus guttatus | Apiaceae | Mediterranean | Therophyte | Da.gu | Β |
| Dianthus diffusus * | Caryophyllaceae | Balkan-Anatolian | Hemicryptophyte | Di.di | A, B |
| Dianthus serratifolius subsp. serratifolius | Caryophyllaceae | Greek endemic | Hemicryptophyte | Di.se | A, B |
| Echium italicum | Boraginaceae | Mediterranean-SW Asian | Hemicryptophyte | Ec.it | Β |
| Erica manipuliflora | Ericaceae | Mediterranean | Chamaephyte | Er.ma | A |
| Erodium cicutarium | Geraniaceae | Circumtemperate | Therophyte | Er.ci | Β |
| Erysimum graecum | Brassicaceae | Greek endemic | Hemicryptophyte | Er.gr | B |
| Euphorbia peplus | Euphorbiaceae | Cosmopolitan | Therophyte | Eu.pe | A, B |
| Filago gallica | Asteraceae | Mediterranean-Atlantic | Therophyte | Fi.ga | A |
| Fumana arabica | Cistaceae | Mediterranean | Chamaephyte | Fu.ar | A |
| Fumana thymifolia * | Cistaceae | Mediterranean | Chamaephyte | Fu.th | A |
| Gagea graeca | Liliaceae | Balkan-Anatolian | Geophyte | Ga.gr | A |
| Gastridium ventricosum | Poaceae | Mediterranean | Therophyte | Ga.ve | B |
| Genista acanthoclada | Fabaceae | Mediterranean | Chamaephyte, Phanerophyte | Ge.ac | A |
| Glaucium flavum * | Papaveraceae | Mediterranean-European | Hemicryptophyt | Gl.fl | B |
| Glebionis coronaria | Asteraceae | Mediterranean | Therophytes | Gl.co | B |
| Helichrysum stoechas subsp. barrelieri * | Asteraceae | Mediterranean | Chamaephyte | He.st | A |
| Hippocrepis ciliata | Fabaceae | Mediterranean | Therophyte | Hi.ci | Β |
| Hymenocarpos circinnatus | Fabaceae | Mediterranean | Therophyte | Hy.ci | Β |
| Knautia integrifolia * | Dipsacaceae | Mediterranean | Therophyte | Kn.in | A, B |
| Lactuca tuberosa * | Asteraceae | European-SW Asian | Hemicryptophyte | La.tu | A |
| Lagurus ovatus | Poaceae | Mediterranean | Therophyte | La.ov | Β |
| Leotondon tuberosus | Asteraceae | Mediterranean | Hemicryptophyte | Le.tu | A |
| Limonium sinuatum | Plumbaginaceae | Mediterranean | Hemicryptophyte | Li.si | Β |
| Linum strictum | Linaceae | Mediterranean | Therophyte | Li.st | A, B |
| Medicago ciliaris | Fabaceae | Mediterranean | Therophyte | Me.ci | B |
| Muscari commutatum | Hyacinthaceae | Balkan-Italian | Geophyte | Mu.co | Β |
| Onobrychis ebenoides | Fabaceae | Greek endemic | Hemicryptophyte | On.eb | A |
| Papaver rhoeas | Papaveraceae | Mediterranean | Therophyte | Pa.rh | Β |
| Paronychia macrosepala | Caryophyllaceae | Mediterranean | Hemicryptophyte | Pa.ma | A, Β |
| Phagnalon rupestre subsp. graecum * | Asteraceae | Mediterranean | Chamaephyte | Ph.gr | A, B |
| Phleum exaratum | Poaceae | Mediterranean | Therophyte | Ph.ex | B |
| Pinus halepensis | Pinaceae | Mediterranean | Phanerophyte | Pi.ha | A, B |
| Pistacia lentiscus | Anacardiaceae | Mediterranean | Phanerophyte | Pi.le | A, B |
| Plantago afra | Plantaginaceae | Mediterranean | Therophyte | Pl.af | Β |
| Plantago albicans | Plantaginaceae | Mediterranean | Hemicryptophyte | Pl.al | Β |
| Plantago bellardii | Plantaginaceae | Mediterranean | Therophyte | Pl.be | A, B |
| Plantago weldenii | Plantaginaceae | Mediterranean | Therophyte | Pl.we | A |
| Poa bulbosa | Poaceae | Paleotemperate | Hemicryptophyte | Po.bu | A |
| Polygala venulosa | Polygalaceae | Mediterranean | Hemicryptophyte | Po.ve | A |
| Reichardia picroides | Asteraceae | Mediterranean | Hemicryptophyte | Re.pi | Β |
| Reseda alba * | Resedaceae | Mediterranean | Therophyte | Re.al | Β |
| Satureja thymbra | Lamiaceae | Mediterranean | Chamaephyte | Sa.th | A |
| Scolymus hispanicus | Asteraceae | Mediterranean | Hemicryptophyte | Sc.hi | Β |
| Scorzonera crocifolia * | Asteraceae | Greek endemic | Hemicryptophyte | Sc.cr | A, B |
| Silene colorata | Caryophyllaceae | Mediterranean | Therophyte | Si.co | Β |
| Silene conica | Caryophyllaceae | European-SW Asian | Therophyte | Si.co | A |
| Silene corinthiaca * | Caryophyllaceae | Greek endemic | Therophyte | Si.co | B |
| Teucrium capitatum | Lamiaceae | Mediterranean | Chamaephyte | Te.ca | A |
| Thesium bergeri | Santalaceae | Mediterranean | Hemicryptophyte | Th.be | A |
| Thymelaea tartonraira * | Thymelaeaceae | Mediterranean | Phanerophyte | Th.ta | A |
| Thymbra capitata * | Lamiaceae | Mediterranean | Chamaephyte | Th.ca | A |
| Tordylium apulum | Apiaceae | Mediterranean | Therophyte | To.ap | Β |
| Tragopogon porrifolius | Asteraceae | Mediterranean | Hemicryptophyte | Tr.po | Β |
| Trifolium stellatum | Fabaceae | Mediterranean | Therophyte | Tr.st | Β |
| Trigonella spruneriana | Fabaceae | Mediterranean | Therophyte | Tr.sp | B |
| Tuberaria guttata | Cistaceae | Mediterranean-Atlantic | Therophyte | Tu.gu | A |
| Urospermun picroides | Asteraceae | Mediterranean | Therophyte | Ur.pi | Β |
| Valantia hispida | Rubiaceae | Mediterranean | Therophyte | Va.hi | Β |
| Vulpia ciliata | Poaceae | Mediterranean-SW Asian | Therophyte | Vu.ci | A |
| Plant Code | As | Cd | Co | Cr | Cu | Fe | Mg | Mn | Ni | Pb | Sb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site A (mining site) | ||||||||||||
| As.lu | 3.13 ± 0.45 a | 6.71 ± 0.52 abcd | 0.24 ± 0.06 ab | 4.24 ± 0.55 fg | 3.73 ± 0.07 abc | 589.46 ± 87.71 cde | 1262.65 ± 7.74 cd | 16.40 ± 0.35 abcde | 2.42 ± 0.39 cde | 241.71 ± 9.67 bcd | 2.70 ± 0.10 c | 575.54 ± 73.52 c |
| Br.re.A | 3.15 ± 0.44 a | 2.86 ± 0.48 abc | 1.08 ± 1.40 b | 5.64 ± 0.07 g | 3.95 ± 0.20 abc | 523.88 ± 87.40 bcd | 765.15 ± 24.94 abc | 20.04 ± 1.30 bcde | 4.04 ± 1.22 f | 153.55 ± 12.21 abc | 1.46 ± 0.20 abc | 254.84 ± 32.29 ab |
| Ce.la | 1.54 ± 0.14 a | 1.35 ± 0.06 ab | 1.00 ± 0.01 a | 1.18 ± 0.21 abcd | 5.38 ± 0.43 cd | 146.96 ± 68.23 a | 1411.22 ± 10.79 cde | 7.89 ± 0.74 abc | 1.07 ± 0.06 abc | 61.44 ± 19.38 ab | 0.97 ± 0.22 abc | 269.84 ± 54.86 ab |
| Ci.cr | 1.06 ± 0.21 a | 16.13 ± 3.45 bcde | 0.17 ± 0.03 a | 1.57 ± 0.51 abcde | 4.25 ± 0.36 abc | 225.26 ± 80.48 abc | 1708.07 ± 215.51 def | 18.07 ± 3.92 abcde | 1.40 ± 0.25 abc | 47.13 ± 9.09 ab | 0.90 ± 0.18 abc | 252.42 ± 25.40 ab |
| Ci.sa | 1.42 ± 0.19 a | 18.40 ± 0.94 de | 0.17 ± 0.03 a | 1.38 ± 0.11 abcde | 2.53 ± 0.13 abc | 233.44 ± 2.17 abc | 1380.04 ± 96.22 cde | 30.31 ± 1.92 e | 3.28 ± 0.03 ef | 49.14 ± 2.21 ab | 0.87 ± 0.25 abc | 272.02 ± 17.40 ab |
| Di.di.A | 2.34 ± 0.34 a | 12.71 ± 0.51 abcde | 0.18 ± 0.02 a | 1.44 ± 0.26 abcde | 3.62 ± 0.26 abc | 226.91 ± 41.24 abc | 1848.24 ± 113.41 def | 29.39 ± 2.43 de | 1.76 ± 0.20 bcde | 80.34 ± 22.28 abc | 1.57 ± 0.14 abc | 619.66 ± 29.76 c |
| Fu.th | 1.09 ± 0.21 a | 5.56 ± 0.195 abcd | 0.4 ± 0.01 a | 2.00 ± 0.09 cde | 2.88 ± 0.05 abc | 221.22 ± 15.47 abc | 882.37 ± 11.17 bc | 11.35 ± 0.40 abcd | 1.71 ± 0.07 bcd | 192.30 ± 15.38 abc | 1.12 ± 0.18 abc | 170.63 ± 4.17 ab |
| He.st | 6.24 ± 2.10 a | 16.74 ± 13.61 cde | 0.24 ± 0.16 ab | 2.62 ± 2.25 de | 7.01 ± 5.43 ab | 612.99 ± 524.76 de | 817.28 ± 683.69 abc | 23.90 ± 20.21 cde | 2.40 ± 1.90 cde | 463.60 ± 389.08 d | 2.66 ± 1.714b c | 573.69 ± 421.58 c |
| Kn.in.A | 1.16 ± 0.33 a | 1.47 ± 0.05 ab | 0.07 ± 0.02 a | 0.94 ± 0.14 abc | 3.66 ± 0.02 abc | 95.92 ± 13.98 a | 2116.39 ± 21.07 ef | 9.83 ± 0.05 abc | 1.33 ± 0.11 abc | 33.57 ± 7.50 ab | 0.88 ± 0.21 abc | 218.40 ± 9.20 ab |
| Ph.gr.A | 1.17 ± 0.49 a | 25.54 ± 10.96 e | 0.27 ± 0.13 ab | 1.20 ± 1.09 abcd | 7.06 ± 5.47 ab | 252.02 ± 27.34 abcd | 1255.98 ± 1063.72 cd | 25.12 ± 21.78 cde | 1.12 ± 0.84 abc | 17.28 ± 14.93 ab | 0.54 ± 0.34 abc | 240.75 ± 18.92 ab |
| Sc.cr | 0.95 ± 0.16 a | 19.39 ± 1.76 de | 0.06 ± 0.03 a | 0.36 ± 0.03 ab | 6.81 ± 0.49 ab | 46.39 ± 4.44 a | 2386.17 ± 214.35 f | 23.58 ± 4.32 a | 1.20 ± 0.19 a | 6.64 ± 0.10 a | 0.87 ± 0.00 abc | 145.55 ± 6.87 ab |
| Th.ca | 1.06 ± 0.22 a | 0 ± 0.00 a | 0.11 ± 0.01 a | 1.23 ± 0.03 abcd | 3.57 ± 0.36 abc | 162.55 ± 25.83 ab | 1456.08 ± 237.19 cde | 7.29 ± 0.51 abc | 1.10 ± 0.09 abc | 29.53 ± 0.86 ab | 0.78 ± 0.03 abc | 55.84 ± 9.44 ab |
| Th.ta | 1.25 ± 0.29 a | 2.25 ± 0.23 abc | 0.15 ± 0.03 a | 1.69 ± 0.01 bcde | 3.65 ± 0.03 abc | 269.45 ± 45.92 abcd | 1347.18 ± 143.83 cde | 8.19 ± 0.73 abc | 1.14 ± 0.01 abc | 24.79 ± 0.65 ab | 0.75 ± 0.11 abc | 58.83 ± 5.45 ab |
| Site B (metallurgical site) | ||||||||||||
| Ai.el | 2.44 ± 0.46 a | 0.13 ± 0.01 a | 0.07 ± 0.00 a | 0.02 ± 0.01 a | 0.26 ± 0.02 a | 2.98 ± 0.42 a | 13.60 ± 0.26 a | 0.21 ± 0.01 a | 0.07 ± 0.02 a | 2.09 ± 0.18 a | 0.41 ± 0.05 abc | 8.32 ± 0.48 a |
| Al.ti | 31.02 ± 7.02 b | 4.37 ± 0.01 abcd | 0.27 ± 0.02 ab | 2.86 ± 0.04 ef | 11.22 ± 0.28 d | 930.81 ± 169.36 e | 1738.68 ± 11.17 def | 52.72 ± 3.44 f | 3.08 ± 0.01 def | 304.89 ± 26.06 cd | 6.75 ± 3.42 d | 336.88 ± 0.22 bc |
| An.vu | 1.44 ± 0.29 a | 0.11 ± 0.01 a | 0.08 ± 0.04 a | 0.02 ± 0.00 a | 0.28 ± 0.01 a | 1.43 ± 0.18 a | 32.92 ± 1.413 a | 0.29 ± 0.00 a | 0.10 ± 0.02 a | 0.71 ± 0.13 a | 0.42 ± 0.13 abc | 12.54 ± 0.30 a |
| Ba.ac | 2.11 ± 0.38 a | 0.06 ± 0.01 a | 0.09 ± 0.01 a | 0.01 ± 0.01 a | 0.53 ± 0.05 a | 6.23 ± 2.06 a | 46.49 ± 4.01 a | 0.37 ± 0.07 a | 0.11 ± 0.04 a | 0.80 ± 0.15 a | 0.24 ± 0.09 a | 5.56 ± 1.76 a |
| Br.re.B | 1.17 ± 0.27 a | 0.07 ± 0.01 a | 0.08 ± 0.05 a | 0.02 ± 0.00 a | 0.26 ± 0.05 a | 4.99 ± 1.58 a | 9.51 ± 1.68 a | 0.33 ± 0.06 a | 0.134 ± 0.01 a | 0.42 ± 0.26 a | 0.62 ± 0.14 abc | 11.12 ± 1.80 a |
| Da.vi | 0.93 ± 0.10 a | 0.07 ± 0.01 a | 0.07 ± 0.05 a | 0.02 ± 0.01 a | 0.22 ± 0.02 a | 0.51 ± 0.05 a | 11.28 ± 0.07 a | 0.18 ± 0.01 a | 0.06 ± 0.03 a | 0.54 ± 0.31 a | 0.33 ± 0.10 a | 4.41 ± 0.19 a |
| Di.di.B | 2.88 ± 1.66 a | 2.24 ± 0.42 abc | 0.11 ± 0.02 a | 0.86 ± 0.42 abc | 2.72 ± 0.59 abc | 151.76 ± 84.70 ab | 1543.46 ± 330.79 cde | 23.23 ± 7.88 cde | 1.27 ± 0.66 abc | 14.62 ± 8.16 ab | 0.85 ± 0.10 abc | 98.27 ± 39.26 ab |
| Gl.fl | 2.48 ± 0.85 a | 0.10 ± 0.01 a | 0.11 ± 0.05 a | 0.60 ± 0.37 abc | 3.44 ± 0.62 abc | 40.78 ± 20.56 a | 191.43 ± 33.56 ab | 0.90 ± 0.28 a | 0.44 ± 0.22 ab | 1.09 ± 0.26 a | 0.57 ± 0.13 abc | 28.08 ± 3.87 a |
| Kn.in.B | 0.78 ± 0.40 a | 0.07 ± 0.01 a | 0.05 ± 0.04 a | 0.03 ± 0.01 a | 0.34 ± 0.01 a | 1.25 ± 0.33 a | 42.24 ± 1.84 a | 0.16 ± 0.02 a | 0.08 ± 0.01 a | 0.27 ± 0.12 a | 0.43 ± 0.10 abc | 12.40 ± 0.34 a |
| La.tu | 1.19 ± 0.12 a | 0.34 ± 0.02 a | 0.10 ± 0.05 a | 0.02 ± 0.01 a | 0.567 ± 0.01 a | 2.64 ± 0.79 a | 60.14 ± 1.68 a | 0.34 ± 0.01 a | 0.07 ± 0.03 a | 1.22 ± 0.21 a | 0.29 ± 0.26 a | 6.12 ± 0.20 a |
| Ph.gr.B | 1.77 ± 0.18 a | 0.20 ± 0.01 a | 0.08 ± 0.01 a | 0.02 ± 0.01 a | 0.70 ± 0.03 a | 4.03 ± 0.59 a | 23.37 ± 1.51 a | 0.47 ± 0.06 a | 0.12 ± 0.04 a | 0.62 ± 0.04 a | 0.43 ± 0.01 abc | 3.62 ± 0.09 a |
| Re.al.B1 | 2.57 ± 0.46 a | 0.09 ± 0.02 a | 0.05 ± 0.02 a | 0.03 ± 0.01 a | 0.27 ± 0.01 a | 4.98 ± 0.10 a | 45.04 ± 1.45 a | 0.587 ± 0.03 a | 0.160 ± 0.03 a | 0.916 ± 0.09 a | 0.34 ± 0.10 ab | 11.585 ± 3.74 a |
| Re.al.B2 | 6.59 ± 7.62 a | 0.21 ± 0.06 a | 0.10 ± 0.02 a | 0.05 ± 0.04 a | 0.55 ± 0.09 a | 9.42 ± 1.22 a | 41.56 ± 0.68 a | 0.31 ± 0.06 a | 0.12 ± 0.05 a | 1.56 ± 1.38 a | 0.59 ± 0.27 abc | 27.61 ± 3.89 a |
| Sc.cr | 1.04 ± 0.11 a | 0.16 ± 0.01 a | 0.10 ± 0.02 a | 0.05 ± 0.02 a | 0.41 ± 0.05 a | 2.04 ± 1.06 a | 49.15 ± 10.89 a | 0.84 ± 0.27 cde | 0.12 ± 0.03 abc | 0.35 ± 0.09 a | 0.36 ± 0.24 ab | 4.38 ± 1.27 a |
| Si.co | 4.75 ± 1.53 a | 0.07 ± 0.00 a | 0.10 ± 0.05 a | 0.25 ± 0.15 ab | 0.76 ± 0.24 ab | 7.47 ± 6.67 a | 44.84 ± 4.51 a | 2.08 ± 1.36 ab | 0.12 ± 0.06 a | 2.48 ± 1.62 a | 0.32 ± 0.07 a | 64.42 ± 42.02 ab |
| Normal concentrations ‡ | 0.02−0.1 | 0.1−0.5 | 0.05−0.5 | 0.02−1.0 | 5.0−20.0 | 50.0−250.0 | 1000.0–5000.0 | 20.0−200.0 | 0.2−2.0 | 1.0−5.0 | <1.0 | 20.0−100.0 |
| Code | As | Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Sb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site A (mining site) | |||||||||||
| As.lu | 0.0063 ± 0.0009 a | 0.0485 ± 0.0038 abcd | 0.018 ± 0.0045 ab | 0.0119 ± 0.0015 ef | 0.0221 ± 0.0004 abc | 0.0150 ± 0.0022 de | 0.0091 ± 0.0002 abcd | 0.0127 ± 0.002 ef | 0.0089 ± 0.0004 bcd | 0.0097 ± 0.0004 c | 0.0300 ± 0.0038 c |
| Br.re.A | 0.0063 ± 0.0008 a | 0.0207 ± 0.0035 abc | 0.0824 ± 0.1067 b | 0.0157 ± 0.0002 f | 0.0234 ± 0.0011 abc | 0.0133 ± 0.0022 bcde | 0.0111 ± 0.0007 bcd | 0.0201 ± 0.006 g | 0.0056 ± 0.0004 abc | 0.0052 ± 0.0007 abc | 0.0132 ± 0.0016 ab |
| Ce.la | 0.003 ± 0.0002 a | 0.0097 ± 0.0004 ab | 0.0073 ± 0.001 a | 0.0033 ± 0.0005 abcd | 0.0319 ± 0.0025 bc | 0.0037 ± 0.0017 a | 0.0043 ± 0.0004 ab | 0.0053 ± 0.0003 abcde | 0.0022 ± 0.0007 ab | 0.0034 ± 0.0007 abc | 0.014 ± 0.0028 ab |
| Ci.cr | 0.0021 ± 0.0004 a | 0.1168 ± 0.0249 bcde | 0.0127 ± 0.002 a | 0.0044 ± 0.0014 abcd | 0.0252 ± 0.0021 abc | 0.0057 ± 0.002 abc | 0.01 ± 0.0021 abcd | 0.0069 ± 0.0012 abcde | 0.0017 ± 0.0003 ab | 0.0032 ± 0.0006 abc | 0.0131 ± 0.0013 ab |
| Ci.sa | 0.0028 ± 0.0003 a | 0.1333 ± 0.0068 de | 0.0132 ± 0.0022 a | 0.0038 ± 0.0003 abcd | 0.015 ± 0.0007 abc | 0.0059 ± 0.0 abcd | 0.0168 ± 0.001 d | 0.0163 ± 0.0001 fg | 0.0018 ± 0.0 ab | 0.0031 ± 0.0008 abc | 0.0141 ± 0.0009 ab |
| Di.di. A | 0.0046 ± 0.0006 a | 0.092 ± 0.0036 abcde | 0.014 ± 0.0014 a | 0.004 ± 0.0007 abcd | 0.0214 ± 0.0015 abc | 0.0057 ± 0.001 abcd | 0.0163 ± 0.0013 cd | 0.0087 ± 0.0009 def | 0.0029 ± 0.0008 ab | 0.0056 ± 0.0005 abc | 0.0322 ± 0.0015 c |
| Fu.th | 0.0022 ± 0.0004 a | 0.0402 ± 0.0014 abcd | 0.0103 ± 0.0007 a | 0.0055 ± 0.0002 bcd | 0.0171 ± 0.0002 abc | 0.0056 ± 0.0003 abc | 0.0063 ± 0.0002 abc | 0.0085 ± 0.0003 bcdef | 0.0071 ± 0.0005 abc | 0.004 ± 0.0006 abc | 0.0088 ± 0.0002 ab |
| He.st | 0.0125 ± 0.0042 a | 0.1212 ± 0.0985 cde | 0.0181 ± 0.0123 ab | 0.0073 ± 0.0063 de | 0.0416 ± 0.0322 c | 0.0156 ± 0.0133 e | 0.0132 ± 0.0112 bcd | 0.0119 ± 0.0094 ef | 0.0171 ± 0.0143 d | 0.0095 ± 0.0061 bc | 0.0298 ± 0.0219 c |
| Kn.in.A | 0.0022 ± 0.0006 a | 0.0106 ± 0.0003 ab | 0.005 ± 0.0014 a | 0.0026 ± 0.0003 abcd | 0.0217 ± 0.0001 abc | 0.0024 ± 0.0003 a | 0.0054 ± 0.0 ab | 0.0066 ± 0.0005 abcde | 0.0012 ± 0.0002 ab | 0.0031 ± 0.0007 abc | 0.0113 ± 0.0004 ab |
| Ph.gr.A | 0.0023 ± 0.0009 a | 0.1849 ± 0.1518 e | 0.0203 ± 0.0096 ab | 0.0033 ± 0.003 abcd | 0.0419 ± 0.0325 c | 0.0064 ± 0.0058 abcde | 0.0139 ± 0.0121 bcd | 0.0055 ± 0.0041 abcde | 0.0006 ± 0.0005 ab | 0.0019 ± 0.0012 a | 0.0125 ± 0.0095 ab |
| Sc.cr.A | 0.0019 ± 0.0003 a | 0.1405 ± 0.0128 de | 0.0047 ± 0.0019 a | 0.001 ± 0.0001 a | 0.0404 ± 0.003 c | 0.0012 ± 0.0001 a | 0.0131 ± 0.0024 bcd | 0.006 ± 0.001 abcde | 0.0002 ± 0.0 a | 0.0031 ± 0.0000 abc | 0.0076 ± 0.0004 ab |
| Th.ca | 0.0021 ± 0.0004 a | 0.0042 ± 0.0006 a | 0.0084 ± 0.0007 a | 0.0034 ± 0.0 abcd | 0.0212 ± 0.0021 abc | 0.0041 ± 0.0006 ab | 0.004 ± 0.0002 ab | 0.0054 ± 0.0004 abcde | 0.001 ± 0.0 ab | 0.0028 ± 0.0001 ab | 0.0029 ± 0.0004 a |
| Th.ta | 0.0025 ± 0.000 3 a | 0.0163 ± 0.0011 a | 0.0116 ± 0.0012 a | 0.0047 ± 0.0 abcd | 0.0216 ± 0.0001 abc | 0.0068 ± 0.0007 abcde | 0.0045 ± 0.0002 ab | 0.0056 ± 0.0000 abcde | 0.0009 ± 0.0 ab | 0.0026 ± 0.0002 a | 0.003 ± 0.0001 a |
| Site B (metallurgical site) | |||||||||||
| Ai. el | 0.0007 ± 0.0001 a | 0.0009 ± 0.0 a | 0.0094 ± 0.0005 a | 0.0001 ± 0.0 a | 0.0003 ± 0.0 a | − | − | 0.0004 ± 0.0001 a | − | 0.0011 ± 0.0001 a | 0.0004 ± 0.0 a |
| Al. ti | 0.0722 ± 0.0632 b | 0.0329 ± 0.0007 abcd | 0.0358 ± 0.0025 ab | 0.0224 ± 0.0003 g | 0.0154 ± 0.0003 abc | 0.0147 ± 0.0026 cde | 0.0178 ± 0.0011 d | 0.0209 ± 0.0 g | 0.0117 ± 0.001 cd | 0.0182 ± 0.0092 d | 0.02 ± 0.0 bc |
| An.vu | 0.0004 ± 0.0 a | 0.0008 ± 0.0 a | 0.0111 ± 0.0045 a | 0.0001 ± 0.0 a | 0.0003 ± 0.0 a | − | − | 0.0006 ± 0.0001 ab | − | 0.0011 ± 0.0003 a | 0.0007 ± 0.0 a |
| Ba.ac | 0.0006 ± 0.0001 a | 0.0004 ± 0.0 a | 0.0112 ± 0.001 a | − | 0.0007 ± 0.0 a | − | 0.0001 ± 0.0 a | 0.0007 ± 0.0002 ab | − | 0.0006 ± 0.0002 a | 0.0003 ± 0.0001 a |
| Br.re.B | 0.0003 ± 0.0 a | 0.0005 ± 0.0 a | 0.0109 ± 0.0064 a | 0.0001 ± 0.0 a | 0.0003 ± 0.0 a | − | 0.0001 ± 0.0 a | 0.0009 ± 0.0 abcd | − | 0.0016 ± 0.0003 a | 0.0006 ± 0.0001 a |
| Da.vi | 0.0002 ± 0.0 a | 0.0005 ± 0.0 a | 0.0092 ± 0.0069 a | 0.0001 ± 0.0 a | 0.0003 ± 0.0 a | − | − | 0.0003 ± 0.0002 a | − | 0.0008 ± 0.0002 a | 0.0002 ± 0.0 a |
| Di.di.B | 0.0008 ± 0.0004 a | 0.0168 ± 0.0032 abc | 0.0143 ± 0.003 a | 0.0067 ± 0.0032 cde | 0.0037 ± 0.0008 a | 0.0024 ± 0.0013 a | 0.0078 ± 0.0026 abcd | 0.0086 ± 0.0045 cdef | 0.0005 ± 0.0003 ab | 0.0023 ± 0.0002 a | 0.0058 ± 0.0023 ab |
| Gl.fl | 0.0007 ± 0.0002 a | 0.0007 ± 0.0 a | 0.0142 ± 0.0063 a | 0.0047 ± 0.0029 abcd | 0.0047 ± 0.0008 ab | 0.0006 ± 0.0003 a | 0.0003 ± 0.0 a | 0.003 ± 0.0015 abcd | − | 0.0015 ± 0.0003 a | 0.0016 ± 0.0002 a |
| Kn.in.B | 0.0002 ± 0.0001 a | 0.0005 ± 0.0 a | 0.0061 ± 0.0049 a | 0.0002 ± 0.0001 a | 0.0004 ± 0.0 a | − | − | 0.0005 ± 0.0 a | − | 0.0011 ± 0.0002 a | 0.0007 ± 0.0 a |
| La. tu | 0.0003 ± 0.0 a | 0.0025 ± 0.0001 a | 0.0131 ± 0.0064 a | 0.0001 ± 0.0001 a | 0.0007 ± 0.0 a | − | 0.0001 ± 0.0 a | 0.0005 ± 0.0001 a | − | 0.0007 ± 0.0007 a | 0.0003 ± 0.0 a |
| Ph.gr.B | 0.0005 ± 0.0 a | 0.0014 ± 0.0 a | 0.0109 ± 0.0016 a | 0.0001 ± 0.0 a | 0.0009 ± 0.0 a | − | 0.0001 ± 0.0 a | 0.0008 ± 0.0003 abc | − | 0.0011 ± 0.0 a | 0.0002 ± 0.0 a |
| Re.al.B1 | 0.0007 ± 0.0001 a | 0.0007 ± 0.0001 a | 0.007 ± 0.0030 a | 0.0002 ± 0.0 a | 0.0003 ± 0.0 a | − | 0.0001 ± 0.0 a | 0.001 ± 0.0002 abcd | − | 0.0009 ± 0.0002 a | 0.0006 ± 0.0002 a |
| Re.al.B2 | 0.0019 ± 0.0018 a | 0.0016 ± 0.0003 a | 0.0127 ± 0.0019 a | 0.0004 ± 0.0002 a | 0.0007 ± 0.0001 a | 0.0001 ± 0.0 a | 0.0001 ± 0.0 a | 0.0008 ± 0.0002 abc | − | 0.0015 ± 0.0005 a | 0.0016 ± 0.0001 a |
| Sc.cr.B | 0.0003 ± 0.0 a | 0.0012 ± 0.0 a | 0.0125 ± 0.0027 a | 0.0003 ± 0.0001 ab | 0.0005 ± 0.0 a | − | 0.0002 ± 0.0 a | 0.0008 ± 0.0001 abc | − | 0.0009 ± 0.0006 a | 0.0002 ± 0.0 a |
| Si.co | 0.0013 ± 0.0004 a | 0.0005 ± 0.0 a | 0.0129 ± 0.0061 a | 0.0019 ± 0.0011 abc | 0.001 ± 0.0003 a | 0.0001 ± 0.0001 a | 0.0007 ± 0.0004 a | 0.0008 ± 0.0004 abc | − | 0.0008 ± 0.0001 a | 0.0038 ± 0.0024 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papazoglou, E.G.; Zine, H.; Trigas, P.; Wójcik, M.; Vangronsveld, J. Native Plant Responses and Elemental Accumulation in Mining and Metallurgical Mediterranean Ecosystems. Plants 2025, 14, 2646. https://doi.org/10.3390/plants14172646
Papazoglou EG, Zine H, Trigas P, Wójcik M, Vangronsveld J. Native Plant Responses and Elemental Accumulation in Mining and Metallurgical Mediterranean Ecosystems. Plants. 2025; 14(17):2646. https://doi.org/10.3390/plants14172646
Chicago/Turabian StylePapazoglou, Eleni G., Hamza Zine, Panayiotis Trigas, Małgorzata Wójcik, and Jaco Vangronsveld. 2025. "Native Plant Responses and Elemental Accumulation in Mining and Metallurgical Mediterranean Ecosystems" Plants 14, no. 17: 2646. https://doi.org/10.3390/plants14172646
APA StylePapazoglou, E. G., Zine, H., Trigas, P., Wójcik, M., & Vangronsveld, J. (2025). Native Plant Responses and Elemental Accumulation in Mining and Metallurgical Mediterranean Ecosystems. Plants, 14(17), 2646. https://doi.org/10.3390/plants14172646








