The Bog Bilberry Enigma: A Phytochemical and Ethnopharmacological Analysis of Vaccinium uliginosum L. Fruits in Regard to Their Alleged Toxicity
Abstract
1. Introduction
- The parasitic fungus Monilinia megalospora (Woronin) Whetzel, which can infect the bog bilberry pericarp, may biosynthesize toxic secondary metabolites. So far, the metabolite profile of Monilinia species remains largely unexplored.
- Allergy or individual intolerance can cause unexpected reactions in some individuals [10].
- There may be a phytochemical difference between isolated populations of bog bilberries, causing some to be toxic. Plant secondary metabolites, which most commonly cause harmful or psychoactive effects in humans, are alkaloids (e.g., scopolamine), amides (e.g., mescaline), monoterpenes (e.g., thujone), sesquiterpenes (e.g., ledol), and diterpenes (e.g., grayanotoxin) [18].
- Is there any significant phytochemical difference between isolated populations of V. uliginosum that would substantiate the reports of toxicity? Are there any metabolites unique to the harvest sites mentioned in the historical literature?
- How common is the infection by Monilinia megalospora? How do the infected “mummy berries” differ from the healthy ones in their chemical makeup? Can any potentially harmful metabolites be identified?
- Can any viability or lifespan-reducing effects be observed on the selected bioassays upon treatment with the berry extracts? If yes, can these be correlated with the content of any secondary metabolites?
- How does the suitability of V. uliginosum for alcoholic fermentation compare to other similar Vaccinium berries? What alcohol percentages can be achieved with or without the addition of sugar and yeast? Are these percentages high enough to justify records of inebriation?
2. Results
2.1. Chemical Profiling of the Nonpolar Bog Bilberry Extracts
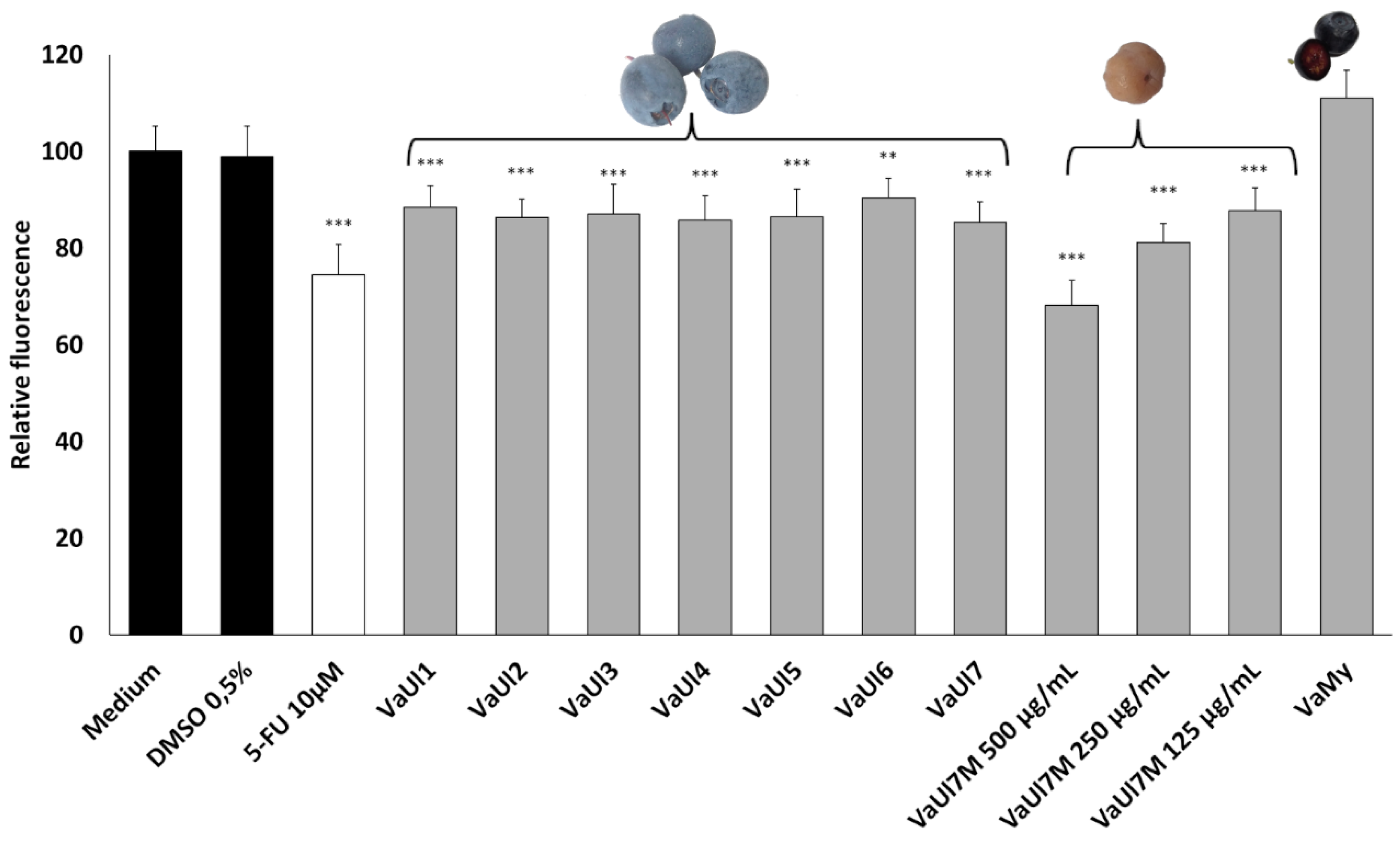
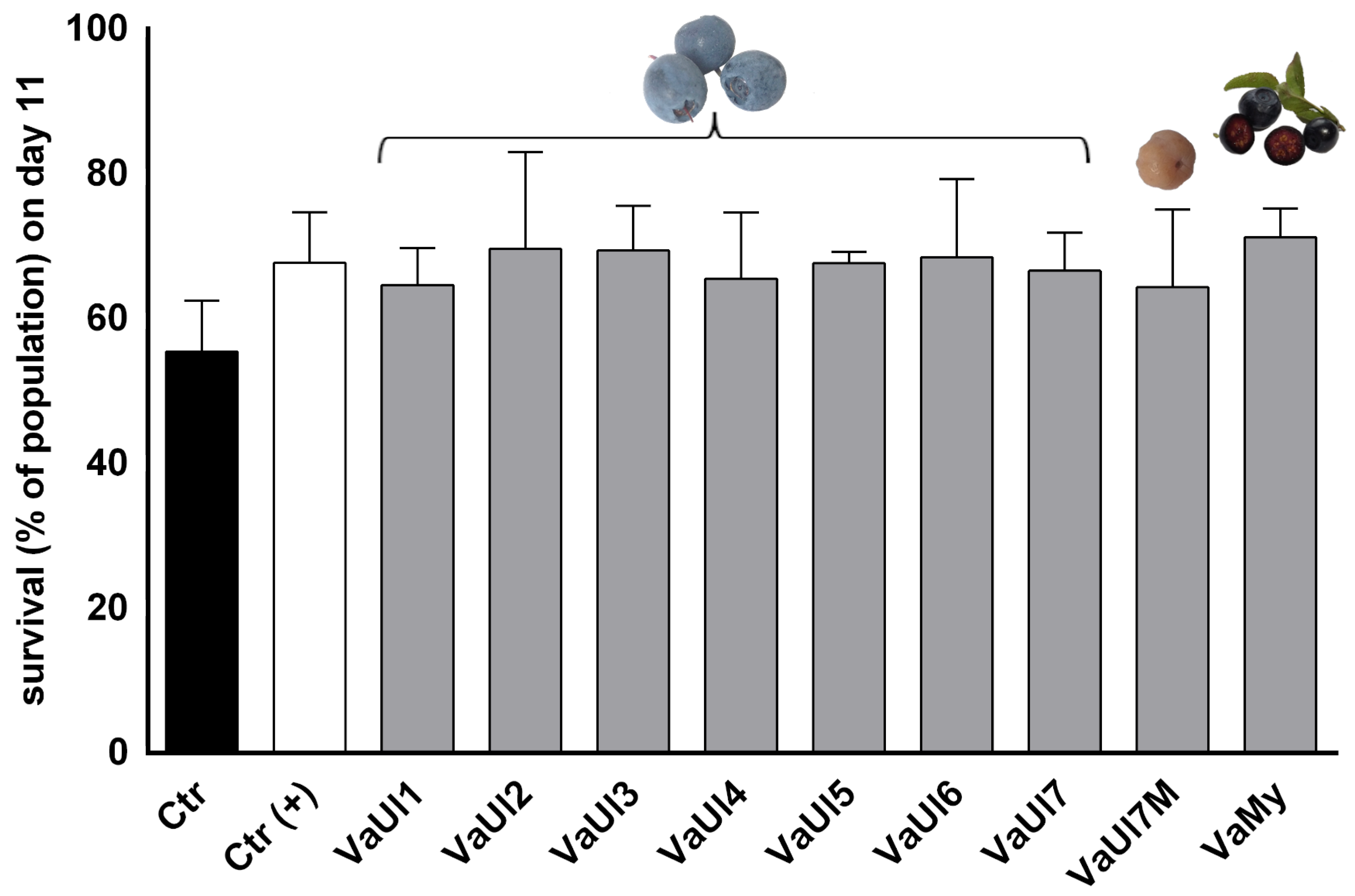
2.2. Bioactivity Testing of LLE Berry Extracts
2.3. Chemical Profiling of the LLE Berry Extracts
2.4. Monilinia Megalospora Influence on the Berry Sample Bioactivity and Chemical Profile
2.5. Fermentation of Vaccinium Berries
3. Discussion
- It is possible that the berries are not sufficiently populated by wild yeast strains to enable successful alcoholic fermentation, and therefore require inoculation.
- The practice of diluting the raw juice would also suggest that V. myrtillus contains metabolites that inhibit bacterial and yeast growth, but not mold. This is the case with cranberries Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. and lingonberries Vaccinium vitis-idaea L., which contain high concentrations of benzoic acid and therefore keep extraordinarily well without any additional conservation [48,49]. A quantitative analysis with GC-MS by Klavins and colleagues compared levels of benzoic acid in several berry species: V. corymbosum 0.64 µg/g, V. uliginosum 0.51 µg/g, and V. myrtillus 6.13 µg/g of fresh berries. The levels of benzoic acid in V. myrtillus were not as high as in cranberry or lingonberry (37.08 and 164.40 µg/g, respectively) [50], but nevertheless were much higher than in the other two species compared in the present work.
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Extraction Methods
4.4. Determination of Anthocyanin Content
4.5. Determination of Total Soluble Polyphenols
4.6. UPLC-DAD-MS
- Concentration of samples: 15 mg/mL for extracts, 1 mg/mL for standard compounds; dissolved in MeOH. Injection volume: 10 µL for extracts, 1 µL for standards.
- 90% H2O + 10% MeOH with 10 mM ammonium formate as ISM solvent, used for an appropriate dilution of the eluate before entering the mass spectrometer.
- Flow rate: 0.3 mL/min for QSM, 0.4 mL/min for ISM.
- Column: Acquity BEH C18, 2.1 × 100 mm, 1.7 μm.
- PDA detector settings: detection wavelength 370 nm for flavonoids, 530 nm for anthocyanins.
- QDa detector settings: capillary voltage 0.8 kV (positive and negative), cone voltages 15 V (positive) and 30 V (negative), mass range 100–1000 m/z, probe temperature 600 °C.
- System 1—column temperature: 45 °C, mobile phase: A: water + 0.1% formic acid, B: acetonitrile + 0.1% formic acid, C: methanol + 0.1% formic acid. Gradient: Initial: A 98.0%, B 0.0%, C 2.0%; 1.0 min: A 98.0%, B 0.0%, C 2.0%; 2.0 min: A 85.0%, B 0.0%, C 15.0%; 15.0 min: A 40.0%, B 0.0%, C 60.0%; 16.0 min: A 5.0%, B 0.0%, C 95.0%; 18.0 min: A 2.0%, B 98.0%, C 0.0%; 22.0 min: A 2.0%, B 98.0%, C 0.0%; 22.5 min: A 98.0%, B 0.0%, C 2.0%; 24.0 min: A 98.0%, B 0.0%, C 2.0%.
- System 2—column temperature: 50 °C, mobile phase: A: water + 1% formic acid, B: methanol + 1% formic acid. Gradient: Initial: A 93.0%, B 7.0%; 43.0 min: A 78.0%, B 22.0%; 43.5 min: A 5.0%, B 95.0%; 48.0 min: A 5.0%, B 95.0%; 48.5 min: A 93.0%, B 7.0%; 50.0 min: A 93.0%, B 7.0%.
4.7. UHPSFC-DAD-ELSD-MS
- Concentration of samples: 4 mg/mL for extracts, 1 mg/mL for standard compounds; dissolved in 4:6 mixture of hexane and isopropanol. Injection volume: 4 µL.
- Mobile phase: supercritical CO2 as A, methanol as B.
- 95% MeOH + 5% H2O with 10 mM ammonium formate as ISM solvent, used for an appropriate dilution of the eluate before entering the mass spectrometer.
- Flow rate: 1 mL/min for both BSM and ISM. Backpressure: 2000 psi.
- Gradient: Initial: A 95.0%, B 5.0%; 3.0 min: A 90.0%, B 10.0%; 10.5 min: A 74.0%, B 26.0%; 11.0 min: A 95.0%, B 5.0%; 12.0 min: A 95.0%, B 5.0%.
- Column: Torus 1-AA (1-Aminoanthracene), 130 Å, 1.7 µm, 3 × 100 mm.
- Column temperature: 50 °C.
- PDA detector settings: detection wavelength 210 nm.
- ELS detector settings: detector gain 100, gas pressure 40.0 psi, nebulizer in heating mode, drift tube temperature 50.0 °C.
- QDa detector settings: capillary voltage 0.8 kV (positive and negative), cone voltages 15 V (positive) and 30 V (negative), mass range 100–1000 m/z, probe temperature 600 °C.
4.8. Cell Viability Assay
4.9. Caenorhabditis Elegans Survival Assay
4.10. Fermentation
- Whole frozen fruits, roughly mashed with no further processing, one bottle per species.
- Whole frozen fruits, mashed and juiced through a cheesecloth, one bottle per species.Subsequently, frozen fruits of V. uliginosum were pureed, filtered through a 0.5 mm sieve, and divided between four sets of conditions, two bottles each:
- 150 mL raw juice with no additions, labeled VUJ_1 and VUJ_2.
- 150 mL raw juice with 10 g added sugar, labeled VUJS_1 and VUJS_2.
- 150 mL raw juice with 0.125 g added brewer’s yeast (Saccharomyces cerevisiae Oenoferm® X-thiol, Erbslöh Geisenheim AG, Geisenheim, Germany), labeled VUJY_1 and VUJY_2.
- 150 mL raw juice with 10 g added sugar and 0.125 g brewer’s yeast, labeled VUJSY_1 and VUJSY_2.
- The same set of analyses was applied to all samples before and after fermentation.
- The pH values were measured with a pH meter (HI223, Hanna Instruments, Germany).
- The °Brix values were measured with a handheld Brix meter (RHB-32ATC, Hong Han GmbH, Germany).
- The alcohol content was determined by the water vapor distillation method adapted from European Pharmacopoeia, 5th ed. [54], and expressed as alcohol by volume (ABV).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacquemart, A.-L. Vaccinium uliginosum L. J. Ecol. 1996, 84, 771–785. [Google Scholar] [CrossRef]
- Alsos, I.G.; Engelskjøn, T.; Gielly, L.; Taberlet, P.; Brochmann, C. Impact of ice ages on circumpolar molecular diversity: Insights from an ecological key species. Mol. Ecol. 2005, 14, 2739–2753. [Google Scholar] [CrossRef]
- Vaneková, Z.; Holloway, P.; Rollinger, J.M. Vaccinium uliginosum L. (bog bilberry) and the search for its alleged toxicity: A review. Front. Toxicol. 2024, 6, 1358840. [Google Scholar] [CrossRef]
- Bogoraz, W. The Chukchee; E. J. Brill: Leiden, The Netherlands, 1909; p. 208. [Google Scholar]
- Jones, A. Plants That We Eat: Nauriat Nigiñaqtuat, 2nd ed.; University of Alaska Press: Fairbanks, AK, USA, 2010. [Google Scholar]
- Wang, Y.; Yang, H.; Zhong, S.; Liu, X.; Li, T.; Zong, C. Variations in sugar and organic acid content of fruit harvested from different Vaccinium uliginosum populations in the Changbai mountains of China. J. Am. Soc. Hortic. Sci. 2019, 144, 420–428. [Google Scholar] [CrossRef]
- Rätsch, C. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications, 1st ed.; Simon & Schuster: New York, NY, USA, 2005; pp. 523–524. [Google Scholar]
- Nevinny, J. Die Rauschbeere (Vaccinium uliginosum L.), ihre Verwechselung mit der Heidelbeere (Vaccinium myrtillus L.) und ihr Nachweis in den Fäces. Z. Hyg. Infekt. 1908, 59, 95–121. [Google Scholar] [CrossRef]
- Feder, E. Über die Heidelbeere und die Rauschbeere. Pharm. Zentralhalle Dtschl. 1912, 53, 1321–1323. [Google Scholar] [CrossRef]
- Frenkiel, H. Ein Fall von Hämoglobinurie bei einem Kinde nach Genuß von Rauschbeeren (Vaccinium uliginosum). Z. Kinderheilkd 1932, 52, 608–612. [Google Scholar] [CrossRef]
- Kreuder, F. Vergiftungserscheinungen nach reichlichem Genuß von Rauschbeeren. Samml. Vergiftungsfällen 1937, 8, 33–34. [Google Scholar] [CrossRef]
- Lendle, L. Vergiftung durch den Genuß von Rauschbeeren in Rußland. Samml. Vergiftungsfällen 1943, 12, 105–106. [Google Scholar] [CrossRef]
- Zipf, K. Vergiftungen durch Rauschbeeren. Samml. Vergiftungsfällen 1944, 13, 139–140. [Google Scholar] [CrossRef]
- Piechocka, J.; Szymczyk, F. Badania nad Vaccinium uliginosum L. Borowka bagienna. Rocz. Panstw. Zakl. Hig. 1955, 6, 109–117. [Google Scholar]
- Nees, H.; Pachaly, P.; Zymalkowski, F. Chemotaxonomy of Ericaceae: Isolation and identification of triterpenes and steroids from Vaccinium uliginosum. Planta Med. 1973, 24, 320–328. [Google Scholar] [CrossRef]
- Lehbert, R. Über die Heidelbeere und die Rauschbeere. Pharm. Zentralhalle Dtschl. 1913, 54, 71–73. [Google Scholar] [CrossRef]
- Kokassaar, U. Mittejoovastad Joovikad. Eest. Lood. 2006, 8. Available online: http://vana.loodusajakiri.ee/eesti_loodus/artikkel1634_1609.html (accessed on 3 July 2025).
- Alrashedy, N.A.; Molina, J. The ethnobotany of psychoactive plant use: A phylogenetic perspective. PeerJ 2016, 4, e2546. [Google Scholar] [CrossRef]
- Netoliczky, F. Die Giftigkeit der “Rauschbeeren” (Vaccinium uliginosum)—ein Mißverständnis. Osterr. Bot. Z. 1914, 64, 43–45. [Google Scholar] [CrossRef]
- Schübeler, F.C. Ericaceae. In Die Pflanzenwelt Norwegens: Ein Beitrag zur Natur und Kulturgeschichte Nord-Europas; IEEE: Christiania, Denmark, 1873; p. 276. [Google Scholar]
- Trivedi, P.; Karppinen, K.; Klavins, L.; Kviesis, J.; Sundqvist, P.; Nguyen, N.; Heinonen, E.; Klavins, M.; Jaakola, L.; Vaananen, J.; et al. Compositional and morphological analyses of wax in northern wild berry species. Food Chem. 2019, 295, 441–448. [Google Scholar] [CrossRef]
- Camp, D.; Davis, R.A.; Campitelli, M.; Ebdon, J.; Quinn, R.J. Drug-like Properties: Guiding Principles for the Design of Natural Product Libraries. J. Nat. Prod. 2012, 75, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Flynn, T.J.; Xia, M.; Wiesenfeld, P.L.; Ferguson, M.S. Evaluation of CYP3A4 inhibition and hepatotoxicity using DMSO-treated human hepatoma HUH-7 cells. Cell Biol. Toxicol. 2015, 31, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Jing, H.; Popovich, D.G. Bog bilberry (Vaccinium uliginosum L.) extract reduces cultured hep-g2, caco-2, and 3t3-l1 cell viability, affects cell cycle progression, and has variable effects on membrane permeability. J. Food Sci. 2010, 75, H103–H107. [Google Scholar] [CrossRef]
- Zwirchmayr, J.; Kirchweger, B.; Lehner, T.; Tahir, A.; Pretsch, D.; Rollinger, J.M. A robust and miniaturized screening platform to study natural products affecting metabolism and survival in Caenorhabditis elegans. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Redl, M.; Shayegan, A.; Rollinger, J.M. Application of 3Rs in Caenorhabditis elegans Research for the Identification of Health-Promoting Natural Products. Planta Med. 2024, 90, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Cui, S.; Chen, W. Anti-aging effects and mechanisms of anthocyanins and their intestinal microflora metabolites. Crit. Rev. Food Sci. Nutr. 2022, 64, 2358–2374. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liu, J.; Guo, Q.; Zhang, N. Transcriptomic approaches to investigate the anti-aging effects of blueberry anthocyanins in a caenorhabditis elegans aging model. Antioxidants 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosín-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog bilberry phenolics, antioxidant capacity and nutrient profile. Food Chem. 2016, 201, 339–349. [Google Scholar] [CrossRef]
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Česoniene, L.; Daubaras, R. Antioxidant properties, phenolic composition and potentiometric sensor array evaluation of commercial and new blueberry (Vaccinium corymbosum) and bog blueberry (Vaccinium uliginosum) genotypes. Food Chem. 2015, 188, 583–590. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef]
- Lätti, A.K.; Jaakola, L.; Riihinen, K.R.; Kainulainen, P.S. Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finland. J. Agric. Food Chem. 2010, 58, 427–433. [Google Scholar] [CrossRef]
- Su, S.; Wang, L.J.; Feng, C.Y.; Liu, Y.; Li, C.H.; Du, H.; Tang, Z.-Q.; Xu, Y.-J.; Wang, L.S. Fingerprints of anthocyanins and flavonols of Vaccinium uliginosum berries from different geographical origins in the Greater Khingan Mountains and their antioxidant capacities. Food Control 2016, 64, 218–225. [Google Scholar] [CrossRef]
- Woronin, M. Über die Sclerotienkrankheit der Vaccinieen-Beeren. In Mémoires de l’Académie Impériale des Sciences de St. Petersburg; Egger et Glasounof: St. Johann in Tirol, Austria, 1888; Volume 36, pp. 1–49. [Google Scholar]
- Gajek, J. Polski Atlas Etnograficzny, 1st ed.; Instytut Historii Kultury Materialnej Polskiej Akademii Nauk: Warszawa, Poland, 1981. [Google Scholar]
- Liu, S.; Marsol-Vall, A.; Laaksonen, O.; Kortesniemi, M.; Yang, B. Characterization and quantification of nonanthocyanin phenolic compounds in white and blue bilberry (Vaccinium myrtillus) juices and wines using UHPLC-dad−ESI-QTOF-MS and UHPLC-Dad. J. Agric. Food Chem. 2020, 68, 7734–7744. [Google Scholar] [CrossRef]
- Behrends, A.; Weber, F. Influence of different fermentation strategies on the phenolic profile of bilberry wine (Vaccinium myrtillus L.). J. Agric. Food Chem. 2017, 65, 7483–7490. [Google Scholar] [CrossRef]
- Kupffer, K.R. Die angebliche Giftigkeit der Blaubeeren und Krähnenbeeren. Korresp. Naturforscher. Ver. Riga 1906, 49, 141. [Google Scholar]
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and Other Non-Polar Compounds in Leaves of Wild and Cultivated Vaccinium Species. Plants 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M. Role of Dietary Fat and Supplementation in Modulating Neurodegenerative Pathology in Two Animal Model Systems. Doctoral Dissertation, University of Alaska, Anchorage, AK, USA, 2018. [Google Scholar]
- Heffels, P.; Müller, L.; Schieber, A.; Weber, F. Profiling of iridoid glycosides in Vaccinium species by UHPLC-MS. Food Res. Int. 2017, 100, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-L.; Kang, J.-H.; Kim, H.-M.; Jeong, S.-H.; Jang, D.-S.; Jang, Y.-P.; Choung, S.-Y. Polyphenol-enriched Vaccinium uliginosum L. fractions reduce retinal damage induced by blue light in A2E-laden arpe19 cell cultures and mice. Nutr. Res. 2016, 36, 1402–1414. [Google Scholar] [CrossRef]
- McArt, S.H.; Miles, T.D.; Rodriguez-Saona, C.; Schilder, A.; Adler, L.S.; Grieshop, M.J. Floral scent mimicry and vector-pathogen associations in a pseudoflower-inducing plant pathogen system. PLoS ONE 2016, 11, e0165761. [Google Scholar] [CrossRef]
- Bansal, K.K. Comparative Genomics and Transcriptomics of the Pollen-Mimicking Floral Infection of Blueberry by Monilinia vaccinia-corymbosi. Doctoral Dissertation, University of Florida, Gainesville, FL, USA, 2020. [Google Scholar]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Li, Y.; Lou, Y.; Feng, X.; Yang, B. Comparison of phenolic profiles of albino bilberry (Vaccinium myrtillus L.) wines fermented by non-saccharomyces yeasts. Food Biosci. 2023, 55, 102980. [Google Scholar] [CrossRef]
- Litwiller, E.M. Nitrogen Deficiency in Relation to Spoilage of the Blueberry, Vaccinium ovatum. Doctoral Dissertation, Oregon State College, Corvallis, OR, USA, 1944. [Google Scholar]
- Visti, A.; Viljakainen, S.; Laakso, S. Preparation of fermentable lingonberry juice through removal of benzoic acid by saccharomyces cerevisiae yeast. Food Res. Int. 2003, 36, 597–602. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Klavins, M. Surface wax composition of wild and cultivated northern berries. Agron. Res. 2019, 17, 1337–1345. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Doumett, S.; Fibbi, D.; Cincinelli, A.; Giordani, E.; Nin, S.; Del Bubba, M. Comparison of nutritional and nutraceutical properties in cultivated fruits of Fragaria vesca L. produced in Italy. Food Res. Int. 2011, 44, 1209–1216. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook. Available online: https://wormbook.org/chapters/www_strainmaintain/strainmaintain.html (accessed on 3 July 2025).
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Ethanol Content and Alcoholimetric tables. In European Pharmacopoeia, 5th ed.; Monograph 01/2005:20910; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2005; pp. 519–530. [Google Scholar]
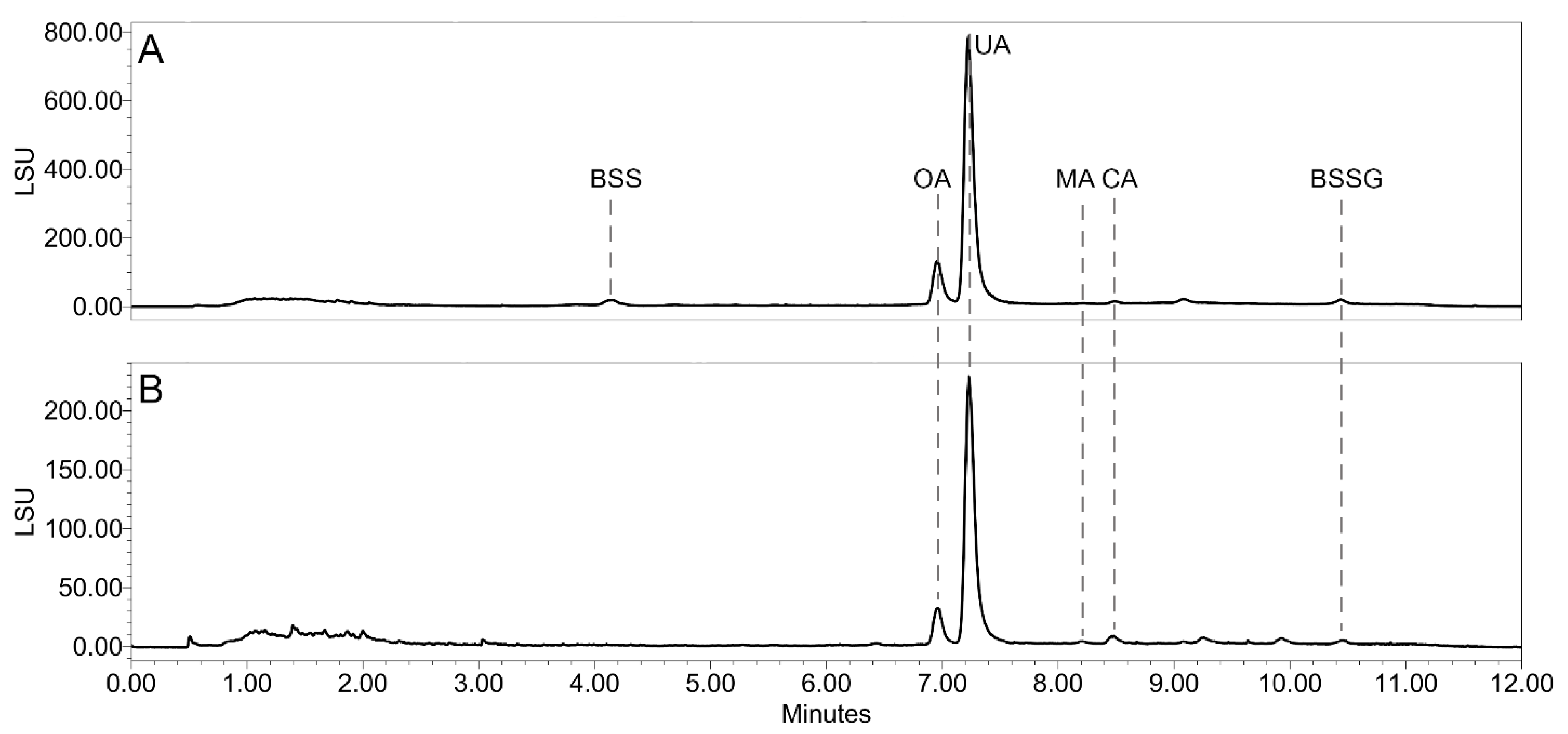
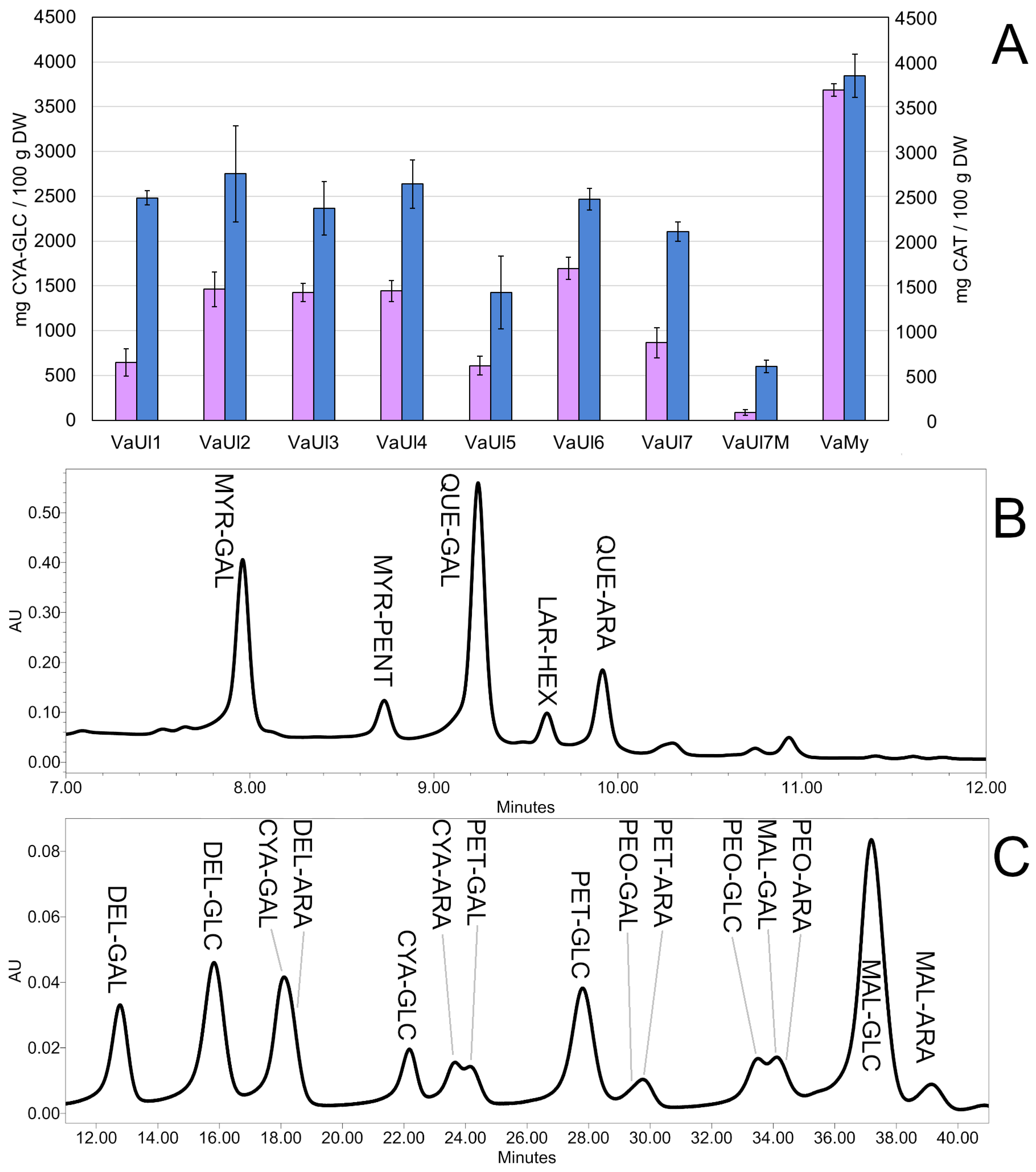
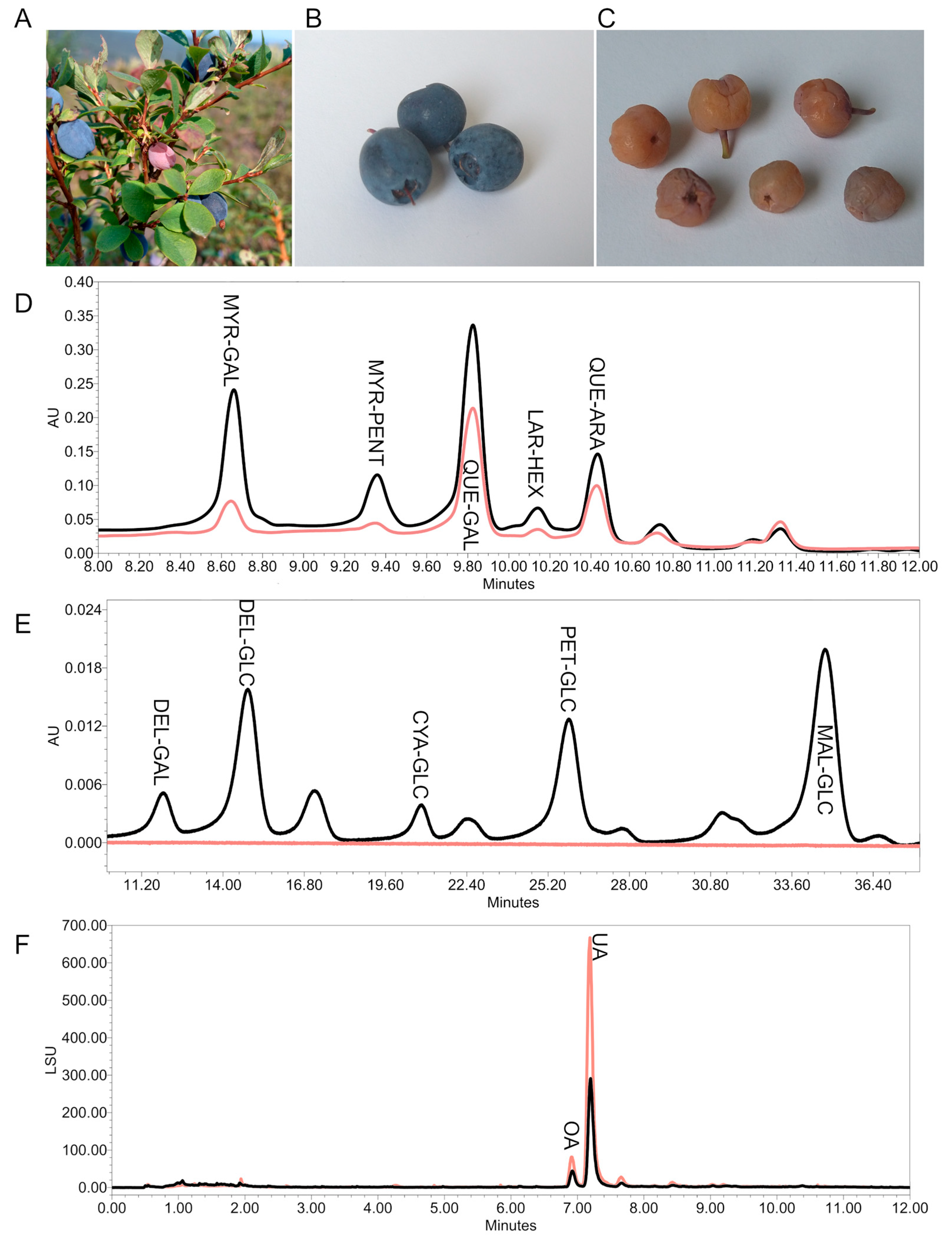
| Sample | TSP [mg CAT/100 g DW] ± SD | % of the Total Peak Area in a UHPLC-DAD Chromatogram at 370 nm | ||||
|---|---|---|---|---|---|---|
| MYR-GAL * | MYR-PENT | QUE-GAL * | LAR-HEX | QUE-ARA * | ||
| VaUl1 | 2481.67 ± 81.10 | 23.77 | 4.93 | 40.41 | 4.99 | 9.88 |
| VaUl2 | 2752.08 ± 537.12 | 23.74 | 4.30 | 42.89 | 4.24 | 11.47 |
| VaUl3 | 2365.47 ± 297.73 | 17.58 | 5.59 | 46.24 | 5.47 | 10.50 |
| VaUl4 | 2636.52 ± 271.80 | 22.90 | 5.18 | 43.61 | 4.34 | 10.66 |
| VaUl5 | 1424.59 ± 404.73 | 20.62 | 5.93 | 42.77 | 6.51 | 10.15 |
| VaUl6 | 2468.18 ± 120.70 | 25.80 | 5.36 | 37.88 | 4.32 | 11.09 |
| VaUl7 | 2103.39 ± 107.23 | 23.17 | 9.12 | 34.22 | 3.88 | 13.27 |
| VaUl7M | 600.13 ± 72.01 | 9.87 | 2.85 | 38.47 | 2.22 | 16.59 |
| VaMy | 3843.57 ± 242.50 | 7.15 | n.d. | 34.69 | n.d. | 5.12 |
| Sample | TMA [mg CYA-GLC/100 g DW] ± SD | % of the Total Peak Area in a UHPLC-DAD Chromatogram at 530 nm | |||||
|---|---|---|---|---|---|---|---|
| DEL-GAL | DEL-GLC * | CYA-GLC * | PET-GLC * | MAL-GLC * | MAL-ARA | ||
| VaUl1 | 646.93 ± 153.16 | 3.94 | 12.93 | 3.05 | 13.48 | 30.52 | 4.56 |
| VaUl2 | 1461.70 ± 193.73 | 8.98 | 10.95 | 2.87 | 10.34 | 31.20 | 4.13 |
| VaUl3 | 1426.07 ± 102.27 | 6.23 | 10.48 | 2.28 | 11.17 | 30.91 | 5.28 |
| VaUl4 | 1442.22 ± 119.67 | 5.74 | 11.06 | 2.12 | 11.52 | 30.84 | 6.08 |
| VaUl5 | 609.40 ± 106.71 | 6.77 | 12.66 | 2.87 | 13.14 | 33.45 | 3.25 |
| VaUl6 | 1694.76 ± 124.79 | 8.54 | 14.16 | 4.43 | 12.36 | 29.22 | 2.24 |
| VaUl7 | 864.23 ± 170.54 | 6.27 | 21.06 | 4.32 | 18.11 | 30.91 | 1.20 |
| VaUl7M | 85.61 ± 33.10 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| VaMy | 3684.15 ± 70.67 | 12.79 | 13.49 | 10.42 | 10.19 | 9.38 | 1.77 |
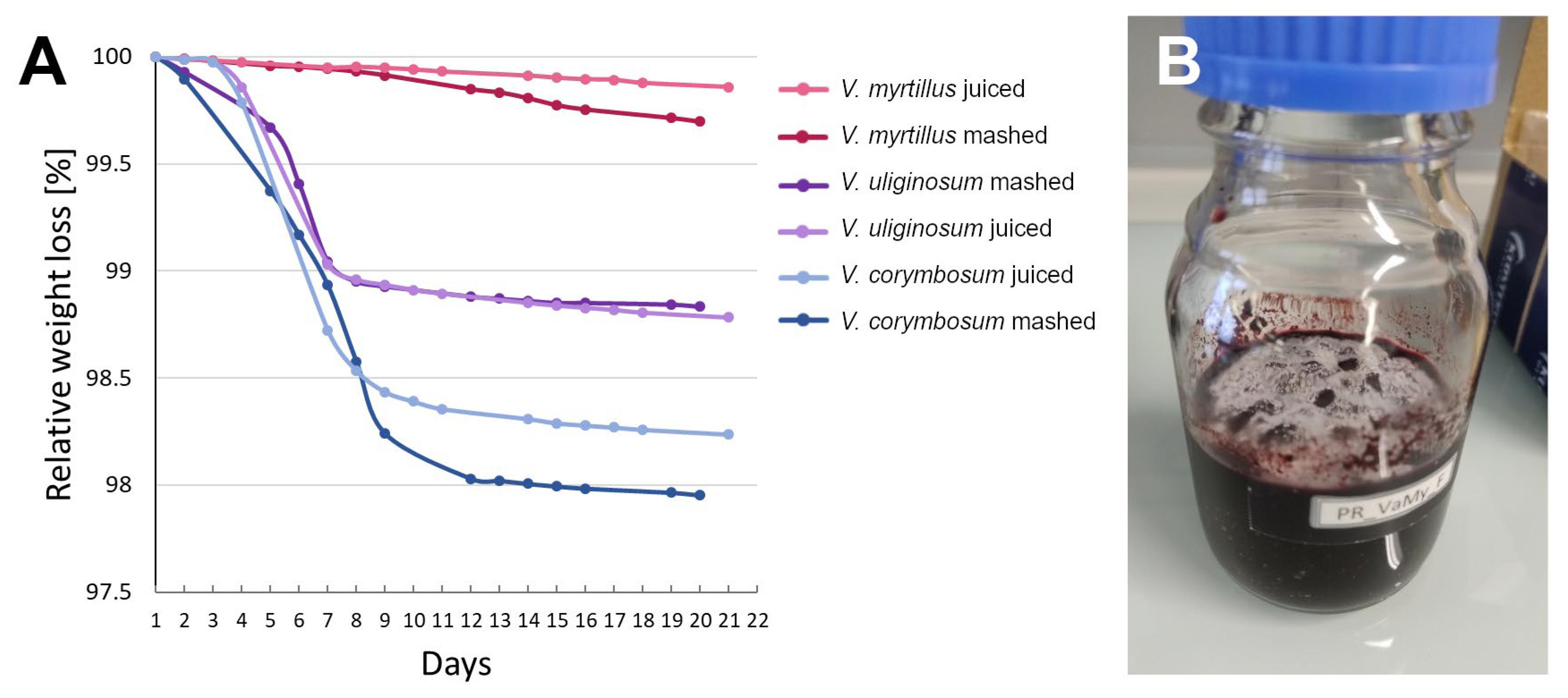
| Species | Sample n = 1 | pH | °Brix | ABV [%] ± SD | TSP [mg CAT/mL] ± SD | TMA [mg CYA-GLC/mL] ± SD |
|---|---|---|---|---|---|---|
| V. uliginosum | before | 2.93 | 8 | - | 3054.07 ± 130.66 | 628.49 ± 23.28 |
| mashed | 2.91 | 5 | 4.8 ± 0.4 | 5035.25 ± 86.40 | 415.49 ± 23.67 | |
| juiced | 2.96 | 5 | 5.8 ± 0.2 | 2922.82 ± 84.55 | 460.97 ± 34.94 | |
| V. corymbosum | before | 2.91 | 14 | - | 269.78 ± 1.02 | 55.56 ± 13.54 |
| mashed | 2.97 | 4.8 | 13.2 ± 0.4 | 809.08 ± 36.20 | 74.43 ± 10.95 | |
| juiced | 2.92 | 4.2 | 10.4 ± 0.8 | 788.40 ± 8.20 | 63.17 ± 10.75 | |
| V. myrtillus | before | 2.96 | 10 | - | 3305.37 ± 15.78 | 1280.76 ± 63.46 |
| mashed | 2.83 | 9 | 1.75 ± 0.25 | 3882.62 ± 64.68 | 450.03 ± 83.17 | |
| juiced | 2.95 | 10.2 | 2.6 ± 0.6 | 3592.13 ± 205.16 | 960.32 ± 75.46 |
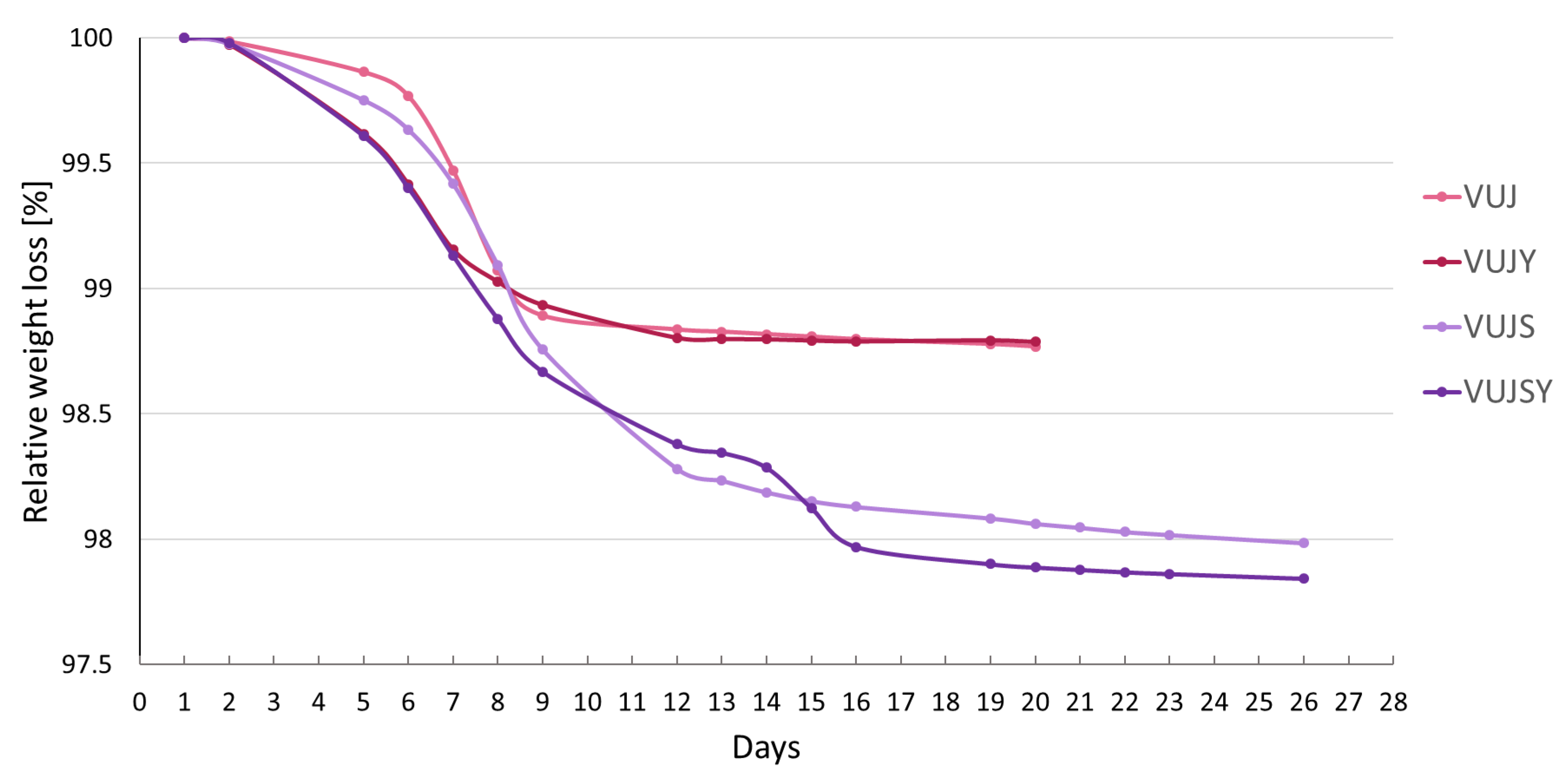
| Sample n = 2 | pH | °Brix | ABV [%] ± SD | TSP [mg CAT/mL] ± SD | TMA [mg CYA-GLC/mL] ± SD |
|---|---|---|---|---|---|
| before | 2.93 | 8 (VUJ and VUJY) 16 (VUJS and VUJSY) | - | 3054.07 ± 130.66 | 628.49 ± 23.28 |
| VUJ | 2.84 | 5 | 5.2 ± 0.28 | 2692.98 ± 136.57 | 419.24 ± 29.14 |
| VUJS | 2.86 | 9.2 | 8.2 ± 0.2 | 3522.15 ± 308.59 | 447.41 ± 44.44 |
| VUJY | 2.81 | 4.8 | 7.3 ± 2.83 | 3219.84 ± 172.98 | 472.75 ± 38.70 |
| VUJSY | 2.95 | 8.2 | 8.2 ± 1.82 | 3611.41 ± 270.35 | 597.30 ± 145.88 |
| Site Characteristics | Collection Date | Voucher Specimen | ||||||
|---|---|---|---|---|---|---|---|---|
| Abbr. | Country | GPS Coordinates | Site Name | Elevation [m a.s.l.] | Biotope | Relevant Plants Present | ||
| VaUl1 1 | Austria | N 47.4591 E 12.3709 | Schwarzsee, Kitzbühel | 780 | Peat bog by an alpine lake | Andromeda polifolia L. | 08.09.2020 | BBE_048 |
| VaUl2 | Austria | N 47.2933 E 11.7618 | Kleiner Gamsstein | 1815 | Alpine blanket bog | - | 11.08.2022 | BBE_058 |
| VaUl3 | Slovakia | N 49.4294 E 19.4996 | Klin bog | 610 | Peat bog | A. polifolia, Rhododendron tomentosum | 02.09.2020 | BBE_006 |
| VaUl4 | Czechia | N 48.8539 E 14.8352 | Kočičí bláto | 460 | Dried peat bog | R. tomentosum | 23.08.2020 | BBE_036 |
| VaUl5 2 | Estonia | N 58.2564 E 27.0559 | Võnnu | 50 | Clearing in pine forest | - | 16.07.2023 | BBE_158 |
| VaUl6 | Norway | N 69.6332 E 18.9950 | Fløya, Tromsø | 450 | Tundra heath | - | 11.09.2023 | BBE_096 |
| VaUl7 | Alaska, USA | N 69.9906 W 147.7928 | Old Murphy Dome Road | 530 | Clearing in spruce forest | R. tomentosum | 27.07.2023 | BBE_088 |
| VaUl7M 3 | Alaska, USA | N 69.9906 W 147.7928 | Old Murphy Dome Road | 530 | Clearing in spruce forest | R. tomentosum | 27.07.2023 | BBE_088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaneková, Z.; Redl, M.; Fischer, L.; Ortmayr, K.; Jaakola, L.; Rollinger, J.M. The Bog Bilberry Enigma: A Phytochemical and Ethnopharmacological Analysis of Vaccinium uliginosum L. Fruits in Regard to Their Alleged Toxicity. Plants 2025, 14, 2645. https://doi.org/10.3390/plants14172645
Vaneková Z, Redl M, Fischer L, Ortmayr K, Jaakola L, Rollinger JM. The Bog Bilberry Enigma: A Phytochemical and Ethnopharmacological Analysis of Vaccinium uliginosum L. Fruits in Regard to Their Alleged Toxicity. Plants. 2025; 14(17):2645. https://doi.org/10.3390/plants14172645
Chicago/Turabian StyleVaneková, Zuzana, Martina Redl, Lorenz Fischer, Karin Ortmayr, Laura Jaakola, and Judith M. Rollinger. 2025. "The Bog Bilberry Enigma: A Phytochemical and Ethnopharmacological Analysis of Vaccinium uliginosum L. Fruits in Regard to Their Alleged Toxicity" Plants 14, no. 17: 2645. https://doi.org/10.3390/plants14172645
APA StyleVaneková, Z., Redl, M., Fischer, L., Ortmayr, K., Jaakola, L., & Rollinger, J. M. (2025). The Bog Bilberry Enigma: A Phytochemical and Ethnopharmacological Analysis of Vaccinium uliginosum L. Fruits in Regard to Their Alleged Toxicity. Plants, 14(17), 2645. https://doi.org/10.3390/plants14172645






