Vasodilatory Effect of n-Butanol Extract from Sanguisorba officinalis L. and Its Mechanism
Abstract
1. Introduction
2. Results
2.1. Results of HPLC-DAD and UPLC-MS for the Identification of Diyu Components
2.2. Effect of BSO on NO Production in HUVECs
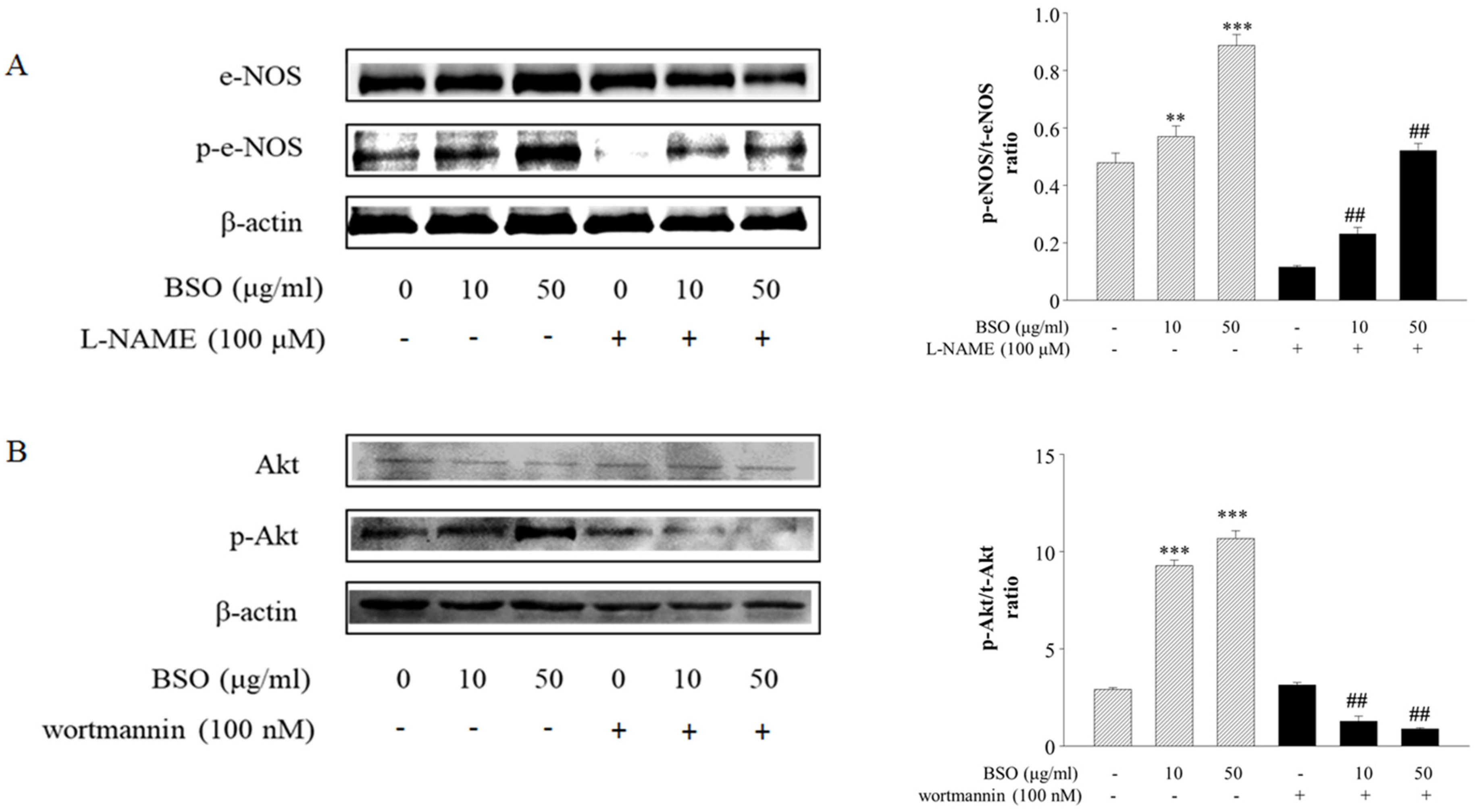
2.3. Effects of BSO-Induced eNOS and Akt Phosphorylation in HUVECs
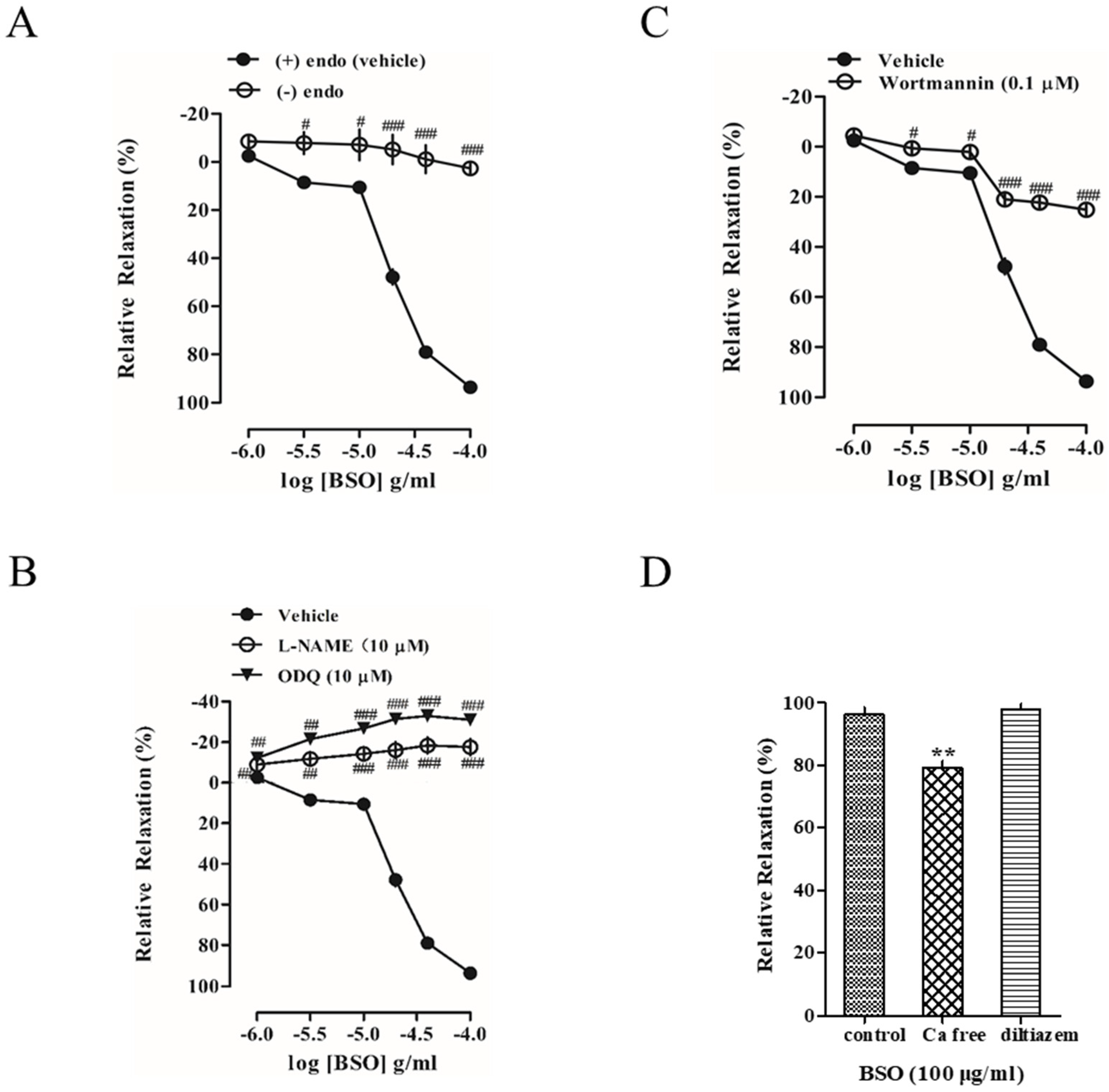
2.4. Effect of BSO on Tension in Endothelium-Intact and Endothelium-Removed Vascular Rings
2.5. Effects of L-NAME and ODQ on BSO-Induced Vasodilatory Effects
2.6. Effect of Akt on BSO-Induced Vasodilatory Effect
2.7. Effect of Extracellular Ca2+ on BSO-Induced Vasodilatory Effect
2.8. Effects of SOCE Modulators on BSO-Induced Vasodilatory Effects
2.9. Effect of Potassium Channels on BSO-Induced Vasodilatory Effects
2.10. Effects of Cyclooxygenase Inhibitor on BSO-Induced Vasodilatory Effect
2.11. Effects of Muscarinic and Adrenergic Receptor Inhibitors on BSO-Induced Vasodilator Effect
2.12. Effect of BSO on the Intensity of the Preconstructed Vasculature of PE in Calcium-Free Fluid
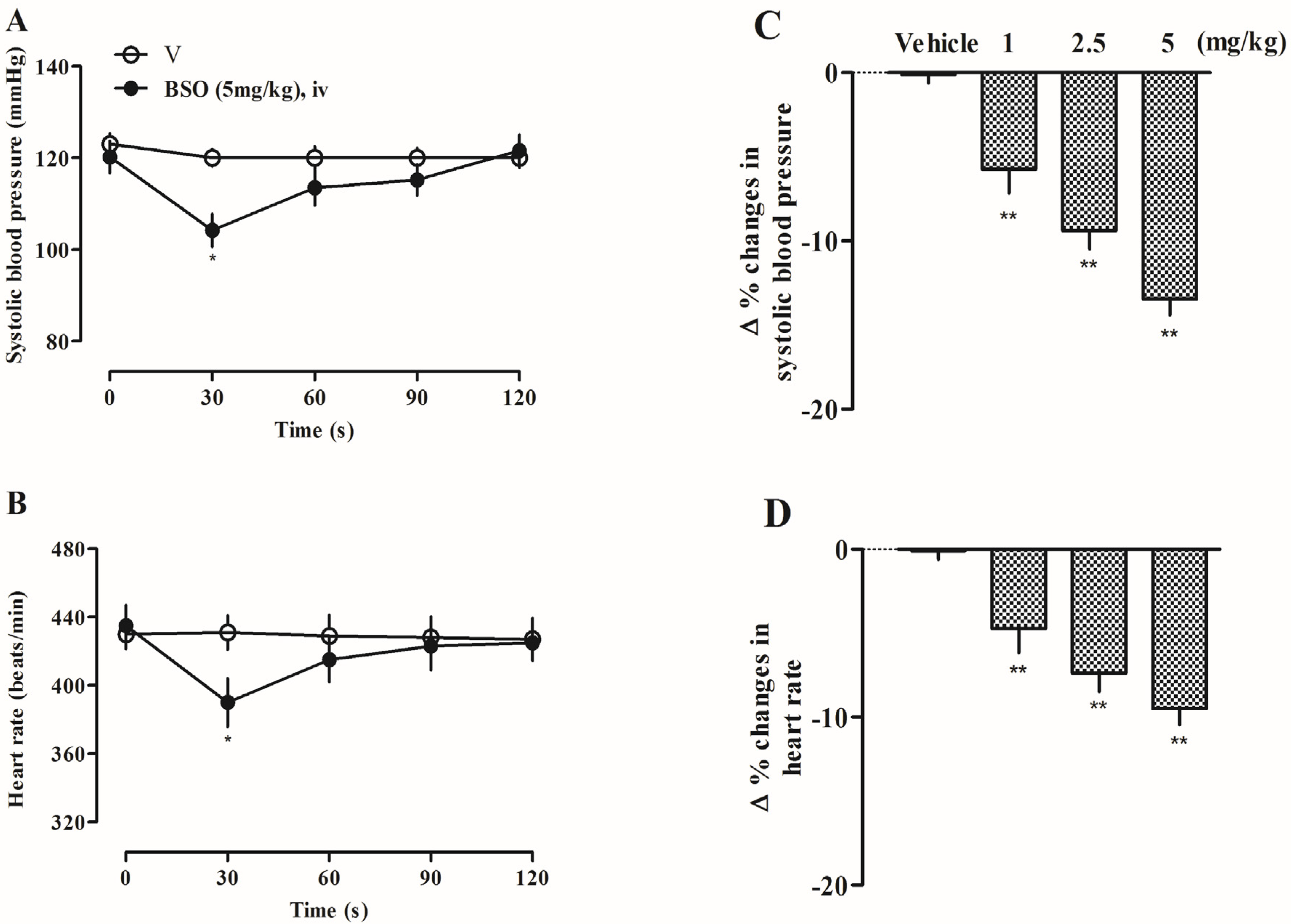
2.13. Effects of BSO on Systolic Blood Pressure and Heart Rate in SD Rats
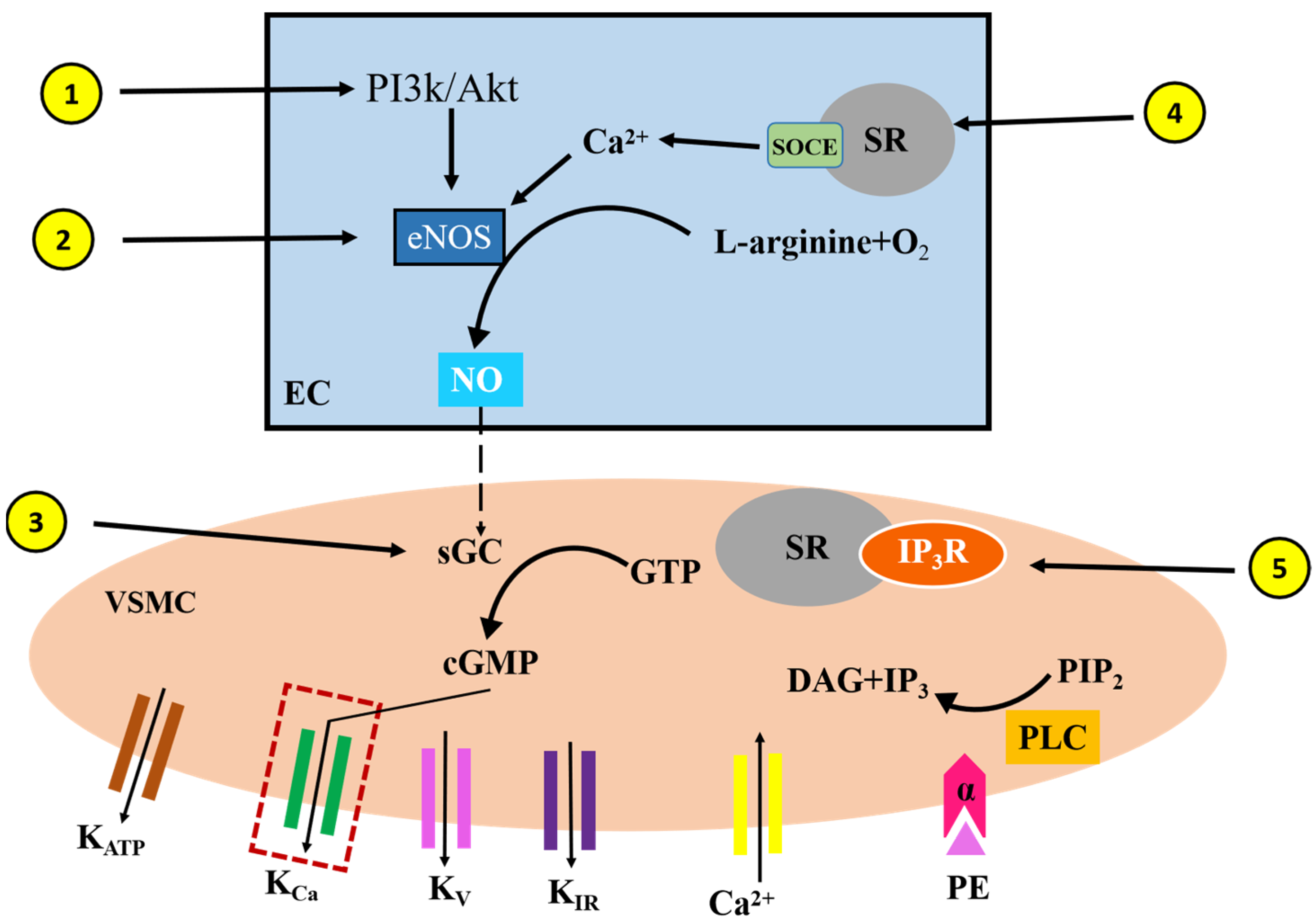
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of n-Butanol Extract of Diyu (BSO)
4.3. Experimental Animals
4.4. HPLC-DAD and UPLC-MS Analysis
4.5. Determination of NO Production in Vascular Endothelial Cells
4.6. Western Blot to Detect Changes in p-eNOS and p-Akt Protein Levels
4.7. Preparation of Isolated Thoracic Aortic Rings
4.8. Determination of Vascular Ring Tone in Isolated Rat Thoracic Aorta
4.9. Measurement of Rat Closing Blood Pressure and Heart Rate
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bkaily, G.; Jacques, D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Abukhodair, A.W.; Abukhudair, W.; Alqarni, M.S. The Effects of L-Arginine in Hypertensive Patients: A Literature Review. Cureus 2021, 13, e20485. [Google Scholar] [CrossRef] [PubMed]
- Piotti, A.; Novelli, D.; Meessen, J.M.T.A.; Ferlicca, D.; Coppolecchia, S.; Marino, A.; Salati, G.; Savioli, M.; Grasselli, G.; Bellani, G.; et al. Endothelial Damage in Septic Shock Patients as Evidenced by Circulating Syndecan-1, Sphingosine-1-Phosphate and Soluble VE-Cadherin: A Substudy of ALBIOS. Crit. Care 2021, 25, 113. [Google Scholar] [CrossRef]
- Knott, A.B.; Bossy-Wetzel, E. Nitric Oxide in Health and Disease of the Nervous System. Antioxid. Redox Signal. 2009, 11, 541–554. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Chakraborti, A.; Gulati, K.; Ray, A. Possible Role of Nitric Oxide (NO) in the Regulation of Gender-Related Differences in Stress-Induced Anxiogenesis in Rats. Nitric Oxide 2014, 43, 74–80. [Google Scholar] [CrossRef]
- Michel, T.; Vanhoutte, P.M. Cellular Signaling and NO Production. Pflügers Arch. 2010, 459, 807–816. [Google Scholar] [CrossRef]
- Morgado, M.; Cairrão, E.; Santos-Silva, A.J.; Verde, I. Cyclic Nucleotide-Dependent Relaxation Pathways in Vascular Smooth Muscle. Cell Mol. Life Sci. 2012, 69, 247–266. [Google Scholar] [CrossRef]
- Nava, E.; Llorens, S. The Local Regulation of Vascular Function: From an Inside–Outside to an Outside–Inside Model. Front. Physiol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Saqib, F.; Ali, A.; Ahmedah, H.T.; Irimie, C.A.; Toma, S.I.; Popovici, B.E.; Moga, M.; Irimie, M. Cardioprotective, Hypotensive and Toxicological Studies of Populus Ciliata (Wall. ex Royle). Biomed. Pharmacother. 2021, 142, 112065. [Google Scholar]
- Halim, M.A.; Gillberg, L.; Boghus, S.; Sundbom, M.; Karlbom, U.; Webb, D.L.; Hellstm, P.M. Nitric Oxide Regulation of Migrating Motor Complex: Randomized Trial of N(G)-Monomethyl-L-Arginine Effects in Relation to Muscarinic and Serotonergic Receptor Blockade. Acta Physiol. 2015, 215, 105–118. [Google Scholar]
- Nam, Y.; Kim, M.; Erdenebileg, S.; Cha, K.H.; Ryu, D.H.; Kim, H.Y.; Lee, S.H.; Jung, J.H.; Nho, C.W. Sanguisorba officinalis L. Ameliorates Hepatic Steatosis and Fibrosis by Modulating Oxidative Stress, Fatty Acid Oxidation, and Gut Microbiota in CDAHFD-Induced Mice. Nutrients 2023, 15, 3779. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, S.Y.; Lee, Y.; Lee, T.K.; Kim, J.E.; Kim, T.H.; Kang, I.J. Standardized Sanguisorba officinalis L. Extract Inhibits Adipogenesis and Promotes Thermogenesis via Reducing Oxidative Stress. Antioxidants 2023, 12, 882. [Google Scholar] [CrossRef]
- Jung, J.; Shin, S.; Park, J.; Lee, K.; Choi, H.Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients 2023, 15, 4510. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, A.; Giangiobbe, S.; Bonora, E.; Hemminki, K.; Försti, A.; Bandapalli, O.R. Whole Genome Sequencing of Familial Non-Medullary Thyroid Cancer Identifies Germline Alterations in MAPK/ERK and PI3K/AKT Signaling Pathways. Biomolecules 2019, 9, 605. [Google Scholar] [CrossRef]
- Ohkita, M.; Tawa, M.; Kitada, K.; Matsumura, Y. Pathophysiological Roles of Endothelin Receptors in Cardiovascular Diseases. J. Pharmacol. Sci. 2012, 119, 302–313. [Google Scholar]
- Wang, Y.; Wang, S.; Wier, W.G.; Zhang, Q.; Jiang, H.; Li, Q.; Chen, S.; Tian, Z.; Li, Y.; Yu, X.; et al. Exercise Improves the Dilatation Function of Mesenteric Arteries in Postmyocardial Infarction Rats via a PI3K/Akt/eNOS Pathway-Mediated Mechanism. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H2097–H2106. [Google Scholar]
- Sun, Y.Y.; Su, X.H.; Jin, J.Y.; Zhou, Z.Q.; Sun, S.S.; Wen, J.F.; Kang, D.G.; Lee, H.S.; Cho, K.W.; Jin, S.N. Rumex acetosa L. Induces Vasorelaxation in Rat Aorta via Activation of PI3-Kinase/Akt-and Ca(2+)-eNOS-NO Signaling in Endothelial Cells. J. Physiol. Pharmacol. 2015, 66, 907–915. [Google Scholar]
- Kim, H.Y.; Oh, H.; Li, X.; Cho, K.W.; Kang, D.G.; Lee, H.S. Ethanol Extract of Seeds of Oenothera Odorata Induces Vasorelaxation via Endothelium-Dependent NO-cGMP Signaling through Activation of Akt-eNOS-sGC Pathway. J. Ethnopharmacol. 2011, 133, 315–323. [Google Scholar]
- Mason, R.P.; Corbalan, J.J.; Jacob, R.F.; Dawoud, H.; Malinski, T. Atorvastatin Enhanced Nitric Oxide Release and Reduced Blood Pressure, Nitroxidative Stress and Rantes Levels in Hypertensive Rats with Diabetes. J. Physiol. Pharmacol. 2015, 66, 65–72. [Google Scholar] [PubMed]
- Zhao, L.Y.; Li, J.; Huang, X.Q.; Wang, G.H.; Lv, X.F.; Meng, W.F.; Chen, W.L.; Pang, J.Y.; Lin, Y.C.; Sun, H.S.; et al. Xyloketal B Exerts Antihypertensive Effect in Renovascular Hypertensive Rats via the NO-sGC-cGMP Pathway and Calcium Signaling. Acta Pharmacol. Sin. 2018, 39, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, S.; Tang, Y.; Zeng, C.; Cai, S. The Effect of A2E on the Ca2+-PKC Signaling Pathway in Human RPE Cells Exposed to Blue Light. J. Ophthalmol. 2022, 2022, 2233223. [Google Scholar] [CrossRef] [PubMed]
- Ampey, A.C.; Dahn, R.L.; Grummer, M.A.; Bird, I.M. Differential Control of Uterine Artery Endothelial Monolayer Integrity by TNF and VEGF Is Achieved through Multiple Mechanisms Operating Inside and Outside the Cell—Relevance to Preeclampsia. Mol. Cell Endocrinol. 2021, 534, 111368. [Google Scholar] [CrossRef]
- Neymotin, S.A.; McDougal, R.A.; Sherif, M.A.; Fall, C.P.; Hines, M.L.; Lytton, W.W. Neuronal Calcium Wave Propagation Varies with Changes in Endoplasmic Reticulum Parameters: A Computer Model. Neural Comput. 2015, 7, 898–924. [Google Scholar] [CrossRef]
- Xia, C.; Liu, C.; Ren, S.; Cai, Y.; Zhang, Q.; Xia, C. Potassium Channels, Tumorigenesis and Targeted Drugs. Biomed. Pharmacother. 2023, 162, 114673. [Google Scholar] [CrossRef]
- Barrera, P.; Skorka, C.; Boktor, M.; Dave, N.; Jimenez, V. A Novel Calcium-Activated Potassium Channel Controls Membrane Potential and Intracellular pH in Trypanosoma Cruzi. Front. Cell Infect. Microbiol. 2020, 9, 464. [Google Scholar] [CrossRef]
- Wrzosek, A.; Gałecka, S.; Žochowska, M.; Olszewska, A.; Kulawiak, B. Alternative Targets for Modulators of Mitochondrial Potassium Channels. Molecules 2022, 27, 299. [Google Scholar] [CrossRef]
- Morita, H.; Zaima, K.; Koga, I.; Saito, A.; Tamamoto, H.; Okazaki, H.; Kaneda, T.; Hashimoto, T.; Asakawa, Y. Vasorelaxant Effects of Macrocyclicbis (Bibenzyls) from Liverworts. Bioorg. Med. Chem. 2011, 19, 4051. [Google Scholar] [CrossRef]
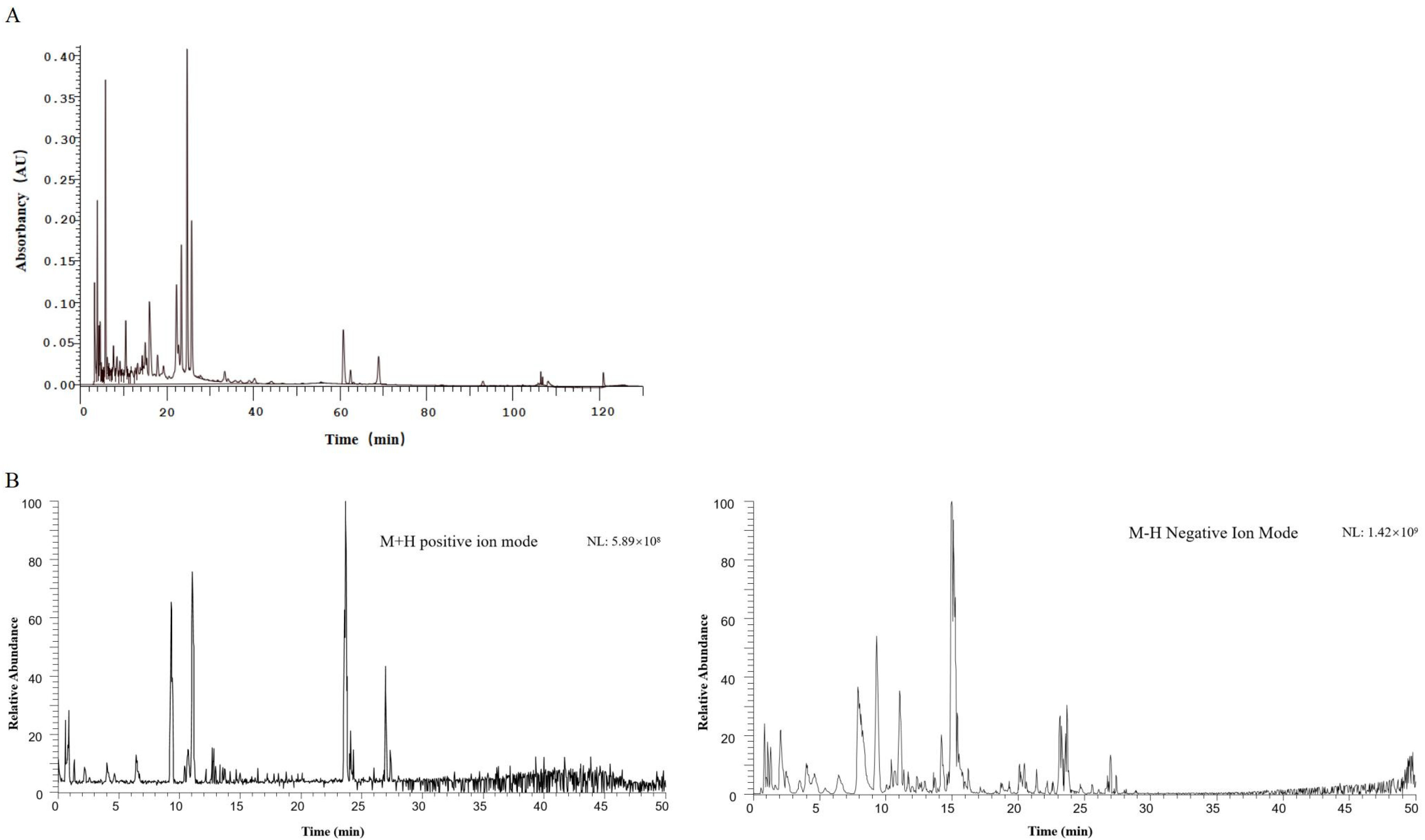


| Comp. | tR (min) | Calc. MW | Formula | ppm | Mode | MS2 | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 0.67 | 174.11032 | C6H14N4O2 | −7.77 | [M+H]+ | 116.07001, 72.93724, 70.06530, 55.93479 | (2S)-2-amino-5-(diaminomethylideneamino)pentanoic acid |
| 2 | 0.676 | 103.06291 | C4H9NO2 | −4.02 | [M+H]+ | 87.04392, 69.03365, 60.08109, 58.06548 | (2R)-2-aminobutanoic acid |
| 3 | 0.814 | 180.06376 | C6H12O6 | 2.09 | [M−H]− | 113.07639, 72.99217, 71.01303, 59.01295 | (3R,4S,5R,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
| 4 | 0.835 | 115.06268 | C5H9NO2 | −5.59 | [M+H]+ | 71.06862, 70.06528, 68.04958, 58.06544 | (2R)-pyrrolidine-2-carboxylic acid |
| 5 | 0.861 | 342.11776 | C12H22O11 | 4.53 | [M−H]− | 113.02408, 101.02386, 89.02372, 71.01302, 59.01295 | α-D-Glucopyranosyl-α-D-glucopyranosid |
| 6 | 0.903 | 290.12013 | C10H18N4O6 | −8.62 | [M+H]+ | 175.23624, 130.08569, 112.08637, 70.06529 | (2S)-2-[[N′-[(4S)-4-amino-4-carboxybutyl]carbamimidoyl]amino]butanedioic acid |
| 7 | 1.94 | 267.09462 | C10H13N5O4 | −7.98 | [M+H]+ | 153.01714, 136.06078, 119.03455, 92.02407, 79.01784 | (2R,3R,4S,5R)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol |
| 8 | 2.479 | 170.02162 | C7H6O5 | 0.57 | [M−H]− | 134.72574, 125.02406, 95.01319, 67.01817 | 3,4,5-trihydroxybenzoic acid |
| 9 | 5.934 | 264.18163 | C15H24N2O2 | −8.14 | [M+H]+ | 205.13214, 148.11089, 136.11107, 120.08011, 91.05392 | (1R,2R,9S,17S)-13-oxido-7-aza-13-azoniatetracyclo [7.7.1.02,7.013,17]heptadecan-6-one |
| 10 | 11.074 | 290.08044 | C15H14O6 | 4.83 | [M−H]− | 203.07140, 159.04532, 123.04472, 109.02902, 83.01304 | (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol |
| 11 | 11.28 | 484.08754 | C20H20O14 | 4.61 | [M−H]− | 271.04730, 211.02528, 169.01433, 125.02402, 107.01330 | 1,6-Bis-O-(3,4,5-trihydroxybenzoyl)hexopyranose |
| 12 | 12.307 | 458.08107 | C22H18O11 | −8.38 | [M+H]+ | 289.06839, 153.01712, 139.03793, 79.01785 | [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate |
| 13 | 12.908 | 450.11827 | C21H22O11 | 4.57 | [M−H]− | 269.04678, 259.06226, 151.00346, 125.02404 | (2R,3R)-7-[(2R,4R,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one |
| 14 | 13.401 | 197.11893 | C14H15N | −7.71 | [M+H]+ | 131.97343, 97.00727, 91.05400, 72.93723 | N-benzyl-1-phenylmethanamine |
| 15 | 13.725 | 304.05587 | C15H12O7 | −8 | [M+H]+ | 231.06354, 153.01709, 123.04327, 65.03883 | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one |
| 16 | 14.211 | 198.05324 | C9H10O5 | 2.08 | [M−H]− | 166.99828, 123.00845, 95.01302, 67.01812 | 4-hydroxy-3,5-dimethoxybenzoic acid |
| 17 | 14.566 | 788.11073 | C34H28O22 | 4.45 | [M−H]− | 635.09155, 465.06940, 313.05804, 169.01428 | 1,2,3,6-Tetrakis-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranose |
| 18 | 14.965 | 302.00383 | C14H6O8 | −8.06 | [M+H]+ | 257.00586, 201.01660, 145.01725, 89.03841 | 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo [6.6.2.04,16.011,15]hexadeca-1(15),4,6,8(16),11,13-hexaene-3,10-dione |
| 19 | 16.239 | 428.17057 | C20H28O10 | 5.43 | [M+H]+ | 133.06563, 89.02371, 71.01303, 59.01295 | (2E)-3-Phenyl-2-propen-1-yl 6-O-β-D-arabinofuranosyl-β-D-glucopyranoside |
| 20 | 17.549 | 274.08183 | C15H14O5 | −8.35 | [M+H]+ | 107.04868, 95.04886, 79.05424, 53.03899 | 3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one |
| 21 | 24.764 | 405.34206 | C22H47NO5 | −8.3 | [M+H]+ | 300.28726, 256.26157, 135.00594, 70.06528 | (2S,3S,5R,10R,12S,14S,15R,16R)-2-Amino-12,16-dimethyl-3,5,10,14,15-icosanepentol |
| 22 | 24.955 | 255.25405 | C16H33NO | −8.5 | [M+H]+ | 212.23561, 93.03658, 88.07549, 53.00249 | hexadecanamide |
| 23 | 49 | 390.27373 | C24H38O4 | −8.41 | [M+H]+ | 167.03261, 149.02219, 121.02760, 65.03879 | dioctyl benzene-1,2-dicarboxylate |
| Q-Orbitrap MS Conditions Polarity | Positive and Negative |
|---|---|
| AGC target | 2 × 105 |
| Sheath gas flow rate | 35 mL/min |
| Sweep gas flow rate | 10 mL/min |
| Spray voltage | 3.50 kV |
| Capillary temperature | 320 °C |
| S-lens RF level | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Li, J.; Wang, S.; Jin, E.; Min, J.Z.; Li, G.; Lee, Y.J.; Cao, L. Vasodilatory Effect of n-Butanol Extract from Sanguisorba officinalis L. and Its Mechanism. Plants 2025, 14, 1095. https://doi.org/10.3390/plants14071095
Jin H, Li J, Wang S, Jin E, Min JZ, Li G, Lee YJ, Cao L. Vasodilatory Effect of n-Butanol Extract from Sanguisorba officinalis L. and Its Mechanism. Plants. 2025; 14(7):1095. https://doi.org/10.3390/plants14071095
Chicago/Turabian StyleJin, Hangyu, Jiaze Li, Shuyuan Wang, Enyi Jin, Jun Zhe Min, Gao Li, Yun Jung Lee, and Lihua Cao. 2025. "Vasodilatory Effect of n-Butanol Extract from Sanguisorba officinalis L. and Its Mechanism" Plants 14, no. 7: 1095. https://doi.org/10.3390/plants14071095
APA StyleJin, H., Li, J., Wang, S., Jin, E., Min, J. Z., Li, G., Lee, Y. J., & Cao, L. (2025). Vasodilatory Effect of n-Butanol Extract from Sanguisorba officinalis L. and Its Mechanism. Plants, 14(7), 1095. https://doi.org/10.3390/plants14071095







