Ethylene-Mediated Drought Tolerance in the Critically Endangered Artocarpus nanchuanensis: Insights from Physiological and Transcriptomic Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Conditions
2.2. Scanning Electron Microscopy of Leaf Stomata
2.3. Determination of Leaf Relative Water Content (RWC)
2.4. Determination of Antioxidant Enzyme Activities
2.5. Determination of Soluble Sugar, Soluble Protein, Proline (Pro), Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA) Contents
2.6. Determination of Photosynthetic Pigment Content
2.7. Determination of Ethylene and Its Biosynthetic Enzyme Concentrations
2.8. Transcriptome Sequencing and Data Analysis
2.9. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
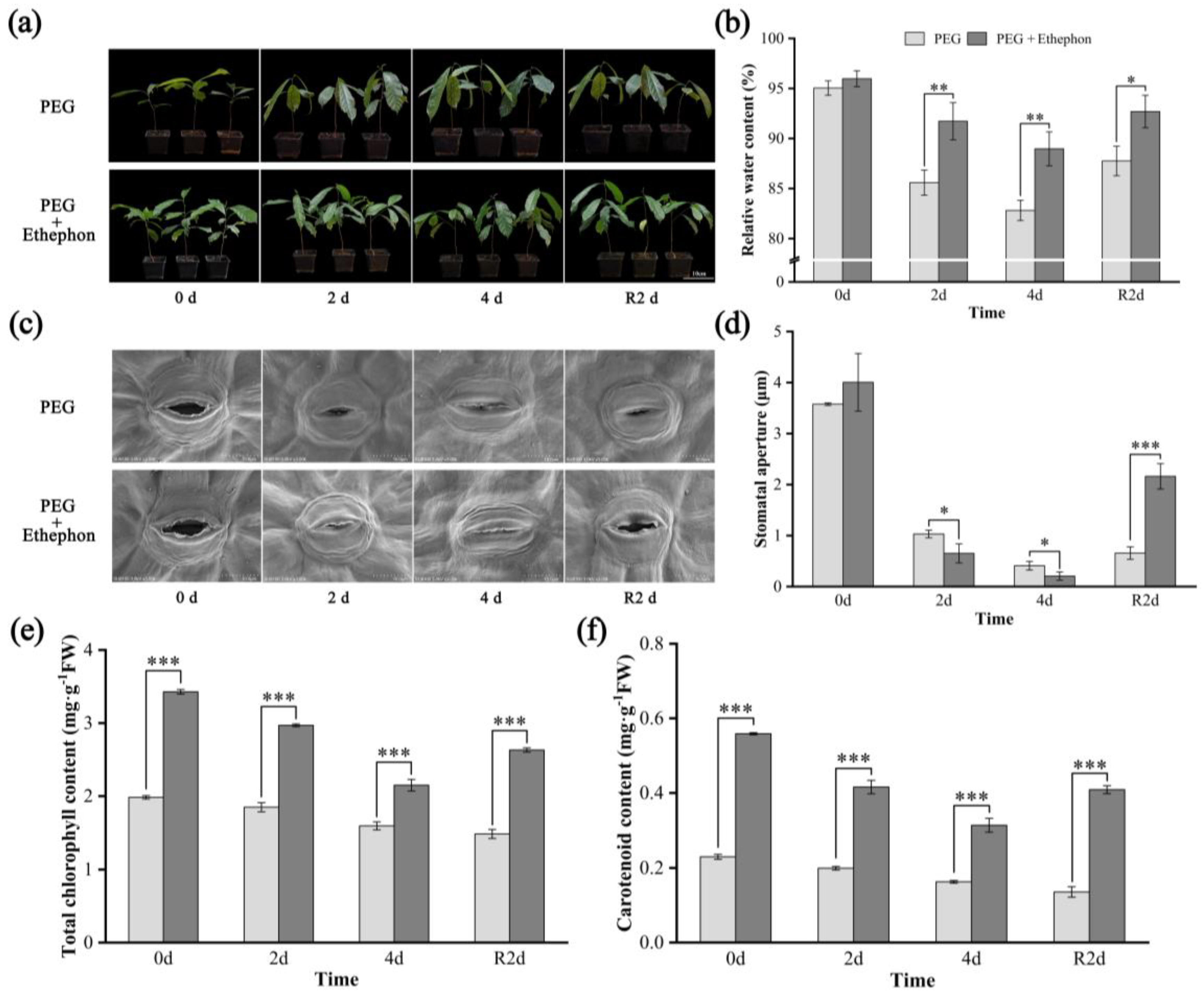
3.1. Morphological Characteristics
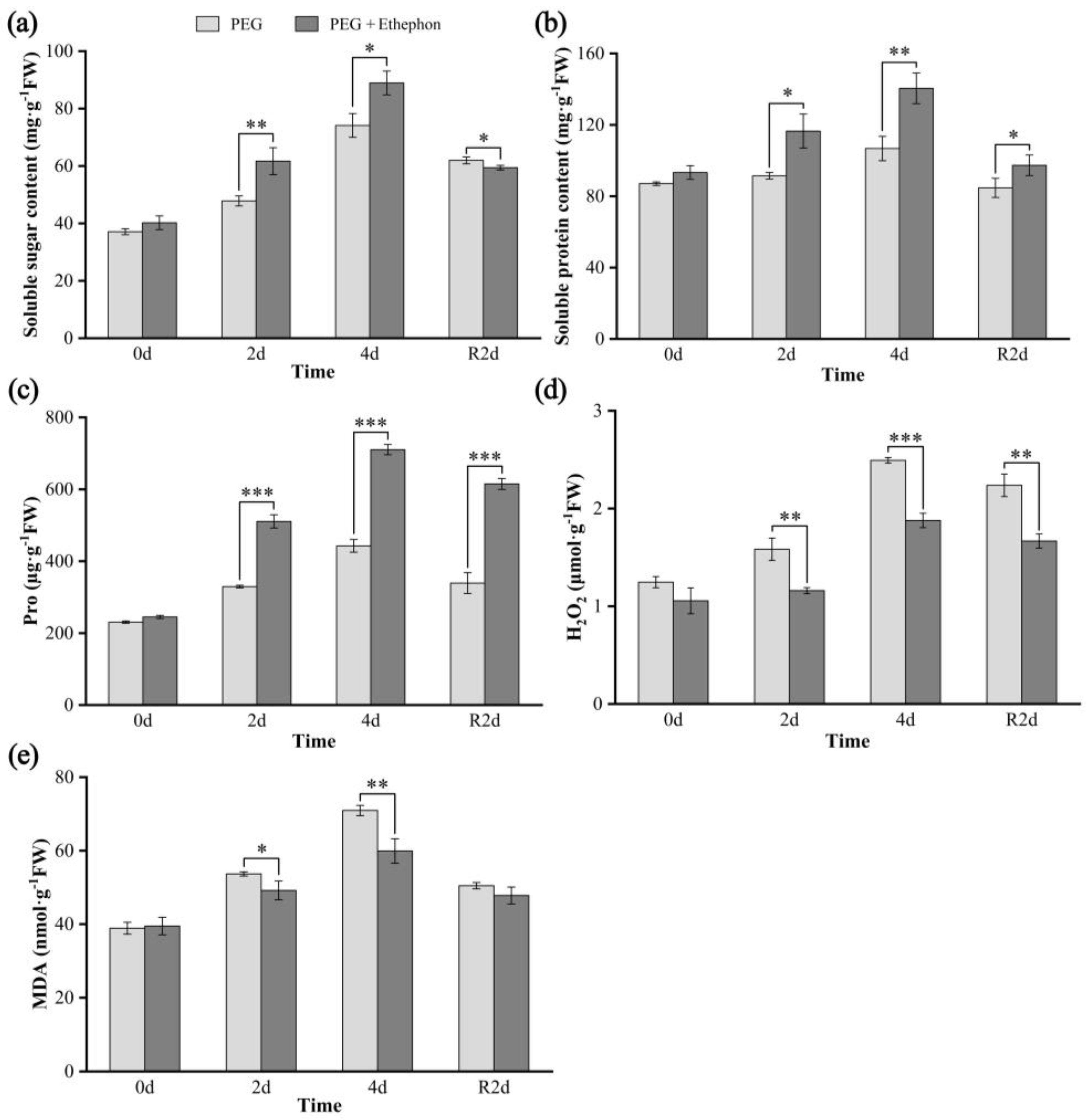
3.2. Osmolyte, H2O2, and MDA Contents
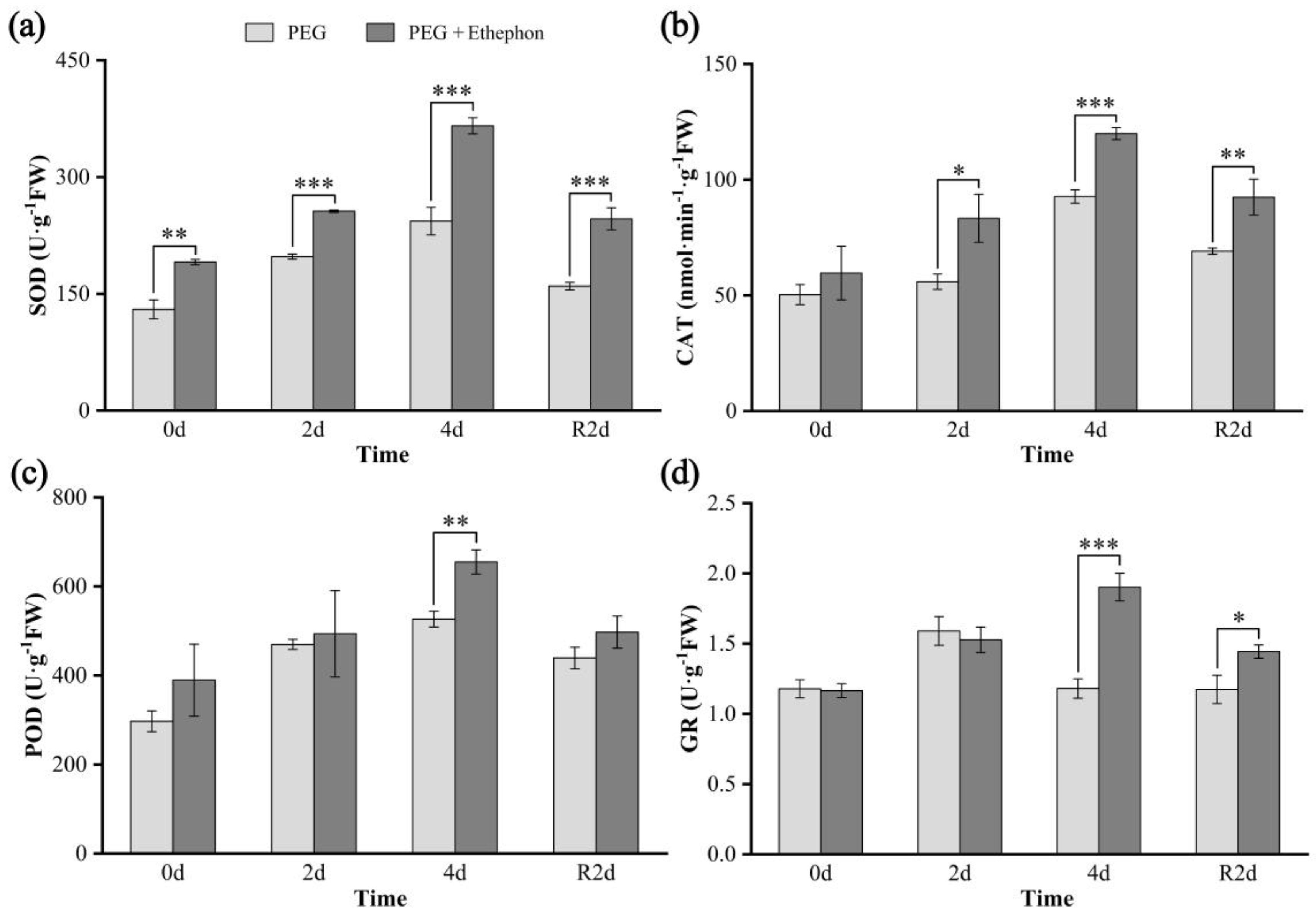
3.3. Antioxidant Enzyme Activities
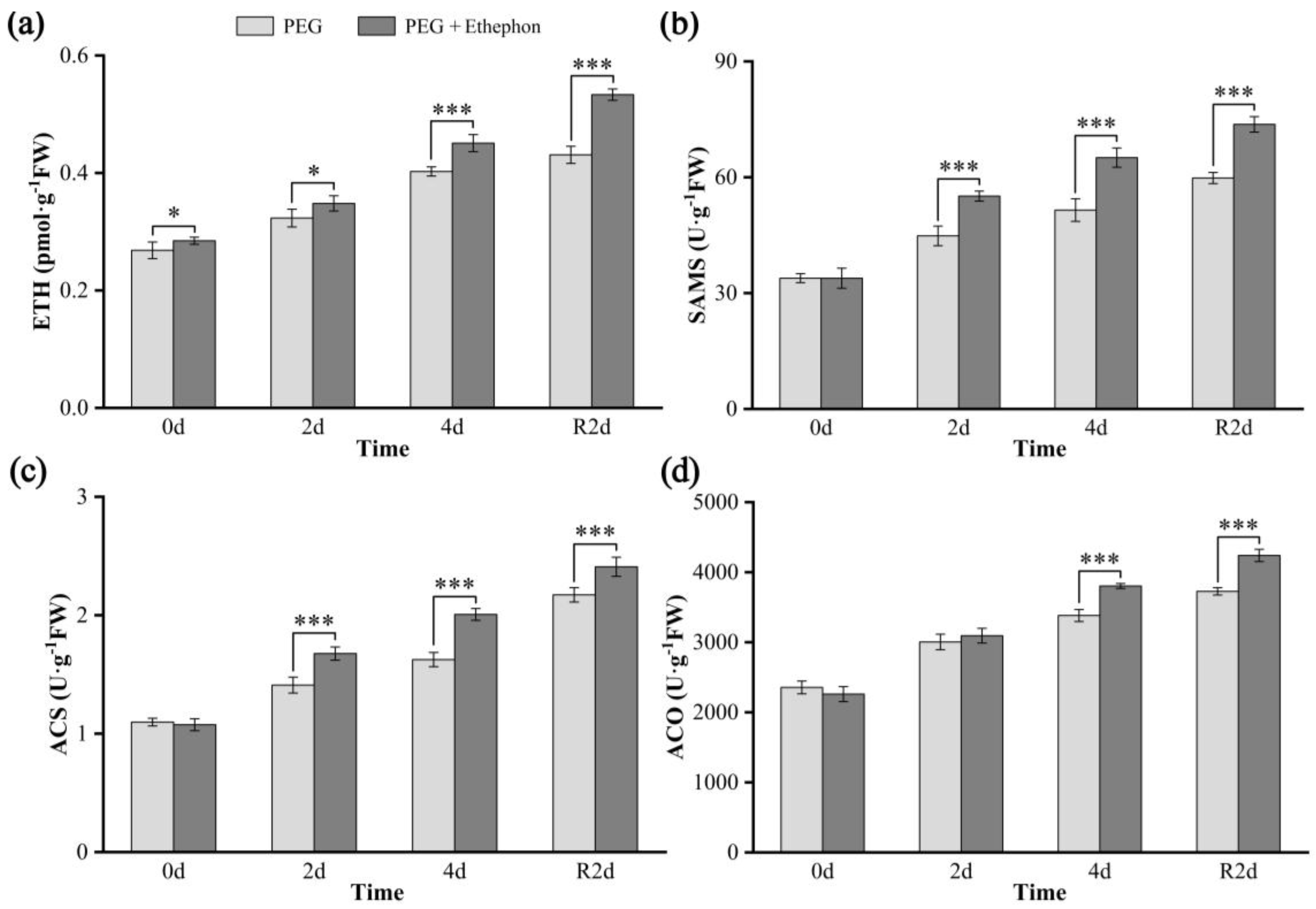
3.4. Concentrations of Ethylene and Its Key Biosynthetic Enzymes
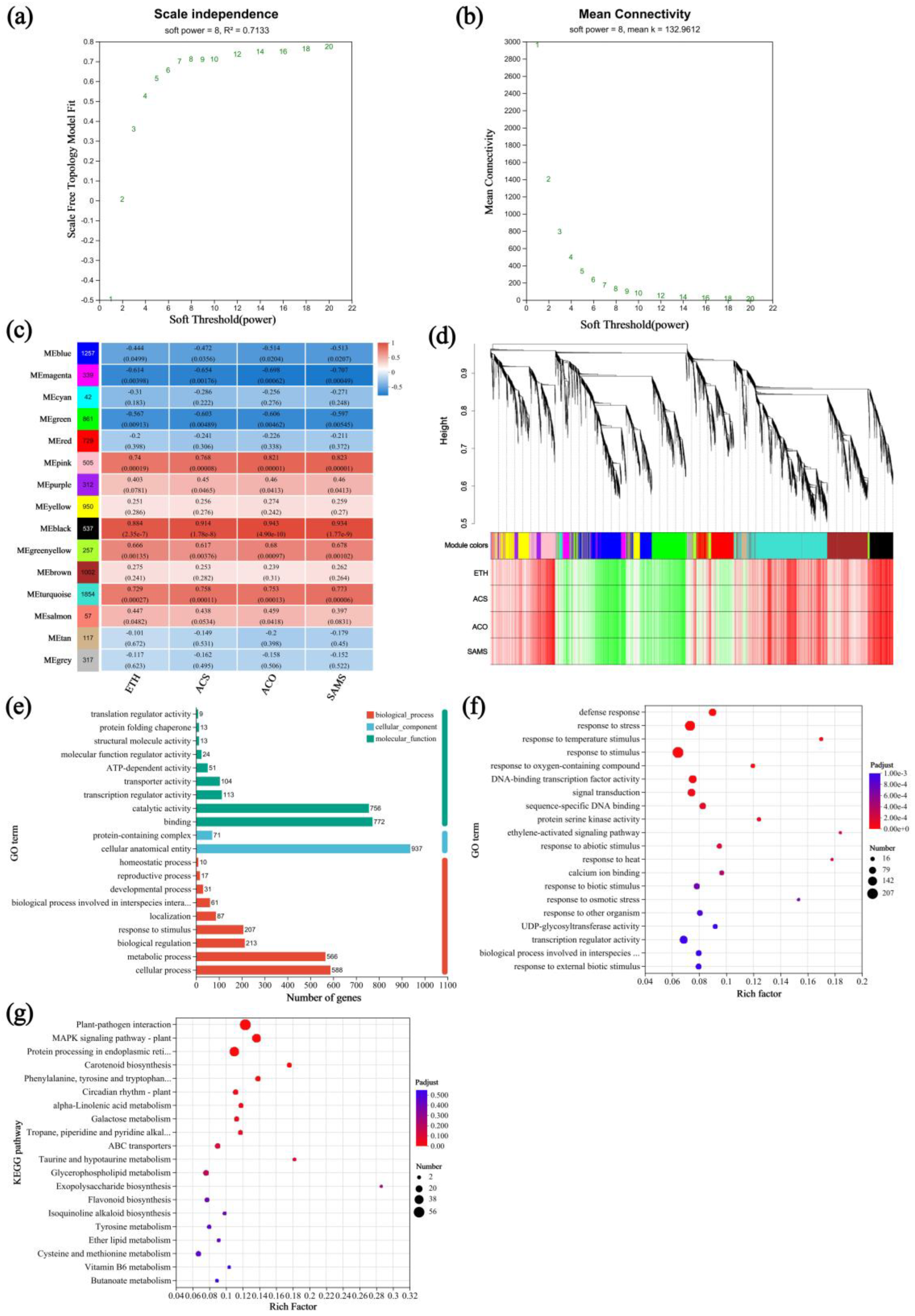
3.5. Expression Patterns and Functional Annotation of DEGs
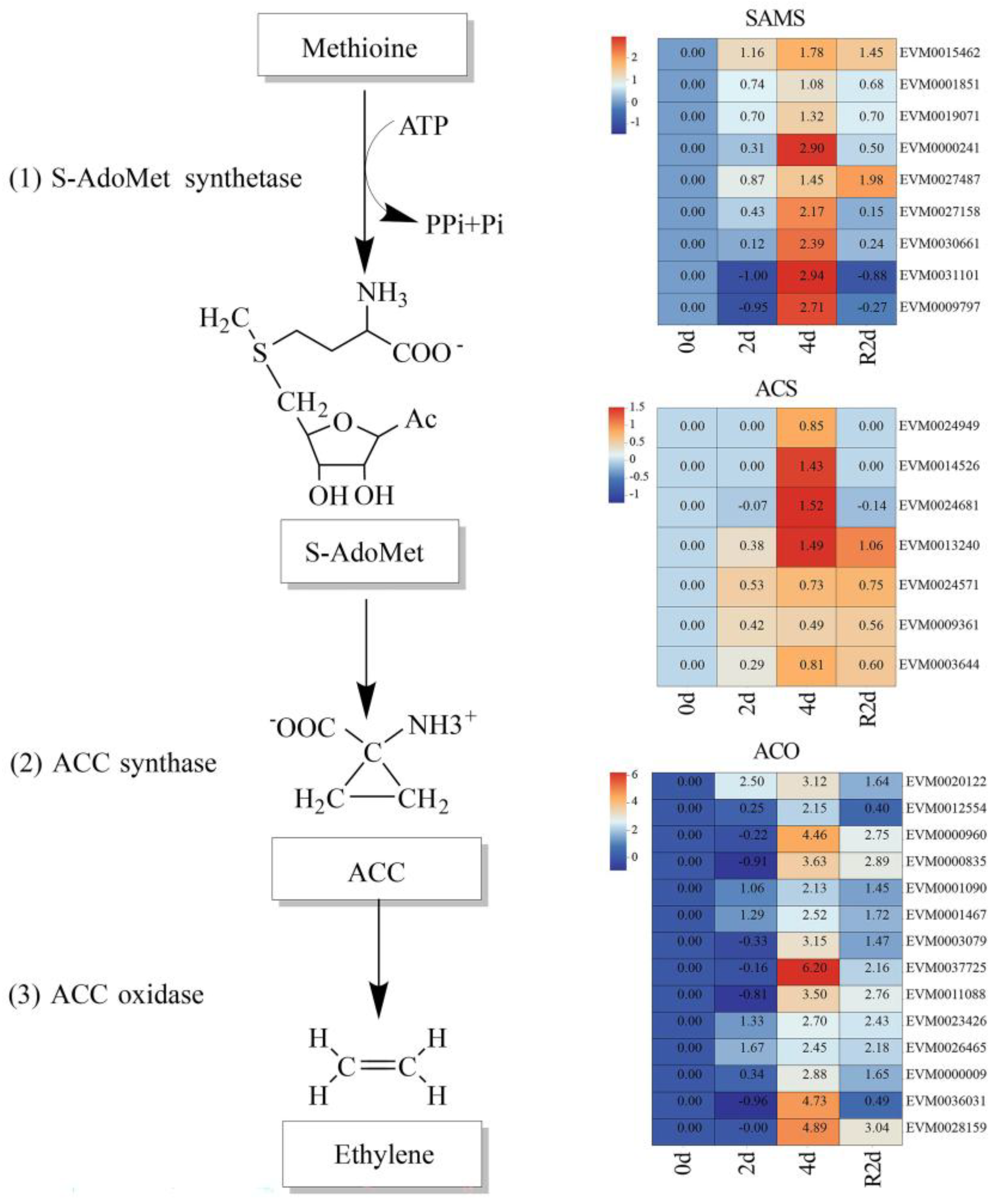
3.6. Expression Profiles of the Ethylene Biosynthesis Pathway and Its Key Genes
3.7. Expression and Screening of Drought-Resistance Genes
4. Discussion
4.1. Phenotypic Responses of A. nanchuanensis to Drought Stress
4.2. Effects of Drought on Osmolytes and ROS Markers in A. nanchuanensis
4.3. Effects of Drought on the Antioxidant System of A. nanchuanensis
4.4. Effects of Drought on Ethylene Production and Key Biosynthetic Enzymes in A. nanchuanensis
4.5. Effects of Drought on the Transcriptome of A. nanchuanensis
5. Conclusions
6. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenstein, M. Plant breeding: Discovery in a dry spell. Nature 2013, 501, 7–9. [Google Scholar] [CrossRef]
- Yi, J.; Li, H.J.; Zhao, Y.; Shao, M.A.; Zhang, H.L.; Liu, M.X. Assessing soil water balance to optimize irrigation schedules of flood-irrigated maize fields with different cultivation histories in the arid region. Agric. Water Manag. 2022, 265, 107543. [Google Scholar] [CrossRef]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.M.; Rambal, S. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: Revision of current hypotheses? Glob. Change Biol. 2002, 8, 999–1017. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, X.; Zou, Z.; Dong, J.; Qin, Y.; Doughty, R.B.; Li, B. Gainers and losers of surface and terrestrial water resources in China during 1989–2016. Nat. Commun. 2020, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene signaling in rice and Arabidopsis: New regulators and mechanisms. J. Integr. Plant Biol. 2021, 63, 102–125. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Zhao, G.J.; Xu, X.L.; Ma, H.C.; Huang, D.; Yang, J.J.; Shu, Y.Y.; Ge, L.; Ping, P. Causes of difficulties with natural regeneration of a Bombax ceiba population in Hong-He dry-hot valleys (DHV). Acta Ecol. Sin. 2016, 36, 1342–1351. [Google Scholar] [CrossRef]
- Öztürk, L.; Küfrevioğlu, Ö.I.; Demir, Y. In vivo and in vitro effects of ethephon on some oxidative enzymes in spinach leaves. Acta Physiol. Plant. 2007, 30, 105–110. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Xie, Y.; Wang, Y.; Duan, L.; Zhang, M.; Li, Z. Ethephon improved drought tolerance in maize seedlings by modulating cuticular wax biosynthesis and membrane stability. J. Plant Physiol. 2017, 214, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981, 68, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.A.L.V.; Cozijnsen, T.; Danhash, N.; Wit, P.J.D. Induction of tomato stress protein mRNAs by ethephon,2,6-dichloroisonicotinic acid and salicylate. Plant Mol. Biol. 1995, 27, 1205–1213. [Google Scholar] [CrossRef]
- Ali, S.; Tahir, S.; Hassan, S.S.; Lu, M.; Wang, X.; Quyen, L.T.Q.; Zhang, W.; Chen, S. The Role of Phytohormones in Mediating Drought Stress Responses in Populus Species. Int. J. Mol. Sci. 2025, 26, 3884. [Google Scholar] [CrossRef]
- Qin, H.N.; Yang, Y.; Dong, S.Y.; He, Q.; Jia, Y.; Zhao, L.N.; Yu, S.X.; Liu, H.Y.; Liu, B.; Yan, Y.H. Threatened species list of China’s higher plants. Biodiv. Sci. 2007, 25, 696–744. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, X.S. Some new taxa of Moraceae in China. Acta Bot. Yunnanica 1989, 11, 24–34. [Google Scholar]
- Liu, W.; Jiang, L.; Liu, B.; Liu, R.; Xiao, Z. Monitoring the evolution process of karst desertification and quantifying its drivers in the karst area of Southwest China. Environ. Sci. Pollut. Res. 2023, 30, 123259–123273. [Google Scholar] [CrossRef]
- Mo, P.L. The Effects of Foliar Spray of Ethephon and Salicylic Acid on Drought Resistance in Different Sugarcane Varieties. Bachelor’s thesis, Guangxi University, Nanning, China, 2003; p. 6. [Google Scholar]
- Mohorović, P.; Geldhof, B.; Holsteens, K.; Rinia, M.; Ceusters, J.; Van de Poel, B. Effect of ethylene pretreatment on tomato plant responses to salt, drought, and waterlogging stress. Plant Direct. 2023, 7, e548. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.A. Exogenous abscisic acid (ABA) and jasmonate (JA) promote metabolic regulation in Jacarandá-Pardo (Machaerium villosum Vog.) seedlings under PEG-induced water deficit. Plant Stress 2023, 9, 100174. [Google Scholar] [CrossRef]
- Sun, M.; Peng, F.; Xiao, Y.; Yu, W.; Zhang, Y.; Gao, H. Exogenous phosphatidylcholine treatment alleviates drought stress and maintains the integrity of root cell membranes in peach. Sci. Hortic. 2020, 259, 108821. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Li, C.; Hao, Y.; Ma, F.; Shu, H. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, K.; Deng-Wang, M.Y.; Dai, C.C. The mechanism of ethylene signaling induced by endophytic fungus Gilmaniella sp. AL12 mediating sesquiterpenoids biosynthesis in Atractylodes lancea. Front. Plant Sci. 2016, 7, 361. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Y.; Zhang, M.; Du, G.; Wang, J. Selection and evaluation of candidate reference genes for quantitative Real-Time PCR in aboveground tissues and drought conditions in Rhododendron delavayi. Front. Genet. 2022, 13, 876482. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Zhan, J.; Thakare, D.; Ma, C.; Lloyd, A.; Nixon, N.M.; Arakaki, A.M.; Burnett, W.J.; Logan, K.O.; Wang, D.; Wang, X.; et al. RNA sequencing of laser-capture microdissected compartments of the maize kernel identifies regulatory modules associated with endosperm cell differentiation. Plant Cell 2015, 27, 513–531. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Akram, Z.M.; Libutti, A.; Rivelli, R.A. Drought Stress in Quinoa: Effects, Responsive Mechanisms, and Management through Biochar Amended Soil: A Review. Agriculture 2024, 14, 1418. [Google Scholar] [CrossRef]
- Haworth, M.; Carli, A.; Montesano, V.; Killi, D.; Fabbri, A.; Balestrini, R.; Centritto, M. Cannabis sativa genotypes with larger leaf areas have higher potential to adjust stomatal size and density in response to water deficit: The effect on stomatal conductance and physiological stomatal behaviour. Plant Stress 2024, 14, 100649. [Google Scholar] [CrossRef]
- Li, H.J.; Yang, M.; Zhao, C.F.; Wang, Y.F.; Zhang, R.H. Physiological and proteomic analyses revealed the response mechanisms of two different drought-resistant maize varieties. BMC Plant Biol. 2021, 21, 513. [Google Scholar] [CrossRef]
- Monteoliva, I.M.; Guzzo, C.M.; Posada, A.G. Breeding for drought tolerance by monitoring chlorophyll content. Gene Technol. 2021, 10, 10–35248. [Google Scholar]
- Uarrota, V.G.; Stefen, D.L.V.; Leolato, L.S.; Gindri, D.M.; Nerling, D. Revisiting carotenoids and their role in plant stress responses: From biosynthesis to plant signaling mechanisms during stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 207–232. [Google Scholar]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.J.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Cai, L.; Abbey, L.; MacDonald, M. Changes in endogenous carotenoids, flavonoids, and phenolics of drought-stressed broccoli seedlings after ascorbic acid preconditioning. Plants 2024, 13, 3513. [Google Scholar] [CrossRef]
- Zakar, T.; Laczko-Dobos, H.; Toth, T.N.; Gombos, Z. Carotenoids Assist in Cyanobacterial Photosystem II Assembly and Function. Front. Plant Sci. 2016, 10, 295. [Google Scholar] [CrossRef]
- Gajendramurthy, H.G.; Brijesh, S.S.; Natarajamurthy, S.; Mohammed, A.; Kalegowda, N.; Arakere Chunchegowda, U. Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: A review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2025, 138, 2337–2343. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Luan, Q.F.; Jiang, J.M.; Li, Y.J. Prediction and utilization of malondialdehyde in exotic pine under drought stress using near-infrared spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Bi, M.H.; Jiang, C.; Brodribb, T.; Yang, Y.J.; Yao, G.Q.; Jiang, H.; Fang, X.W. Ethylene constrains stomatal reopening in Fraxinus chinensis post moderate drought. Tree Physiol. 2023, 43, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Liu, X.D.; Yao, G.Q.; Liu, J.; Fang, X.W. Ethylene-mediated stomatal responses to dehydration and rehydration in seed plants. J. Exp. Bot. 2024, 75, 6719–6732. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.H.; Wu, J.Y. Research progress on leaf anatomical structures of plants under drought stress. Agric. Sci. Technol. 2016, 17, 4–7. [Google Scholar] [CrossRef]
- Pearce, D.W.; Millard, S.; Bray, D.F.; Rood, S.B. Stomatal characteristics of riparian poplar in a semiarid environment. Tree Physiol. 2006, 26, 211–218. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Valtat, A.; Abiven, S.; Studer, M.S.; Ong, R.; Schmid, B. The role of soluble sugars during drought in tropical tree seedlings with contrasting tolerances. J. Plant Ecol. 2020, 13, 389–397. [Google Scholar] [CrossRef]
- Caprioli, M.; Katholm, A.K.; Melone, G.; Ramlv, H.; Santo, N. Trehalose in desiccated rotifers: A comparison between a bdelloid and a monogonont species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 139, 527–532. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, Z.Q.; Zhao, Y.D.; Shen, W.J.; Chen, M.S.; Gao, H.Q.; He, J.M. Ethylene-induced stomatal closure is mediated via MKK1/3-MPK3/6 cascade to EIN2 and EIN3. J. Integr. Plant Biol. 2021, 63, 1324–1340. [Google Scholar] [CrossRef]
- Doron, S.; Roye, N.; Cristina, B.M.; Alex, C.; Hillel, F. MIZ1 regulates ECA1 to generate a slow, long-distance phloem-transmitted Ca2+ signal essential for root water tracking in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 8031–8036. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Novo, E.; Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 2008, 1, 5. [Google Scholar] [CrossRef]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Harshavardhan, V.T.; Wu, T.M.; Hong, C.Y. Glutathione Reductase and Abiotic Stress Tolerance in Plants; Springer: Cham, Switzerland, 2017; pp. 265–286. [Google Scholar] [CrossRef]
- Viktoriya, A.; Hamada, A.; Ivanina, V.; Petrova, A.S.; Anna, H.; Joachim, M.; Han, A.; Beemster, G.T.S. High antioxidant activity facilitates maintenance of cell division in leaves of drought tolerant maize hybrids. Front. Plant Sci. 2017, 8, 884. [Google Scholar] [CrossRef]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 3, 194. [Google Scholar] [CrossRef]
- Rina, M.; Sonia, P.; Joseph, R.; Yosepha, S.; Kira, R. Chilling-induced leaf abscission of Ixora coccinea plants. III. Enhancement by high light via increased oxidative processes. Physiol Plant. 2001, 113, 338–345. [Google Scholar] [CrossRef]
- Shanazari, M.; Golkar, P.; Mirmohammady Maibody, A.M. Effects of drought stress on some agronomic and bio-physiological traits of Trititicum aestivum, Triticale, and Tritipyrum genotypes. Arch. Agron. Soil Sci. 2018, 64, 2005–2018. [Google Scholar] [CrossRef]
- Pastroi, G.M.; Trippi, V.S. Oxidative stress induces high rate of glutathione reductase synthesis in drought-resistant maize strain. Plant Cell Physiol. 1992, 33, 957–961. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Saxena, D.C. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol. Plant. 1998, 41, 387–394. [Google Scholar] [CrossRef]
- Wang, X.; Oh, W.M.; Komatsu, S. Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses. J. Proteom. 2016, 114, 161–181. [Google Scholar] [CrossRef]
- Habibi, N.; Terada, N.; Sanada, A.; Kamata, A.; Koshio, K. Impact of limited irrigation on fruit quality and ethylene biosynthesis in tomato: A comprehensive analysis of physical, biochemical, and metabolomic traits. Plants 2005, 14, 406. [Google Scholar] [CrossRef]
- Wanjala, J.K.; Jawad, M.U.; Wei, Y.Y.; Gereziher, M.T.; Odongo, M.R.; Xu, Y.C.; Liu, F. Genome-wide characterization of the SAMS gene family in cotton unveils the putative role of GhSAMS2 in enhancing abiotic stress tolerance. Agronomy 2023, 13, 612. [Google Scholar] [CrossRef]
- Young, T.E.; Meeley, R.B.; Gallie, D.R. ACC synthase expression regulates leaf performance and drought tolerance in maize: ACC synthase regulates maize leaf performance. Plant J. 2004, 40, 813–825. [Google Scholar] [CrossRef]
- Htay, A.N.; Riza, J.C.; Hyunhee, K.; Xu, J.P.; Chung, M.Y.; Kil, K.C. Role of ethylene biosynthesis genes in the regulation of salt stress and drought stress tolerance in Petunia. Front. Plant Sci. 2022, 13, 844449. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Saify, S.; Iqbal, N.; Khan, N.A. The ethylene biosynthetic enzymes, 1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS) and acc oxidase (ACO): The less explored players in abiotic stress tolerance. Biomolecules 2024, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Geisler, M. HSP90 and co-chaperones: A multitaskers’ view on plant hormone biology. FEBS Lett. 2019, 593, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, P.; Tafreshi, S.A.H. Central role of 70-kDa heat shock protein in adaptation of plants to drought stress. Cell Stress Chaperones 2020, 25, 1071–1081. [Google Scholar] [CrossRef]
- Verma, A.K.; Tamadaddi, C.; Tak, Y.; Lal, S.S.; Cole, S.J.; Hines, J.K.; Sahi, C. The expanding world of plant J-domain proteins. CRC Crit. Rev. Plant Sci. 2019, 38, 382–400. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Iusem, N.D.; Morris, P.C. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol. 2007, 173, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef] [PubMed]
- Azoulay-Shemer, T.; Schulze, S.; Nissan-Roda, D.; Bosmans, K.; Shapira, O.; Weckwerth, P.; Zamora, O.; Yarmolinsky, D.; Trainin, T.; Kollist, H.; et al. A role for ethylene signaling and biosynthesis in regulating and accelerating CO2—And abscisic acid-mediated stomatal movements in Arabidopsis. New Phytol. 2023, 238, 2460–2475. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, Y.; Yang, F.; Yang, K.; Li, W.; Zhang, X.; Liu, W.; Deng, H. Ethylene-Mediated Drought Tolerance in the Critically Endangered Artocarpus nanchuanensis: Insights from Physiological and Transcriptomic Analyses. Plants 2025, 14, 2636. https://doi.org/10.3390/plants14172636
Zhang Z, Chen Y, Yang F, Yang K, Li W, Zhang X, Liu W, Deng H. Ethylene-Mediated Drought Tolerance in the Critically Endangered Artocarpus nanchuanensis: Insights from Physiological and Transcriptomic Analyses. Plants. 2025; 14(17):2636. https://doi.org/10.3390/plants14172636
Chicago/Turabian StyleZhang, Zhe, Yunli Chen, Fang Yang, Kunjian Yang, Wenqiao Li, Xiao Zhang, Wanhong Liu, and Hongping Deng. 2025. "Ethylene-Mediated Drought Tolerance in the Critically Endangered Artocarpus nanchuanensis: Insights from Physiological and Transcriptomic Analyses" Plants 14, no. 17: 2636. https://doi.org/10.3390/plants14172636
APA StyleZhang, Z., Chen, Y., Yang, F., Yang, K., Li, W., Zhang, X., Liu, W., & Deng, H. (2025). Ethylene-Mediated Drought Tolerance in the Critically Endangered Artocarpus nanchuanensis: Insights from Physiological and Transcriptomic Analyses. Plants, 14(17), 2636. https://doi.org/10.3390/plants14172636





