Microorganisms as Potential Accelerators of Speed Breeding: Mechanisms and Knowledge Gaps
Abstract
1. Introduction
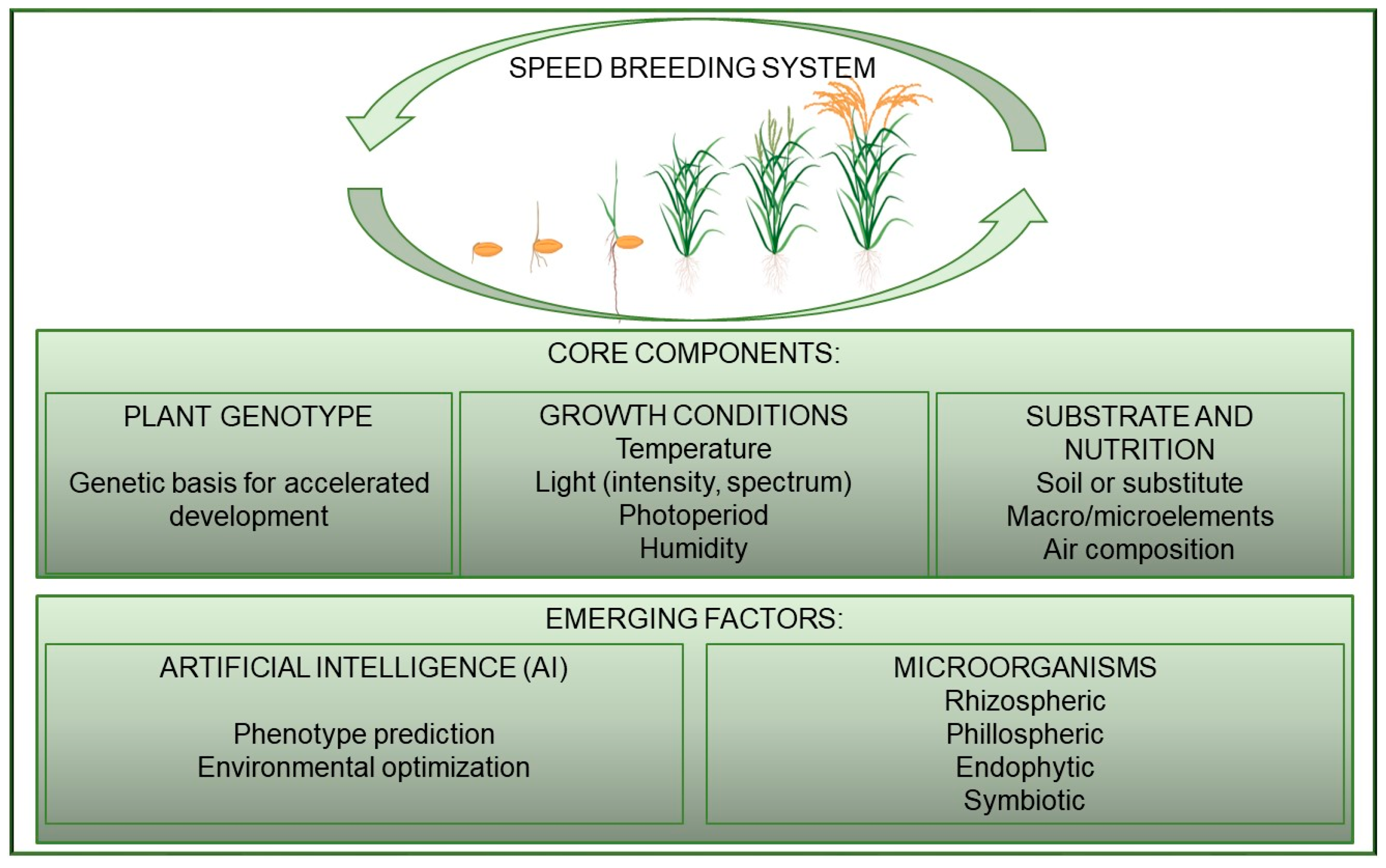
2. Principal Aspects of Speed Breeding Technology
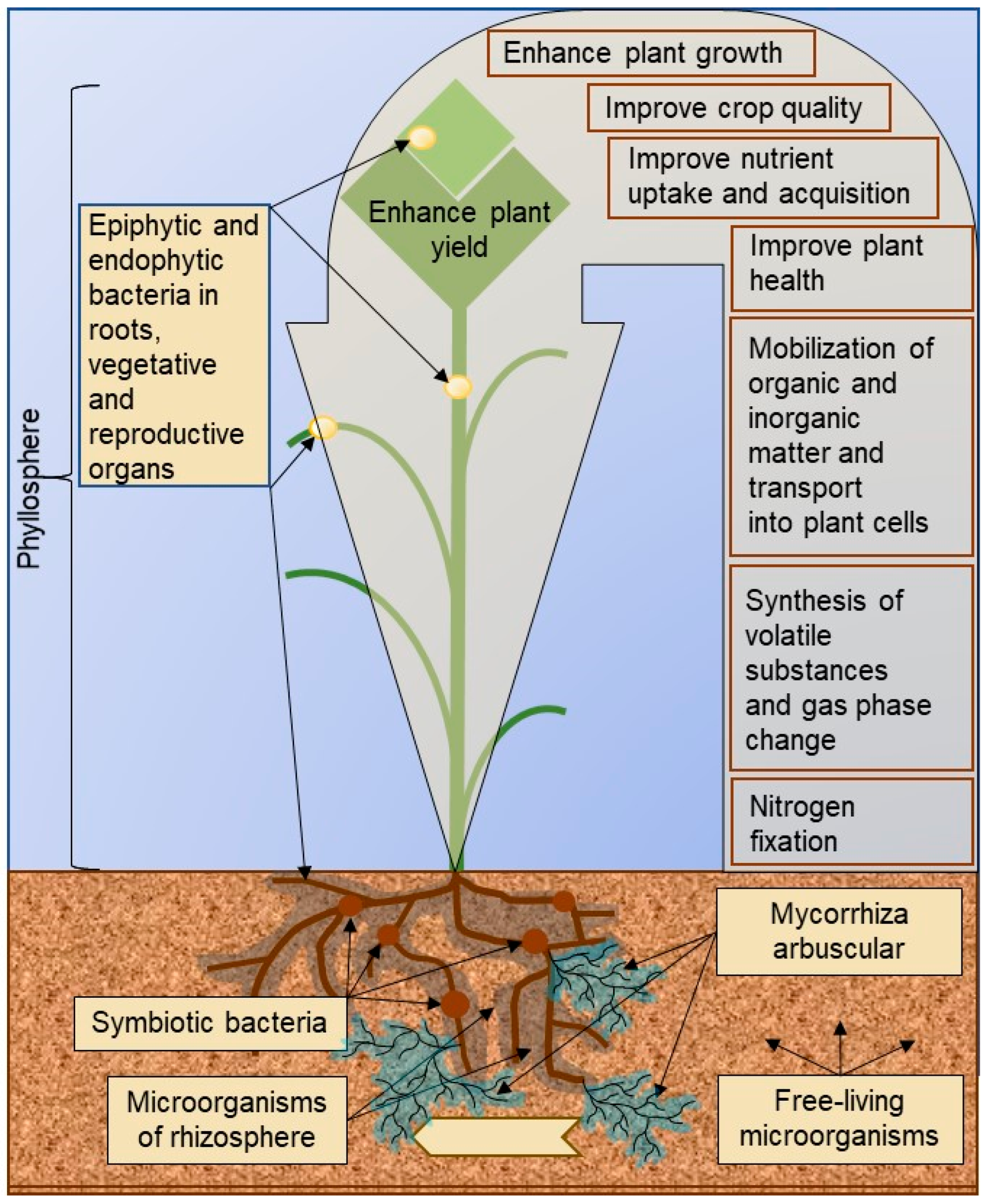
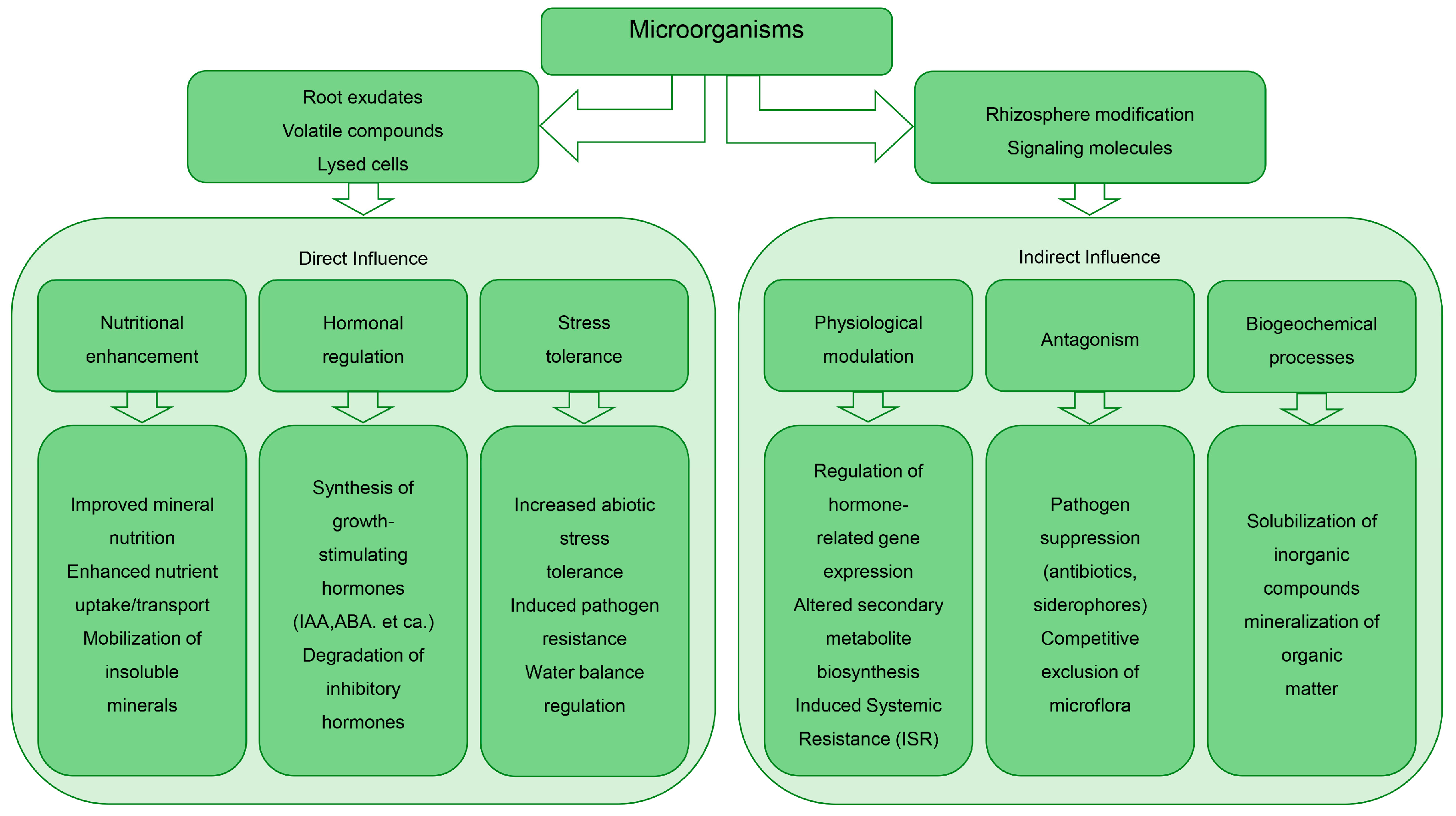
3. Rhizosphere Microbiome: Mechanisms of Plant Growth Promotion and Stress Mitigation in Speed Breeding
4. Symbiotic Systems in Speed Breeding Technology
5. Arbuscular Mycorrhizal Fungi (AMF)
6. Endophytes
7. Epiphytes and Endophytes of Phyllosphere
8. Discussion and Problems
9. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | ABscisic Acid |
| ACC | 1-AminoCyclopropane-1-Carboxylate |
| AI | Artificial Intelligence |
| AMF | Arbuscular Mycorrhizal Fungi |
| APX | Ascorbate Peroxidase |
| CAT | Catalase |
| EPSs | ExoPolySaccharides |
| GWAS | Genome-Wide Association Studies |
| IAA | Indole-3-acetic acid |
| MAS | Marker-Assisted Selection |
| NGS | Next-Generation Sequencing |
| PGPR | Plant-Growth-Promoting Rhizobacteria |
| SOD | Superoxide dismutase |
| QTLs | Quantitative Trait Loci |
References
- Kabade, P.G.; Kumar, S.; Kohli, A.; Singh, U.M.; Sinha, P.; Singh, V.K. Speed Breeding 3.0: Mainstreaming Light-Driven Plant Breeding for Sustainable Genetic Gains. Trends Biotechnol. 2025, 23. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Bashir, A.; Abbas, A.; Li, X.; Shi, Q.; Niu, D.; Zhang, L. Harnessing Light, Photoperiod and Temperature for Accelerated Flowering in Speed Breeding: Mechanisms, Applications and Crop Diversity. J. Plant Physiol. 2025, 311, 154548. [Google Scholar] [CrossRef]
- Williams, K.; Subramani, M.; Lofton, L.W.; Penney, M.; Todd, A.; Ozbay, G. Tools and Techniques to Accelerate Crop Breeding. Plants 2024, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Teressa, T.; Semahegn, Z.; Bejiga, T. Multi Environments and Genetic-Environmental Interaction (GxE) in Plant Breeding and Its Challenges: A Review Article. Int. J. Res. Stud. Agric. Sci. 2021, 7, 11–18. [Google Scholar] [CrossRef]
- Hudson, A.I.; Odell, S.G.; Dubreuil, P.; Tixier, M.-H.; Praud, S.; Runcie, D.E.; Ross-Ibarra, J. Analysis of Genotype-by-Environment Interactions in a Maize Mapping Population. G3 Genes|Genomes|Genet. 2022, 12, jkac013. [Google Scholar] [CrossRef] [PubMed]
- Egea-Gilabert, C.; Pagnotta, M.A.; Tripodi, P. Genotype × Environment Interactions in Crop Breeding. Agronomy 2021, 11, 1644. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, K.; Jun, X.; Bo, L. Role and Functions of Beneficial Microorganisms in Sustainable Aquaculture. Bioresour. Technol. 2009, 100, 3780–3786. [Google Scholar] [CrossRef]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of Plant Growth Promoting Rhizobacteria (PGPR) as a Plant Growth Enhancer for Sustainable Agriculture: A Review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Lopes, M.J.D.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- De Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Igiehon, B.C.; Babalola, O.O.; Hassen, A.I. Rhizosphere Competence and Applications of Plant Growth-Promoting Rhizobacteria in Food Production—A Review. Sci. Afr. 2024, 23, e02081. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. IJMS 2021, 22, 10529. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Veselov, D. Water Relations in Plants Treated with Growth Promoting Rhizosphere Bacteria. Plant Soil 2024, 494, 51–72. [Google Scholar] [CrossRef]
- Smith, J.E. Mycorrhizal Symbiosis (Third Edition). Soil Sci. Soc. Am. J. 2009, 73, 694. [Google Scholar] [CrossRef]
- Koltai, H.; Kapulnik, Y. Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-9488-9. [Google Scholar]
- White, P.J.; Hammond, J.P. Plant Ecophysiology. In The Ecophysiology of Plant-Phosphorus Interactions; Plant Ecophysiology Series; De Kok, L.J., Hawkesford, M.J., Stulen, I., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 7, ISBN 978-1-4020-8434-8. [Google Scholar]
- Bakker, P.A.H.M.; Raaijmakers, J.M.; Bloemberg, G.; Höfte, M.; Lemanceau, P.; Cooke, B.M. New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Bakker, P.A.H.M., Raaijmakers, J.M., Bloemberg, G., Höfte, M., Lemanceau, P., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-6775-4. [Google Scholar]
- Dilworth, M.J.; James, E.K.; Sprent, J.I.; Newton, W.E. Nitrogen-Fixing Leguminous Symbioses; Dilworth, M.J., James, E.K., Sprent, J.I., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-3545-6. [Google Scholar]
- Patel, S.; Gurjar, D. Speed Breeding: A Transformative Approach to Accelerate Crop Improvement. Plant Cell Biotech. Mol. Biol. 2024, 25, 24–35. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, A.; Dutta, S. Rapid Generation Advance Methods to Fast-Track Crop Breeding: A Review. Agric. Rev. 2022, 45, 693. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. IJMS 2020, 21, 2590. [Google Scholar] [CrossRef]
- Kumar, V. Speed Breeding: Accelerated Plant Breeding. J. Agric. Res. Technol. 2022, 47, 36–39. [Google Scholar] [CrossRef]
- Mobini, S.H.; Lulsdorf, M.; Warkentin, T.D.; Vandenberg, A. Plant Growth Regulators Improve in Vitro Flowering and Rapid Generation Advancement in Lentil and Faba Bean. Vitr. Cell. Dev. Biol. Plant 2015, 51, 71–79. [Google Scholar] [CrossRef]
- Marenkova, A.G.; Blinkov, A.O.; Radzeniece, S.; Kocheshkova, A.A.; Karlov, G.I.; Lavygina, V.A.; Patrushev, M.V.; Divashuk, M.G. Testing and Modification of the Protocol for Accelerated Growth of Malting Barley under Speed Breeding Conditions. Nanotechnol. Russ. 2024, 19, 808–814. [Google Scholar] [CrossRef]
- Poulet, L.; Massa, G.D.; Morrow, R.C.; Bourget, C.M.; Wheeler, R.M.; Mitchell, C.A. Significant Reduction in Energy for Plant-Growth Lighting in Space Using Targeted LED Lighting and Spectral Manipulation. Life Sci. Space Res. 2014, 2, 43–53. [Google Scholar] [CrossRef]
- Alahmad, S.; Dinglasan, E.; Leung, K.M.; Riaz, A.; Derbal, N.; Voss-Fels, K.P.; Able, J.A.; Bassi, F.M.; Christopher, J.; Hickey, L.T. Speed Breeding for Multiple Quantitative Traits in Durum Wheat. Plant Methods 2018, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed Breeding in Growth Chambers and Glasshouses for Crop Breeding and Model Plant Research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef]
- Leal Filho, W.; Wall, T.; Rui Mucova, S.A.; Nagy, G.J.; Balogun, A.-L.; Luetz, J.M.; Ng, A.W.; Kovaleva, M.; Safiul Azam, F.M.; Alves, F.; et al. Deploying Artificial Intelligence for Climate Change Adaptation. Technol. Forecast. Soc. Change 2022, 180, 121662. [Google Scholar] [CrossRef]
- Godwin, I.D.; Rutkoski, J.; Varshney, R.K.; Hickey, L.T. Technological Perspectives for Plant Breeding. Theor. Appl. Genet. 2019, 132, 555–557. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding Crops to Feed 10 Billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Cairns, J.E. Field High-Throughput Phenotyping: The New Crop Breeding Frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Rai, K.K. Integrating Speed Breeding with Artificial Intelligence for Developing Climate-Smart Crops. Mol. Biol. Rep. 2022, 49, 11385–11402. [Google Scholar] [CrossRef]
- Van Dijk, A.D.J.; Kootstra, G.; Kruijer, W.; De Ridder, D. Machine Learning in Plant Science and Plant Breeding. iScience 2021, 24, 101890. [Google Scholar] [CrossRef]
- Ashkani, S.; Rafii, M.Y.; Shabanimofrad, M.; Miah, G.; Sahebi, M.; Azizi, P.; Tanweer, F.A.; Akhtar, M.S.; Nasehi, A. Molecular Breeding Strategy and Challenges Towards Improvement of Blast Disease Resistance in Rice Crop. Front. Plant Sci. 2015, 6, 886. [Google Scholar] [CrossRef]
- Niazian, M.; Niedbała, G. Machine Learning for Plant Breeding and Biotechnology. Agriculture 2020, 10, 436. [Google Scholar] [CrossRef]
- Parmley, K.A.; Higgins, R.H.; Ganapathysubramanian, B.; Sarkar, S.; Singh, A.K. Machine Learning Approach for Prescriptive Plant Breeding. Sci. Rep. 2019, 9, 17132. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic Root Exudate Chemistry and Microbial Substrate Preferences Drive Patterns in Rhizosphere Microbial Community Assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Terán, F.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Facing Climate Change: Plant Stress Mitigation Strategies in Agriculture. Physiol. Plant. 2024, 176, e14484. [Google Scholar] [CrossRef]
- Khan, N.A.; Owens, L.; Nuñez, M.A.; Khan, A.L. Complexity of Combined Abiotic Stresses to Crop Plants. Plant Stress 2025, 17, 100926. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere Bacterial Interactions and Impact on Plant Health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef]
- Beltrán-García, M.J.; Martínez-Rodríguez, A.; Beltran-García, C.; Miranda-Rivera, J.V.; Valdez-Salas, B.; Di Mascio, P.; White, J.F. Multifactorial Stress Combination, Plant Microbiome Recruitment, and Reactive Oxygen Species/Antioxidant Feedbacks for Plant Stress Alleviation. In Sustainable Agricultural Practices; Elsevier: Newark, NJ, USA, 2024; pp. 1–32. ISBN 978-0-443-19150-3. [Google Scholar]
- Sardar, N.; Bibi, Y.; Iriti, M.; Hassan, A. Crop Microbiomes Enhance Antioxidant Defense in Plants. In Plant Holobiome Engineering for Climate-Smart Agriculture; Sayyed, R.Z., Ilyas, N., Eds.; Sustainable Plant Nutrition in a Changing World; Springer Nature: Singapore, 2024; pp. 345–365. ISBN 978-981-99-9387-1. [Google Scholar]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Nivetha, N.; Lavanya, A.K.; Vikram, K.V.; Asha, A.D.; Sruthi, K.S.; Bandeppa, S.; Annapurna, K.; Paul, S. PGPR-Mediated Regulation of Antioxidants: Prospects for Abiotic Stress Management in Plants. In Antioxidants in Plant-Microbe Interaction; Springer: Singapore, 2021; pp. 471–497. ISBN 978-981-16-1349-4. [Google Scholar]
- Macabuhay, A.; Arsova, B.; Watt, M.; Nagel, K.A.; Lenz, H.; Putz, A.; Adels, S.; Müller-Linow, M.; Kelm, J.; Johnson, A.A.T.; et al. Plant Growth Promotion and Heat Stress Amelioration in Arabidopsis Inoculated with Paraburkholderia Phytofirmans PsJN Rhizobacteria Quantified with the GrowScreen-Agar II Phenotyping Platform. Plants 2022, 11, 2927. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, P.; Ali, N.; Saini, S.; Pati, P.K.; Pati, A.M. Physiological and Molecular Insight of Microbial Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2023, 14, 1041413. [Google Scholar] [CrossRef]
- Chen, L.; Heng, J.; Qin, S.; Bian, K. A Comprehensive Understanding of the Biocontrol Potential of Bacillus Velezensis LM2303 against Fusarium Head Blight. PLoS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef]
- Nam, H.S.; Anderson, A.J.; Kim, Y.C. Biocontrol Efficacy of Formulated Pseudomonas Chlororaphis O6 against Plant Diseases and Root-Knot Nematodes. Plant Pathol. J. 2018, 34, 241–249. [Google Scholar] [CrossRef]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Biocontrol of Plant Pathogens Using Plant Growth Promoting Bacteria. In Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2013; pp. 319–360. ISBN 978-94-007-5960-2. [Google Scholar]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated Induction of Systemic Resistance and Physiochemical Alterations in Plants against the Pathogens: Current Perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Zanon, M.S.A.; Cavaglieri, L.R.; Palazzini, J.M.; Chulze, S.N.; Chiotta, M.L. Bacillus Velezensis RC218 and Emerging Biocontrol Agents against Fusarium Graminearum and Fusarium Poae in Barley: In Vitro, Greenhouse and Field Conditions. Int. J. Food Microbiol. 2024, 413, 110580. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS Line with Gene Btcry1Ia Encoding Cry1Ia Toxin from Bacillus thuringiensis Promotes Integrated Wheat Defense against Pathogen Stagonospora nodorum Berk. and Greenbug Schizaphis graminum Rond. Biol. Control. 2020, 144, 104242. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; De Vos, P.; Kromann, P.; Declerck, S.; Prestwich, B.D. Biological Control Agents: From Field to Market, Problems, and Challenges. Trends Biotechnol. 2014, 32, 493–496. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and Dogmas of Biocontrol Changes in Perceptions Derived from Research on Trichoderma Harzinum T-22. Plant Disease 2000, 84, 377–393. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gao, S.; Hassan, M.A.; Huang, Z.; Rasheed, A.; Hearne, S.; Prasanna, B.; Li, X.; Li, H. Artificial Intelligence in Plant Breeding. Trends Genet. 2024, 40, 891–908. [Google Scholar] [CrossRef]
- Eftekhari, M.; Ma, C.; Orlov, Y.L. Editorial: Applications of Artificial Intelligence, Machine Learning, and Deep Learning in Plant Breeding. Front. Plant Sci. 2024, 15, 1420938. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Zhang, H.; Li, L. Big Data and Artificial Intelligence-aided Crop Breeding: Progress and Prospects. J. Integr. Plant Biol. 2025, 67, 722–739. [Google Scholar] [CrossRef]
- Harfouche, A.L.; Jacobson, D.A.; Kainer, D.; Romero, J.C.; Harfouche, A.H.; Scarascia Mugnozza, G.; Moshelion, M.; Tuskan, G.A.; Keurentjes, J.J.B.; Altman, A. Accelerating Climate Resilient Plant Breeding by Applying Next-Generation Artificial Intelligence. Trends Biotechnol. 2019, 37, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular Information Technology with DNA. Trends Biotechnol. 2023, 41, 851–852. [Google Scholar] [CrossRef]
- Mueller, S.M.; Messina, C.D.; Vyn, T.J. Simultaneous Gains in Grain Yield and Nitrogen Efficiency over 70 Years of Maize Genetic Improvement. Sci. Rep. 2019, 9, 9095. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, W.; Xu, J.; Lei, Y.; Song, X.; Liang, Z.; Zhu, T.; Liang, Y.; Hao, Y.; Yuan, L.; et al. Bromodomain-Containing Proteins BRD1, BRD2, and BRD13 Are Core Subunits of SWI/SNF Complexes and Vital for Their Genomic Targeting in Arabidopsis. Mol. Plant 2021, 14, 888–904. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Shen, E.; Yang, K.; Fan, Z.; Zhu, Q.-H.; Fan, L.; Ye, C.-Y. BreedingAIDB: A Database Integrating Crop Genome-to-Phenotype Paired Data with Machine Learning Tools Applicable to Breeding. Plant Commun. 2024, 5, 100894. [Google Scholar] [CrossRef] [PubMed]
- Rumetshofer, T.; Inglese, F.; De Bresser, J.; Mannfolk, P.; Strandberg, O.; Jönsen, A.; Bengtsson, A.; Nilsson, M.; Knutsson, L.; Lätt, J.; et al. Tract-Based White Matter Hyperintensity Patterns in Patients with Systemic Lupus Erythematosus Using an Unsupervised Machine Learning Approach. Sci. Rep. 2022, 12, 21376. [Google Scholar] [CrossRef] [PubMed]
- Lareen, A.; Burton, F.; Schäfer, P. Plant Root-Microbe Communication in Shaping Root Microbiomes. Plant Mol. Biol. 2016, 90, 575–587. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-Acetic Acid (IAA) Production Trait, a Useful Screening to Select Endophytic and Rhizosphere Competent Bacteria for Rice Growth Promoting Agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Khalid, M.; Nazli, F.; Arshad, M. Efficacy of Rhizobium and Pseudomonas Strains to Improve Physiology, Ionic Balance and Quality of Mung Bean under Salt-Affected Conditions on Farmer’s Fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial Mediation of Plant Hormone Status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Bursakov, S.A.; Kroupin, P.Y.; Karlov, G.I.; Divashuk, M.G. Tracing the Element: The Molecular Bases of Molybdenum Homeostasis in Legumes. Agronomy 2023, 13, 2300. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Kha, T.Z.; Kanarskii, A.V.; Kanarskaia, Z.A.; Shcherbakov, A.V.; Shcherbakova, E.N. The Key Plant Growth Stimulator—Rhizobacteria. Vestn. Volga State Univ. Technol. Ser For. Ecol. Nat. Manag. 2020, 3, 58–73. [Google Scholar] [CrossRef]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted Beneficial Effects of Rhizosphere Microorganisms on Plant Health and Productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.-S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.-M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial Volatile Emissions Regulate Auxin Homeostasis and Cell Expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of Auxin Producing and Phosphate Solubilizing Bacteria on Mobility of Soil Phosphorus, Growth Rate, and P Acquisition by Wheat Plants. Acta Physiol. Plant 2017, 39, 253. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Ponmurugan, P.; Gopi, C. In Vitro Production of Growth Regulators and Phosphatase Activity by Phosphate Solubilizing Bacteria. Afr. J. Biotechnol. 2016, 5, 348–350. [Google Scholar]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Mishra, P.; Nanda, S.R.; Barpanda, T.; Dash, M.; Dash, S.; Choudhury, S.; Roul, S.; Mishra, A. The Complexity of Kodo Millet: Genomic Analysis and Implications in Crop Improvement. Planta 2025, 261, 15. [Google Scholar] [CrossRef]
- Hernandes, V. Gabriela Breaking the Microbiome Players That Influence Leaf Heat Tolerance. Which Microbe Can Help Plants Be More Tolerant to Heat? Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2024. [Google Scholar]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–Microbiome Interactions under a Changing World: Responses, Consequences and Perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Pramanick, B.; Dey, P.; Bhadra, P.; Shankar, T.; Anand, K. Thermotolerant Soil Microbes and Their Role in Mitigation of Heat Stress in Plants. In Soil Microbiomes for Sustainable Agriculture; Yadav, A.N., Ed.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, Switzerland, 2021; Volume 27, pp. 203–242. ISBN 978-3-030-73506-7. [Google Scholar]
- Dastogeer, K.M.G.; Zahan, M.I.; Rhaman, M.S.; Sarker, M.S.A.; Chakraborty, A. Microbe-Mediated Thermotolerance in Plants and Pertinent Mechanisms- A Meta-Analysis and Review. Front. Microbiol. 2022, 13, 833566. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular Mycorrhizal Fungi Mitigate Negative Effects of Combined Drought and Heat Stress on Tomato Plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Ahmad, M.; Imtiaz, M.; Shoib Nawaz, M.; Mubeen, F.; Imran, A. What Did We Learn From Current Progress in Heat Stress Tolerance in Plants? Can Microbes Be a Solution? Front. Plant Sci. 2022, 13, 794782. [Google Scholar] [CrossRef]
- Amoo, A.E.; Olanrewaju, O.S.; Babalola, O.O.; Ajilogba, C.F.; Chukwuneme, C.F.; Ojuederie, O.B.; Omomowo, O.I. The Functionality of Plant-Microbe Interactions in Disease Suppression. J. King Saud Univ. Sci. 2023, 35, 102893. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Chet, I.; Inbar, J. Biological Control of Fungal Pathogens. Appl. Biochem. Biotechnol. 1994, 48, 37–43. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Teixeira, P.J.P.L.; Berendsen, R.L.; Pieterse, C.M.J.; Zamioudis, C. Editorial: Beneficial Microbiota Interacting With the Plant Immune System. Front. Plant Sci. 2021, 12, 698902. [Google Scholar] [CrossRef]
- Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Applied Bacteriology and Biotechnology, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-31330-1. [Google Scholar]
- Trivedi, P.; Schenk, P.M.; Wallenstein, M.D.; Singh, B.K. Tiny Microbes, Big Yields: Enhancing Food Crop Production with Biological Solutions. Microb. Biotechnol. 2017, 10, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Liu, Y. Hybrid Seed Microorganism: A New Driving Force for Breeding. Appl. Soil Ecol. 2024, 199, 105422. [Google Scholar] [CrossRef]
- Nelson, L.M. Plant Growth Promoting Rhizobacteria (PGPR): Prospects for New Inoculants. Crop Manag. 2004, 3, 1–7. [Google Scholar] [CrossRef]
- Higa, T. Microbiological Method for Disposing of Organic Waste Materials. U.S. Patent No 5,707,856, 13 January 1998. [Google Scholar]
- Higa, T. Effective Microorganisms—A New Dimension for Nature Farming. In Proceedings of the Second International Conference on Kyusei Nature Farming, Piracicaba, Brazil; US Department of Agriculture: Washington, DC, USA, 1994; pp. 20–22. [Google Scholar]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial Fertilizers: A Comprehensive Review of Current Findings and Future Perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Olle, M.; Williams, I.H. Effective Microorganisms and Their Influence on Vegetable Production—A Review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef]
- Real-Santillán, R.O.; del-Val, E.; Cruz-Ortega, R.; Contreras-Cornejo, H.Á.; González-Esquivel, C.E.; Larsen, J. Increased Maize Growth and P Uptake Promoted by Arbuscular Mycorrhizal Fungi Coincide with Higher Foliar Herbivory and Larval Biomass of the Fall Armyworm Spodoptera frugiperda. Mycorrhiza 2019, 29, 615–622. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Wang, B.; Yeun, L.H.; Xue, J.; Liu, Y.; Ané, J.; Qiu, Y. Presence of Three Mycorrhizal Genes in the Common Ancestor of Land Plants Suggests a Key Role of Mycorrhizas in the Colonization of Land by Plants. New Phytol. 2010, 186, 514–525. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed Breeding Short-Day Crops by LED-Controlled Light Schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. [Google Scholar] [CrossRef]
- Balachandar, D.; Raja, P.; Kumar, K.; Sundaram, S. Non-Rhizobial Nodulation in Legumes. Biotechnol. Mol. Biol. Rev. 2007, 2, 49–57. [Google Scholar] [CrossRef]
- Hirsch, P.; Sprent, J.I. Nodulation in Legumes. Ann. Bot. 2002, 89, 797–798. [Google Scholar] [CrossRef]
- Nandigam, S.; Mahendrakar, M.D.; Srungarapu, R.; Chand, U.; Gopalakrishnan, S.; Thati, S.; Vatluri, S.R.; Vadlamudi, S.; Vemula, A.; Kudapa, H.; et al. Rapid Generation Advancement of RIL Population and Assessing the Impact of Rhizobium Nodulation on Crop Yields in Chickpea. Sci. Rep. 2025, 15, 13945. [Google Scholar] [CrossRef]
- Schüβler, A.; Schwarzott, D.; Walker, C. A New Fungal Phylum, the Glomeromycota: Phylogeny and Evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Jones, C.S. Metabolomics of Forage Plants: A Review. Ann. Bot. 2012, 110, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Graham, P.H.; Hungria, M.; Tlusty, B. Breeding for Better Nitrogen Fixation in Grain Legumes: Where Do the Rhizobia Fit In? Crop Manag. 2004, 3, 1–6. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.R.; Perez-Limon, S.; Li, M.; Barrales-Gamez, B.; Albinsky, D.; Paszkowski, U.; Olalde-Portugal, V.; Sawers, R.J. The Genetic Architecture of Host Response Reveals the Importance of Arbuscular Mycorrhizae to Maize Cultivation. eLife 2020, 9, e61701. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, A.A.; Babalola, O.O. Genomic Mechanisms of Plant Growth-Promoting Bacteria in the Production of Leguminous Crops. Front. Genet. 2023, 14, 1276003. [Google Scholar] [CrossRef]
- Coale, F.J.; Meisinger, J.J.; Wiebold, W.J. Effects of Plant Breeding and Selection on Yields and Nitrogen Fixation in Soybeans under Two Soil Nitrogen Regimes. Plant Soil 1985, 86, 357–367. [Google Scholar] [CrossRef]
- Bliss, F.A. Breding Common Bean for Improved Biological Nitrogen Fixation. Plant Soil 1993, 152, 71–79. [Google Scholar] [CrossRef]
- Hungria, M.; Franchini, J.C.; Campo, R.J.; Graham, P.H. The Importance of Nitrogen Fixation to Soybean Cropping in South America. In Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment; Werner, D., Newton, W.E., Eds.; Nitrogen Fixation: Origins, Applications, and Research Progress; Springer: Berlin/Heidelberg, Germany, 2005; Volume 4, pp. 25–42. ISBN 978-1-4020-3542-5. [Google Scholar]
- Shtark, O.Y.; Borisov, A.U.; Zhukov, V.A.; Nemankin, T.A.; Tikhonovich, I.A. Multi-Component Symbiosis of Legumes with Beneficial Soil Microbes: Genetic and Evolutionary Basis of Application in Sustainable Crop Production. Ecol. Genet. 2011, 9, 80–94. [Google Scholar] [CrossRef]
- Leppyanen, I.V. Analysis of The Effects of Joint Inoculation by Arbuscular Mycorrhizal Fungi and Rhizobia on The Growth and Development of Pea Plants Pisum sativum L. Agric. Biol. 2021, 56, 475–486. [Google Scholar] [CrossRef]
- Shtark, O.Y.; Danilova, T.N.; Naumkina, T.S.; Vasilchikov, A.G.; Chebotar, V.K.; Kazakov, A.E.; Zhernakov, A.I.; Nemankin, T.A.; Prilepskaya, N.A.; Borisov, A.U.; et al. Analysis Of Pea (Pisum sativum L.) Source Material For Breeding Of Cultivars With High Symbiotic Potential And Choice Of Criteria For Its Evaluation. Ecol. Genet. 2006, 4, 22–28. [Google Scholar] [CrossRef]
- Samineni, S.; Sen, M.; Sajja, S.B.; Gaur, P.M. Rapid Generation Advance (RGA) in Chickpea to Produce up to Seven Generations per Year and Enable Speed Breeding. Crop J. 2020, 8, 164–169. [Google Scholar] [CrossRef]
- Kelvin, K. Varietal Differences in Symbiotic Nitrogen Fixation and Nitrogen Redistribution in Common Bean. J. Plant Breed. Crop Sci. 2023, 15, 86–89. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Gaude, N.; Bortfeld, S.; Duensing, N.; Lohse, M.; Krajinski, F. Arbuscule-containing and Non-colonized Cortical Cells of Mycorrhizal Roots Undergo Extensive and Specific Reprogramming during Arbuscular Mycorrhizal Development. Plant J. 2012, 69, 510–528. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.-C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones Stimulate Arbuscular Mycorrhizal Fungi by Activating Mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Harrison, M.J.; Paszkowski, U. Reprogramming Plant Cells for Endosymbiosis. Science 2009, 324, 753–754. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; García-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First Indications for the Involvement of Strigolactones on Nodule Formation in Alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Draper, J.; Rasmussen, S.; Zubair, H. Metabolite Analysis and Metabolomics in the Study of Biotrophic Interactions between Plants and Microbes. In Annual Plant Reviews; Hall, R.D., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 25–59. ISBN 978-1-4051-9954-4. [Google Scholar]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago Truncatula Phosphate Transporter Indispensable for the Arbuscular Mycorrhizal Symbiosis. Proc. Acad. Natl. Sci. USA. 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Shachar-Hill, Y.; Pfeffer, P.E.; Douds, D.; Osman, S.F.; Doner, L.W.; Ratcliffe, R.G. Partitioning of Intermediary Carbon Metabolism in Vesicular-Arbuscular Mycorrhizal Leek. Plant Physiol. 1995, 108, 7–15. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Tripathi, S.; Bahuguna, R.N.; Shrivastava, N.; Singh, S.; Chatterjee, A.; Varma, A.; Jagadish, S.K. Microbial Biofortification: A Sustainable Route to Grow Nutrient-Rich Crops under Changing Climate. Field Crops Res. 2022, 287, 108662. [Google Scholar] [CrossRef]
- Barea, J.M. Future Challenges and Perspectives for Applying Microbial Biotechnology in Sustainable Agriculture Based on a Better Understanding of Plant-Microbiome Interactions. J. Soil Sci. Plant Nutr. 2015, 15, 261–282. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and Nutrient Cycling in Ecosystems—A Journey towards Relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Cappellazzo, G.; Lanfranco, L.; Fitz, M.; Wipf, D.; Bonfante, P. Characterization of an Amino Acid Permease from the Endomycorrhizal Fungus Glomus mosseae. Plant Physiol. 2008, 147, 429–437. [Google Scholar] [CrossRef]
- Facelli, E.; Smith, S.E.; Smith, F.A. Mycorrhizal Symbiosis—Overview and New Insights into Roles of Arbuscular Mycorrhizas in Agro- and Natural Ecosystems. Austral. Plant Pathol. 2009, 38, 338. [Google Scholar] [CrossRef]
- Ercoli, L.; Schüßler, A.; Arduini, I.; Pellegrino, E. Strong Increase of Durum Wheat Iron and Zinc Content by Field-Inoculation with Arbuscular Mycorrhizal Fungi at Different Soil Nitrogen Availabilities. Plant Soil 2017, 419, 153–167. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular Mycorrhizal Fungi for the Biocontrol of Plant-Parasitic Nematodes: A Review of the Mechanisms Involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef]
- Ceustermans, A.; Van Hemelrijck, W.; Van Campenhout, J.; Bylemans, D. Effect of Arbuscular Mycorrhizal Fungi on Pratylenchus Penetrans Infestation in Apple Seedlings under Greenhouse Conditions. Pathogens 2018, 7, 76. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Jiang, L.; Chen, C.; Wu, L.; Liu, Z. Different Pathogen Defense Strategies in Arabidopsis: More than Pathogen Recognition. Cells 2018, 7, 252. [Google Scholar] [CrossRef]
- Singh, I.; Giri, B. Arbuscular Mycorrhiza Mediated Control of Plant Pathogens. In Mycorrhiza—Nutrient Uptake, Biocontrol, Ecorestoration; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 131–160. ISBN 978-3-319-68866-4. [Google Scholar]
- Hilou, A.; Zhang, H.; Franken, P.; Hause, B. Do Jasmonates Play a Role in Arbuscular Mycorrhiza-Induced Local Bioprotection of Medicago Truncatula against Root Rot Disease Caused by Aphanomyces Euteiches? Mycorrhiza 2014, 24, 45–54. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Siddiqui, Z.A. Arbuscular Mycorrhizal Fungi as Potential Bioprotectants Against Plant Pathogens. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z.A., Akhtar, M.S., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–97. ISBN 978-1-4020-8769-1. [Google Scholar]
- Zhu, Y. Carbon Cycling by Arbuscular Mycorrhizal Fungi in Soil–Plant Systems. Trends Plant Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef]
- Bago, B.; Pfeffer, P.E.; Abubaker, J.; Jun, J.; Allen, J.W.; Brouillette, J.; Douds, D.D.; Lammers, P.J.; Shachar-Hill, Y. Carbon Export from Arbuscular Mycorrhizal Roots Involves the Translocation of Carbohydrate as Well as Lipid. Plant Physiol. 2003, 131, 1496–1507. [Google Scholar] [CrossRef]
- Alvarado-López, C.J.; Dasgupta-Schubert, N.; Ambriz, J.E.; Arteaga-Velazquez, J.C.; Villegas, J.A. Lead Uptake by the Symbiotic Daucus Carota L.–Glomus Intraradices System and Its Effect on the Morphology of Extra- and Intraradical Fungal Microstructures. Environ. Sci. Pollut. Res. 2019, 26, 381–391. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Maimaitiaili, B.; Kafle, A.; Khan, K.S.; Feng, G. Breeding Practice Improves the Mycorrhizal Responsiveness of Cotton (Gossypium spp. L.). Front. Plant Sci. 2021, 12, 780454. [Google Scholar] [CrossRef]
- Sendek, A.; Karakoç, C.; Wagg, C.; Domínguez-Begines, J.; Do Couto, G.M.; Van Der Heijden, M.G.A.; Naz, A.A.; Lochner, A.; Chatzinotas, A.; Klotz, S.; et al. Drought Modulates Interactions between Arbuscular Mycorrhizal Fungal Diversity and Barley Genotype Diversity. Sci. Rep. 2019, 9, 9650. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Xie, M.-M.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi and Rhizobia Synergistically Promote Root Colonization, Plant Growth, and Nitrogen Acquisition. Plant Growth Regul. 2023, 100, 691–701. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wang, W.; Mo, F.; Ren, A.-T.; Wang, Z.-Y.; Zhu, Y.; Xiong, Y.-C. Seven-Year Long-Term Inoculation with Funneliformis Mosseae Increases Maize Yield and Soil Carbon Storage Evidenced by in Situ 13C-Labeling in a Dryland. Sci. Total Environ. 2024, 944, 173975. [Google Scholar] [CrossRef]
- Elliott, A.J.; Daniell, T.J.; Cameron, D.D.; Field, K.J. A Commercial Arbuscular Mycorrhizal Inoculum Increases Root Colonization across Wheat Cultivars but Does Not Increase Assimilation of Mycorrhiza-acquired Nutrients. Plants People Planet 2021, 3, 588–599. [Google Scholar] [CrossRef]
- O’Brien, A.M.; Ginnan, N.A.; Rebolleda-Gómez, M.; Wagner, M.R. Microbial Effects on Plant Phenology and Fitness. Am. J. Bot. 2021, 108, 1824–1837. [Google Scholar] [CrossRef]
- Chialva, M.; Zouari, I.; Salvioli, A.; Novero, M.; Vrebalov, J.; Giovannoni, J.J.; Bonfante, P. Gr and Hp-1 Tomato Mutants Unveil Unprecedented Interactions between Arbuscular Mycorrhizal Symbiosis and Fruit Ripening. Planta 2016, 244, 155–165. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Chow, C.; Padda, K.P.; Puri, A.; Chanway, C.P. An Archaic Approach to a Modern Issue: Endophytic Archaea for Sustainable Agriculture. Curr. Microbiol. 2022, 79, 322. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic Microbes and Their Potential Applications in Crop Management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Khan, A.L.; Ullah, I.; Hussain, J.; Kang, S.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I. Regulations of Essential Amino Acids and Proteomics of Bacterial Endophytes Sphingomonas sp. L K11 during Cadmium Uptake. Environ. Toxicol. 2016, 31, 887–896. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily Light Integral: A Research Review and High-Resolution Maps of the United States. Horts 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.A.; Hassan, S.E.-D. Plant Growth-Promoting Endophytic Bacterial Community Inhabiting the Leaves of Pulicaria incisa (Lam.) DC Inherent to Arid Regions. Plants 2021, 10, 76. [Google Scholar] [CrossRef]
- Tanaka, A.; Takemoto, D.; Chujo, T.; Scott, B. Fungal Endophytes of Grasses. Curr. Opin. Plant Biol. 2012, 15, 462–468. [Google Scholar] [CrossRef]
- Dunleavy, J.M. Curtobacterium plantarum sp. Nov. Is Ubiquitous in Plant Leaves and Is Seed Transmitted in Soybean and Corn. Int. J. Syst. Bacteriol. 1989, 39, 240–249. [Google Scholar] [CrossRef]
- Tyc, O.; Putra, R.; Gols, R.; Harvey, J.A.; Garbeva, P. The Ecological Role of Bacterial Seed Endophytes Associated with Wild Cabbage in the United Kingdom. MicrobiologyOpen 2020, 9, e00954. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; Von Maltzahn, G.; et al. A New Approach to Modify Plant Microbiomes and Traits by Introducing Beneficial Bacteria at Flowering into Progeny Seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.-A.; Barret, M. Ecological Patterns of Seed Microbiome Diversity, Transmission, and Assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Cope-Selby, N.; Cookson, A.; Squance, M.; Donnison, I.; Flavell, R.; Farrar, K. Endophytic Bacteria in Miscanthus Seed: Implications for Germination, Vertical Inheritance of Endophytes, Plant Evolution and Breeding. GCB Bioenergy 2017, 9, 57–77. [Google Scholar] [CrossRef]
- Rodríguez, C.E.; Mitter, B.; Barret, M.; Sessitsch, A.; Compant, S. Commentary: Seed Bacterial Inhabitants and Their Routes of Colonization. Plant Soil 2018, 422, 129–134. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.P.; Van Overbeek, L.S.; Van Elsas, J.D. Dynamics of Seed-Borne Rice Endophytes on Early Plant Growth Stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial Seed Endophytes: Genera, Vertical Transmission and Interaction with Plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Gerna, D.; Clara, D.; Allwardt, D.; Mitter, B.; Roach, T. Tailored Media Are Key to Unlocking the Diversity of Endophytic Bacteria in Distinct Compartments of Germinating Seeds. Microbiol. Spectr. 2022, 10, e00172-22. [Google Scholar] [CrossRef]
- Frank, A.; Saldierna Guzmán, J.; Shay, J. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Barret, M.; Guimbaud, J.; Darrasse, A.; Jacques, M. Plant Microbiota Affects Seed Transmission of Phytopathogenic Microorganisms. Mol. Plant Pathol. 2016, 17, 791–795. [Google Scholar] [CrossRef]
- Chesneau, G.; Torres-Cortes, G.; Briand, M.; Darrasse, A.; Preveaux, A.; Marais, C.; Jacques, M.-A.; Shade, A.; Barret, M. Temporal Dynamics of Bacterial Communities during Seed Development and Maturation. FEMS Microbiol. Ecol. 2020, 96, fiaa190. [Google Scholar] [CrossRef]
- Bintarti, A.F.; Sulesky-Grieb, A.; Stopnisek, N.; Shade, A. Endophytic Microbiome Variation Among Single Plant Seeds. Phytobiomes J. 2022, 6, 45–55. [Google Scholar] [CrossRef]
- Klaedtke, S.; Jacques, M.; Raggi, L.; Préveaux, A.; Bonneau, S.; Negri, V.; Chable, V.; Barret, M. Terroir Is a Key Driver of Seed-associated Microbial Assemblages. Environ. Microbiol. 2016, 18, 1792–1804. [Google Scholar] [CrossRef]
- Wassermann, B.; Müller, H.; Berg, G. An Apple a Day: Which Bacteria Do We Eat with Organic and Conventional Apples? Front. Microbiol. 2019, 10, 1629. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl Environ Microbiol 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Tokas, D.; Singh, S.; Yadav, R.; Singh, A.N. Plant-Microbe Interactions: Role in Sustainable Agriculture and Food Security in a Changing Climate. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Elsevier: Cambridge, MA, USA, 2023; pp. 363–391. ISBN 978-0-323-91876-3. [Google Scholar]
- Wagner, M.R.; Lundberg, D.S.; Del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host Genotype and Age Shape the Leaf and Root Microbiomes of a Wild Perennial Plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant Microbial Diversity Is Suggested as the Key to Future Biocontrol and Health Trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant Growth-Promoting Microorganisms for Environmental Sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of Inoculation with Plant Growth-Promoting Bacteria (PGPB) on Amelioration of Saline Stress in Maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhou, L.-G.; Wu, J.-Y. Promotion of Salvia Miltiorrhiza Hairy Root Growth and Tanshinone Production by Polysaccharide–Protein Fractions of Plant Growth-Promoting Rhizobacterium Bacillus Cereus. Process Biochem. 2010, 45, 1517–1522. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using Plant Growth-Promoting Rhizobacteria (PGPR) to Improve Plant Growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of Commercial Bio-Inoculants to Increase Agricultural Production through Improved Phosphrous Acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Provorov, N.A.; Vorobyev, N.I. Adaptive and Progressive Evolution of Plant-Microbe Symbiosis. Ecol. Genet. 2013, 11, 12–22. [Google Scholar] [CrossRef]
- Vorobyov, N.I.; Provorov, N.A. Simulation of Evolution of the Legume-Rhizobia Symbiosis for an Improved Functional Integrity of Partners and for Ecological Efficiency of Their Interaction. Ecol. Genet. 2010, 8, 16–26. [Google Scholar] [CrossRef]
- Provorov, N.A. Agricultural Microbiology and Symbiogenetics: Synthesis of Classical Ideas and Construction of Highly Productive Agrocenoses (Review). Sel’skokhozyaistvennaya Biol. [Agric. Biol.] 2022, 57, 821–831. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Brockwell, J.; Bottomley, P.J.; Thies, J.E. Manipulation of Rhizobia Microflora for Improving Legume Productivity and Soil Fertility: A Critical Assessment. Plant Soil 1995, 174, 143–180. [Google Scholar] [CrossRef]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Tikhonovich, I.A.; Provorov, N.A. Simbiogenetics of Microbe-Plant Interactions. Ecol. Genet. 2003, 1, 36–46. [Google Scholar] [CrossRef]
- Koulman, A.; Tapper, B.A.; Fraser, K.; Cao, M.; Lane, G.A.; Rasmussen, S. High-throughput Direct-infusion Ion Trap Mass Spectrometry: A New Method for Metabolomics. Rapid Comm. Mass Spectrom. 2007, 21, 421–428. [Google Scholar] [CrossRef]
- Johnson, R.D.; Lane, G.A.; Koulman, A.; Cao, M.; Fraser, K.; Fleetwood, D.J.; Voisey, C.R.; Dyer, J.M.; Pratt, J.; Christensen, M.; et al. A Novel Family of Cyclic Oligopeptides Derived from Ribosomal Peptide Synthesis of an in Planta-Induced Gene, gigA, in Epichloë Endophytes of Grasses. Fungal Genet. Biol. 2015, 85, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Koulman, A.; Johnson, L.J.; Lane, G.A.; Rasmussen, S. Advanced Data-Mining Strategies for the Analysis of Direct-Infusion Ion Trap Mass Spectrometry Data from the Association of Perennial Ryegrass with Its Endophytic Fungus, Neotyphodium lolii. Plant Physiol. 2008, 146, 1501–1514. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bursakov, S.A.; Karlov, G.I.; Kroupin, P.Y.; Divashuk, M.G. Microorganisms as Potential Accelerators of Speed Breeding: Mechanisms and Knowledge Gaps. Plants 2025, 14, 2628. https://doi.org/10.3390/plants14172628
Bursakov SA, Karlov GI, Kroupin PY, Divashuk MG. Microorganisms as Potential Accelerators of Speed Breeding: Mechanisms and Knowledge Gaps. Plants. 2025; 14(17):2628. https://doi.org/10.3390/plants14172628
Chicago/Turabian StyleBursakov, Sergey A., Gennady I. Karlov, Pavel Yu. Kroupin, and Mikhail G. Divashuk. 2025. "Microorganisms as Potential Accelerators of Speed Breeding: Mechanisms and Knowledge Gaps" Plants 14, no. 17: 2628. https://doi.org/10.3390/plants14172628
APA StyleBursakov, S. A., Karlov, G. I., Kroupin, P. Y., & Divashuk, M. G. (2025). Microorganisms as Potential Accelerators of Speed Breeding: Mechanisms and Knowledge Gaps. Plants, 14(17), 2628. https://doi.org/10.3390/plants14172628




