Brassinosteroids Enhance Low-Temperature Resistance by Promoting the Formation of Sugars in Maize Mesocotyls
Abstract
1. Introduction
2. Results
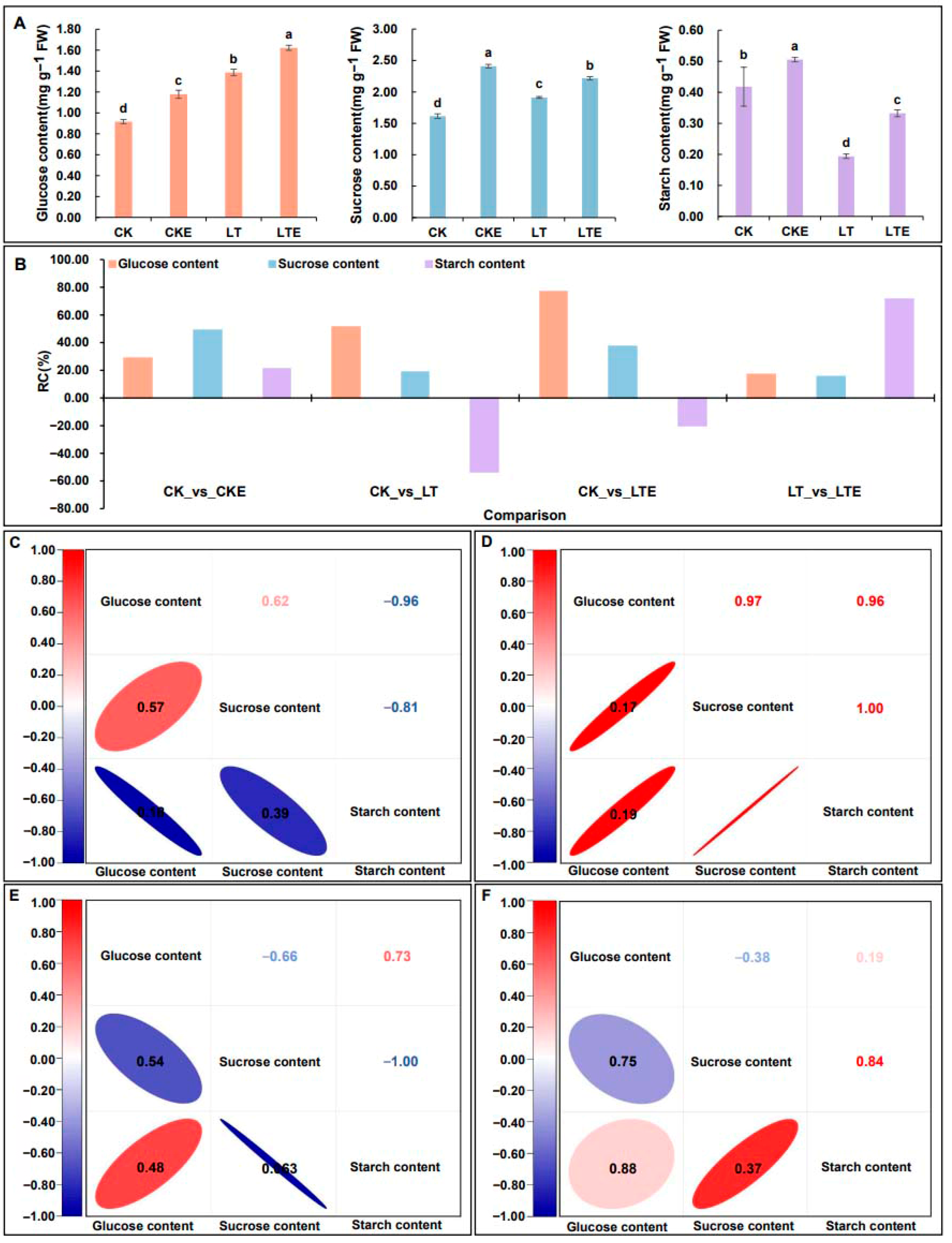
2.1. The Changes and Correlation Analysis of Glucose, Sucrose, and Starch Contents in the Mesocotyls Under Four Treatments
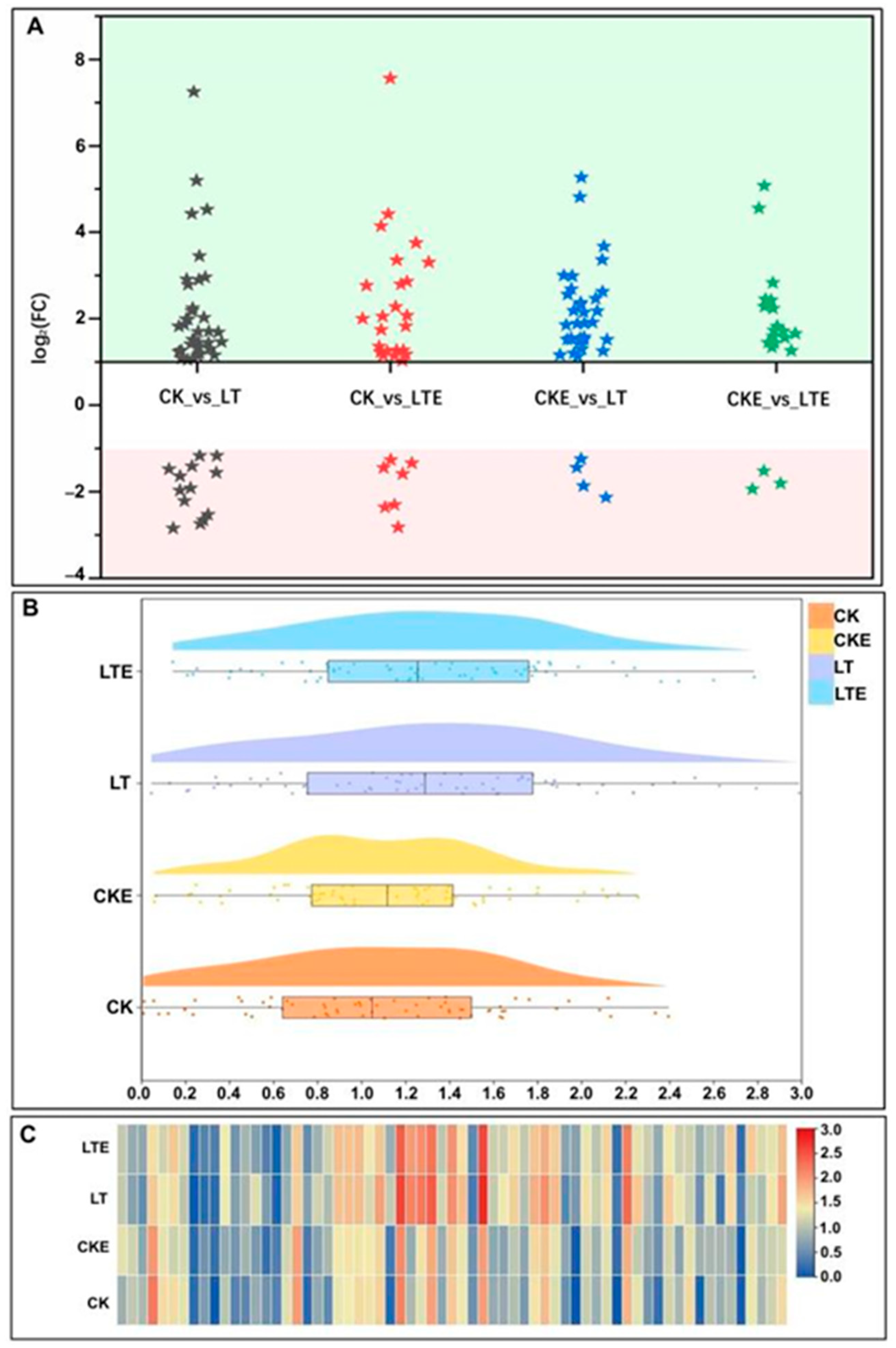
2.2. Overview of RNA-Seq Quality and DEG Identification
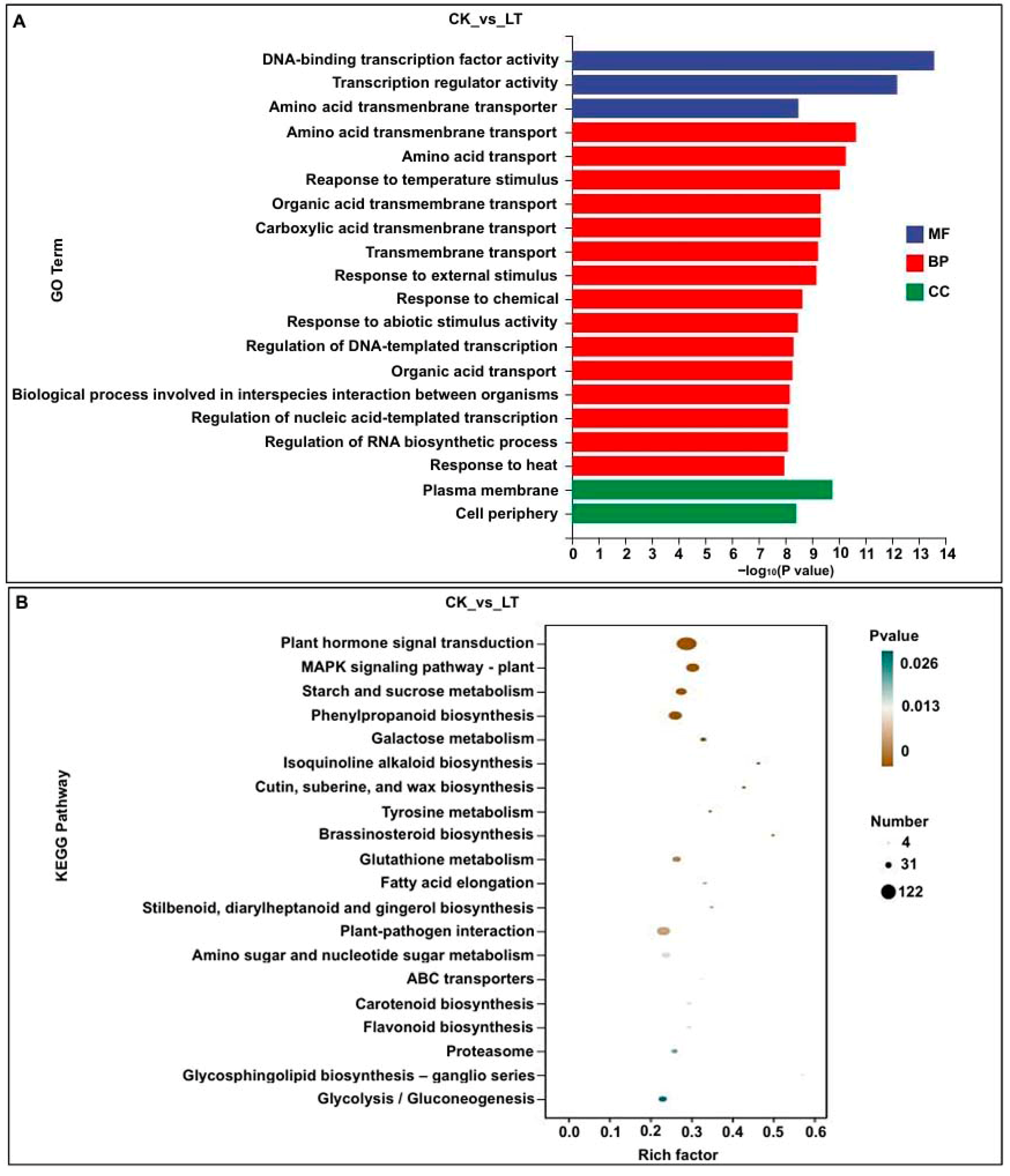
2.3. Functional Annotation and Enrichment Analysis of DEGs
2.4. DEGs Involved in Starch and Sucrose Metabolism
2.5. DEGs Involved in Glycolysis/Gluconeogenesis
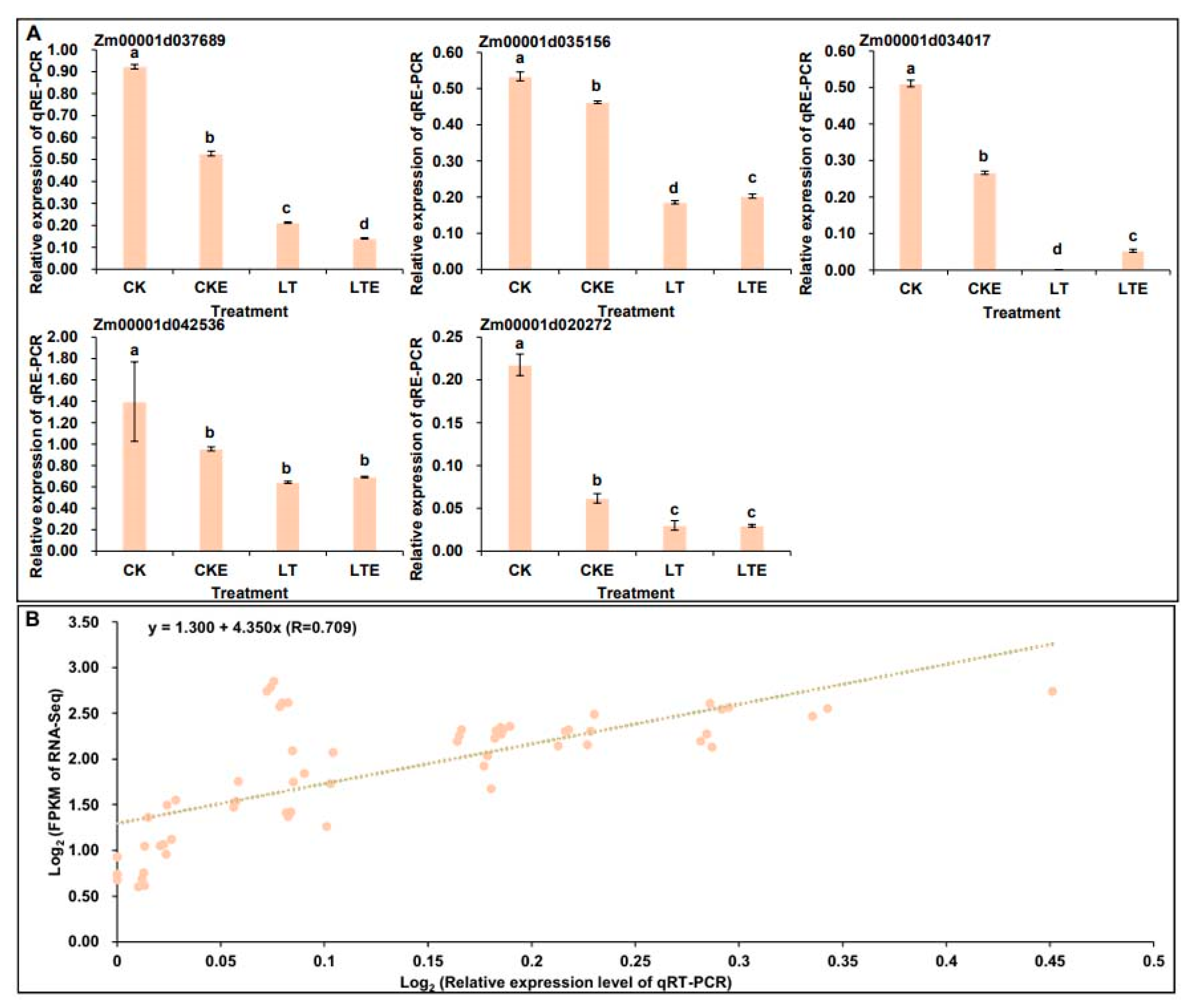
2.6. Verification of RNA-Seq Data by Quantitative Real-Time PCR (qRT-PCR)
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Treatments
4.2. Physiological Metabolism Measurements
4.3. Statistical Analyses
4.4. RNA-Seq Analysis and DEG Identification
4.5. qRT-PCR Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, Q.H.; Ren, Z.; Zhu, Q.X.; Zhou, Y.; Zhu, M.Y.; He, J.G.; Wang, X.M.; Zhao, G.W. Maize SERRATE 1B positively regulates seed germinability under low-temperature. Plant Sci. 2025, 355, 112458. [Google Scholar] [CrossRef]
- Széles, A.; Horváth, É.; Simon, K.; Zagyi, P.; Huzsvai, L. Maize production under drought stress: Nutrient supply, yield prediction. Plants 2023, 12, 3301. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, T.T.; Zhang, Q.G.; Wang, J.H.; Guo, J.J.; Wang, L.W.; Song, L.; Yan, Y.Y.; Zhang, D.M.; Wei, J.F.; et al. Genetic and molecular characterization of maize landraces from central China. Agronomy 2024, 14, 1278. [Google Scholar] [CrossRef]
- Gao, J.; Liu, N.G.; Wang, X.Q.; Niu, Z.Y.; Liao, Q.; Ding, R.S.; Du, T.S.; Kang, S.Z.; Tong, L. Maintaining grain number by reducing grain abortion is the key to improve water use efficiency of maize under deficit irrigation and salt stress. Agric. Water Manag. 2024, 294, 108727. [Google Scholar] [CrossRef]
- He, F.Q.; Zhao, X.Q.; Qi, G.X.; Sun, S.Q.; Shi, Z.Z.; Niu, Y.N.; Wu, Z.F.; Zhou, W.Q. Exogenous melatonin alleviates NaCl injury by influencing stomatal morphology, photosynthetic performance, and antioxidant balance in maize. Int. J. Mol. Sci. 2024, 25, 10077. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Schumaker, K.S.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Liu, S.; Guo, N.; Ma, H.X.; Sun, H.; Zheng, X.; Shi, J. First report of root rot caused by bipolaris zeicola on maize in hebei province. Plant Dis. 2021, 105, 2247. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, J.G.; Cao, J.S.; Ma, X.N.; Li, W.Y.; Yang, G.B. Hub gene mining and co-expression network construction of low-temperature response in maize of seedling by WGCNA. Genes 2023, 14, 1598. [Google Scholar] [CrossRef]
- Zhang, J.G.; Li, S.J.; Cai, Q.; Wang, Z.H.; Cao, J.S.; Yu, T.; Xie, T.L. Exogenous diethyl aminoethyl hexanoate ameliorates low temperature stress by improving nitrogen metabolism in maize seedlings. PLoS ONE 2020, 15, e0232294. [Google Scholar] [CrossRef]
- Meng, X.K.; Liang, Z.K.; Dai, X.R.; Zhang, Y.; Mahboub, S.; Ngu, D.W.; Roston, R.L.; Schnable, J.C. Predicting transcriptional responses to cold stress across plant species. Proc. Natl. Acad. Sci. USA 2021, 118, e2026330118. [Google Scholar] [CrossRef]
- Sobkowiak, A.; Jo ’nczyk, M.; Jarochowska, E.; Biecek, P.; Trzcinska-Danielewicz, J.; Leipner, J.; Fronk, J.; Sowinski, P. Genome-wide transcriptiomic analysis of response to low temperature reveals camdidate genes determining divergent cold-sensitivity of maize inbred lines. Plant Mol. Biol. 2014, 85, 317–331. [Google Scholar] [CrossRef]
- Friero, I.; Larriba, E.; MartínezMelgarejo, P.A.; Justamante, M.D.; Alarcón, M.V.; Albacete, A.; Salguero, J.; Pérez-Pérez, J. Transcriptomic and hormonal analysis of the roots of maize seedlings grown hydroponically at low temperature. Plant Sci. 2022, 326, 111525. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.A.; Wang, X.; Zafar, S.A.; Noor, M.A.; Hussain, H.A.; Azher, N.M.; Farooq, M. Thermal stresses in maize: Effects and management strategies. Plants 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Enders, T.A.; Dennis, S.S.; Oakland, J.; Callen, S.T.; Gehan, M.A.; Miller, N.D.; Spalding, E.P.; Springer, N.M.; Hirsch, C.D. Classifying cold-stress responses of inbred maize seedlings using RGB imaging. Plant Direct 2019, 3, e00104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Hu, H.R.; Hu, X.M.; Wang, G.H.; Du, X.M.; Li, L.; Wang, F.; Fu, J.J.; Wang, G.Y.; Wang, J.H.; et al. Transcriptome analysis of near-Isogenic lines provides novel insights into genes associated with seed low-temperature germination ability inmaize (Zea mays L.). Plants 2022, 11, 887. [Google Scholar] [CrossRef]
- Gong, F.; Yang, L.; Tai, F.; Hu, X.; Wang, W. “Omics” of Maize stress response for sustainable food production: Opportunities and challenges. Omics 2014, 18, 714–732. [Google Scholar] [CrossRef]
- Ma, S.; Xi, Z.; Wang, Q. Risk evaluation of cold damage to corn in northeast China. J. Nat. Disasters 2003, 12, 137–141. [Google Scholar]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Frascaroli, E.; Salvi, S.; Stamp, P. QTL controlling root and shoot traits of maize seedlings under cold stress. Theor. Appl. Genet. 2004, 109, 618–629. [Google Scholar] [CrossRef]
- Mauro, D.F.; Bridget, H.; Jim, G.; Susanne, B. Transcriptomic response of maize primary roots to low temperatures at seedling emergence. PeerJ 2017, 5, e2839. [Google Scholar] [CrossRef]
- Zhou, X.M.; Muhammad, I.; Lan, H.; Xia, C. Recent advances in the analysis of cold tolerance in maize. Front. Plant Sci. 2022, 13, 866034. [Google Scholar] [CrossRef]
- Manja, B.; Dragana, I.M.; Violeta, A.; Nenad, D.; Ana, N. Maize transcriptome profiling reveals low temperatures affect photosynthesis during the emergence stage. Front. Plant Sci. 2025, 16, 1527447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Q.S.; Zhang, B.R.; Zhang, F.; Liu, P.F.; Zhou, S.L.; Liu, X.L. Hormonal and enzymatic responses of maize seedlings to chilling stress as affected by triazoles seed treatments. Plant Physiol. Biochem. 2020, 148, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Qazi, H.A.; Dar, Z.A.; John, R. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiol. Mol. Biol. Plants 2021, 27, 1395–1412. [Google Scholar] [CrossRef]
- He, R.Y.; Zheng, J.J.; Chen, Y.; Pan, Z.Y.; Yang, T.; Zhou, Y.; Li, X.F.; Nan, X.Y.; Li, Y.Z.; Cheng, M.C.; et al. QTL-seq and transcriptomic integrative analyses reveal two positively regulated genes that control the low-temperature germination ability of MTP-maize introgression lines. Theor. Appl. Genet. 2023, 136, 116. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhao, C.; Niu, Y.N.; Chao, W.; He, W.; Wang, Y.F.; Mao, T.T.; Bai, X.D. Understanding and comprehensive evaluation of cold resistance in the seedlings of multiple maize genotypes. Plants 2022, 11, 1881. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Sun, S.Q.; Zhao, X.Q.; Shi, Z.Z.; He, F.Q.; Qi, G.X.; Li, X.; Niu, Y.N.; Zhou, W.Q. Exogenous 24-epibrassinolide improves low-temperature tolerance of maize seedlings by influencing sugar signaling and metabolism. Int. J. Mol. Sci. 2025, 26, 585. [Google Scholar] [CrossRef]
- Dong, T.Y.; Hao, T.Y.; Hakeem, A.; Ren, Y.H.; Fang, J.G. Synergistic variation in abscisic acid and brassinolide treatment signaling component alleviates fruit quality of ‘Shine Muscat’ grape during cold storage. Food Chem. 2025, 464, 141584. [Google Scholar] [CrossRef]
- Xia, X.J.; Fang, P.P.; Guo, X.; Qian, X.J.; Zhou, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid-mediated apoplastic H2O2—Glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato plant. Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal andacquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, 5982–5991. [Google Scholar] [CrossRef]
- Ye, K.; Li, H.; Ding, Y.l.; Shi, Y.T.; Song, C.P.; Gong, Z.Z.; Yang, S.H. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in arabidopsis. Plant Cell 2019, 31, 2682–2696. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Cebrián, G.; Gautam, K.; Moya, J.I.; Martínez, C.; Jamilena, M. A mutation in the brassinosteroid biosynthesis gene CpDWF5 disrupts vegetative and reproductive development and the salt stress response in squash (Cucurbita pepo). Hortic. Res. 2024, 11, uhae050. [Google Scholar] [CrossRef]
- Kloc, Y.; Boguta, M.D.; Paulina, Z.R.; Łukasz, Ł.; Anna, N.O.; Wacław, O. HvGSK1.1 controls salt tolerance and yield through the brassinosteroid signaling pathway in barley. Int. J. Mol. Sci. 2024, 25, 998. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Liu, Y.; Sang, K.Q.; Wang, T.; Yu, J.Q.; Zhou, Y.H.; Xia, X.J. Brassinosteroid signaling positively regulates abscisic acid biosynthesis in response to chilling stress in tomato. J. Integr. Plant Biol. 2022, 65, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Yusuf, M.; Chalkoo, S.; Hayat, S.; Ahmad, A. 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 2011, 49, 55–64. [Google Scholar] [CrossRef]
- Ding, M.Y.; Wang, L.Y.; Sun, Y.T.; Zhang, J.B.; Chen, Y.S.; Wang, X.S.; Liu, L.J. Transcriptome analysis of brassinolide under low temperature stress in winter wheat. AoB Plants 2023, 15, plad005. [Google Scholar] [CrossRef]
- Dong, F.X.; Li, X.Y.; Liu, C.; Zhao, B.X.; Ma, Y.; Ji, W. Exogenous 24-epibrassinolide mitigates damage in grape seedlings under low-temperature stress. Front. Plant Sci. 2025, 16, 1487680. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderon-Urrea, A.; Lyu, J.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome analysis of pepper (Capsicum annuum) revealed a role of 24-epibrassinolide in response to chilling. Front. Plant Sci. 2016, 7, 1281. [Google Scholar] [CrossRef]
- Wu, X.X.; Ding, H.D.; Chen, J.L.; Zhu, Z.W.; Zha, D.S. Amelioration of oxidative damage in Solanum melongena seedlings by 24-epibrassinolide during chilling stress and recovery. Biol. Plant. 2015, 59, 350–356. [Google Scholar] [CrossRef]
- Zhou, Y.L.; You, X.Y.; Wang, X.Y.; Cui, L.H.; Jiang, Z.H.; Zhang, K.P. Exogenous 24-epibrassinolide enhanced drought tolerance and promoted BRASSINOSTEROID-INSENSITIVE2 expression of quinoa. Plants 2024, 13, 873. [Google Scholar] [CrossRef]
- Evandro, F.M.; Francisco, V.S.S.; Antônio, M.P.B.; Lourival, F.C.; Emanoela, P.P.; Nubia, M.F. Effect of soil conditioners on the chemical attributes of a saline-sodic soil and on the initial growth of the castor bean plant. Semin. Cienc. Agrar. 2015, 36, 2527–2538. [Google Scholar]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Jiang, G.Q.; Wang, S.C.; Xie, J.; Tan, P.; Han, L.P. Discontinuous low temperature stress and plant growth regulators during the germination period promote roots growth in alfalfa (Medicago sariva L.). Plant Physiol. Biochem. 2023, 197, 107624. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Sun, S.Q.; Shi, Z.Z.; He, F.Q.; Qi, G.X.; Li, X.; Niu, Y.N. Characterization of cytoskeletal profilin genes in plasticity elongation of mesocotyl and coleoptile of maize under diverse abiotic stresses. Int. J. Mol. Sci. 2024, 25, 11693. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Anna, B.K.; Jennifer, M.; Szymon, S.; Justyna, M.; Piotr, O.; Jacek, Z. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of miscanthus × giganteus and Zea mays at low temperature. Planta 2020, 252, 23. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.X.; Tao, Y.F.; Lu, S.Q.; Li, Y.; Dong, Y.L.; Jiang, B.J.; Xi, B.W.; Qiang, J. Integration of transcriptome, histopathology, and physiological indicators reveals regulatory mechanisms of largemouth bass (Micropterus salmoides) in response to carbonate alkalinity stress. Aquaculture 2025, 596, 741883. [Google Scholar] [CrossRef]
- Li, Y.M.; Ye, Y.C.; Zhu, X.Y.; Wei, Y.X.; Li, Y.; Sun, Z.; Zhou, K.; Gao, P.C.; Yao, Z.L.; Lai, Q.F. Transcriptional analysis reveals antioxidant, ion transport, and glycolysis mechanisms in Litopenaeus vannamei gills involved in the response to high alkali stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2025, 306, 111868. [Google Scholar] [CrossRef]
- Han, Q.H.; Zhu, Q.X.; Shen, Y.; Lee, M.; Lübberstedt, T.; Zhao, G.W. QTL mapping low-temperature germination ability in the maize IBM Syn10 DH population. Plants 2022, 11, 214. [Google Scholar] [CrossRef]
- Lee, H.S.; Sasaki, K.; Higashitani, A.; Ahn, S.N.; Sato, T. Mapping and characterization of quantitative trait loci for mesocotyl elongation in rice (Oryza sativa L.). Rice 2012, 5, 13. [Google Scholar] [CrossRef]
- Lee, H.S.; Sasaki, K.; Kang, J.W.; Sato, T.; Song, W.Y.; Ahn, S.N. Mesocotyl elongation is essential for seedling emergence under deep-seeding condition in rice. Rice 2017, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C. Molecular mechanisms supporting rice germination and coleoptile elongation under low oxygen. Plants 2020, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Lu, X.; Liu, H.; Zhao, Q.; Ye, G. Mesocotyl elongation, an essential trait for dry-seeded rice (Oryza sativa L.): A review of physiological and genetic basis. Planta 2020, 251, 27. [Google Scholar] [CrossRef]
- Narsai, R.; Edwards, J.; Roberts, T.; Whelan, J.; Joss, G.; Atwell, B. Mechanisms of growth and patterns of gene expression in oxygen-deprived rice coleoptiles. Plant J. 2015, 82, 25–40. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, P.; Zhao, Z.; Zhao, G.; Tian, B.; Wang, J.; Wang, G. Mapping QTL controlling maize deep-seeding tolerance-related traits and confirmation of a major QTL for mesocotyl length. Theor. Appl. Genet. 2012, 124, 223–232. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.R.; Wang, Y.T.; Ma, M.J.; Zhang, Q.F.; Bai, T.; Li, Z.C.; Zhang, Z.Y.; Yin, H.Q.; Wang, Y. Association mapping of mesocotyl and coleoptile length in rice using various genome-wide association study models. Crop Sci. 2024, 64, 3417–3429. [Google Scholar] [CrossRef]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef]
- Liu, H.M.; Zhang, Y.; Lu, S.; Chen, H.; Wu, J.W.; Zhu, X.; Zou, B.H.; Hua, J. HsfA1d promotes hypocotyl elongation under chilling via enhancing expression of ribosomal protein genes in Arabidopsis. New Phytol. 2021, 231, 646–660. [Google Scholar] [CrossRef]
- Jin, J.J.; Sun, W.C.; Wu, J.Y.; Fang, Y.; Li, X.C.; Ma, L.; Liu, L.J.; Zeng, R. Hypocotyl elongation based on HY5 transcription factor in cold resistant winter rapeseed (Brassica napus L.). Oil Crop Sci. 2022, 7, 40–52. [Google Scholar] [CrossRef]
- Zhou, J.X.; Min, D.D.; Li, Z.L.; Fu, X.D.; Zhao, X.M.; Wang, J.H.; Zhang, X.H.; Li, F.J.; Li, X.A. Effects of chilling acclimation and methyl jasmonate on sugar metabolism in tomato fruits during cold storage. Sci. Hortic. 2021, 289, 110495. [Google Scholar] [CrossRef]
- Wang, H.B.; Gong, M.; Xin, H.; Tang, L.Z.; Dai, D.Q.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857–865. [Google Scholar] [CrossRef]
- Du, J.J.; Peng, J.J.; Chen, T.T.; Shui, X.; Liao, H.Z.; Lin, X.K.; Zhou, K.B. Respiratory mechanism of calcium magnesium foliar fertilizer in alleviating sugar-dropping in Feizixiao litchi pulp. J. South. Agric. 2023, 54, 356–364. [Google Scholar]
- Zhou, W.; Cao, X.N.; Li, H.Y.; Cui, X.K.; Diao, X.M.; Qiao, Z.J. Genomic analysis of hexokinase genes in foxtail millet (Setaria italica): Haplotypes and expression patterns under abiotic stresses. Int. J. Mol. Sci. 2025, 26, 1962. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.L.; He, X.X.; Li, L.; Liu, Z.C.; Zhang, G.B.; Lv, J.; Yu, J.H. Regulatory role of exogenous 24-epibrassinolide on tomato fruit quality. BMC Plant Biol. 2025, 25, 703. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.H.; Tang, X.D.; Cai, J.X.; Tan, R.Y.; Gao, X. Effects of exogenous EBR on the physiology of cold resistance and the expression of the VcCBF3 gene in blueberries during low-temperature stress. PLoS ONE 2025, 20, e0313194. [Google Scholar] [CrossRef]
- Wang, C.L.; Chen, S.; Dong, Y.P.; Ren, R.J.; Chen, D.F.; Chen, X.W. Chloroplastic Os3BGlu6 contributes significantly to cellular ABA pools and impacts drought tolerance and photosynthesis in rice. New Phytol. 2020, 226, 1042–1054. [Google Scholar] [CrossRef]
- Wei, J.P.; Chen, Q.S.; Lin, J.X.; Chen, F.Q.; Chen, R.N.; Liu, H.L.; Chu, P.Y.; Lu, Z.Y.; Li, S.Z.; Yu, G.B. Genome-wide identification and expression analysis of tomato glycoside hydrolase family 1 β-glucosidase genes in response to abiotic stresses. Biotechnol. Biotechnol. Equip. 2022, 36, 268–280. [Google Scholar] [CrossRef]
- Huang, X.Y.; Guo, W.J.; Yang, L.; Zou, Z.G.; Zhang, X.Y.; Addo-Danso, S.D.; Zhou, L.L.; Li, S.B. Effects of drought stress on non-structural carbohydrates in different organs of cunninghamia lanceolata. Plants 2023, 12, 2477. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N.; Hossain, Z.; Zhao, B.Y.; Bai, X.D.; Mao, T.T. New insights into light spectral quality inhibits the plasticity elongation of maize mesocotyl and coleoptile during seed germination. Front. Plant Sci. 2023, 14, 1152399. [Google Scholar] [CrossRef]

| Sample | Raw_Bases | Clean_Bases | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|
| CK | 7,190,234,245 | 7,060,135,638 | 54.80 | 97.57 | 93.36 |
| CKE | 7,831,246,761 | 7,684,241,030 | 53.78 | 97.62 | 93.49 |
| LT | 7,102,597,973 | 6,979,600,983 | 54.32 | 97.63 | 93.51 |
| LTE | 7,645,088,626 | 7,513,833,740 | 54.36 | 97.70 | 93.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhao, X.; Li, X.; Niu, Y. Brassinosteroids Enhance Low-Temperature Resistance by Promoting the Formation of Sugars in Maize Mesocotyls. Plants 2025, 14, 2612. https://doi.org/10.3390/plants14172612
Sun S, Zhao X, Li X, Niu Y. Brassinosteroids Enhance Low-Temperature Resistance by Promoting the Formation of Sugars in Maize Mesocotyls. Plants. 2025; 14(17):2612. https://doi.org/10.3390/plants14172612
Chicago/Turabian StyleSun, Siqi, Xiaoqiang Zhao, Xin Li, and Yining Niu. 2025. "Brassinosteroids Enhance Low-Temperature Resistance by Promoting the Formation of Sugars in Maize Mesocotyls" Plants 14, no. 17: 2612. https://doi.org/10.3390/plants14172612
APA StyleSun, S., Zhao, X., Li, X., & Niu, Y. (2025). Brassinosteroids Enhance Low-Temperature Resistance by Promoting the Formation of Sugars in Maize Mesocotyls. Plants, 14(17), 2612. https://doi.org/10.3390/plants14172612






