Emerging Invasive Weeds in Iran: Occurrence, Ecological Impacts, and Sustainable Management
Abstract
1. Introduction
2. Concept and Criteria of Weed Invasiveness
3. Detailed Species Profiles
3.1. Agricultural Field Invasive Weeds
3.1.1. Ambrosia psilostachya DC
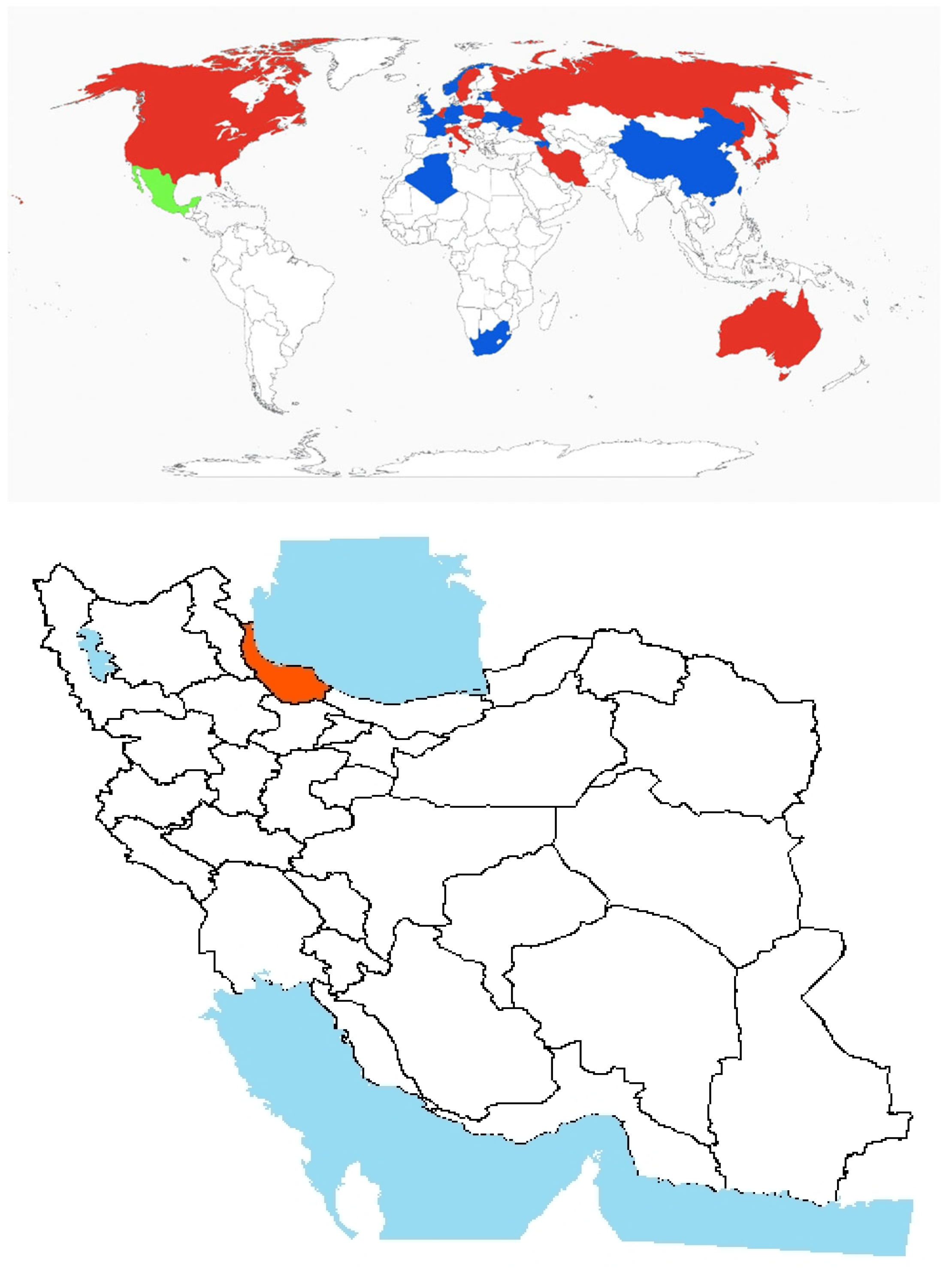
- Origin and global invasiveness: The genus Ambrosia includes approximately 40 species and numerous subspecies, many of which are known for their allergenic properties [7]. A. psilostachya DC. was first recorded in Europe in the 1800s [18] and has since expanded its distribution to include North America, Europe, Asia, and other parts of the Americas [19]. It is an herbaceous perennial plant native to Mexico, where it plays a role in local ecosystems. It disrupts native biodiversity and agricultural productivity across the United States, Canada, Australia, Russia, and parts of Europe. Its presence has also been documented in China, France, Germany, and South Africa, reflecting its widespread introduction across Europe, Asia, and Africa (Figure 1) [7]. In Iran, the species was initially identified in 1991 in Anzali, Guilan Province (Figure 1). Subsequent investigations by the Guilan Research Institute confirmed its establishment in the region [20]. Since its first detection, the species has spread to several surrounding areas, including Rezvanshahr, Talesh, Astara counties, and the Rasht Trench (Figure 1).
- Morphological description, biology, and ecology: A. psilostachya is an erect herbaceous perennial characterized by horizontal running rootstocks, allowing it to propagate both vegetatively via rhizomes and sexually through seed production. The stems are rigidly upright and may be unbranched or heavily branched in the upper parts of the plant. Leaves are thick, hairy, and oval-lanceolate in outline, typically reaching lengths of up to 5 inches and widths of 2 inches. They are deeply dissected into narrow lobes, often with secondary lobing. The leaf surface, covered in fine hairs, tends to accumulate dust, which can hinder herbicide adhesion and reduce control efficacy [21,22]. The inflorescence is composed of unisexual flower heads, with male (staminate) and female flowers located on different parts of the same plant. Staminate flowers are small, bead-like, yellow to greenish, and short-stalked, initially densely clustered and later spreading during maturation. Pistillate (female) flowers are solitary and sparse, typically found at the base of the floral cluster, along the stem, or in axils, accompanied by large leaf-like bracts [23].
- Agricultural impact and management strategies: A. psilostachya poses considerable ecological and agricultural challenges due to its allelopathic properties and prolific spread along roadsides and non-agricultural lands [26]. The species releases toxic compounds that inhibit the growth of surrounding vegetation, adversely impacting crops such as peanuts, sunflowers, corn, soybeans, and wheat [27]. In agricultural systems, high densities of A. psilostachya have been linked to notable yield reductions. Additionally, the species produces highly allergenic pollen, ranking as the second most significant allergen in the United States, affecting nearly 25% of the population [28]. The impact of pollen on public health has also been highlighted in earlier work [29]. In New Zealand, risk assessments have identified A. psilostachya as a high-risk invasive species, emphasizing the need for robust control strategies [30].
3.1.2. Boreava orientalis: Jaub. & Spach
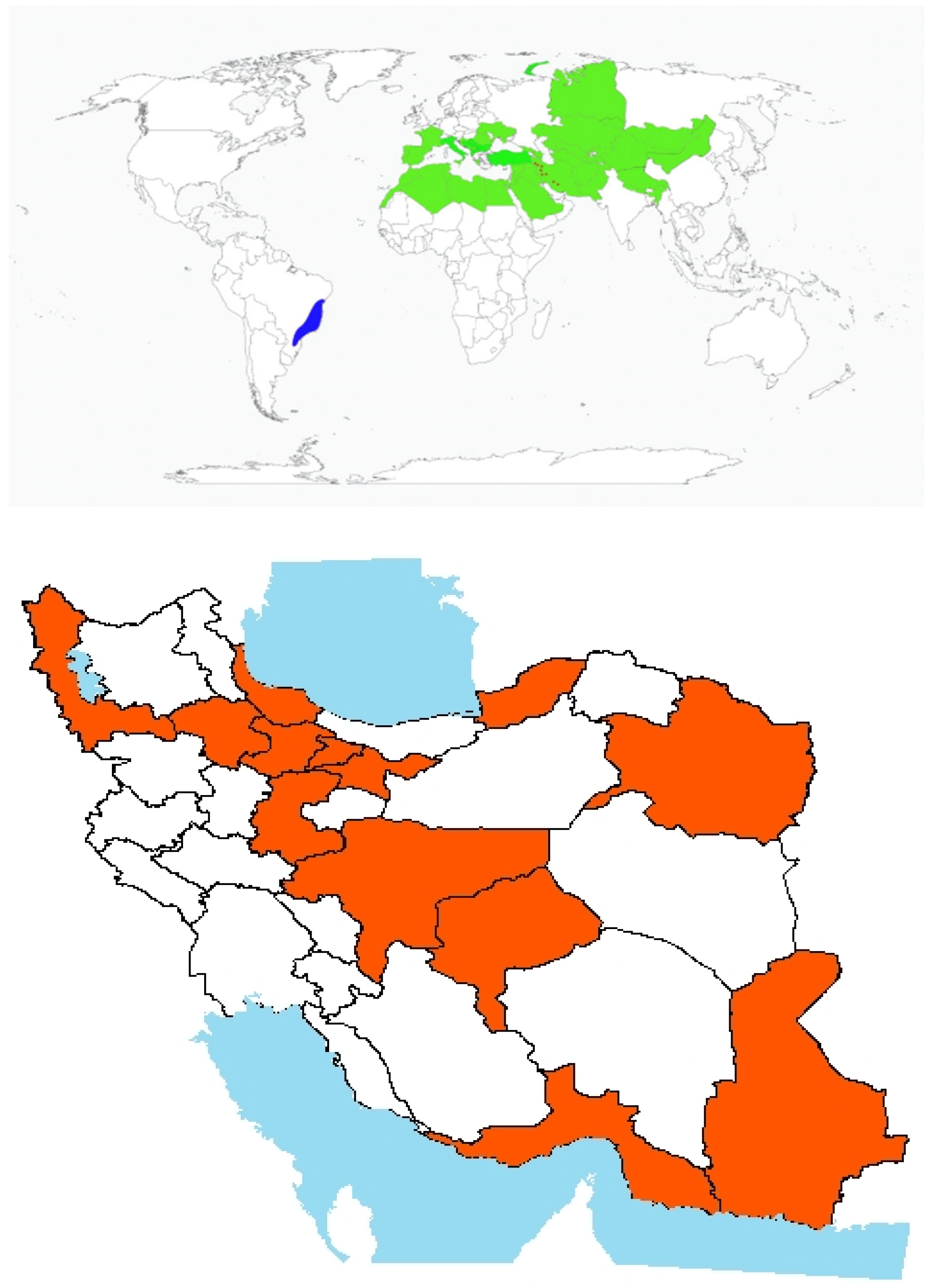
- Origin and global invasiveness: It is native to parts of western Asia, including Iran, Pakistan, Syria, and Turkey (Figure 2). It is widespread from Kütahya to Istanbul in Turkey and from Armenia to the Euphrates [38]. In Iran, it was initially reported from Khorasan and Chaharmahal Bakhtiari (not as a weed), but it has been more recently identified in Kurdistan croplands [39]. According to the Manual of Alien Plants of Belgium records also document its presence in Belgium, Germany, France, the United Kingdom, and Pakistan [40].
- Morphological description, biology, and ecology: B. orientalis Jaub. & Spach is an annual glabrous herb that reaches 15–30 cm in height and typically branches above the middle of the plant into a loose, ebracteate inflorescence [41]. It exhibits a glaucous appearance with yellow flowers and simple leaves. Basal leaves are oblong-lanceolate, clasping, auriculate or sagittate, with entire margins and acute apices, while cauline leaves are also sagittate. The petals are approximately 5 mm long, and the calyx is open, not or scarcely saccate. Petals are spathulate to oblong–lanceolate, filaments are slightly dilated, and the ovary is 1–2-ovulate with a capitate stigma. The slender pedicels, 6–8 mm long in fruit, are erect or spreading. The fruit is indehiscent, one-seeded, beaked, ovate, and may be winged or wingless; it is 8–10 mm long, somewhat tubercled, and plicate between the wings. The radicle is incumbent, and the species has a chromosome number of 14n with small-sized chromosomes [41].
- Agricultural impact and management challenges: B. orientalis is a winter annual weed that commonly infests irrigated and rain-fed fields of wheat, barley, chickpeas, and rapeseed [39,46]. It is rarely found in non-arable lands such as pastures and gardens, likely due to the absence of annual tillage. This species has also been reported in winter wheat fields on the Anatolian Plateau of Turkey [47]. Notably, B. orientalis exhibits allelopathic activity through the release of isothiocyanates derived from glucosinolate breakdown, which may contribute to its competitiveness and render it unpalatable or toxic to livestock [48].
3.1.3. Cynanchum acutum L.
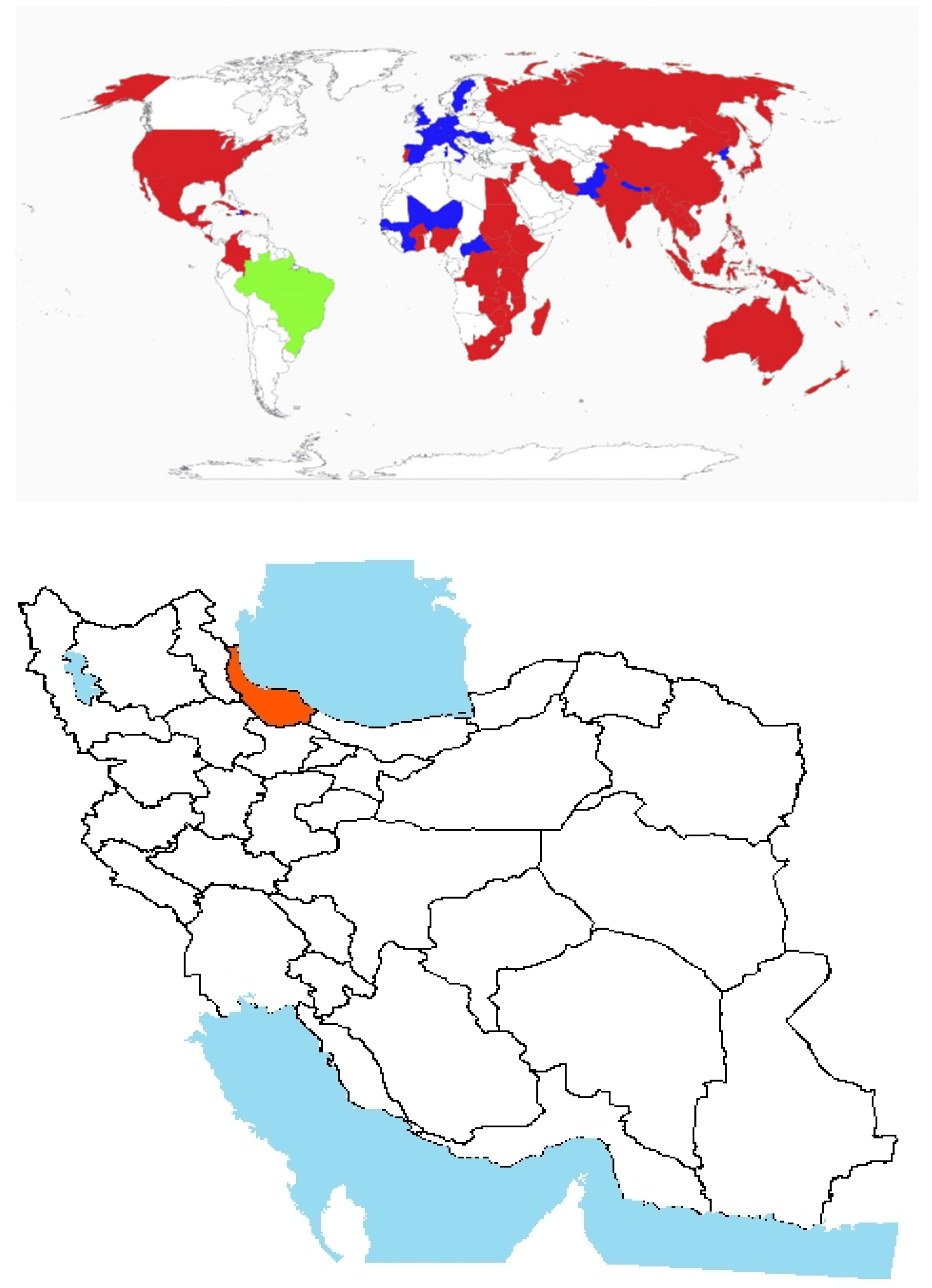
- Origin and global invasiveness: The genus name “Cynanchum” is derived from the Greek meaning “dog strangler”, likely referencing its twining growth habit, while “acutum” alludes to its sharply pointed leaves [51]. Its origin lies within the Mediterranean Basin, encompassing Southern Europe, North Africa, and parts of Western Asia (Figure 3) [52]. The perennial vine thrives in warm, arid climates and is frequently found in coastal regions, on sandy dunes, and in disturbed habitats [53]. In addition to its invasive potential, Cynanchum L. has been used in traditional medicinal practices in some cultures.
- Morphological description, biology, and ecology: C. acutum is a perennial species distinguished by either glabrous surfaces or multicellular hairs, exhibiting fibrous, fleshy, or woody roots with greenish stems. Its leaves are opposite, petiolate, and typically cordate, elliptical, ovate, or obovate, with entire margins and acute apices. Occasionally, small leafy stipules are present. The plant produces extra-axillary inflorescences that can be raceme-like, corymbose, or umbel-like. Flowers range in diameter from 3 to 15 mm, displaying imbricate or contorted aestivation with dextrorse orientation. They are nectariferous and have a divided corolla that is contorted in the bud stage, appearing rotate, sub-rotate, or tubular in shape. Flower coloration varies from white to green and yellow, and occasionally reddish. The calyx is free to the base, composed of erect sepals often containing basal glands. The seeds are oval, flat, and brown, well-adapted for wind dispersal, facilitating long-distance propagation [41,56].
- Ecological impact and management strategies: C. acutum possesses a robust root system and an ascending, twining stem that wraps around tree trunks, extending into branches and obstructing light penetration to the canopy. This growth behavior damages tree buds and inhibits photosynthesis. It produces flowers and seeds on exposure to light [57]. Control methods include mechanical removal and herbicide application. This deep-rooting behavior enhances its ability to persist and regenerate, making it a challenging invasive species. Effective herbicides include glufosinate ammonium [58], paraquat, and glyphosate [56], while picloram and nicosulfuron have shown limited efficacy [59]. As such, the development of effective, long-term management strategies is essential for mitigating its ecological and agricultural impact.
3.1.4. Ibicella lutea (Lindl.) Van Eselt.
- Origin and global invasiveness: The family Martyniaceae comprises five genera and 16 species globally, with the greatest concentration in Argentina and other parts of South America (Figure 4) [60]. A distinguishing morphological trait of this family is the presence of fruits with a curved beak, which may be longer or shorter than the fruit body [61]. The genus Ibicella, part of Martyniaceae, includes eight species that are naturally distributed from South America to Mexico [62]. I. lutea (Lindl.) Van Eselt., commonly known as the yellow unicorn plant, has previously been misidentified in various studies. For instance, a study on Lesos Island in the eastern Aegean region misclassified a population of I. lutea, later clarified to be aligned with Proboscidea louisianica (Mill.) Thell. ssp. louisianica based on Bretting’s interspecific classification [63,64]. While I. lutea has been recorded in neighboring countries such as Turkey, no members of the genus Proboscidea have yet been documented there [65].
- Morphological description, biology, and ecology: I. lutea, a member of the Martyniaceae family in the order Lamiales, is an annual plant with tuberous roots, native to the New World. The family includes both annual and perennial herbaceous plants, noted for their glandular hairs and strong fragrance [69]. I. lutea typically reaches heights of 20–120 cm and is characterized by hairy, nearly circular leaves and clusters of bright yellow flowers with red spots internally. The inflorescences bear long petioles that may either terminate or split, with broad, scaly bracts situated just below the flowers [64]. The flowers are bisexual, composed of five unequal sepals and a bilabiate corolla, with stamens arranged epipetally—two long, two short, and one vestigial. The superior ovary exhibits parietal placentation and terminates in a two-lobed stigma that often closes upon contact. The fruit, initially fleshy, matures into a woody capsule with a prominent beaked endocarp, culminating in two curved, horn-like projections longer than the fruit body itself [69]. The seeds are dark, flattened, and uneven in texture, while the fruit morphology varies from smooth to rough, resembling the spiny capsules of Datura stramonium L.
- Agricultural impact and management strategies: Field observations and research indicate that I. lutea poses a growing threat to both agricultural and natural ecosystems, particularly in arid and semi-arid regions across the southern United States. Its large size and vigorous growth habit have demonstrated significant competitive ability against both pasture and row crops. Studies show that I. lutea can reduce cotton (Gossypium hirsutum L.) yields by 60 to 74% [72], with weekly interference resulting in incremental yield losses of up to 5% [73]. Furthermore, its competitive effect has been observed to extend as far as 0.5 m from neighboring plants by the season’s end [72]. Although previously undocumented in Iran, recent reports confirm its invasion in the Ilam and Kermanshah Provinces, indicating a recent introduction [68].
3.1.5. Picnomon acarna (L.) Cass.
- Origin and global invasiveness: P. acarna (L.) Cass. is a spiny, annual, and herbaceous plant native to the Mediterranean region (Figure 5). Initially prevalent in neglected areas and rangelands, P. acarna has become a major invasive weed in rainfed agricultural fields, especially in western Iran [77]. It has also been reported in the western regions of Victoria and the southeastern parts of South Australia (Figure 5). The species’ non-palatability and resilience to soil compaction and erosion have enabled its proliferation in Iranian forests and pastures (Figure 5) [78]. The increasing adoption of conservation tillage and reduced soil disturbance in rainfed systems [79] has further facilitated the spread of P. acarna.

- Morphological description, biology, and ecology: Belonging to the Asteraceae family, it exhibits typical morphological traits such as spiny, hairy leaves that can accumulate dust and reduce herbicide absorption [22]. A notable characteristic of this plant is its height, reaching approximately 50 cm, with leaves bearing spines that range from 10 to 15 cm in length [80]. The species produces seeds with a pappus measuring 1 to 2 cm in length, aiding in wind dispersal over long distances [81]. Notably, its seeds are primarily photoblastic, meaning that they require light for germination [82]. This germination strategy contributes to its success in open and disturbed habitats. Its effective seed dispersal and environmental adaptability enhance its invasive potential across diverse habitats [82].
- Agricultural impact and management strategies: The proliferation of P. acarna has intensified in rainfed agricultural systems due to favorable environmental and management conditions [83]. This invasive species poses a significant threat to key rainfed crops, such as wheat (Triticum aestivum L.) and chickpeas (Cicer arietinum L.). Its rigid, spinous structure severely complicates harvest operations, particularly for crops like chickpeas and lentils, which are traditionally harvested by hand. In fields heavily infested with P. acarna, farmers often abandon cultivation altogether due to the high risk of injury posed by the weed’s long spines [84]. Up to 25% yield reduction can occur at a density of 16 plants of P. acarna per square meter [82].
3.1.6. Physalis divaricata D. Don
- Origin and global invasiveness: P. divaricata D. Don, commonly known as Annual Groundcherry, is primarily native to Latin America and South America (Figure 6). Vargas et al. (2001) classify it as native to Mexico, while Ramírez and Davenport (2016) recognize Colombia as a major centre of its natural distribution [86,87].
- Morphological description, biology and ecology: The Solanaceae family includes approximately 2500 species across 90 genera, predominantly found in warm regions such as Central and South America, and temperate climates worldwide. This diverse plant family ranges from herbaceous annuals to woody perennials. Their leaves are typically alternate with variable shapes and sizes, while the flowers are mostly unisexual and occasionally hermaphroditic. Floral structures usually exhibit pentamerous symmetry, although variability exists. Fruits are commonly berries or capsules [67]. As a member of this family, it is an annual plant that produces berry-type fruits which transition in color from green to purple black upon ripening. The immature berries are known to be toxic [89]. Fruiting generally occurs by late June, with seed germination peaking approximately four weeks later. Seed viability remains high when buried at depths of 10–30 cm, whereas surface-level seeds are more susceptible to desiccation and mortality. Germination typically begins in March following winter moisture absorption in January, with full fruit development occurring by April [90]. The seeds can exhibit a germination rate of 93% under alternating temperatures of 10/20 °C (night/day), although germination drops by 41% under dark conditions at the same temperature. Salinity also affects germination: a concentration of 22.85 mM reduces the maximum germination by 50%. Additionally, soil pH levels between 6 and 7 are optimal for germination, indicating the species’ sensitivity to environmental factors such as temperature, light, salinity, and acidity [91].
- Agricultural impact and management strategies: P. divaricata is recognized as a problematic weed across various agricultural systems in Iran due to its entangling growth habit, which complicates mechanical harvesting, particularly in fine grain and sugar beet crops [90,93]. In potato fields and sugar beet systems, its synchronous growth cycle exacerbates competition with crops [94,95]. Its competitive presence is most pronounced during the summer months in summer crops across the Lorestan and Chaharmahal Bakhtiari Provinces [94]. The weed has also been reported in orchards in Lorestan [94] and Dare Shahr in Ilam Province [68]. Notably, high-density infestations of P. divaricata can lead to crop yield reductions of 50–60% [96]. Additionally, P. divaricata has been observed in tomato fields in southeastern Iran [97], highlighting its broad ecological range and significant agricultural impact.
3.1.7. Vicia hyrcanica Fisch. & C.A. Mey.
- Origin and global invasiveness: V. hyrcanica Fisch. & C.A. Mey. is a climbing annual winter legume characterized by weak stems and seed-based dispersal. It is native to northern Iran and has progressively extended its range into western parts of the country [80]. The genus Vicia is made up of herbaceous legumes, primarily distributed across temperate regions, particularly in Mediterranean areas (Figure 7) [101,102]. Despite their wide distribution, comprehensive information on the Vicia genus’s natural distribution, taxonomy, and agricultural potential remains underexplored. In Iran, V. hyrcanica is commonly found in rainfed fields, pastures, and perennial horticultural systems. In recent years, the species has rapidly spread into both disturbed and undisturbed habitats across the country (Figure 7).
- Morphological description, biology, and ecology: V. hyrcanica typically germinates in late winter, aligning its life cycle with that of cultivated legume crops in Iran. The stem is erect and can reach a length of up to 80 cm. Its leaves consist of three to eight pairs of linear to narrowly elliptic leaflets, each measuring between 1.5 and 3.0 cm. The species produces solitary, yellow flowers that range from 1.0 to 1.8 cm in length. The mature legume is circular in cross-section, approximately 4 cm long, and dark brown to black in color. Upon ripening, the pod dehisces into two twisted halves, facilitating seed dispersal [103].
- Agricultural impact and management strategies: V. hyrcanica is widely recognized as an important cover crop species in Iran [106]. Despite efforts in domestication and breeding, many cover crops, including V. hyrcanica, retain seed dormancy mechanisms inherited from their wild ancestors [107,108]. These dormancy traits can limit their effective integration into cropping systems and raise concerns about their potential weediness. The presence of seed dormancy allows V. hyrcanica to persist in the soil seed bank and germinate unpredictably, potentially disrupting future crop cycles. Studies have shown that both genotype and environmental factors during seed development, as well as postharvest storage conditions, influence dormancy levels and germination timing [108,109,110]. Additionally, climatic conditions during the parental plant’s development significantly impact seed germination potential [111], increasing the species’ risk of becoming invasive in new areas.
3.2. Aquatic Invasive Weeds
3.2.1. Azolla filiculoides Lam. and Azolla pinnata
- Origin and global invasiveness: Azolla spp., commonly referred to as mosquitofern, represent a genus of small aquatic ferns with a rich evolutionary history. The first recorded collection of Azolla was made by Baptiste-Jean Lamarck in South America, although genetic differentiation among species indicates varied origins [113]. Fossil evidence suggests that Azolla has existed for over 70 million years, tracing its origins back to the early Cenozoic era [114]. Historically believed to have originated in the Americas, subsequent genetic studies have revealed a more complex and widespread origin. Today, Azolla species are recognized as having diverse evolutionary roots across tropical, subtropical, and warm temperate regions of Africa, Asia, and the Americas (Figure 8) [115], with some species naturally occurring in parts of Europe and Asia [116]. Due to its rapid vegetative growth and nitrogen-fixing ability, Azolla has been introduced to several regions, including Australia and parts of Africa, for agricultural and ecological purposes. However, its widespread introduction has led to invasiveness in multiple countries, such as the United States, the United Kingdom, France, Iran, and South Africa (Figure 8).
- Morphological description, biology, and ecology: Azolla spp. (Salviniaceae) are small, floating aquatic ferns characterized by polygonal or triangular shapes [117]. Most species measure between 3 and 4 cm, except for Azolla nilotica, which may exceed this size. The stems are covered with minute, alternate, scaly leaves arranged in two overlapping ranks. Roots are unbranched but bear fine lateral rootlets that give a feathery appearance when submerged [118]. Color and size can vary significantly depending on environmental conditions, such as light and nutrient availability [119]. Azolla spp. reproduce both vegetatively and sexually. While vegetative propagation is the predominant method [120], sexual reproduction occurs, involving the formation of megaspores and microspores; however, it is considered a rare phenomenon in natural populations [113].
- Ecological impact and management challenges: Azolla spp. can severely disrupt aquatic ecosystems by reducing oxygen levels, altering water acidity, limiting light penetration, and disturbing aquatic food webs [125]. In northern Iran, particularly in the Guilan and Mazandaran Provinces, the dense proliferation of Azolla in Anzali Wetland has led to increased toxicity and mortality of fish and other aquatic organisms, pushing the ecosystem toward collapse [126] (Figure 8). In rice paddies, the rapid surface growth of Azolla forms a thick mat that interferes with rice transplanting by bending and submerging seedlings, thus impeding early crop establishment [126].
3.2.2. Eichhornia crassipes (Mart.) Solms
- Origin and global invasiveness: E. crassipes (Mart.) Solms (Family: Pontederiaceae), commonly known as water hyacinth, is a floating aquatic plant native to the Amazon Basin in South America, where it naturally inhabits rivers, lakes, and wetlands (Figure 9) [130]. It is well adapted to tropical and subtropical climates, thriving in nutrient-rich freshwater environments. The species was first described in 1842 by the German botanist Kunth. Initially introduced outside its native range as an ornamental plant in the late 19th and early 20th centuries, E. crassipes quickly gained popularity due to its attractive appearance. However, its ability to reproduce rapidly through both vegetative and sexual means has made it one of the most invasive aquatic plants globally [131].
- Morphological description, biology, and ecology: The plant is characterized by its circular to oval-shaped leaves with flexible, spongy-covered petioles and lilac to blue flowers. The roots are fibrous and unbranched [136], facilitating nutrient absorption while minimizing resistance in aquatic conditions. Mature plants exhibit pendant roots, rhizomes, stolons, leaves, inflorescences, and fruit. The air-filled sacs in their leaves and stems allow it to remain on the water’s surface [137].
- Ecological impact and management challenges: E. crassipes poses severe threats to aquatic ecosystems by reducing water pH and oxygen levels, obstructing waterways, accelerating sedimentation [143], and disrupting native biodiversity and activities such as fishing, irrigation, and transportation [142]. Its dense mats significantly hinder light penetration, degrading water quality and aquatic health, which, in turn, threatens food security [144]. Furthermore, it depletes essential nutrients in water bodies [145], contributing to the ecological decline of wetlands. The unchecked proliferation of E. crassipes often leads to habitat monopolization, outcompeting native flora and fauna [146]. In some wetlands, the increased biomass of this invasive species has resulted in ecosystem collapse. The economic burden of managing E. crassipes is substantial, with global control efforts costing an estimated USD 124 million annually [147]. Despite its invasive nature, it has also been investigated for beneficial applications such as phytoremediation and bioenergy production [148].
4. Policy and Stakeholder Engagement
5. Conclusions
6. Future Research Direction
- Research should prioritize the development of herbicide formulations specifically tailored to the physiological and ecological traits of invasive species.
- The introduction of non-native plants such as Azolla spp., I. lutea, and P. acarna into new habitats, including Iran, underscores the urgent need for comprehensive risk assessments and monitoring systems. Remote sensing and GIS technologies can significantly enhance early detection, spatial analysis, and targeted control strategies.
- For managing E. crassipes, studies should investigate the biotic and abiotic factors in its native range that naturally regulate its growth to inform new management tactics.
- Research should focus on developing tillage methods, particularly nocturnal tillage, to inhibit P. acarna seed germination. As its photoblastic seeds require light, nighttime soil disturbance may prevent emergence by keeping seeds in darkness. Studies must identify optimal tillage depth, timing, and frequency, while assessing impacts on soil health, biodiversity, and crop yield.
- Future research should focus on the weed’s germination biology under various environmental conditions. Germination rates of 93% of P. divaricata at 10/20 °C (night/day) and the 50% reduction under salinity levels of 22.85 mM indicate that environmental manipulation could be leveraged for control.
- For B. orientalis, there is a need to assess the effectiveness of shifting from post-emergence to pre-emergence herbicide strategies. Field trials across diverse agro-ecological zones are required to evaluate the efficacy of pre-emergent applications in suppressing germination and reducing the risk of resistance.
- Managing invasive species like B. orientalis and A. psilostachya calls for coordinated action involving adaptive management, public awareness, and continuous monitoring. While chemical control remains common, biological methods, such as the use of O. communa against A. psilostachya, offer sustainable alternatives.
7. Methodology
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasena, N.R. ‘Seeing Weeds with New Eyes’ Part II–Some Historical Perspectives and ‘Proto Weeds’. Weeds—J. Asian-Pac. Weed Sci. Soc. 2020, 2, 1–16. [Google Scholar]
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An Ecological Future for Weed Science to Sustain Crop Production and the Environment. A Review. Agron. Sustain. Dev. 2020, 40, 28. [Google Scholar] [CrossRef]
- Montagnani, C.; Gentili, R.; Brundu, G.; Caronni, S.; Citterio, S. Accidental Introduction and Spread of Top Invasive Alien Plants in the European Union through Human-Mediated Agricultural Pathways: What Should We Expect? Agronomy 2022, 12, 423. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Panzavolta, T.; Bracalini, M.; Benigno, A.; Moricca, S. Alien Invasive Pathogens and Pests Harming Trees, Forests, and Plantations: Pathways, Global Consequences and Management. Forests 2021, 12, 1364. [Google Scholar] [CrossRef]
- Enders, M.; Havemann, F.; Ruland, F.; Bernard-Verdier, M.; Catford, J.A.; Gómez-Aparicio, L.; Haider, S.; Heger, T.; Kueffer, C.; Kühn, I.; et al. A Conceptual Map of Invasion Biology: Integrating Hypotheses into a Consensus Network. Glob. Ecol. Biogeogr. 2020, 29, 978–991. [Google Scholar] [CrossRef]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Cuthbert, R.N.; Essl, F.; Haubrock, P.J.; Ricciardi, A.; Courchamp, F. Biological Invasions Are as Costly as Natural Hazards. Perspect. Ecol. Conserv. 2023, 21, 143–150. [Google Scholar] [CrossRef]
- Westbrooks, R.G. Invasive Plants: Changing the Landscape of America: Fact Book; Federal Interagency Committee for the Management of Noxious and Exotic Weeds: Washington, DC, USA, 1998.
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Sohrabi, S.; Vilà, M.; Zand, E.; Gherekhloo, J.; Hassanpour-bourkheili, S. Alien Plants of Iran: Impacts, Distribution and Managements. Biol. Invasions 2023, 25, 97–114. [Google Scholar] [CrossRef]
- Heshmati, I.; Khorasani, N.; Shams-Esfandabad, B.; Riazi, B. Forthcoming Risk of Prosopis Juliflora Global Invasion Triggered by Climate Change: Implications for Environmental Monitoring and Risk Assessment. Environ. Monit. Assess. 2019, 191, 72. [Google Scholar] [CrossRef]
- Stastny, M.; Schaffner, U.; Elle, E. Do Vigour of Introduced Populations and Escape from Specialist Herbivores Contribute to Invasiveness? J. Ecol. 2005, 93, 27–37. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Power, A.G. Release of Invasive Plants from Fungal and Viral Pathogens. Nature 2003, 421, 625–627. [Google Scholar] [CrossRef]
- Weber, E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Hallett, S.G. Dislocation from Coevolved Relationships: A Unifying Theory for Plant Invasion and Naturalization? Weed Sci. 2006, 54, 282–290. [Google Scholar] [CrossRef]
- Zimdahl, R.L.; Basinger, N.T. Fundamentals of Weed Science; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Chauvel, B.; Dessaint, F.; Cardinal-Legrand, C.; Bretagnolle, F. The Historical Spread of Ambrosia artemisiifolia L. in France from Herbarium Records. J. Biogeogr. 2006, 33, 665–673. [Google Scholar] [CrossRef]
- Cheraghian, A. A Guide for Diagnosis and Detection of Quarantine Pests: Perennial Ragweed. Ambrosia Psilostachya DC. Asterales: Asteraceae; Bureau of Plant Pest Surveillance and Pest Risk Analysis; Plant Protection Organization: Tehran, Iran, 2016. [Google Scholar]
- Montagnani, C.; Gentili, R.; Smith, M.; Guarino, M.F.; Citterio, S. The Worldwide Spread, Success, and Impact of Ragweed (Ambrosia Spp.). CRC Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Sharifi Kaliani, F.; Babaei, S.; ZafarSohrabpour, Y. Study of the Effects of Dusts on the Morphological and Physiological Traits of Some Crops. J. Plant Prod. Res. 2021, 28, 205–220. [Google Scholar] [CrossRef]
- Sharifi Kalyani, F.; Babaei, S.; Zafarsohrabpour, Y.; Nosratti, I.; Gage, K.; Sadeghpour, A. Investigating the Impacts of Airborne Dust on Herbicide Performance on Amaranthus retroflexus. Sci. Rep. 2024, 14, 3785. [Google Scholar] [CrossRef]
- Bassett, I.J.; Crompton, C.W. The Biology of Canadian Weeds: 11. Ambrosia artemisiifolia L. and A. Psilostachya DC. Can. J. Plant Sci. 1975, 55, 463–476. [Google Scholar] [CrossRef]
- Fried, G.; Belaud, A.; Chauvel, B. Ecology and Impact of an Emerging Invasive Species in France: Western Ragweed (Ambrosia psilostachya DC.). Rev. d’Écologie (Terre Vie) 2015, 70, 53–67. [Google Scholar] [CrossRef]
- Ardenghi, N.; Polani, F. The Flora of the Province of Pavia (Lombardy, Northern Italy). 1. The Oltrepò Pavese. Nat. Hist. Sci. 2016, 3, 51–79. [Google Scholar] [CrossRef]
- Béres, I.; Kazinczi, G.; Narwal, S. Allelopathic Plants. 4. Common Ragweed (Ambrosia elatior L. Syn A. Artemisiifolia). Allelopath. J. 2002, 9, 27–34. [Google Scholar]
- Leather, G.R. Weed Control Using Allelopathic Crop Plants. J. Chem. Ecol. 1983, 9, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.S.W.; Batterman, S.A. Allergenic Pollen Production across a Large City for Common Ragweed (Ambrosia artemisiifolia). Landsc. Urban. Plan. 2019, 190, 103615. [Google Scholar] [CrossRef] [PubMed]
- Wodehouse, R.P. Hayfever Plants. Their Appearance, Distribution, Time of Flowering, and Their Role in Hayfever, with Special Reference to North America. Madroño 1946, 8, 201–204. [Google Scholar]
- Gerber, E.; Schaffner, U.; Gassmann, A.; Hinz, H.; Seier, M.; Müller-Schärer, H. Prospects for Biological Control of Ambrosia artemisiifolia in Europe: Learning from the Past. Weed Res. 2011, 51, 559–573. [Google Scholar] [CrossRef]
- Milakovic, I.; Fiedler, K.; Karrer, G. Management of roadside populations of invasive A mbrosia artemisiifolia by mowing. Weed Res. 2014, 54, 256–264. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Sandell, L.D.; Jugulam, M.; Kruger, G.R.; Marx, D.B.; Jhala, A.J. Integrated Management of Glyphosate-Resistant Giant Ragweed (Ambrosia trifida) with Tillage and Herbicides in Soybean. Weed Technol. 2016, 30, 45–56. [Google Scholar] [CrossRef]
- Moradi, M.; Babaei, S.; Tahmasebi, I.; Nosratti, I. Assessing Resistance to Clodinafop-Propargyl Herbicide in Wild Oat Avena Ludoviciana Populations in Wheat Fields of Some Western and Southwestern Regions of Iran. J. Appl. Res. Plant Prot. 2023, 12, 461–473. [Google Scholar]
- Moradi, M.; Babaei, S.; Nosratti, I.; Tahmasebi, I. Evaluation of the Resistance of Galium aparine L. to Common Herbicides in Wheat Fields of Western and Southwestern Iran. J. Appl. Res. Plant Prot. 2024, 13, 373–385. [Google Scholar]
- Soltani, N.; Shropshire, C.; Sikkema, P. Giant Ragweed (Ambrosia trifida L.) Control in Corn. Can. J. Plant Sci. 2011, 91, 577–581. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Guo, J.Y.; Zheng, X.W.; Luo, M.; Chen, H.S.; Wan, F.H. Reevaluation of Biosecurity of Ophraella communa against Sunflower (Helianthus annuus). Biocontrol Sci. Technol. 2011, 21, 1147–1160. [Google Scholar] [CrossRef]
- Bonini, M.; Gentili, R.; Müller-Schärer, H. Ragweed Management and the Potential Benefits and Risks of Ophraella communa in Northern Italy: Researchers Meet Their Stakeholders. Not. Soc. Bot. Ital. 2017, 26, 1–4. [Google Scholar]
- Moazzeni, H.; Zarre, S.; Al-Shehbaz, I.A.; Mummenhoff, K. Phylogeny of Isatis (Brassicaceae) and Allied Genera Based on Its Sequences of Nuclear Ribosomal DNA and Morphological Characters. Flora—Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 337–343. [Google Scholar] [CrossRef]
- Babaei, S.; Adeli, T.; Tahmasebi, I.; Mozafarian, V. Introducing Waxy Leaved Mustard (Boreava orientalis Jaub. and Spach.) as a Problematic Weed in Wheat Fields of Kurdistan Province. Cereal Res. 2019, 9, 261–269. [Google Scholar] [CrossRef]
- Verloove, F.; Groom, Q.; Brosens, D.; Desmet, P.; Reyserhove, L. Manual of the Alien Plants of Belgium. 2020. Available online: https://alienplantsbelgium.myspecies.info/ (accessed on 2 April 2024).
- Davis, P.H.; Cullen, J.; Coode, M.J.E. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1965. [Google Scholar]
- McConnell, E.G.; Evans, J.O.; Dewey, S.A. Dyer’s Woad. In Biology and Management of Noxious Rangeland Weeds; Sheley, R.L., Petroff, J.K., Eds.; OSU Press: Corvallis, OR, USA, 1999; pp. 231–237. [Google Scholar]
- Loik, M.E.; Redar, S.P. Microclimate, Freezing Tolerance, and Cold Acclimation along an Elevation Gradient for Seedlings of the Great Basin Desert Shrub, Artemisia tridentata. J. Arid. Environ. 2003, 54, 769–782. [Google Scholar] [CrossRef]
- Loik, M.E.; Harte, J. High-Temperature Tolerance of Artemisia tridentata and Potentilla gracilis under a Climate Change Manipulation. Oecologia 1996, 108, 224–231. [Google Scholar] [CrossRef]
- Young, J.A.; Evans, R.A. Germination of Dyers Woad. Weed Sci. 1971, 19, 76–78. [Google Scholar] [CrossRef]
- Adeli, T.; Tahmasebi, I.; Babaei, S.; Sadeghpour, A. Assessing Allelopathic Potential: Boreava orientalis Impact on Triticum Aestivum. J. Crop Prot. 2023, 12, 389–401. [Google Scholar]
- Tezel, C. Competitive Effects and Control of Weeds in Winter Wheat on the Anatolian Plateau, Turkey. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1975. [Google Scholar]
- Oleszek, W. Allelopathic Effects of Volatiles from Some Cruciferae Species on Lettuce, Barnyard Grass and Wheat Growth. Plant Soil. 1987, 102, 271–273. [Google Scholar] [CrossRef]
- Naghib Alsadati, M.; Babaei, S.; Tahmasebi, I.; Kiani, H. Evaluation of Airborne Dust Effect on the Efficiency of Atlantis OD, Clodinafop Propargyl and 2,4-D+MCPA Herbicides on Weed Control in Wheat. Iran. J. Field Crop Sci. 2020, 50, 4. [Google Scholar] [CrossRef]
- Babaei, S.; Ahmadi, S.; Tahmasebi, I.; Sarseyfi, M. Evaluation the Efficacy of Chemical and Non-Chemical Weed Control Approaches in Strawberry (Fragaria Ananassa) Fields in Kurdistan. Iran. J. Weed Sci. 2022, 18, 11–22. [Google Scholar] [CrossRef]
- CABI Compendium Invasive Species | CABI Digital Library. Available online: https://www.cabidigitallibrary.org/product/QI (accessed on 9 June 2025).
- Plants of the World Online | Kew Science. Available online: https://powo.science.kew.org/ (accessed on 9 June 2025).
- Wilson, B.G. Mediterranean Wild Flowers; Harpercollins Publishers Ltd.: London, UK, 2001. [Google Scholar]
- Lawlor, F.M. Herbicidal Treatment of the Invasive Plant Cynanchum rossicum and Experimental Post-Control Restoration of Infested Sites. Master’s Thesis, State University of New York College of Environmental Science and Forestry, New York, NY, USA, 2000. [Google Scholar]
- Ar, B.; Tuttu, G.; Gülçin, D.; Özcan, A.U.; Kara, E.; Sürmen, M.; Çiçek, K.; Velázquez, J. Response of an invasive plant species (Cynanchum acutum L.) to changing climate conditions and its impact on agricultural land-scapes. Land 2022, 11, 1438. [Google Scholar] [CrossRef]
- Zaifie, M. Flora of Iran (No. 28 of Estabarq Genus); Forest and Rangeland Research Institute: Tehran, Iran, 1999.
- Soteres, J.K.; Murray, D.S. Root Distribution and Reproductive Biology of Honeyvine Milkweed (Cynanchum Laeve). Weed Sci. 1982, 30, 158–163. [Google Scholar] [CrossRef]
- Berti, A.; Sattin, M.; Baldoni, G.; Del Pino, A.M.; Ferrero, A.; Montemurro, P.; Tei, F.; Viggiani, P.; Zanin, G. Relationships between Crop Yield and Weed Time of Emergence/Removal: Modelling and Parameter Stability across Environments. Weed Res. 2008, 48, 378–388. [Google Scholar] [CrossRef]
- Badli, K. Investigation of Sulfonylurea Group Herbicides in Grain Maize; Moghan Pest and Diseases Research Department: Moghan, Iran, 1997. [Google Scholar]
- Ihlenfeldt, H.D. Martyniaceae. In Flowering Plants·Dicotyledons: Lamiales (except Acanthaceae including Avicenniaceae); Springer: Berlin/Heidelberg, Germany, 2004; pp. 283–288. [Google Scholar]
- Thieret, J.W. The Martyniaceae in the Southeastern United States. J. Arnold Arbor. 1977, 58, 25–39. [Google Scholar] [CrossRef]
- Gutierrez, R. A Phylogenetic Study of the Plant Family Martyniaceae (Order Lamiales). Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2011. [Google Scholar]
- Bretting, P. The Taxonomic Relationship between Proboscidea louisianica and Proboscidea Fragrans (Martyniaceae). Southwest. Nat. 1983, 28, 445–449. [Google Scholar] [CrossRef]
- Yannitsardos, A.; Bazos, I. Ibicella (Stapf) Van Eseltine: A Genus of the American Family Martyniaceae New for Greece. Ann. Musei Goulandris 2006, 11, 271–279. [Google Scholar]
- Sevgi, E.; Hançer, Ç.K.; Yılmaz, H.; Akkaya, M. A New Alien Species Record for the Flora of Turkey: Proboscidea louisianica (Miller) Thell. Eurasian J. For. Sci. 2017, 5, 19–25. [Google Scholar] [CrossRef][Green Version]
- Small, E. North American Cornucopia: Top 100 Indigenous Food Plants; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Mozaffarian, V. Plant Classification: Taxonomic Morphology (Vol. 1); Amirkabir Publishing Institute: Tehran, Iran, 2010. [Google Scholar]
- Saboori, A.; Rezapanah, M.; Shirazi, J.; Rahnama, K.; Mounes; Bakhshi; Jahanshahi Afshar, F.; Najafi, H. (Eds.) Introducing the New Invasive Weed of Devils Claw (Ibicella Lutea) in the Landscape of Ilam City and Sugar Sugar Beet Field in Homail-Kermanshah Province. In Proceedings of the 24th Iranian Plant Protection Congress, Tehran, Iran, 3–6 September 2022; Iranian Research Institute of Plant Protection: Tehran, Iran, 2022. [Google Scholar]
- Wetherwax, M.; Heckard, L.R. Martyniaceae. 2012. Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=79174 (accessed on 12 May 2025).
- Morgan, J.W. Effects of Population Size on Seed Production and Germinability in an Endangered, Fragmented Grassland Plant. Conserv. Biol. 1999, 13, 266–273. [Google Scholar] [CrossRef]
- Almazán-Núñez, R.C.; Alvarez-Alvarez, E.A.; Sierra-Morales, P.; Rodríguez-Godínez, R. Fruit Size and Structure of Zoochorous Trees: Identifying Drivers for the Foraging Preferences of Fruit-Eating Birds in a Mexican Successional Dry Forest. Animals 2021, 11, 3343. [Google Scholar] [CrossRef]
- Mercer, K.L.; Pawlak, J.A.; Murray, D.S.; Verhalen, L.M.; Riffle, M.S.; McNew, R.W. Distance-of-Influence of Devil’s-Claw (Proboscidea louisianica) on Cotton (Gossypium hirsutum). Weed Technol. 1990, 4, 87–91. [Google Scholar] [CrossRef]
- Riffle, M.S.; Thilsted, W.E.; Murray, D.S.; Ahring, R.M.; Waller, G.R. Germination and Seed Production of Unicorn-Plant (Proboscidea louisianica). Weed Sci. 1988, 36, 787–791. [Google Scholar] [CrossRef]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Prather, T.; Miller, T.; Hulting, A. Control of Problem Weeds. In Pacific Northwest Weed Management Handbook; Oregon State University Agricultural: Corvallis, OR, USA, 2011. [Google Scholar]
- Lyon, L.L.; Keeling, J.W.; Dotray, P.A. Evaluation and Adaptation of the HADSS® Computer Program in Texas Southern High Plains Cotton. Weed Technol. 2004, 18, 315–324. [Google Scholar] [CrossRef]
- Bagheri, A.; Sohrabi, N.; Mondani, F.; Nosratti, I. Weed Infestation Is Affected by Chickpea Farmer Demographics and Agronomic Practices. Weed Res. 2021, 61, 45–54. [Google Scholar] [CrossRef]
- Mirdavoodi, H.; Marvi Mohadjer, M.R.; Zahedi Amiri, G.; Etemad, V. Disturbance Effects on Plant Diversity and Invasive Species in Western Oak Communities of Iran (Case Study: Dalab Forest, Ilam). Iran. J. For. Poplar Res. 2013, 21, 1–15. [Google Scholar] [CrossRef]
- Hemmat, A.; Eskandari, I. Tillage System Effects upon Productivity of a Dryland Winter Wheat–Chickpea Rotation in the Northwest Region of Iran. Soil. Tillage Res. 2004, 78, 69–81. [Google Scholar] [CrossRef]
- Assadi, M.; Ramak Maassoumi, A.; Khatamsaz, M. Flora of Iran; Ministry of Agriculture, Islamic Republic of Iran: Tehran, Iran, 1989.
- Parsons, W.T.; Parsons, W.T.; Cuthbertson, E. Noxious Weeds of Australia; CSIRO Publishing: Clayton South, Australia, 2001. [Google Scholar]
- Nosratti, I.; Almaleki, S.; Chauhan, B.S. Seed Germination Ecology of Soldier Thistle (Picnomon acarna): An Invasive Weed of Rainfed Crops in Iran. Weed Sci. 2019, 67, 261–266. [Google Scholar] [CrossRef]
- Nosratti, I.; Sabeti, P.; Chaghamirzaee, G.; Heidari, H. Weed Problems, Challenges, and Opportunities in Iran. Crop Prot. 2020, 134, 104371. [Google Scholar] [CrossRef]
- Bagheri, A.; Zargarian, N.; Mondani, F.; Nosratti, I. Artificial Neural Network Potential in Yield Prediction of Lentil (Lens culinaris L.) Influenced by Weed Interference. J. Plant Prot. Res. 2020, 60, 284–295. [Google Scholar] [CrossRef]
- Stanley, K.A.; Shirtliffe, S.J.; Benaragama, D.; Syrovy, L.D.; Duddu, H.S.N. Field Pea and Lentil Tolerance to Interrow Cultivation. Weed Technol. 2018, 32, 205–210. [Google Scholar] [CrossRef]
- Vargas, O.; Martínez, M.; Dávila, P. Two New Species of Physalis (Solanaceae) Endemic to Jalisco, Mexico. Brittonia 2001, 53, 505–510. [Google Scholar] [CrossRef]
- Ramírez, F.; Davenport, T.L. The Phenology of the Capuli Cherry [Prunus Serotina Subsp. Apuli (Cav.) McVaugh] Characterized by the BBCH Scale, Landmark Stages and Implications for Urban Forestry in Bogotá, Colombia. Urban. For. Urban. Green. 2016, 19, 202–211. [Google Scholar] [CrossRef]
- Singh, H.; Singh, T.; Singh, A.P.; Kaur, S.; Arora, S.; Singh, B. Hepatoprotective Effect of Physalis divaricata in Paracetamol Induced Hepatotoxicity: In Vitro, in Silico and in Vivo Analysis. J. Ethnopharmacol. 2022, 290, 115024. [Google Scholar] [CrossRef]
- Defelice, M.S. The Black Nightshades, Solanum nigrum L. et al.—Poison, Poultice, and Pie1. Weed Technol. 2003, 17, 421–427. [Google Scholar] [CrossRef]
- Nazarialam, J.; Alizadeh, H.; Rahimian Mashhadi, H. Aspects of Management and Biology of Annual Ground Cherry (Physalis divaricata) in Sugar Beet Fields. Iran. Crop Sci. 2010, 41, 569–576. [Google Scholar]
- Ghorbani, R.; Zeidali, E.; Hosseini, M. The Effect of Some Environmental Factors on Germination and Emergence of Weeds of Hyoscyamus Niger, Physalis divaricata and Rumex crispus. Crop Ecophysiol. 2016, 36, 661–674. [Google Scholar]
- Harker, K.N.; Blackshaw, R.E.; Clayton, G.W. Wild Oat (Avena fatua) vs. Redstem Filaree (Erodium cicutarium) Interference in Dry Pea. Weed Technol. 2007, 21, 235–240. [Google Scholar] [CrossRef]
- Rashed Mohassel, M.H.; Nadjafi, H. Biology and Control of Weeds; Ferdowsi University of Mashhad: Mashhad, Iran, 2001. [Google Scholar]
- Mousavi, S.K.; Ahmadi, A. Influence of Environmental Factors on Germination of Ground Cherry, Physalis divaricata. Appl. Entomol. Phytopathol. 2008, 76, 59–78. [Google Scholar]
- Amjadi, H.; Heidari, G.; Babaei, S.; Sharifi, Z. Evaluation of Yield, Yield Components and Some Quality Traits of Tuber of Potato (Solanum tuberosum L.) under Different Weed and Nutritional Management Practices. Front. Plant Sci. 2025, 15, 1495541. [Google Scholar] [CrossRef]
- Hoagland, R.E. Microbes and Microbial Products as Herbicides: An Overview; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Shamshiri, M.; Heydarnejad, J.; Kamali, M.; Pouramini, N.; Massumi, H. Identification of Wild Hosts of Tomato Yellow Leaf Curl Virus in South-Eastern Iran. Arch. Phytopathol. Plant Prot. 2019, 52, 917–929. [Google Scholar] [CrossRef]
- Darvishnia, M.; Ziedali, E.; Azadbakht, N.; Darvishnia, F.; Golzardi, F. Biocontrol of Physalis Divericata with Antagonistic Plant Pathogens in Lorestan Province. Weed Res. 2011, 2, 43–59. [Google Scholar]
- Ahmadkhani, E.; Nosratti, I.; Sabeti, P. Evaluation of Various Herbicides for Controlling Annual Ground Cherry Physalis divaricata in Sugar Beet Beta Vulgaris Fields. J. Appl. Res. Plant Prot. 2023, 11, 97–109. [Google Scholar] [CrossRef]
- Zand, A.; Nizamabadi, N.; Baghestani, M.A.; Shimi, P.; Mousavi, S.K. Guidelines for Chemical Control of Weeds in Iran, 6th ed.; Mashhad University Jihad Publications: Mashhad, Iran, 2019. [Google Scholar]
- Frediani, M.; Maggini, F.; Gelati, M.; Cremonini, R. Repetitive DNA Sequences as Probes for Phylogenetic Analysis in Vicia Genus. Caryologia 2004, 57, 379–386. [Google Scholar] [CrossRef][Green Version]
- Jaaska, V. Isozyme Variation and Phylogenetic Relationships in Vicia Subgenus Cracca (Fabaceae). Ann. Bot. 2005, 96, 1085–1096. [Google Scholar] [CrossRef][Green Version]
- Parsa, A. Flora of Iran; Forest and Rangeland Research Institute Publication: Tehran, Iran, 2000; Volume 33. [Google Scholar]
- Honarmand, S.J.; Nosratti, I.; Nazari, K.; Heidari, H. Factors Affecting the Seed Germination and Seedling Emergence of Muskweed (Myagrum perfoliatum). Weed Biol. Manag. 2016, 16, 186–193. [Google Scholar] [CrossRef]
- Amiri Deh Ahmadi, S.; Parsa, M.; Bannayan, M.; Nassiri Mahallati, M.; Deihimfard, R. Yield Gap Analysis of Chickpea under Semi-Arid Conditions: A Simulation Study. Int. J. Plant Prod. 2014, 8, 531–548. [Google Scholar]
- Javanmard, A.; Nikdel, H.; Amani Machiani, M. Evaluation of Forage Quantity and Quality in Domestic Populations of Hairy Vetch (Vicia villosa L.), Vetch (Vicia sativa L.) and Caspian Vetch (Vicia hyrcanica) under Rainfed Condition. J. Agric. Sci. Sustain. Prod. 2019, 29, 15–31. [Google Scholar]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Kissing Kucek, L.; Riday, H.; Rufener, B.P.; Burke, A.N.; Eagen, S.S.; Ehlke, N.; Krogman, S.; Mirsky, S.B.; Reberg-Horton, C.; Ryan, M.R. Pod Dehiscence in Hairy Vetch (Vicia villosa Roth). Front. Plant Sci. 2020, 11, 82. [Google Scholar] [CrossRef]
- Nosratti, I.; Soltanabadi, S.; Honarmand, S.J.; Chauhan, B.S. Environmental Factors Affect Seed Germination and Seedling Emergence of Invasive Centaurea Balsamita. Crop Pasture Sci. 2017, 68, 583–589. [Google Scholar] [CrossRef]
- Parker, T.A.; y Teran, J.C.; Palkovic, A.; Jernstedt, J.; Gepts, P. Genetic Control of Pod Dehiscence in Domesticated Common Bean: Associations with Range Expansion and Local Aridity Conditions. bioRxiv 2019. [Google Scholar] [CrossRef]
- Dürr, C.; Dickie, J.B.; Yang, X.Y.; Pritchard, H.W. Ranges of Critical Temperature and Water Potential Values for the Germination of Species Worldwide: Contribution to a Seed Trait Database. Agric. For. Meteorol. 2015, 200, 222–232. [Google Scholar] [CrossRef]
- Nosratti, I.; Mahdavi-Rad, S.; Heidari, H.; Saeidi, M. Differential Tolerance of Pumpkin Species to Bentazon, Metribuzin, Trifluralin, and Oxyfluorfen. Planta Daninha 2017, 35, e017165650. [Google Scholar] [CrossRef]
- Carrapiço, F. Azolla as a Superorganism. Its Implication in Symbiotic Studies. In Symbioses and Stress: Joint Ventures in Biology; Seckbach, J., Grube, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 225–241. [Google Scholar]
- Kenrick, P. A History of Plants in Fifty Fossils; Smithsonian Institution: Washington, DC, USA, 2020. [Google Scholar]
- Heckman, C.W. Ecoclimatological Survey of the Wetland Biota in the Tropical Wet-and-Dry Climatic Zone. Glob. Ecol. Biogeogr. Lett. 1997, 6, 97–114. [Google Scholar] [CrossRef]
- Costa, N.; Martins, D.; Rodella, R.; Rodrigues-Costa, A. Anatomical Leaf Changes in Eichhornia crassipes Due to Herbicides Application. Planta Daninha 2011, 29, 17–23. [Google Scholar] [CrossRef]
- Lumpkin, T.A.; Plucknett, D.L. Azolla: Botany, Physiology, and Use as a Green Manure. Econ. Bot. 1980, 34, 111–153. [Google Scholar] [CrossRef]
- Lejeune, A.; Peng, J.; Le Boulengé, E.; Larondelle, Y.; Van Hove, C. Carotene Content of Azolla and Its Variations during Drying and Storage Treatments. Anim. Feed. Sci. Technol. 2000, 84, 295–301. [Google Scholar] [CrossRef]
- Hussner, A. Alien Aquatic Plant Species in European Countries. Weed Res. 2012, 52, 297–306. [Google Scholar] [CrossRef]
- Farahpour-Haghani, A.; Jalaeian, M.; Alinia, F.; Majidi-Shilsar, F. First Report of Rush Veneer Nomophila Noctuella (Lepidoptera: Crambidae: Spilomelinae) on Water Fern in Wetlands and Rice Fields. In Proceedings of the 22nd Iranian Plant Protection Congress, Karaj, Iran, 27–30 August 2016. [Google Scholar] [CrossRef]
- Sadeghi, R.; Zarkami, R.; Sabetraftar, K.; Van Damme, P. Application of Classification Trees to Model the Distribution Pattern of a New Exotic Species Azolla filiculoides (Lam.) at Selkeh Wildlife Refuge, Anzali Wetland, Iran. Ecol. Modell. 2012, 243, 8–17. [Google Scholar] [CrossRef]
- Watanabe, I.; Berja, N.S. The Growth of Four Species of Azolla as Affected by Temperature. Aquat. Bot. 1983, 15, 175–185. [Google Scholar] [CrossRef]
- Pirasteh, M. The Protection and Restoration of Anzali Wetland; Department of Environmental Protection: Gilan, Iran, 1995.
- Sabet Raftar, K. Azolla Analysis of the Environmental Impacts on Aquatic Ecosystems of the Wetlands. Ph.D. Thesis, University of Tehran, Tehran, Iran, 1994. [Google Scholar]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Padasht–Dehkaii, F.; Tajadoditalab, K.; Hosseini–Chaleshtare, M.; Rabiei, M.; Sharafi, N.; Shokrivahed, H.; Alizadeh, M.; Alinia, F.; Omrani, M.; Kavosi, M. Paddy Rice Cultivation Guide; Agricultural Research, Education and Extension Organization (AREEO): Tehran, Iran, 2015. [Google Scholar]
- Axelsen, S.; Julian, C. Weed Control in Small Dams. Part II Control of Salvinia, Azolla and of Water Hyacinth. Qld. Agric. J. 1988, 114, 291–298. [Google Scholar]
- Ashton, P.J. Azolla Infestations in South Africa: History of the Introduction, Scope of the Problem and Prospects for Management; Department of Water Affairs and Forestry: Pretoria, South Africa, 1992.
- Winston, R.; Schwarzländer, M.; Hinz, H.L.; Day, M.D.; Cock, M.J.; Julien, M. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th ed.; CABI Databases: Wallingford, UK, 2014. [Google Scholar]
- Lock, J.M.; Gopal, B. Water Hyacinth. (Aquatic Plant Studies 1). J. Trop. Ecol. 1988, 4, 92–93. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2011, 55, 282–298. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.P.; Ruiz-Téllez, T.; Starfinger, U.; Brunel, S. Monographs on Invasive Plants in Europe N° 2: Eichhornia crassipes (Mart.) Solms. Bot. Lett. 2017, 164, 303–326. [Google Scholar] [CrossRef]
- Dagno, K.; Lahlali, R.; Diourté, M.; Jijakli, H. Fungi Occurring on Waterhyacinth (Eichhornia crassipes (Martius) Solms-Laubach) in Niger River in Mali and Their Evaluation as Mycoherbicides. J. Aquat. Plant Manag. 2012, 50, 25–32. [Google Scholar]
- Gunnarsson, C.; Mattsson, C. Water Hyacinth-Trying to Turn an Environmental Problem into an Agricultural Resource. Waste Manag. 1997, 27, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Rohi, A.M.; Naderi Jelodar, M.; Roshan Tabari, M.A.; Afraei, V.; Parakande, F. Ecology of Aquatic Plant Control Plant (Eichhoornia Crassipes) in Aquatic Ecosystems of Mazandaran Province. J. Aquat. Casp. Sea 2017, 2, 25–36. [Google Scholar]
- Ben Bakrim, W.; Ezzariai, A.; Karouach, F.; Sobeh, M.; Kibret, M.; Hafidi, M.; Kouisni, L.; Yasri, A. Eichhornia crassipes (Mart.) Solms: A Comprehensive Review of Its Chemical Composition, Traditional Use, and Value-Added Products. Front. Pharmacol. 2022, 13, 842511. [Google Scholar] [CrossRef]
- Mujere, N. Water Hyacinth: Characteristics, Problems, Control Options, and Beneficial Uses. In Impact of Water Pollution on Human Health and Environmental Sustainability; IGI Global: Hershey, PA, USA, 2016; pp. 343–361. [Google Scholar]
- Moran, P.J. Leaf Scarring by the Weevils Neochetina Eichhorniae and N. bruchi Enhances Infection by the Fungus Cercospora piaropi on Water Hyacinth, Eichhornia crassipes. BioControl 2005, 50, 511–524. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Energy Crops. 1983. Available online: http://www.rain-tree.com/papaya.htm (accessed on 22 October 2024).
- Mirzajani, A.; Naderi, S.; Parvaneh Moghadam, D. Distribution Survey and Some Biological Aspects of Water Hyacinth in Anzali Wetland, Guilan Province. Iran. J. Plant Biol. 2019, 11, 51–62. [Google Scholar]
- Olivares, E.; Colonnello, G. Salinity Gradient in the Mánamo River, a Dammed Distributary of the Orinoco Delta, and Its Influence on the Presence of Eichhornia crassipes and Paspalum repens. Interciencia 2000, 25, 242–248. [Google Scholar]
- Malik, A. Environmental Challenge Vis a Vis Opportunity: The Case of Water Hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Troutman, J.P.; Rutherford, D.A.; Kelso, W. Patterns of Habitat Use among Vegetation-Dwelling Littoral Fishes in the Atchafalaya River Basin, Louisiana. Trans. Am. Fish. Soc. 2007, 136, 1063–1075. [Google Scholar] [CrossRef]
- Schmitz, D.C.; Schardt, J.D.; Leslie, A.J.; Dray, F.J.; Osborne, J.A.; Nelson, B.V. The Ecological Impact and Management History of Three Invasive Alien Aquatic Plant Species in Florida. In Biological Pollution: The Control and Impact of Invasive Exotic Species, Proceedings of a Symposium held at Indianapolis, IN, USA, 25–26 October 1991; Indiana Academy of Science: Indianapolisna, IN, USA, 1993; pp. 173–194. [Google Scholar]
- Brendonck, L.; Maes, J.; Rommens, W.; Dekeza, N.; Nhiwatiwa, T.; Barson, M.; Callebaut, V.; Phiri, C.; Moreau, K.; Gratwicke, B. The Impact of Water Hyacinth (Eichhornia crassipes) in a Eutrophic Subtropical Impoundment (Lake Chivero, Zimbabwe). II. Species Diversity. Arch. Hydrobiol. 2003, 158, 389–405. [Google Scholar] [CrossRef]
- Inderjit, S. Invasive Plants: Ecological and Agricultural Aspects; Birkhäuser: Basel, Switzerland, 2005; ISBN 3764371374. [Google Scholar]
- Wainger, L.A.; Harms, N.E.; Magen, C.; Liang, D.; Nesslage, G.M.; McMurray, A.M.; Cofrancesco, A.F. Evidence-Based Economic Analysis Demonstrates That Ecosystem Service Benefits of Water Hyacinth Management Greatly Exceed Research and Control Costs. PeerJ 2018, 6, e4824. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, C.C.; Petersen, C.M. Water Hyacinths as a Resource in Agriculture and Energy Production: A Literature Review. Waste Manag. 2007, 27, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Khotsa, M.D.; Mkolo, N.M.; Motshudi, M.C.; Mphephu, M.M.; Makhafola, M.A.; Naidoo, C.M. A Comprehensive Review of the Biology, Ecological Impacts, and Control Strategies of Eichhornia crassipes. Diversity 2025, 17, 564. [Google Scholar] [CrossRef]
- Mudge, C.R.; Netherland, M. Response of Giant Bulrush, Water Hyacinth, and Water Lettuce to Foliar Herbicide Applications. J. Aquat. Plant Manag. 2014, 52, 75–80. [Google Scholar]
- FAO. Progress on Water Hyacinth (Eichhornia crassipes) Management. Available online: https://www.fao.org/4/y5031e/y5031e0c.htm (accessed on 9 June 2025).
- ICID Aquatic Weeds and Their Management; International Commission on Irrigation and Drainage: New Delhi, India, 2002.
- Siemering, G.S.; Hayworth, J.D.; Greenfield, B.K. Assessment of Potential Aquatic Herbicide Impacts to California Aquatic Ecosystems. Arch. Environ. Contam. Toxicol. 2008, 55, 415–431. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Mahajan, G. Recent Advances in Weed Management; Springer: New York, NY, USA, 2014; ISBN 978-1-4939-1018-2. [Google Scholar]
- Mirzajani, A.; Nezamabadi, N.; Salavatian, S.; Bagheri, S.; Salahi, M.; Rufchaei, R. Chemical Control of Water hyacinth by Some Herbicides and Their Effect on Some Aquatic Invertebrate. 2022. Available online: https://www.researchsquare.com/article/rs-1242651/v1 (accessed on 12 April 2023).
- Martinez, B.; Reaser, J.K.; Dehgan, A.; Zamft, B.; Baisch, D.; McCormick, C.; Giordano, A.J.; Aicher, R.; Selbe, S. Technology Innovation: Advancing Capacities for the Early Detection of and Rapid Response to Invasive Species. Biol. Invasions 2019, 22, 75–100. [Google Scholar] [CrossRef]
- Williams, F.; Constantine, K.L.; Ali, A.A.; Karanja, T.W.; Kibet, S.; Lingeera, E.K.; Muthike, G.; Rwomushana, I.; Godwin, J.; Day, R. An Assessment of the Capacity and Responsiveness of a National System to Address the Threat of Invasive Species: A Systems Approach. CABI Agric. Biosci. 2021, 2, 42. [Google Scholar] [CrossRef]
- Ballard, H.L.; Fernandez-Gimenez, M.E.; Sturtevant, V.E. Integration of Local Ecological Knowledge and Conventional Science: A Study of Seven Community-Based Forestry Organizations in the USA. Ecol. Soc. 2008, 13, 37. [Google Scholar] [CrossRef]
- Dowsley, M. Community Clusters in Wildlife and Environmental Management: Using TEK and Community Involvement to Improve Co-Management in an Era of Rapid Environmental Change. Polar Res. 2009, 28, 43–59. [Google Scholar] [CrossRef]
- McNeely, J.A.; Mooney, H.A.; Neville, L.E.; Schei, P.J.; Waage, J.K. Global Strategy on Invasive Alien Species; IUCN: Gland, Switzerland; Cambridge, UK, 2001; ISBN 2-8317-0609-2. [Google Scholar]





| Ecological Habitats | Scientific Name | Family | Common Name | Synonym | Origin | Life Cycle | Weed Status in Iran |
|---|---|---|---|---|---|---|---|
| Agricultural field weeds | Ambrosia psilostachya DC. | Asteraceae | Cuman ragweed | Ambrosia californica Rydb.; Ambrosia coronopifolia Torr. & A. Gray; Ambrosia cumanensis auct. non Kunth; Ambrosia rugelii Rydb. | Mexico | Annual Perennial | Serious/Principal Weed |
| Boreava orientalis Jaub. & Spach | Brassicaceae | Waxy leaved mustard or yellow weed | Isatis quadrivalvis | Western Asia | Annual | Serious/Principal Weed | |
| Cynanchum acutum L. | Asclepiadaceae | Swallowwort or climbing milkweed | Solenostemma acutum (L.) Wehmer; Vincetoxicum acutum (L.) Kuntze | Mediterranean region | Perennial | Common Weed | |
| Ibicella lutea (Lindl.) Van Eselt. | Martyniaceae | Devil’s Claw | Martynia lutea Lindl.; Proboscidea lutea (Lindl.) Stapf. | Argentina and South America | Annual | Occasional/Emerging Weed | |
| Picnomon acarna (L.) Cass. | Asteraceae | Soldier thistle | Cirsium acarna (L.) Moench. Carduus acarna L. | Mediterranean region | Annual | Common Weed | |
| Physalis divaricata D. Don | Solanaceae | Annual Groundcherry | Physalis halicacabum | Latin America and South America | Annual | Common Weed | |
| Vicia hyrcanica Fisch. & C.A. Mey. | Fabaceae | Hyrcan vetch | Hypechusa hyrcanica (Fisch. & C.A.Mey.) Alef. | Mediterranean region | Annual | Occasional/Emerging Weed | |
| Aquatic Invasive weeds | Azolla filiculoides Lam. | Salviniaceae | Pacific Mosquitofern | - | Africa, Asia, and America | Annual | Serious/Principal Weed |
| Eichhornia crassipes (Mart.) Solms | Pontederiaceae | Water hyacinth | Eichhornia speciosa Kunth; Piaropus crassipes (Mart.) Raf. | Amazon Basin in South America | Perennial | Serious/Principal Weed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi, A.R.; Babaei, S.; Nosratti, I.; Zeidali, E.; Babaei, M.; Asadi Oskouei, E.; Saberi, H.; Redhu, M.; Sadeghpour, A. Emerging Invasive Weeds in Iran: Occurrence, Ecological Impacts, and Sustainable Management. Plants 2025, 14, 2611. https://doi.org/10.3390/plants14172611
Yousefi AR, Babaei S, Nosratti I, Zeidali E, Babaei M, Asadi Oskouei E, Saberi H, Redhu M, Sadeghpour A. Emerging Invasive Weeds in Iran: Occurrence, Ecological Impacts, and Sustainable Management. Plants. 2025; 14(17):2611. https://doi.org/10.3390/plants14172611
Chicago/Turabian StyleYousefi, Ali Reza, Sirwan Babaei, Iraj Nosratti, Ehsan Zeidali, Masoumeh Babaei, Ebrahim Asadi Oskouei, Hesan Saberi, Mandeep Redhu, and Amir Sadeghpour. 2025. "Emerging Invasive Weeds in Iran: Occurrence, Ecological Impacts, and Sustainable Management" Plants 14, no. 17: 2611. https://doi.org/10.3390/plants14172611
APA StyleYousefi, A. R., Babaei, S., Nosratti, I., Zeidali, E., Babaei, M., Asadi Oskouei, E., Saberi, H., Redhu, M., & Sadeghpour, A. (2025). Emerging Invasive Weeds in Iran: Occurrence, Ecological Impacts, and Sustainable Management. Plants, 14(17), 2611. https://doi.org/10.3390/plants14172611









