pMAGs: A Versatile and Efficient Vector System for Multi-Gene Studies in Plants
Abstract
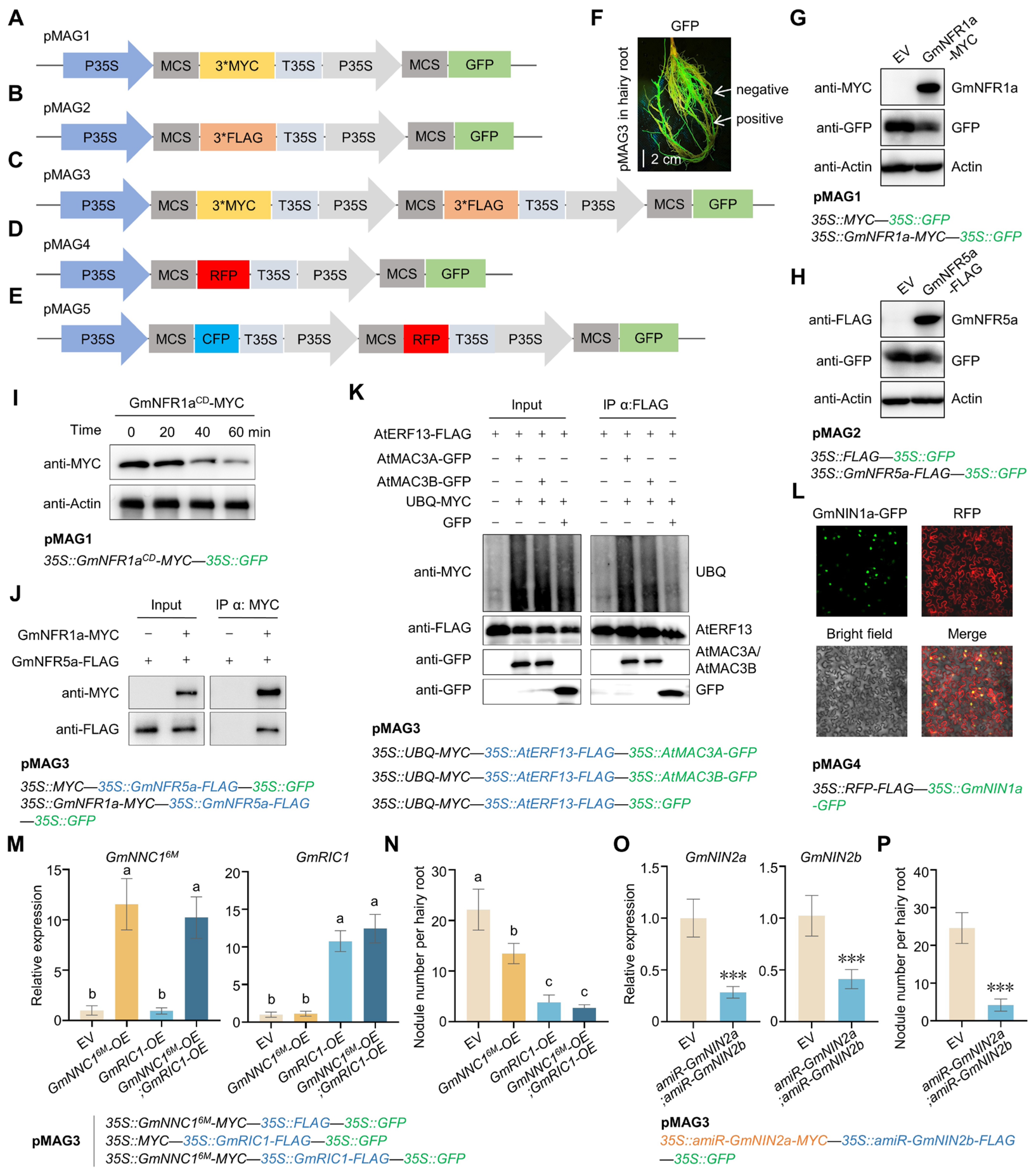
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. pMAG Vectors Construction
4.2. Soybean Hairy Root Transformation and Nodulation Phenotypic Analysis
4.3. RNA Extraction and RT-qPCR
4.4. Western Blot Assay
4.5. Cell-Free Assay
4.6. Co-IP Assay
4.7. In Vivo Ubiquitination Assay
4.8. Agrobacterium Tumefaciens-Mediated Invasion of N. benthamiana Leaves
5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, H.M.; Xu, X.; Liu, X.; Chen, W.B.; Yang, G.H.; Wong, F.L.; Li, M.W.; He, W.M.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.X.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.J.; Thelen, J.J.; Cheng, J.L.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Liu, S.L.; Zhang, M.; Feng, F.; Tian, Z.X. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef]
- Lu, S.J.; Dong, L.D.; Fang, C.; Liu, S.L.; Kong, L.P.; Cheng, Q.; Chen, L.Y.; Su, T.; Nan, H.Y.; Zhang, D.; et al. Stepwise selection on homeologous genes controlling flowering and maturity during soybean domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef]
- Shi, J.C.; Zhao, B.Y.; Zheng, S.; Zhang, X.W.; Wang, X.L.; Dong, W.T.; Xie, Q.J.; Wang, G.; Xiao, Y.P.; Chen, F.; et al. A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 2021, 184, 5527–5540. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Peng, Y.Q.; Lyu, X.G.; Liu, B.; Sun, S.Y.; Wang, X.L. Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science 2021, 374, 65–71. [Google Scholar] [CrossRef]
- Bao, A.L.; Chen, H.F.; Chen, L.M.; Chen, S.L.; Hao, Q.N.; Guo, W.; Qiu, D.Z.; Shan, Z.H.; Yang, Z.L.; Yuan, S.L.; et al. CRISPR/Cas9-mediated targeted mutagenesis of GmSPL9 genes alters plant architecture in soybean. BMC Plant Biol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zhang, X.H.; Zhong, L.J.; Wang, X.Y.; Jin, L.S.; Lyu, S.H. One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. BMC Plant Biol. 2020, 20, 208. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, M.; Zhao, Y.; Liang, N.S.; Li, H.Y.; Li, P.X.; Yang, L.L.; Xu, M.Y.; Bian, X.X.; Wang, M.X.; et al. SHORT-ROOT paralogs mediate feedforward regulation of D-type cyclin to promote nodule formation in soybean. Proc. Natl. Acad. Sci. USA 2022, 119, e2108641119. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.W.; Cui, Y.Y.; Ke, M.Y.; Xu, H.F.; Xu, Q.Z.; Chen, J.M.; Li, Y.; Huang, L.M.; Zhao, H.; et al. GmPIN-dependent polar auxin transport is involved in soybean nodule development. Plant Cell 2021, 33, 2981–3003. [Google Scholar] [CrossRef]
- Han, J.L.; Ma, K.; Li, H.L.; Su, J.; Zhou, L.; Tang, J.T.; Zhang, S.J.; Hou, Y.K.; Chen, L.T.; Liu, Y.G.; et al. All-in-one: A robust fluorescent fusion protein vector toolbox for protein localization and BiFC analyses in plants. Plant Biotechnol. J. 2022, 20, 1098–1109. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Yount, B.; Denison, M.R.; Weiss, S.R.; Baric, R.S. Systematic assembly of a full-length infectious cDNA of mouse hepatitis virus strain A59. J. Virol. 2002, 76, 11065–11078. [Google Scholar] [CrossRef]
- Smith, H.O.; Wilcox, K.W. A Restriction Enzyme from Hemophilus-Influenzae. 1. Purification and General Properties. J. Mol. Biol. 1970, 51, 379–391. [Google Scholar] [CrossRef]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.M.; Werner, S.; Jones, J.D.G.; Patron, N.J.; Marillonnet, S. A Golden Gate Modular Cloning Toolbox for Plants. ACS Synth. Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.X.; Indrasumunar, A.; Nguyen, C.D.T.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes: Mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Chen, S.B.; Tao, L.Z.; Zeng, L.R.; Vega-Sanchez, M.E.; Umemura, K.; Wang, G.L. A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef]
- Dong, W.T.; Zhu, Y.Y.; Chang, H.Z.; Wang, C.H.; Yang, J.; Shi, J.C.; Gao, J.P.; Yang, W.B.; Lan, L.Y.; Wang, Y.R.; et al. An SHR-SCR module specifies legume cortical cell fate to enable nodulation. Nature 2021, 589, 586–590. [Google Scholar] [CrossRef]
- Liu, L.; Qu, J.H.; Wang, C.Y.; Liu, M.; Zhang, C.M.; Zhang, X.Y.; Guo, C.; Wu, C.G.; Yang, G.D.; Huang, J.G.; et al. An efficient genetic transformation system mediated by in fruit trees based on the transgenic hairy root to shoot conversion. Plant Biotechnol. J. 2024, 22, 2093–2103. [Google Scholar] [CrossRef]
- Cao, X.S.; Xie, H.T.; Song, M.L.; Lu, J.H.; Ma, P.; Huang, B.Y.; Wang, M.G.; Tian, Y.F.; Chen, F.; Peng, J.; et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2023, 4, 100345. [Google Scholar] [CrossRef]
- Cao, X.S.; Xie, H.T.; Song, M.L.; Zhao, L.H.; Liu, H.L.; Li, G.F.; Zhu, J.K. Simple method for transformation and gene editing in medicinal plants. J. Integr. Plant Biol. 2024, 66, 17–19. [Google Scholar] [CrossRef]
- Wang, L.X.; Sun, Z.X.; Su, C.; Wang, Y.L.; Yan, Q.Q.; Chen, J.H.; Ott, T.; Li, X. A GmNINa-miR172c-NNC1 Regulatory Network Coordinates the Nodulation and Autoregulation of Nodulation Pathways in Soybean. Mol. Plant 2019, 12, 1211–1226. [Google Scholar] [CrossRef]
- He, Y.B.; Zhang, T.; Sun, H.; Zhan, H.D.; Zhao, Y.D. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.Y.; Zhang, D.; Tang, X.L.; Li, Z.; Shen, C.; Han, X.; Deng, W.H.; Yin, W.L.; Xia, X.L. PtrWRKY75 overexpression reduces stomatal aperture and improves drought tolerance by salicylic acid-induced reactive oxygen species accumulation in poplar. Environ. Exp. Bot. 2020, 176, 104117. [Google Scholar] [CrossRef]
- Yu, Z.P.; Qu, X.Z.; Lv, B.S.; Li, X.X.; Sui, J.X.; Yu, Q.Q.; Ding, Z.J. MAC3A and MAC3B mediate degradation of the transcription factor ERF13 and thus promote lateral root emergence. Plant Cell 2024, 36, 3162–3176. [Google Scholar] [CrossRef] [PubMed]
- Rübsam, H.; Krönauer, C.; Abel, N.B.; Ji, H.T.; Lironi, D.; Hansen, S.B.; Nadzieja, M.; Kolte, M.; Abel, D.; de Jong, N.; et al. Nanobody-driven signaling reveals the core receptor complex in root nodule symbiosis. Science 2023, 379, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.L.; Gao, Z.; Li, L.F.; Chen, J.S.; Ye, K.Z.; Xu, T.; Mai, S.Y.; Han, Q.Q.; Chen, C.F.; Wu, S.W.; et al. Soybean symbiotic-nodule zonation and cell differentiation are defined by NIN2 signaling and GH3-dependent auxin homeostasis. Dev. Cell 2024, 59, 2254–2269. [Google Scholar] [CrossRef]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. cis-Regulatory Elements in Plant Development, Adaptation, and Evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Grotewold, E.; Stam, M. Cis-regulatory sequences in plants: Their importance, discovery, and future challenges. Plant Cell 2022, 34, 718–741. [Google Scholar] [CrossRef]
- Gudynaite-Savitch, L.; Johnson, D.A.; Miki, B.L.A. Strategies to mitigate transgene-promoter interactions. Plant Biotechnol. J. 2009, 7, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Cui, D.; Einstein, J.; Narasimhulu, S.; Vergara, C.E.; Gelvin, S.B. Strength and Tissue-Specificity of Chimeric Promoters Derived from the Octopine and Mannopine Synthase Genes. Plant J. 1995, 7, 661–676. [Google Scholar] [CrossRef]
- Yu, Z.P.; Ma, J.X.; Zhang, M.Y.; Li, X.X.; Sun, Y.; Zhang, M.X.; Ding, Z.J. Auxin promotes hypocotyl elongation by enhancing BZR1 nuclear accumulation in. Sci. Adv. 2023, 9, eade2493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, J.; Zhao, H.; Ding, Z.; Li, X.; Yu, Z. pMAGs: A Versatile and Efficient Vector System for Multi-Gene Studies in Plants. Plants 2025, 14, 2602. https://doi.org/10.3390/plants14162602
Zhang M, Liu J, Zhao H, Ding Z, Li X, Yu Z. pMAGs: A Versatile and Efficient Vector System for Multi-Gene Studies in Plants. Plants. 2025; 14(16):2602. https://doi.org/10.3390/plants14162602
Chicago/Turabian StyleZhang, Mengyue, Jing Liu, Han Zhao, Zhaojun Ding, Xiaoxuan Li, and Zipeng Yu. 2025. "pMAGs: A Versatile and Efficient Vector System for Multi-Gene Studies in Plants" Plants 14, no. 16: 2602. https://doi.org/10.3390/plants14162602
APA StyleZhang, M., Liu, J., Zhao, H., Ding, Z., Li, X., & Yu, Z. (2025). pMAGs: A Versatile and Efficient Vector System for Multi-Gene Studies in Plants. Plants, 14(16), 2602. https://doi.org/10.3390/plants14162602






