Enhancing UV-B Protection and Abiotic Stress Tolerance in Tomato Plants: The Role of Silicon Nanoparticles in Photosynthetic Parameters, Pigments, and Secondary Metabolite Production
Abstract
1. Introduction
2. Results and Discussions
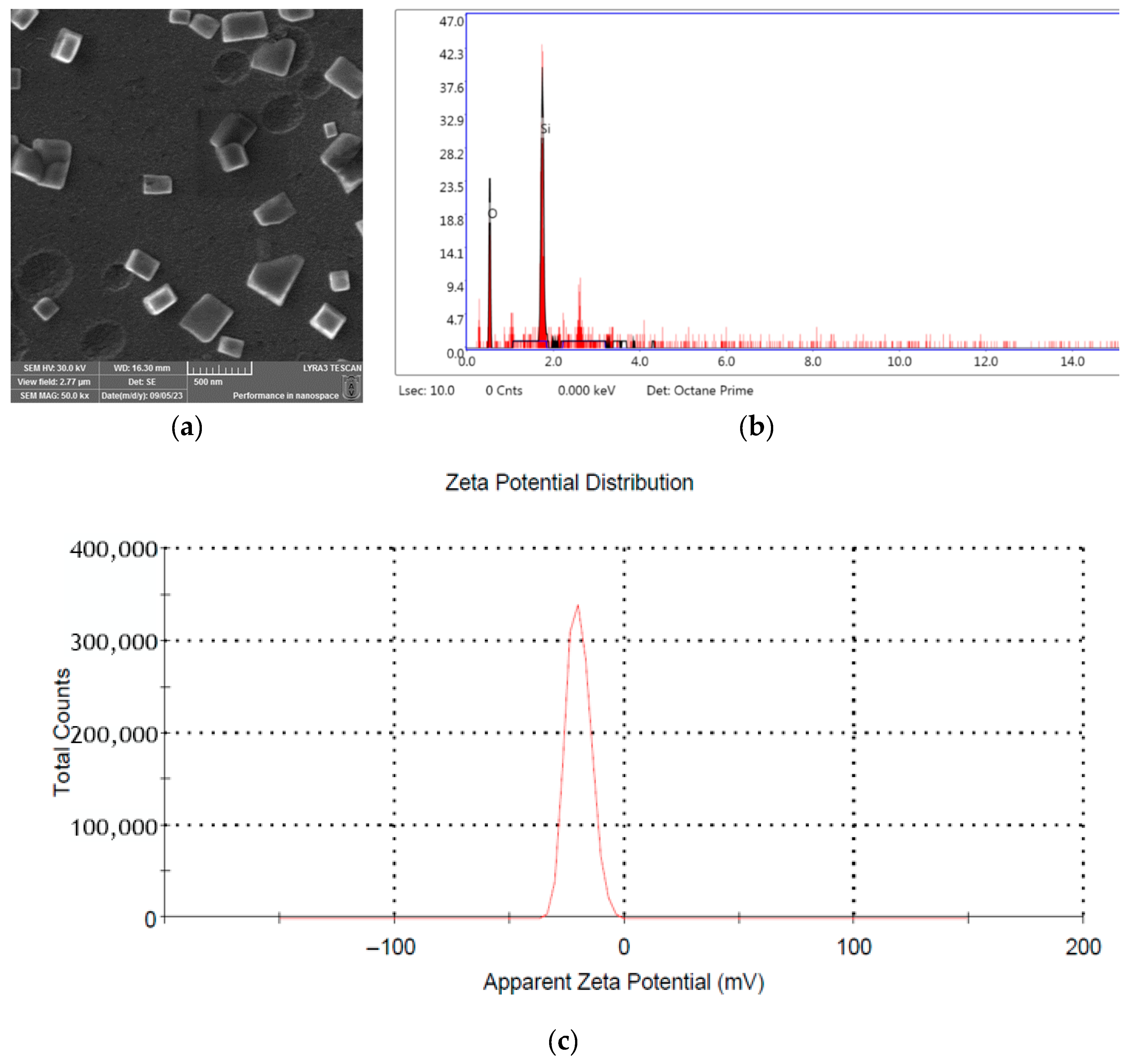
2.1. Characterization of SiNPs Material
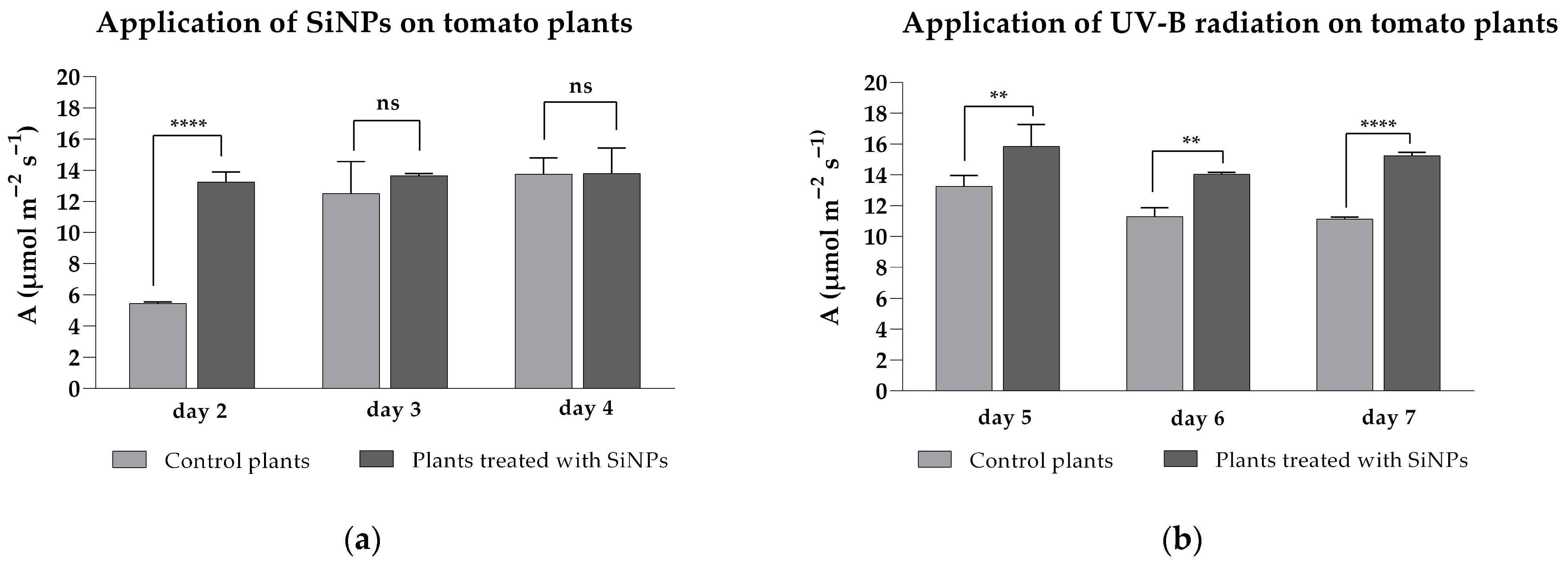
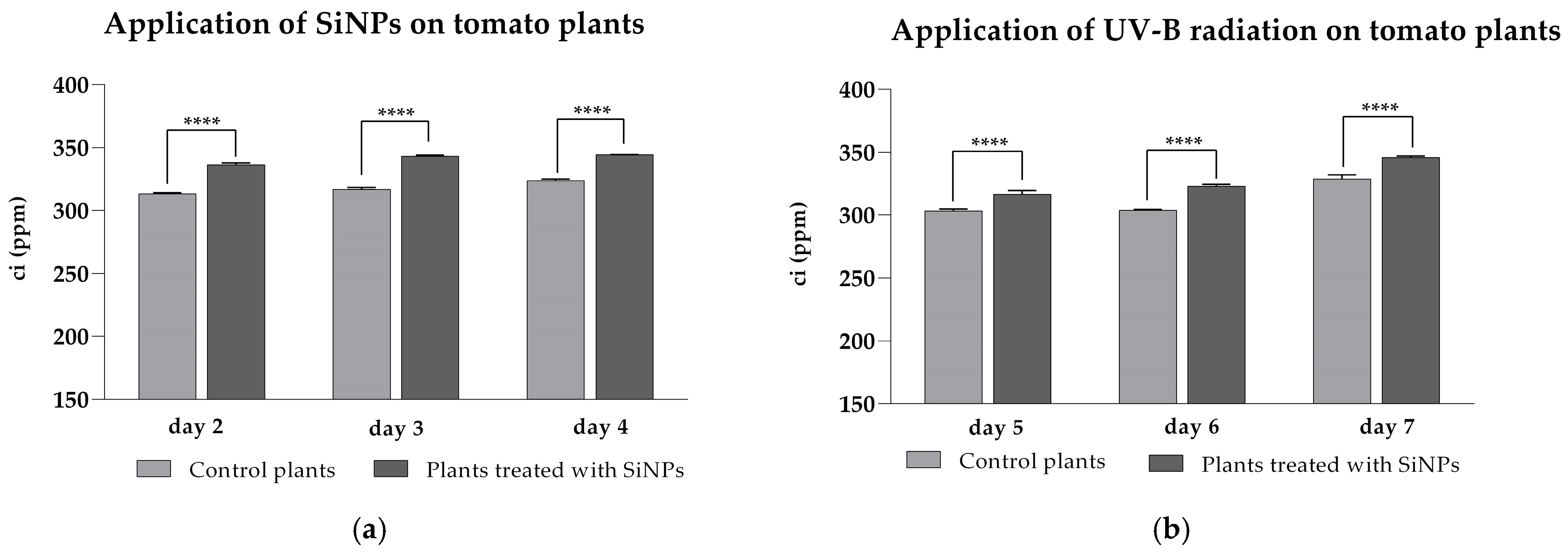
2.2. Influence of SiNP Treatment on Photosynthetic Parameters in Tomato Plants
2.3. Influence of SiNP Treatment on Targeted Photosynthesis-Related Secondary Metabolites
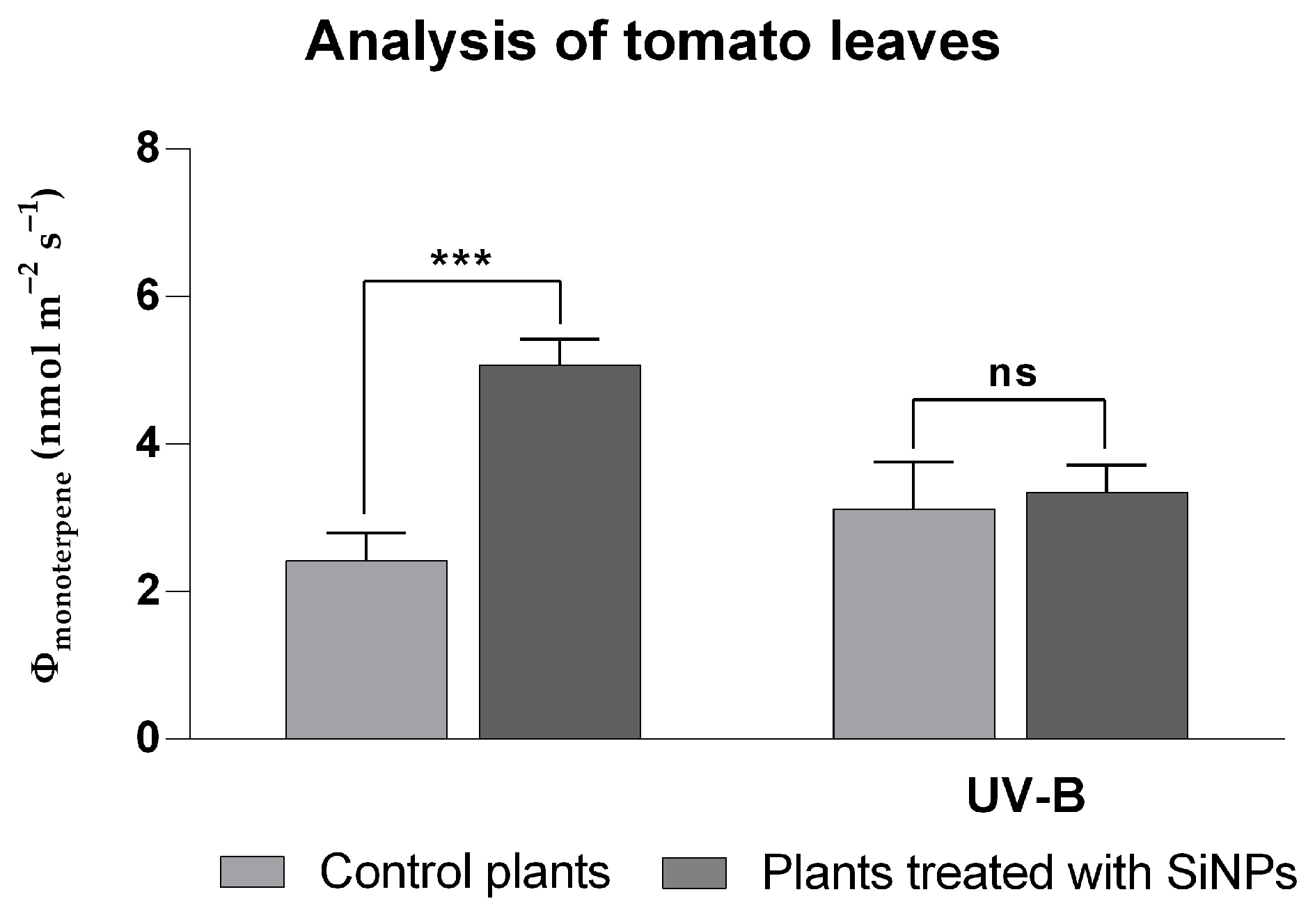
2.3.1. Analysis of BVOCs Emitted for SiNP-Treated and -Untreated Tomato Plants
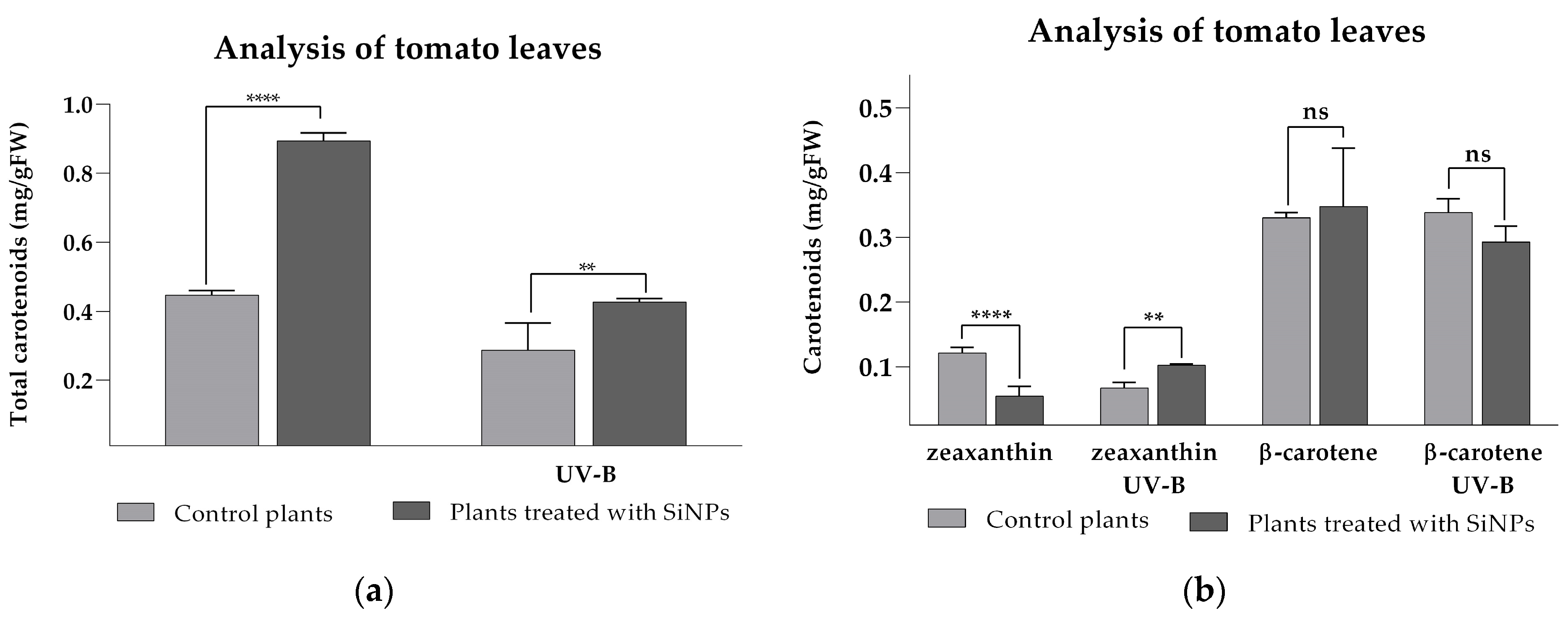
2.3.2. Analysis of Photosynthetic Pigments for SiNP-Treated and -Untreated Tomato Plants
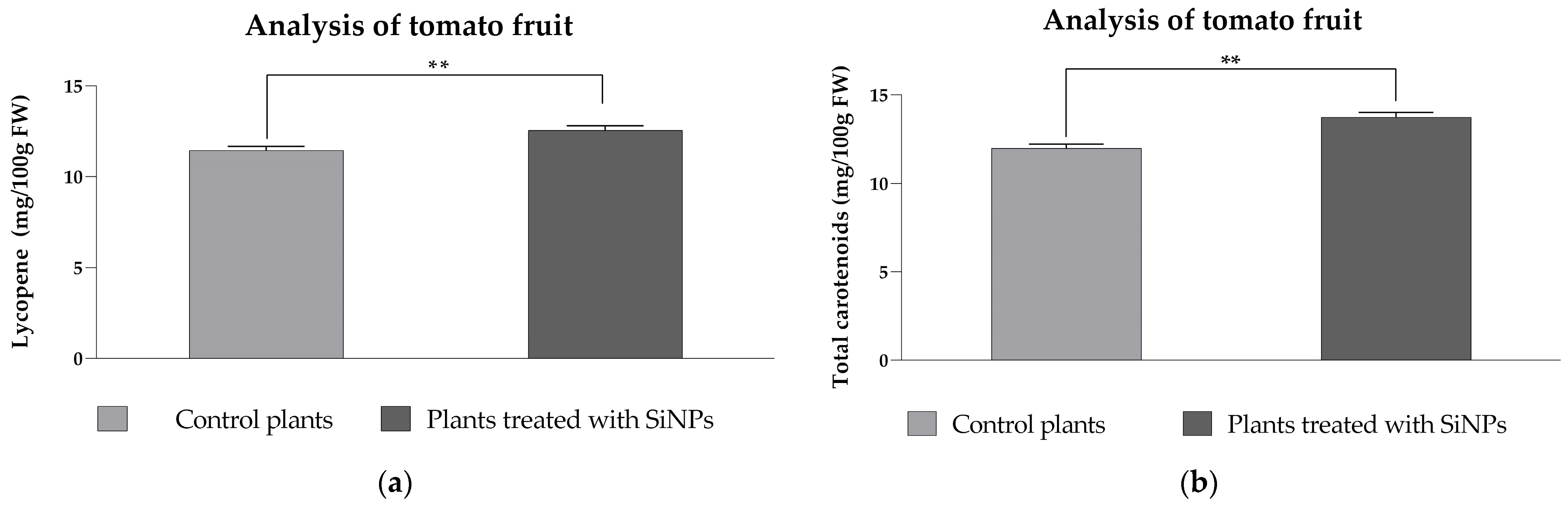
2.3.3. Analysis of Lycopene and Total Carotenoid Content in Tomato Fruit from SiNP-Treated and -Untreated Plants
2.4. Correlations Between Physiological and Biochemical Parameters in Tomato Plants
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Chemical Reagents
3.3. Synthesis and Characterization of Silicon Nanoparticles (SiNPs)
3.4. Experimental Model: SiNP Treatments and UV-B Exposure
3.5. Measurement of Photosynthetic Parameters
3.6. Measurement of Secondary Metabolites in Tomato Leaves
3.6.1. BVOC Sampling and GC-MS Analysis
3.6.2. Chromatographic Analysis of Photosynthetic Pigments
3.6.3. Total Carotenoid and Lycopene Concentrations in Tomato Fruits
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahawar, L.; Ramasamy, K.P.; Suhel, M.; Prasad, S.M.; Živčák, M.; Brestic, M.; Rastogi, A.; Skalický, M. Silicon Nanoparticles: Comprehensive Review on Biogenic Synthesis and Applications in Agriculture. Environ. Res. 2023, 232, 116292. [Google Scholar] [CrossRef] [PubMed]
- Dotto, M.; Casati, P. Developmental Reprogramming by UV-B Radiation in Plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Application of Nanoparticles for Enhanced UV-B Stress Tolerance in Plants. Plant Nano Biol. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Azadi, M.; Siavash Moghaddam, S.; Rahimi, A.; Pourakbar, L.; Popović-Djordjević, J. Biosynthesized Silver Nanoparticles Ameliorate Yield, Leaf Photosynthetic Pigments, and Essential Oil Composition of Garden Thyme (Thymus vulgaris L.) Exposed to UV-B Stress. J. Environ. Chem. Eng. 2021, 9, 105919. [Google Scholar] [CrossRef]
- de Lima Nechet, K.; Heck, D.W.; Terao, D.; Halfeld-Vieira, B.d.A. Effect of the Increase of UV-B Radiation on Strawberry Fruit Quality. Sci. Hortic. 2015, 193, 7–12. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Rice Responses and Tolerance to Ultraviolet-B (UV-B) Radiation. In Advances in Rice Research for Abiotic Stress Tolerance; Woodhead Publishing: Cambridge, UK, 2019; pp. 709–724. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon Nanoparticles More Effectively Alleviated UV-B Stress than Silicon in Wheat (Triticum aestivum) Seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Deng, H.; Sun, X.; Wang, A.; Tang, X.; Gao, Y.; Zhang, N.; Wang, L.; Yang, S.; et al. Tomato UV-B Receptor SlUVR8 Mediates Plant Acclimation to UV-B Radiation and Enhances Fruit Chloroplast Development via Regulating SlGLK2. Sci. Rep. 2018, 8, 6097. [Google Scholar] [CrossRef]
- Moradi Rikabad, M.; Pourakbar, L.; Siavash Moghaddam, S.; Popović-Djordjević, J. Agrobiological, Chemical and Antioxidant Properties of Saffron (Crocus sativus L.) Exposed to TiO2 Nanoparticles and Ultraviolet-B Stress. Ind. Crops Prod. 2019, 137, 137–143. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Feng, J.; Yan, X.; Chen, H.; Han, R. Effects of TiO2-NPs Pretreatment on UV-B Stress Tolerance in Arabidopsis thaliana. Chemosphere 2021, 281, 130809. [Google Scholar] [CrossRef] [PubMed]
- Safshekan, S.; Pourakbar, L.; Rahmani, F. The Effect of Zn NPs on Some Growth, Biochemical and Anatomical Factors of Chickpea Plant Stem under UVB Irradiation. Plant. Nano. Biol. 2025, 12, 100154. [Google Scholar] [CrossRef]
- Kataria, S.; Haque, M.I.; Filacek, A.; Barboricova, M.; Ferencova, J.; Jain, M.; Rastogi, A.; Brestic, M. Enhancing UV-B Tolerance in Radish and Mung Bean Plants Using Magnetic Iron Oxide Nanoparticles Foliar Application. Physiol. Plant 2025, 177, e70285. [Google Scholar] [CrossRef]
- Bhat, J.A.; Rajora, N.; Raturi, G.; Sharma, S.; Dhiman, P.; Sanand, S.; Shivaraj, S.M.; Sonah, H.; Deshmukh, R. Silicon Nanoparticles (SiNPs) in Sustainable Agriculture: Major Emphasis on the Practicality, Efficacy and Concerns. Nanoscale Adv. 2021, 3, 4019–4028. [Google Scholar] [CrossRef] [PubMed]
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon Nanoparticles Decrease Arsenic Translocation and Mitigate Phytotoxicity in Tomato Plants. Environ. Sci. Pollut. Res. 2022, 29, 34147–34163. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Luo, C.; Liu, X.; Wang, S.; Liu, T.; Li, X. Foliar Application of Two Silica Sols Reduced Cadmium Accumulation in Rice Grains. J. Hazard Mater. 2009, 161, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon Nanoparticles (SiNp) Alleviate Chromium (VI) Phytotoxicity in Pisum sativum (L.) Seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Quintarelli, V.; Ben Hassine, M.; Radicetti, E.; Stazi, S.R.; Bratti, A.; Allevato, E.; Mancinelli, R.; Jamal, A.; Ahsan, M.; Mirzaei, M.; et al. Advances in Nanotechnology for Sustainable Agriculture: A Review of Climate Change Mitigation. Sustainability 2024, 16, 9280. [Google Scholar] [CrossRef]
- Santos, P.A.; Biraku, X.; Nielsen, E.; Ozketen, A.C.; Ozketen, A.A.; Hakki, E.E. Agricultural Nanotechnology for a Safe and Sustainable Future: Current Status, Challenges, and Beyond. J. Sci. Food Agric. 2025, 105, 3159–3169. [Google Scholar] [CrossRef]
- El-Moneim, D.A.; Dawood, M.F.A.; Moursi, Y.S.; Farghaly, A.A.; Afifi, M.; Sallam, A. Positive and Negative Effects of Nanoparticles on Agricultural Crops. Nanotechnol. Environ. Eng. 2021, 6, 21. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Chitkara, M.; Singh Sandhu, I. Nanotechnology; Jenny Stanford Publishing: Singapore, 2021; ISBN 9781003120261. [Google Scholar]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle Applications in Agriculture: Overview and Response of Plant-Associated Microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
- Bapat, G.; Zinjarde, S.; Tamhane, V. Evaluation of Silica Nanoparticle Mediated Delivery of Protease Inhibitor in Tomato Plants and Its Effect on Insect Pest Helicoverpa armigera. Colloids Surf. B Biointerfaces 2020, 193, 111079. [Google Scholar] [CrossRef]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.M.A.; Gulfishan, M.; Lux, A. Silicon Nanoparticles in Higher Plants: Uptake, Action, Stress Tolerance, and Crosstalk with Phytohormones, Antioxidants, and Other Signalling Molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Jin, H.; Yin, C.; Hua, Y.; Huang, Q.; Zhou, G.; Xu, Y.; He, Y.; Liang, Y.; Zhu, Z. Comparative Effects of Silicon and Silicon Nanoparticles on the Antioxidant System and Cadmium Uptake in Tomato under Cadmium Stress. Sci. Total Environ. 2023, 904, 166819. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Deng, C.; Eggleston, I.; Gao, S.; Li, A.; Alvarez Reyes, W.R.; Cai, K.; Qiu, R.; Haynes, C.L.; et al. Optimizing SiO2 Nanoparticle Structures to Enhance Drought Resistance in Tomato (Solanum lycopersicum L.): Insights into Nanoparticle Dissolution and Plant Stress Response. J. Agric. Food Chem. 2025, 73, 9983–9993. [Google Scholar] [CrossRef]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Li, Y.; Li, Z.; Lu, C.; Li, P.; et al. Silica Nanoparticles Protect Rice against Biotic and Abiotic Stresses. J. Nanobiotechnol. 2022, 20, 197. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and Physiological Responses of Maize (Zea mays L.) to Porous Silica Nanoparticles in Soil. J. Nanoparticle Res. 2012, 14, 1294. [Google Scholar] [CrossRef]
- El-Serafy, R.S. Silica Nanoparticles Enhances Physio-Biochemical Characters and Postharvest Quality of Rosa Hybrida L. Cut Flowers. J. Hortic. Res. 2019, 27, 47–54. [Google Scholar] [CrossRef]
- Roychoudhury, A. Silicon-Nanoparticles in Crop Improvement and Agriculture. Int. J. Recent Adv. Biotechnol. Nanotechnol. 2020, 3, 1571–2582. [Google Scholar]
- Miao, G.; Han, J.; Han, T. Silicon Nanoparticles and Apoplastic Protein Interaction: A Hypothesized Mechanism for Modulating Plant Growth and Immunity. Plants 2025, 14, 1630. [Google Scholar] [CrossRef] [PubMed]
- Nazim, M.; Li, X.; Anjum, S.; Ahmad, F.; Ali, M.; Muhammad, M.; Shahzad, K.; Lin, L.; Zulfiqar, U. Silicon Nanoparticles: A Novel Approach in Plant Physiology to Combat Drought Stress in Arid Environment. Biocatal. Agric. Biotechnol. 2024, 58, 103190. [Google Scholar] [CrossRef]
- Wang, L.; Pan, T.; Gao, X.; An, J.; Ning, C.; Li, S.; Cai, K. Silica Nanoparticles Activate Defense Responses by Reducing Reactive Oxygen Species under Ralstonia Solanacearum Infection in Tomato Plants. NanoImpact 2022, 28, 100418. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica Nanoparticles Boost Growth and Productivity of Cucumber under Water Deficit and Salinity Stresses by Balancing Nutrients Uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Kurjak, D.; Lux, A.; Corpas, F.J. Silicon Nanoparticles (SiNPs) Restore Photosynthesis and Essential Oil Content by Upgrading Enzymatic Antioxidant Metabolism in Lemongrass (Cymbopogon flexuosus) under Salt Stress. Front. Plant. Sci. 2023, 14, 1116769. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Sunoj, V.S.J.; Wen, Y.; Zhu, J.J.; Muralidharan, G.; Cao, K.F. Foliar Application of Nanoparticles Mitigates the Chilling Effect on Photosynthesis and Photoprotection in Sugarcane. Plant Physiol. Biochem. 2020, 149, 50–60. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and Transport of Silicon in Plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Razmi, J.; Eshaghabadi, A.H.; Pashang, D. Silicon Nanoparticles (SiNP): A Novel and Sustainable Strategy for Mitigating Environmental Stresses in Plants. J. Soil. Sci. Plant Nutr. 2024, 24, 2167–2191. [Google Scholar] [CrossRef]
- Madany, M.M.Y.; Saleh, A.M.; Habeeb, T.H.; Hozzein, W.N.; AbdElgawad, H. Silicon Dioxide Nanoparticles Alleviate the Threats of Broomrape Infection in Tomato by Inducing Cell Wall Fortification and Modulating ROS Homeostasis. Environ. Sci. Nano 2020, 7, 1415–1430. [Google Scholar] [CrossRef]
- Udalova, Z.V.; Folmanis, G.E.; Fedotov, M.A.; Pelgunova, L.A.; Krysanov, E.Y.; Khasanov, F.K.; Zinovieva, S.V. Effects of Silicon Nanoparticles on Photosynthetic Pigments and Biogenic Elements in Tomato Plants Infected with Root-Knot Nematode Meloidogyne incognita. Dokl. Biochem. Biophys. 2020, 495, 329–333. [Google Scholar] [CrossRef]
- Derbalah, A.; Shenashen, M.; Hamza, A.; Mohamed, A.; El Safty, S. Antifungal Activity of Fabricated Mesoporous Silica Nanoparticles against Early Blight of Tomato. Egypt. J. Basic Appl. Sci. 2018, 5, 145–150. [Google Scholar] [CrossRef]
- Naaz, H.; Rawat, K.; Saffeullah, P.; Umar, S. Silica Nanoparticles Synthesis and Applications in Agriculture for Plant Fertilization and Protection: A Review. Environ. Chem. Lett. 2023, 21, 539–559. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of Silicon Nanoparticles in Agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher Stomatal Density Improves Photosynthetic Induction and Biomass Production in Arabidopsis Under Fluctuating Light. Front. Plant Sci. 2020, 11, 589603. [Google Scholar] [CrossRef] [PubMed]
- Mavrič Čermelj, A.; Golob, A.; Vogel-Mikuš, K.; Germ, M. Silicon Mitigates Negative Impacts of Drought and UV-B Radiation in Plants. Plants 2021, 11, 91. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Zeng, Y.; Li, D.-M.; Guo, D.-J.; Rajput, V.D.; Chen, G.-L.; Barakhov, A.; Minkina, T.M.; Li, Y.-R. Characteristics of Leaf Stomata and Their Relationship with Photosynthesis in Saccharum officinarum Under Drought and Silicon Application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- Vandegeer, R.K.; Zhao, C.; Cibils-Stewart, X.; Wuhrer, R.; Hall, C.R.; Hartley, S.E.; Tissue, D.T.; Johnson, S.N. Silicon Deposition on Guard Cells Increases Stomatal Sensitivity as Mediated by K + Efflux and Consequently Reduces Stomatal Conductance. Physiol. Plant 2021, 171, 358–370. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Hãggman, H.; Jaakola, L. Light-Controlled Flavonoid Biosynthesis in Fruits. Front. Plant. Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Deng, X.; Wang, S.; Tanaka, K.; Zhang, S. Aquaporin-Mediated Increase in Root Hydraulic Conductance Is Involved in Silicon-Induced Improved Root Water Uptake under Osmotic Stress in Sorghum bicolor L. J. Exp. Bot. 2014, 65, 4747–4756. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Arshad, M.; Al-Kheraif, A.A.; Azzam, M.A.; Al Balawi, T. Silicon Nanoparticle-Induced Regulation of Carbohydrate Metabolism, Photosynthesis, and ROS Homeostasis in Solanum lycopersicum Subjected to Salinity Stress. ACS Omega 2022, 7, 31834–31844. [Google Scholar] [CrossRef]
- Eghlima, G.; Mohammadi, M.; Ranjabr, M.-E.; Nezamdoost, D.; Mammadov, A. Foliar Application of Nano-Silicon Enhances Drought Tolerance Rate of Pot Marigold (Calendula officinalis L.) by Regulation of Abscisic Acid Signaling. BMC Plant. Biol. 2024, 24, 1220. [Google Scholar] [CrossRef] [PubMed]
- Saja-Garbarz, D.; Libik-Konieczny, M.; Janowiak, F. Silicon Improves Root Functioning and Water Management as Well as Alleviates Oxidative Stress in Oilseed Rape under Drought Conditions. Front. Plant. Sci. 2024, 15, 1359747. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of Plants to Overcome Abiotic and Biotic Stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, S.; Seco, R.; Musaev, K.; Basu, C.; Kim, S.; Guenther, A. Impact of Heat Stress on Foliar Biogenic Volatile Organic Compound Emission and Gene Expression in Tomato (Solanum lycopersicum) Seedlings. Elem. Sci. Anthr. 2022, 10, 00096. [Google Scholar] [CrossRef]
- Kralova, K.; Jampilek, J. Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles. Appl. Sci. 2021, 11, 1813. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.-J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Gankema, P. UV-B Radiation Affects Plant Volatile Emissions and Shade Avoidance Responses. Ph.D. Thesis, Utrecht University Repository, Utrecht, The Netherlands, 2015. [Google Scholar]
- Fang, J.; Wan, X.; Ma, X. Nanoscale Silicas in Oryza sativa L. and Their UV Absorption. Guang Pu Xue Yu Guang Pu Fen Xi 2006, 26, 2315–2318. [Google Scholar]
- Schaller, J.; Brackhage, C.; Bäucker, E.; Dudel, E.G. UV-Screening of Grasses by Plant Silica Layer? J. Biosci. 2013, 38, 413–416. [Google Scholar] [CrossRef]
- Pinedo-Guerrero, Z.H.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; González-Morales, S.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Form of Silica Improves Yield, Fruit Quality and Antioxidant Defense System of Tomato Plants under Salt Stress. Agriculture 2020, 10, 367. [Google Scholar] [CrossRef]
- Liu, L.; Lin, N.; Liu, X.; Yang, S.; Wang, W.; Wan, X. From Chloroplast Biogenesis to Chlorophyll Accumulation: The Interplay of Light and Hormones on Gene Expression in Camellia Sinensis Cv. Shuchazao Leaves. Front. Plant. Sci. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The Biochemistry and Molecular Biology of Chlorophyll Breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- Parveen, A.; Siddiqui, Z.A. Impact of Silicon Dioxide Nanoparticles on Growth, Photosynthetic Pigments, Proline, Activities of Defense Enzymes and Some Bacterial and Fungal Pathogens of Tomato. Vegetos 2022, 35, 83–93. [Google Scholar] [CrossRef]
- Grewal, S.; Boora, R.; Kumari, S.; Thakur, R.; Goel, S. Fascinating Aspects of Nanosilicon Enabled Plant Stress Tolerance—A Comprehensive Review. Plant. Nano. Biol. 2024, 8, 100077. [Google Scholar] [CrossRef]
- Mushinskiy, A.A.; Aminova, E.V.; Korotkova, A.M. Evaluation of Tolerance of Tubers Solanum tuberosum to Silica Nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 34559–34569. [Google Scholar] [CrossRef]
- Hu, W.; Su, Y.; Yang, R.; Xie, Z.; Gong, H. Effect of Foliar Application of Silicon and Selenium on the Growth, Yield and Fruit Quality of Tomato in the Field. Horticulturae 2023, 9, 1126. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy 2022, 12, 2366. [Google Scholar] [CrossRef]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of Transgenic Tomato Plants Expressing an Additional Phytoene Synthase in a Fruit-Specific Manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-Carotene Hydroxylase Enhances Stress Tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Stamatakis, K.; Tsimilli-Michael, M.; Papageorgiou, G.C. On the Question of the Light-Harvesting Role of β-Carotene in Photosystem II and Photosystem I Core Complexes. Plant Physiol. Biochem. 2014, 81, 121–127. [Google Scholar] [CrossRef]
- Oyarburo, N.S.; Machinandiarena, M.F.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Potassium Phosphite Increases Tolerance to UV-B in Potato. Plant Physiol. Biochem. 2015, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- von Caemmerer, S.; Farquhar, G.D. Some Relationships between the Biochemistry of Photosynthesis and the Gas Exchange of Leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Niinemets, Ü. Gas Chromatography-Mass Spectrometry Method for Determination of Monoterpene and Sesquiterpene Emissions from Stressed Plants. Stud. Univ. Babes-Bolyai Chem. 2009, 54, 329–339. [Google Scholar]
- Toome, M.; Randjärv, P.; Copolovici, L.; Niinemets, Ü.; Heinsoo, K.; Luik, A.; Noe, S.M. Leaf Rust Induced Volatile Organic Compounds Signalling in Willow during the Infection. Planta 2010, 232, 235–243. [Google Scholar] [CrossRef]
- Opriş, O.; Copaciu, F.; Loredana Soran, M.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of Nine Antibiotics on Key Secondary Metabolites and Physiological Characteristics in Triticum aestivum: Leaf Volatiles as a Promising New Tool to Assess Toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Schwack, W. Determination of Free and Bound Carotenoids in Paprika (Capsicum annuum L.) by LC/MS. Eur. Food Res. Technol. 2000, 211, 52–55. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 12 July 2025).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copaciu, F.; Faur, C.-A.; Bunea, A.; Leopold, L.; Sima, R.M.; Lăcătuș, M.A.; Lupitu, A.; Moisa, C.; Copolovici, D.M.; Copolovici, L. Enhancing UV-B Protection and Abiotic Stress Tolerance in Tomato Plants: The Role of Silicon Nanoparticles in Photosynthetic Parameters, Pigments, and Secondary Metabolite Production. Plants 2025, 14, 2599. https://doi.org/10.3390/plants14162599
Copaciu F, Faur C-A, Bunea A, Leopold L, Sima RM, Lăcătuș MA, Lupitu A, Moisa C, Copolovici DM, Copolovici L. Enhancing UV-B Protection and Abiotic Stress Tolerance in Tomato Plants: The Role of Silicon Nanoparticles in Photosynthetic Parameters, Pigments, and Secondary Metabolite Production. Plants. 2025; 14(16):2599. https://doi.org/10.3390/plants14162599
Chicago/Turabian StyleCopaciu, Florina, Cosmin-Alin Faur, Andrea Bunea, Loredana Leopold, Rodica Maria Sima, Mihai Andrei Lăcătuș, Andreea Lupitu, Cristian Moisa, Dana Maria Copolovici, and Lucian Copolovici. 2025. "Enhancing UV-B Protection and Abiotic Stress Tolerance in Tomato Plants: The Role of Silicon Nanoparticles in Photosynthetic Parameters, Pigments, and Secondary Metabolite Production" Plants 14, no. 16: 2599. https://doi.org/10.3390/plants14162599
APA StyleCopaciu, F., Faur, C.-A., Bunea, A., Leopold, L., Sima, R. M., Lăcătuș, M. A., Lupitu, A., Moisa, C., Copolovici, D. M., & Copolovici, L. (2025). Enhancing UV-B Protection and Abiotic Stress Tolerance in Tomato Plants: The Role of Silicon Nanoparticles in Photosynthetic Parameters, Pigments, and Secondary Metabolite Production. Plants, 14(16), 2599. https://doi.org/10.3390/plants14162599









