Unveiling the Petunia hybrida Virome: Metatranscriptomic Profiling from the Bulgarian Market and In Vitro Cultures
Abstract
1. Introduction
2. Results
2.1. Phenotypic and Photosynthetic Alterations in Symptomatic and Asymptomatic Petunia Plants
2.2. In Vitro Culture of Symptomatic and Asymptomatic Plantlets
2.3. Virus Detection by Long Non-Coding RNA Sequencing
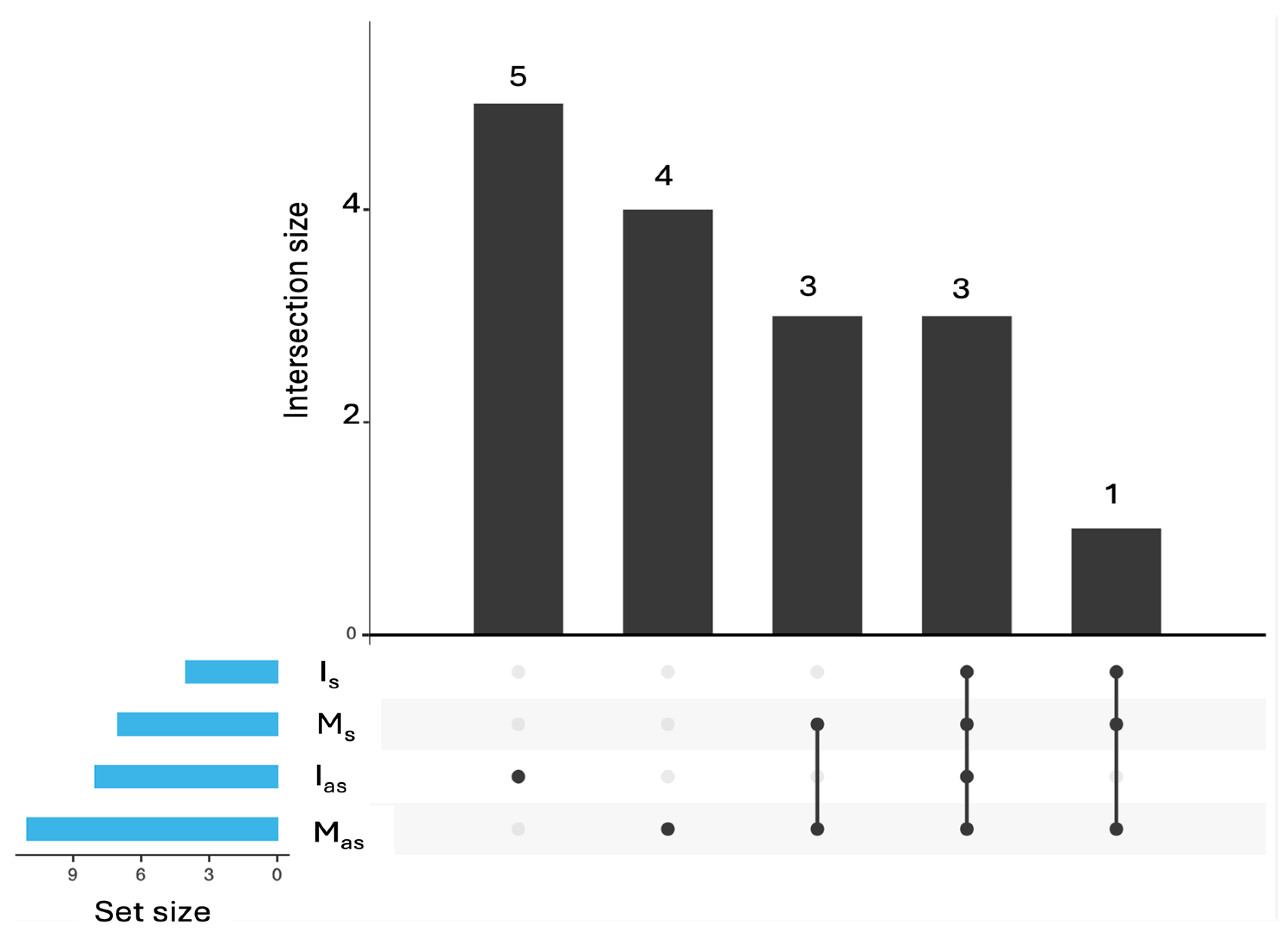
2.4. Taxonomic Composition of the Petunia Virome Across Market and In Vitro Samples
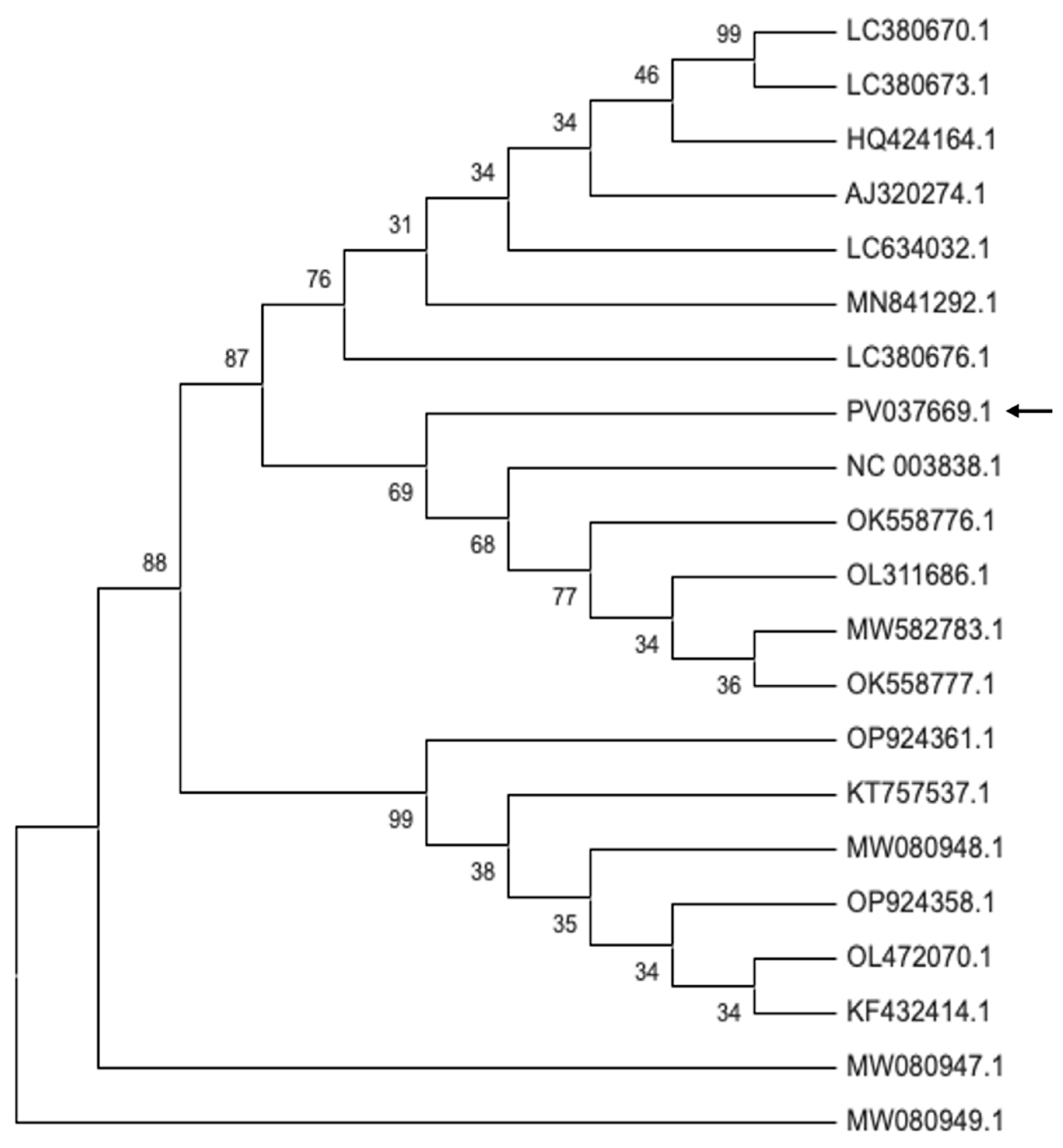
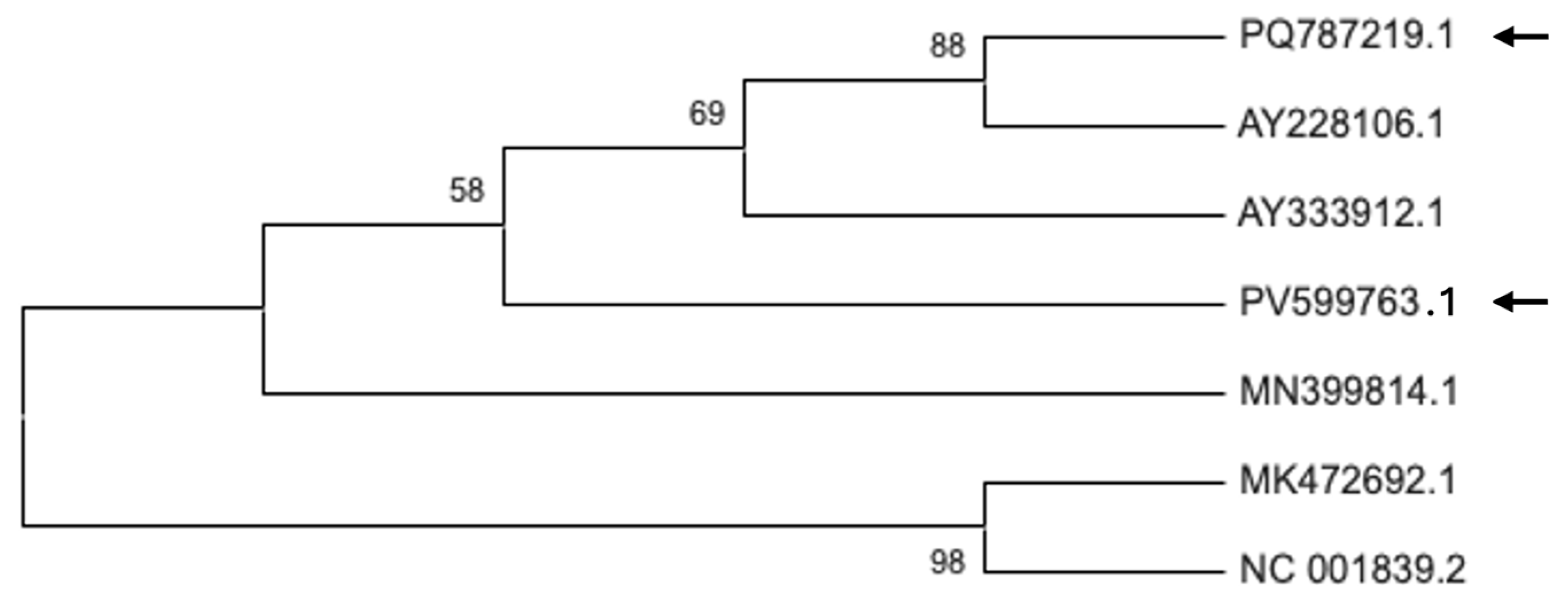
2.5. Phylogenetic Analysis of Plant Viruses in Bulgarian Petunia Isolates
3. Discussion
4. Materials and Methods
4.1. Plant Sampling
4.2. Chlorophyll a Fluorescence Assay and Chlorophyll Content
4.3. Establishment of In Vitro Cultures from Axillary Buds
4.4. Long Non-Coding RNA Sequencing and Bioinformatics Analysis
4.5. PCR and RT-PCR Virus Amplification and Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bombarely, A.; Moser, M.; Amrad, A.; Bao, M.; Bapaume, L.; Barry, C.S.; Bliek, M.; Boersma, M.R.; Borghi, L.; Bruggmann, R.; et al. Insight into the Evolution of the Solanaceae from the Parental Genomes of P. hybrida. Nat. Plants 2016, 2, 16074. [Google Scholar] [CrossRef] [PubMed]
- de Vlaming, P.; Gerats, A.G.M.; Wiering, H.; Wijsman, H.J.W.; Cornu, A.; Farcy, E.; Maizonnier, D.P. hybrida: A Short Description of the Action of 91 Genes, Their Origin and Their Map Location. Plant Mol. Biol. Rep. 1984, 2, 21–42. [Google Scholar] [CrossRef]
- Galliot, C.; Hoballah, M.E.; Kuhlemeier, C.; Stuurman, J. Genetics of Flower Size and Nectar Volume in Petunia Pollination Syndromes. Planta 2006, 225, 203–212. [Google Scholar] [CrossRef]
- Morita, Y.; Hoshino, A. Recent Advances in Flower Color Variation and Patterning of Japanese Morning Glory and Petunia. Breed. Sci. 2018, 68, 128–138. [Google Scholar] [CrossRef]
- Cao, Z.; Guo, Y.; Yang, Q.; He, Y.; Fetouh, M.I.; Warner, R.M.; Deng, Z. Genome-Wide Identification of Quantitative Trait Loci for Important Plant and Flower Traits in Petunia Using a High-Density Linkage Map and an Interspecific Recombinant Inbred Population Derived from Petunia integrifolia and P. axillaris. Hortic. Res. 2019, 6, 27. [Google Scholar] [CrossRef]
- Geitmann, A. Petunia. Evolutionary, Developmental and Physiological Genetics. Ann. Bot. 2011, 107, vi–vii. [Google Scholar] [CrossRef]
- National Agricultural Statistics Service. Floriculture Crops 2018 Summary; USDA: Washington, DC, USA, 2019.
- Lesemann, D.-E. Viruses Recently Detected In Vegetatively Propagated Petunia. Acta Hortic. 1996, 88–95. [Google Scholar] [CrossRef]
- Hammond, J.; Huang, Q.; Jordan, R.; Meekes, E.; Fox, A.; Vazquez-Iglesias, I.; Vaira, A.M.; Copetta, A.; Delmiglio, C. International Trade and Local Effects of Viral and Bacterial Diseases in Ornamental Plants. Annu. Rev. Phytopathol. 2023, 61, 73–95. [Google Scholar] [CrossRef]
- Boonham, N.; Hims, M.; Barker, I.; Spence, N. Potato Virus Y from Petunia Can Cause Symptoms of Potato Tuber Necrotic Ringspot Disease (PTNRD). Eur. J. Plant Pathol. 1999, 105, 617–621. [Google Scholar] [CrossRef]
- Chung, B.-N.; Kim, J.-S.; Cho, J.-D.; Cheong, S.-R.; Jeong, M.-I. Tobacco Mosaic Virus Detected in Vegetatively Propagated Petunia Hybrids “Surfinia”. Plant Pathol. J. 2007, 23, 34–36. [Google Scholar] [CrossRef]
- Richert-Poggeler, K.R. Induction of Infectious Petunia Vein Clearing (Pararetro) Virus from Endogenous Provirus in Petunia. EMBO J. 2003, 22, 4836–4845. [Google Scholar] [CrossRef] [PubMed]
- Tollenaere, C.; Susi, H.; Laine, A.-L. Evolutionary and Epidemiological Implications of Multiple Infection in Plants. Trends Plant Sci. 2016, 21, 80–90. [Google Scholar] [CrossRef]
- Hu, G.; Dong, Y.; Zhang, Z.; Fan, X.; Ren, F. Effect of In vitro Culture of Long Shoot Tip on Variant Structure and Titer of Grapevine Viruses. Plants 2022, 11, 1907. [Google Scholar] [CrossRef] [PubMed]
- White, P.R. Multiplication of the Viruses of Tobacco and Aucuba Mosaics in Growing Excised Tomato Root-Tips. Phytopathology 1934, 24, 1003–1011. [Google Scholar]
- Marton, L.; Duran-Vila, N.; Lin, J.J.; Semancik, J.S. Properties of Cell Cultures Containing the Citrus Exocortis Viroid. Virology 1982, 122, 229–238. [Google Scholar] [CrossRef]
- Lin, J.J.; Semancik, J.S. Coordination between Host Nucleic Acid Metabolism and Citrus Exocortis Viroid Turnover. Virus Res. 1985, 3, 213–230. [Google Scholar] [CrossRef]
- Mühlbach, H.-P.; Sänger, H.L. Continous Replication of Potato Spindle Tuber Viroid (PSTV) in Permanent Cell Cultures of Potato and Tomato. Biosci. Rep. 1981, 1, 79–87. [Google Scholar] [CrossRef]
- Wang, M.C.; Lin, J.J.; Duran-Vila, N.; Semancik, J.S. Alteration in Cell Wall Composition and Structure in Viroid-Infected Cells. Physiol. Mol. Plant Pathol. 1986, 28, 107–124. [Google Scholar] [CrossRef]
- Haegeman, A.; Foucart, Y.; De Jonghe, K.; Goedefroit, T.; Al Rwahnih, M.; Boonham, N.; Candresse, T.; Gaafar, Y.Z.A.; Hurtado-Gonzales, O.P.; Kogej Zwitter, Z.; et al. Looking beyond Virus Detection in RNA Sequencing Data: Lessons Learned from a Community-Based Effort to Detect Cellular Plant Pathogens and Pests. Plants 2023, 12, 2139. [Google Scholar] [CrossRef]
- Pecman, A.; Kutnjak, D.; Gutiérrez-Aguirre, I.; Adams, I.; Fox, A.; Boonham, N.; Ravnikar, M. Next Generation Sequencing for Detection and Discovery of Plant Viruses and Viroids: Comparison of Two Approaches. Front. Microbiol. 2017, 8, 1998. [Google Scholar] [CrossRef] [PubMed]
- Bömer, M.; Rathnayake, A.I.; Visendi, P.; Sewe, S.O.; Sicat, J.P.A.; Silva, G.; Kumar, P.L.; Seal, S.E. Tissue Culture and Next-Generation Sequencing: A Combined Approach for Detecting Yam (Dioscorea Spp.) Viruses. Physiol. Mol. Plant Pathol. 2019, 105, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Maclot, F.; Candresse, T.; Filloux, D.; Malmstrom, C.M.; Roumagnac, P.; van der Vlugt, R.; Massart, S. Illuminating an Ecological Blackbox: Using High Throughput Sequencing to Characterize the Plant Virome Across Scales. Front. Microbiol. 2020, 11, 578064. [Google Scholar] [CrossRef]
- Ismajli, B.; Galbács, Z.N.; Takács, A.P.; Várallyay, É. The First High-Throughput Sequencing-Based Study of Viruses Infecting Solanaceous Crops in Kosovo Reveals Multiple Infections in Peppers by Six Plant Viruses. Plants 2025, 14, 1273. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cuevas, M.-C.; Nameth, S.G.P. Virus-Associated Diseases of Double Petunia: Frequency and Distribution in Ohio Greenhouses. HortScience 2002, 37, 543–546. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis Group: London, UK, 2000; pp. 443–480. [Google Scholar]
- Bakardjieva, N.; Denkova, S.; Hristova, D.; Hristoval, D. Tomato Spotted Wilt Virus on Ornamental Species in Bulgaria. Biotechnol. Biotechnol. Equip. 1998, 12, 49–52. [Google Scholar] [CrossRef]
- Shih, S.M.-H.; Doran, P.M. In vitro Propagation of Plant Virus Using Different Forms of Plant Tissue Culture and Modes of Culture Operation. J. Biotechnol. 2009, 143, 198–206. [Google Scholar] [CrossRef]
- Noreen, F.; Akbergenov, R.; Hohn, T.; Richert-Pöggeler, K.R. Distinct Expression of Endogenous Petunia Vein Clearing Virus and the DNA Transposon DTph1 in Two P. hybrida Lines Is Correlated with Differences in Histone Modification and SiRNA Production. Plant J. 2007, 50, 219–229. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring Fast Fluorescence Transients to Address Environmental Questions: The JIP-Test. In Photosynthesis: From Light to Biosphere; Springer: Dordrecht, The Netherlands, 1995; pp. 4869–4872. [Google Scholar]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable Chlorophyll Fluorescence and Its Use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Yang, M.; Chen, T.; Liu, Y.-X.; Huang, L. Visualizing Set Relationships: EVenn’s Comprehensive Approach to Venn Diagrams. iMeta 2024, 3, e184. [Google Scholar] [CrossRef]
- Khan, A.; Mathelier, A. Intervene: A Tool for Intersection and Visualization of Multiple Gene or Genomic Region Sets. BMC Bioinform. 2017, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for MacOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]






| Market | In Vitro | |||||||||||
|
Sympt.
plants | 41 Ms | 42 Ms | 43 Ms | 47 Ms | 48 Ms | 49 Ms | 41 Is | 42 Is | 43 Is | 47 Is | 48 Is | 49 Is |
| TAV | + | + | − | − | + | − | + | + | − | − | + | − |
| CMV | + | + | − | − | + | + | + | + | + | + | + | + |
| PVCV | + | + | − | + | + | + | + | + | + | + | + | + |
| Market | In vitro | |||||||||||
| Asympt. plants | 31 Mas | 33 Mas | 35 Mas | 40 Mas | 51 Mas | 53 Mas | 31 Ias | 33 Ias | 35 Ias | 40 Ias | 51 Ias | 53 Ias |
| TAV | − | + | + | − | − | − | − | + | + | − | − | − |
| CMV | + | + | + | + | + | + | + | + | + | + | + | + |
| PVCV | − | + | + | + | + | + | + | + | + | + | + | + |
| Intersection | Viruses |
|---|---|
| Ias & Is & Mas & Ms | cucumber mosaic vírus; tomato aspermy vírus; petunia vein clearing vírus |
| Mas & Ms | Petunia exserta mitovirus 1, * Partitiviridae sp.; cherry leaf roll virus |
| Is & Mas & Ms | * Bacteriophage sp. |
| Mas | apple mosaic virus; Botrytis cinerea alpha-like virus 1; Botrytis cinerea mitovirus 1; grapevine associated narnavirus-1 |
| Ias | Erysiphe necator associated partitivirus 2; Erysiphales ourmia-like virus 2; Erysiphe necator ourmia-like virus 82; Erysiphe necator associated ourmia-like virus 82; * Caudoviricetes sp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valkova, R.; Jurak, S.; Apostolova-Kuzova, E.; Baev, V.; Nacheva, L.; Yahubyan, G.; Škorić, D.; Gozmanova, M. Unveiling the Petunia hybrida Virome: Metatranscriptomic Profiling from the Bulgarian Market and In Vitro Cultures. Plants 2025, 14, 2597. https://doi.org/10.3390/plants14162597
Valkova R, Jurak S, Apostolova-Kuzova E, Baev V, Nacheva L, Yahubyan G, Škorić D, Gozmanova M. Unveiling the Petunia hybrida Virome: Metatranscriptomic Profiling from the Bulgarian Market and In Vitro Cultures. Plants. 2025; 14(16):2597. https://doi.org/10.3390/plants14162597
Chicago/Turabian StyleValkova, Rumyana, Stoyanka Jurak, Elena Apostolova-Kuzova, Vesselin Baev, Lilyana Nacheva, Galina Yahubyan, Dijana Škorić, and Mariyana Gozmanova. 2025. "Unveiling the Petunia hybrida Virome: Metatranscriptomic Profiling from the Bulgarian Market and In Vitro Cultures" Plants 14, no. 16: 2597. https://doi.org/10.3390/plants14162597
APA StyleValkova, R., Jurak, S., Apostolova-Kuzova, E., Baev, V., Nacheva, L., Yahubyan, G., Škorić, D., & Gozmanova, M. (2025). Unveiling the Petunia hybrida Virome: Metatranscriptomic Profiling from the Bulgarian Market and In Vitro Cultures. Plants, 14(16), 2597. https://doi.org/10.3390/plants14162597










