Converging Patterns of Heterotrophic Respiration Between Growing and Non-Growing Seasons in Northern Temperate Grasslands
Abstract
1. Introduction
2. Result
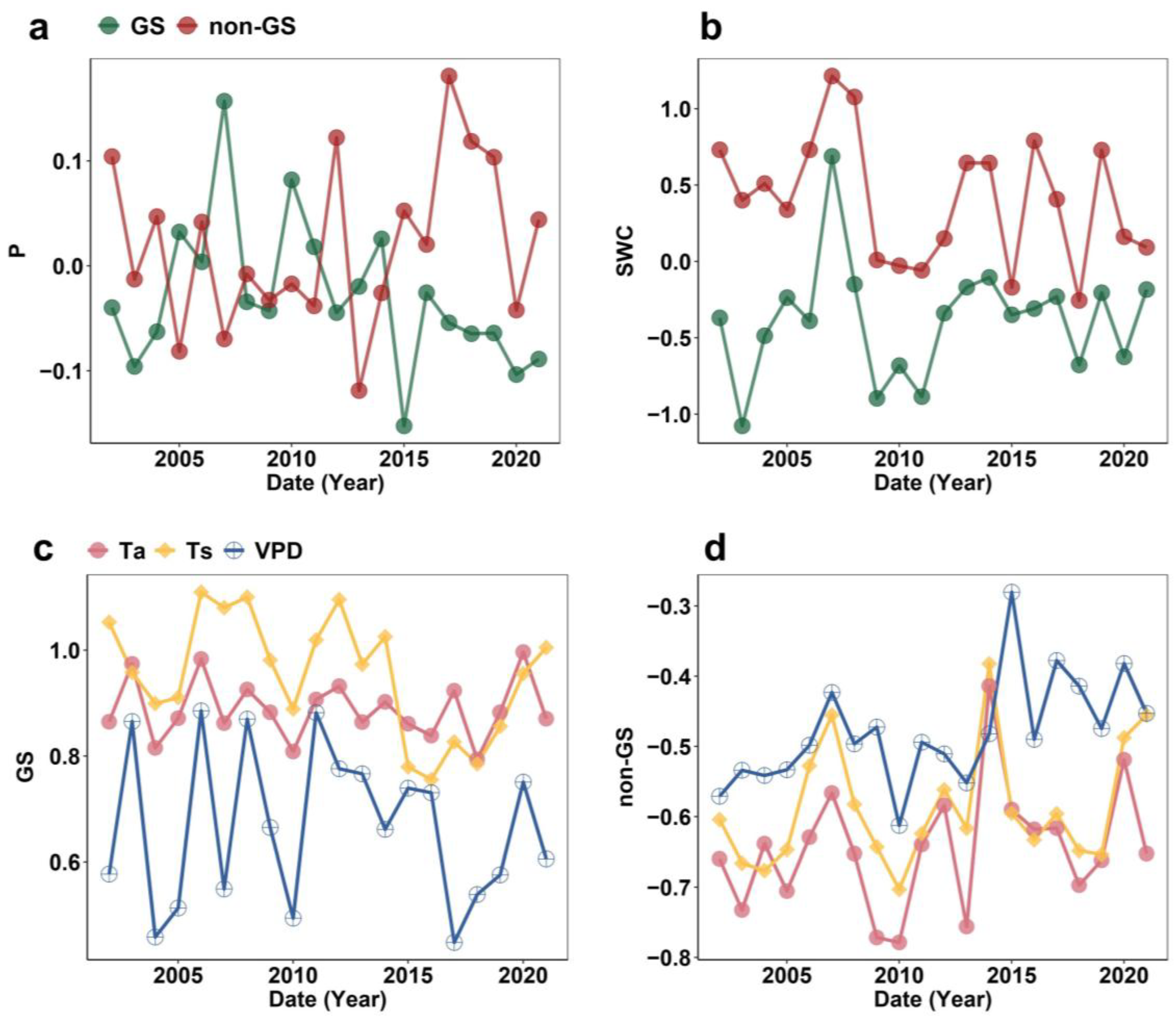
2.1. Seasonal Pattern of Hydrothermal Variables
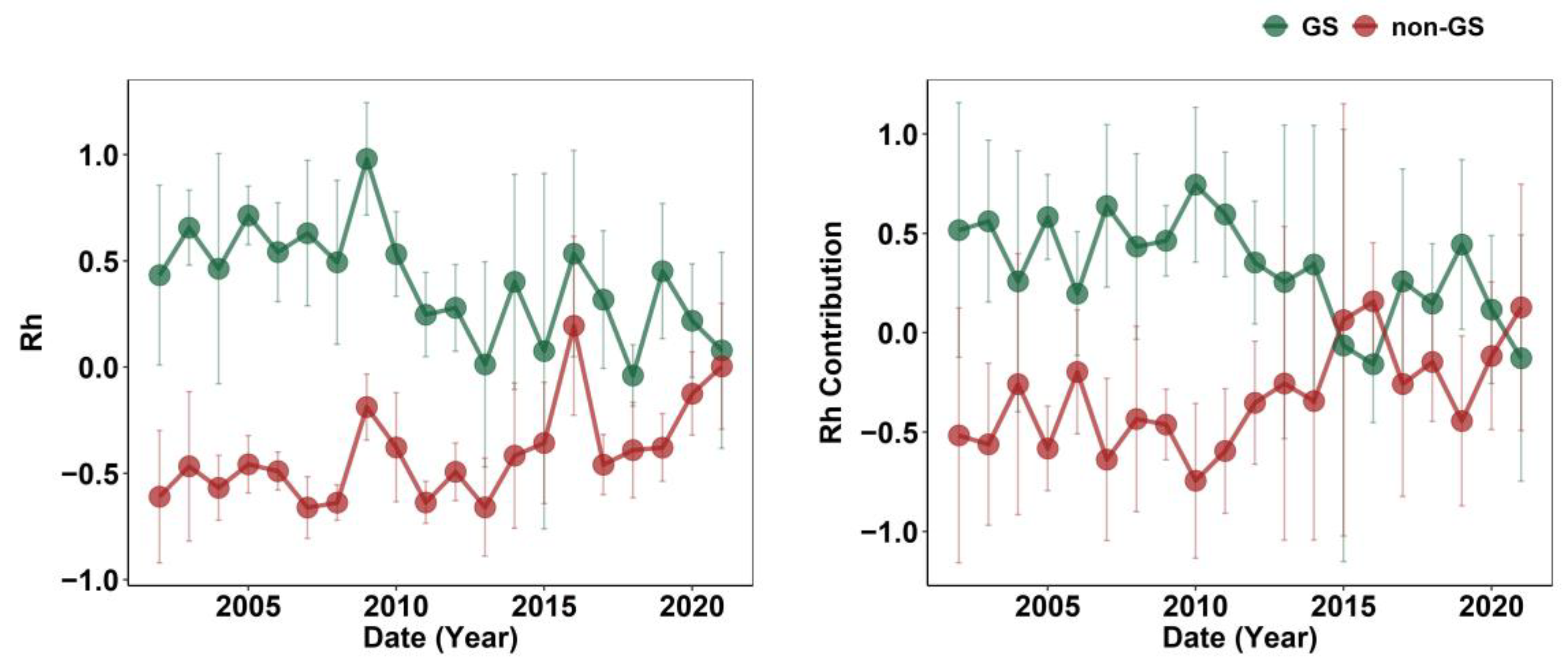
2.2. Seasonal Rh and Its Contribution to Annual Rh
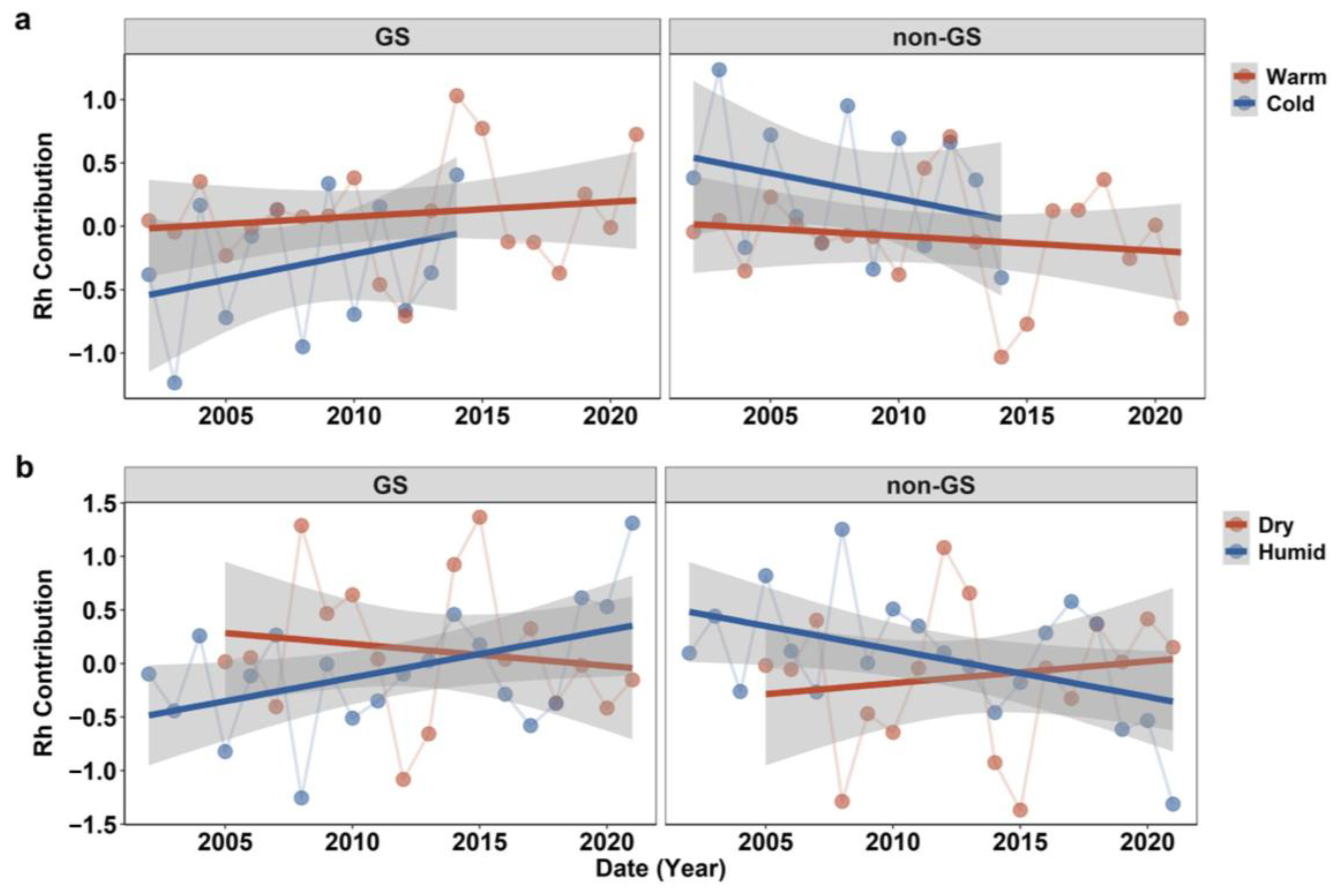
2.3. Seasonal Rh Contribution to Annual Rh in Spatial Difference
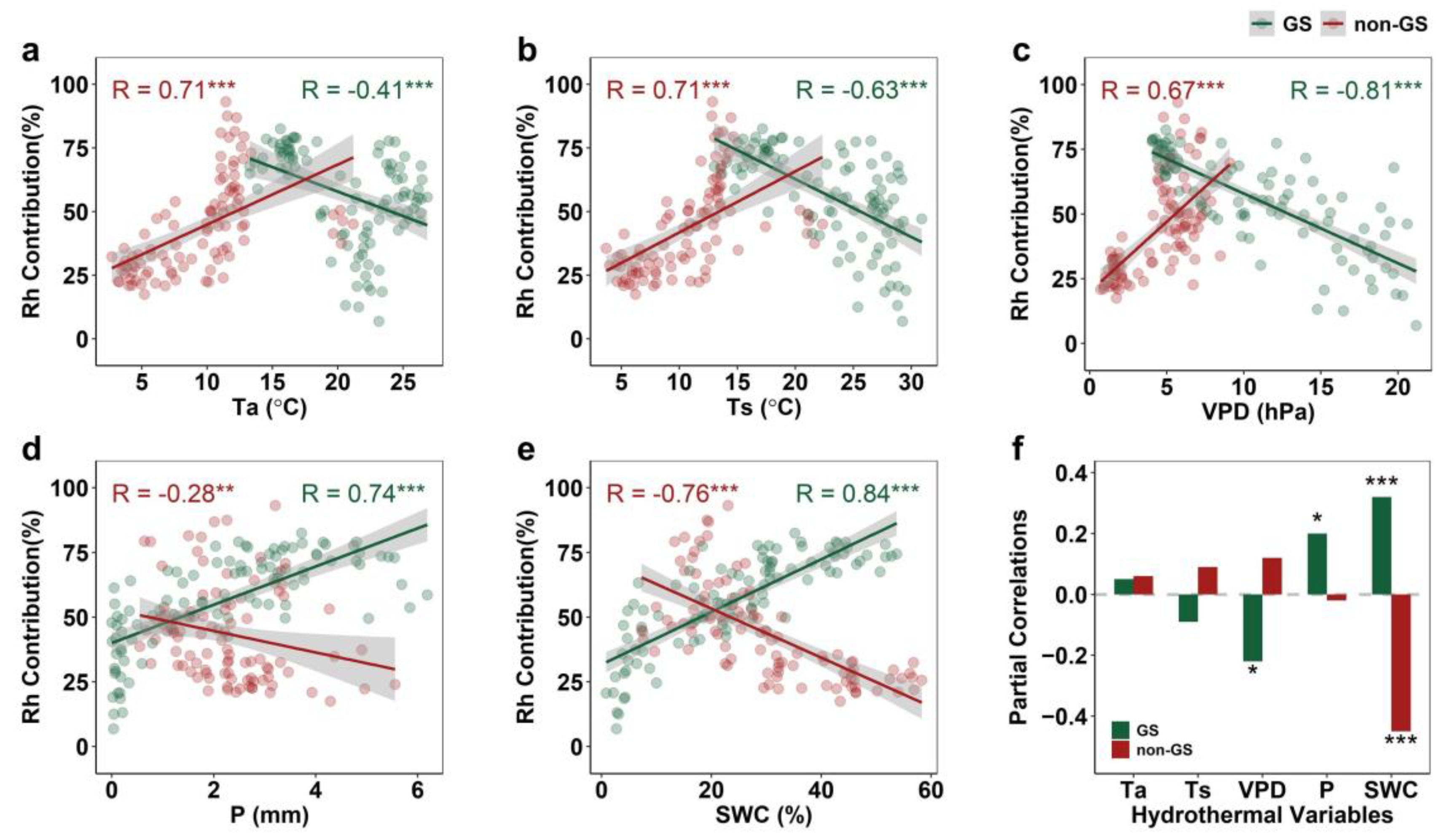
2.4. Seasonal Rh Response to Hydrothermal Variables
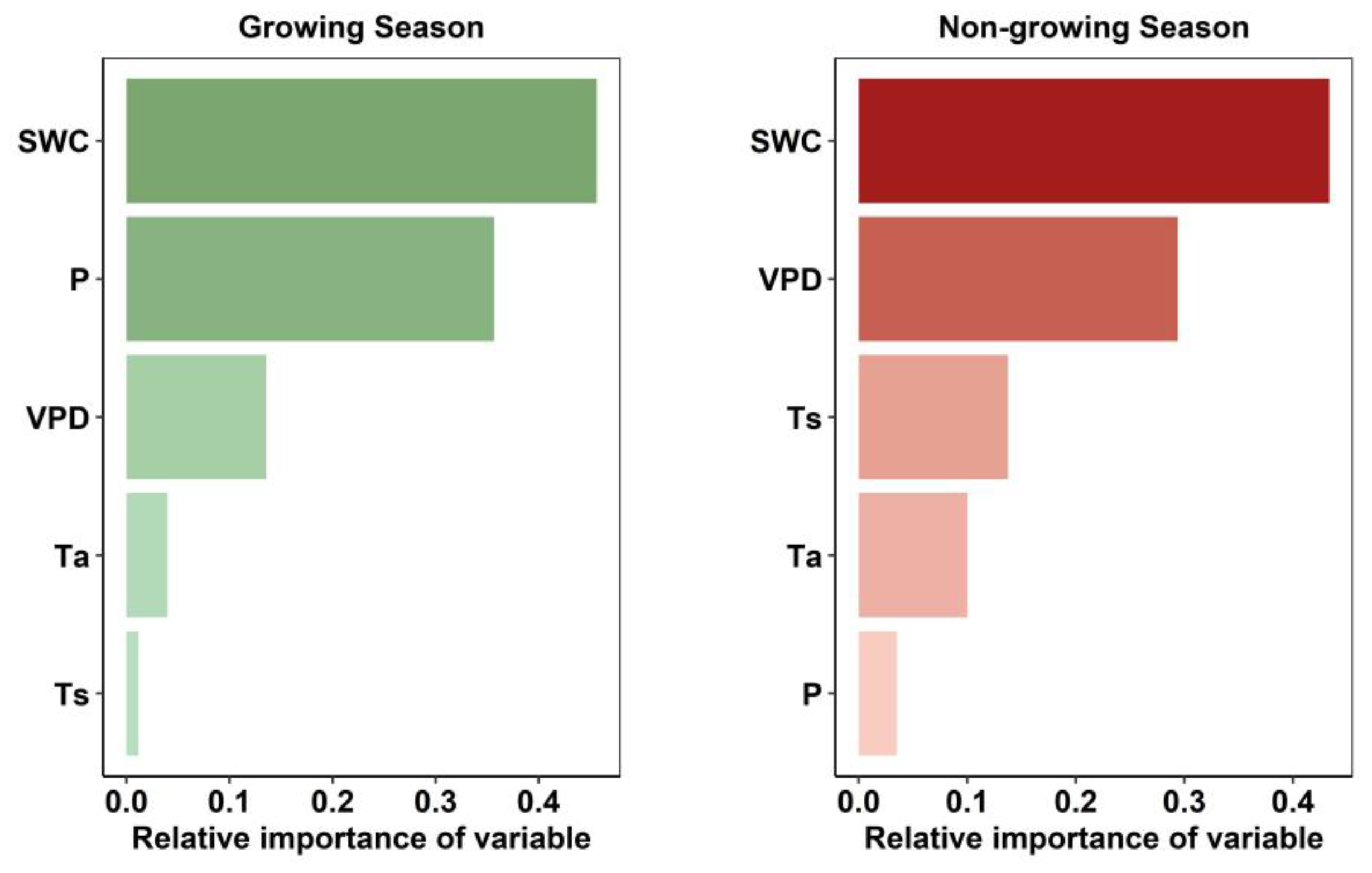
2.5. Importance of Hydrothermal Drivers for Seasonal Rh
3. Discussion
3.1. Seasonality and Magnitude of Soil Rh
3.2. Growing-Season Rh and Its Drivers
3.3. Non-Growing Season Rh and Its Drivers
4. Materials and Methods
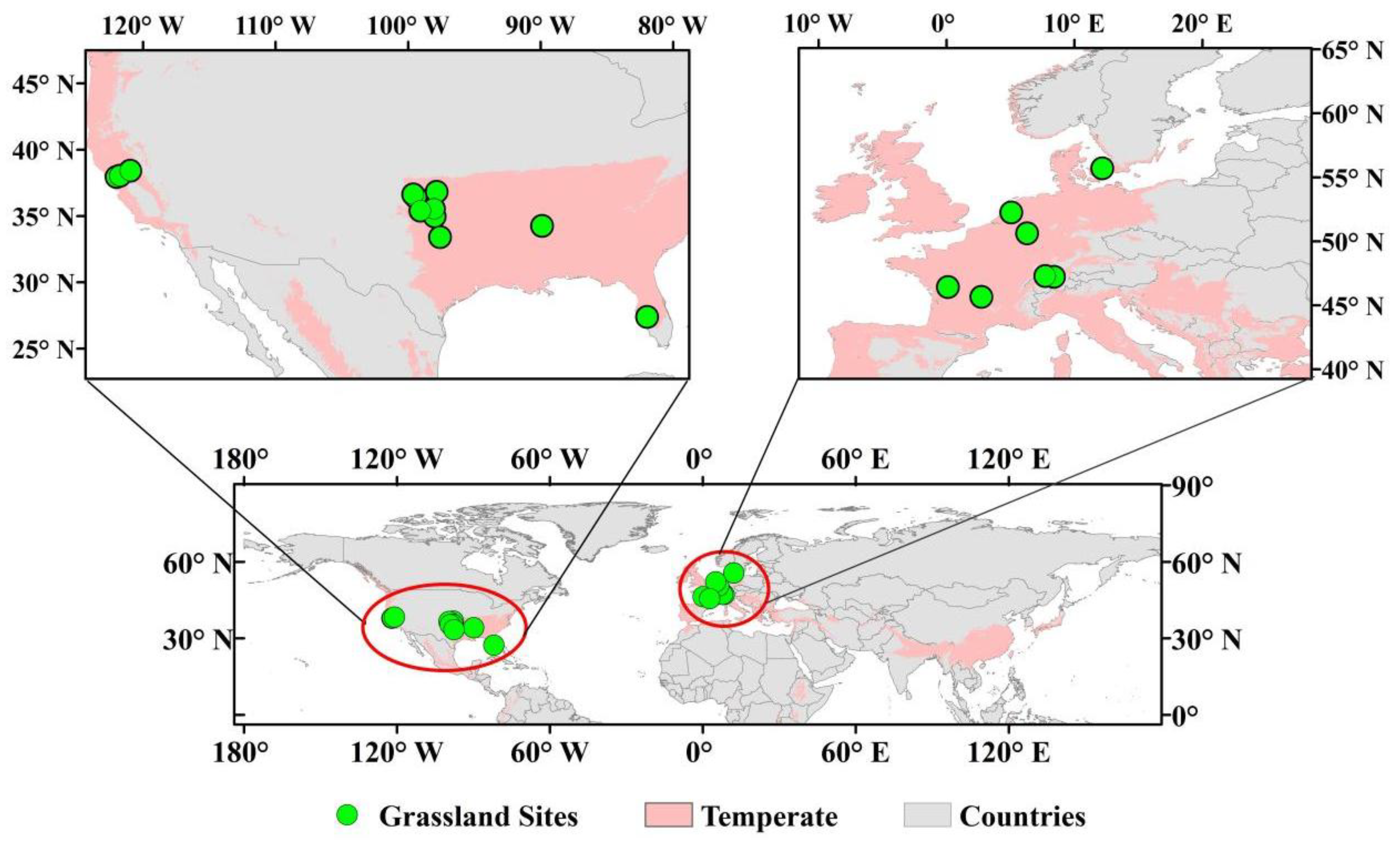
4.1. Study Area
4.2. Calculation of Seasonal Rh
4.3. Data Processing of Hydrothermal Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPBES. Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- He, Y.; Kong, Z.; Hu, X.; Zhang, J.; Wang, M.; Peng, C.; Zhu, Q. Water and heat conditions separately controlled inter-annual variation and growth trend of NDVI in the temperate grasslands in China. Acta Ecol. Sin. 2022, 42, 766–777. [Google Scholar] [CrossRef]
- Seddon, A.W.R.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Kawamura, K. Grassland degradation in China: Methods of monitoring, management and restoration. Grassl. Sci. 2007, 53, 1–17. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; IPCC: Cambridge, UK, 2021. [Google Scholar]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Huang, N.; Wang, L.; Song, X.; Black, T.A.; Jassal, R.S.; Myneni, R.B.; Wu, C.; Wang, L.; Song, W.; Ji, D.; et al. Spatial and temporal variations in global soil respiration and their relationships with climate and land cover. Sci. Adv. 2020, 6, eabb8508. [Google Scholar] [CrossRef]
- Hashimoto, S.; Carvalhais, N.; Ito, A.; Migliavacca, M.; Nishina, K.; Reichstein, M. Global spatiotemporal distribution of soil respiration modeled using a global database. Biogeosciences 2015, 12, 4121–4132. [Google Scholar] [CrossRef]
- Laffitte, B.; Zhou, T.; Yang, Z.; Ciais, P.; Jian, J.; Huang, N.; Seyler, B.C.; Pei, X.; Tang, X. Timescale matters: Finer temporal resolution influences driver contributions to global soil respiration. Glob. Change Biol. 2025, 31, e70118. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef]
- Nissan, A.; Alcolombri, U.; Peleg, N.; Galili, N.; Jimenez-Martinez, J.; Molnar, P.; Holzner, M. Global warming accelerates soil heterotrophic respiration. Nat. Commun. 2023, 14, 3452. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Song, Y.; Zhu, B. Patterns of soil respiration and its temperature sensitivity in grassland ecosystems across China. Biogeosciences 2018, 15, 5329–5341. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, M.; Xuan, J.; Xu, W.; Wang, S.; Miao, R.; Wang, D.; Wu, W.; Liu, Y.; Han, S. Effects of warming on soil respiration during the non-growing seasons in a semiarid temperate steppe. J. Plant Ecol. 2020, 13, 288–294. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Chung, H.; Yu, L.; Mi, Z.; Geng, Y.; Jing, X.; Wang, S.; Zeng, H.; Cao, G.; et al. Non-growing-season soil respiration is controlled by freezing and thawing processes in the summer monsoon-dominated Tibetan alpine grassland. Glob. Biogeochem. Cycles 2014, 28, 1081–1095. [Google Scholar] [CrossRef]
- Hu, T.; Hu, H.; Li, F.; Dou, X.; Sun, L. Changes in the non-growing season soil heterotrophic respiration rate are driven by environmental factors after fire in a cold temperate forest ecosystem. Ann. For. Sci. 2021, 78, 38. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X. Contrasting rhizospheric and heterotrophic components of soil respiration during growing and non-growing seasons in a temperate deciduous forest. Forests 2019, 10, 8. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Y.; Gao, C.; Verburg, P.S.J.; Iii, J.A.A.; Darrouzet-Nardi, A.; Schimel, D.S. Concurrent and lagged impacts of an anomalously warm year on autotrophic and heterotrophic components of soil respiration: A deconvolution analysis. New Phytol. 2010, 187, 184–198. [Google Scholar] [CrossRef]
- Jin, C.; Jian, J.; Bourque, C.P.-A.; Zha, T.; Dai, L.; Yang, Y.; Fu, R.; Chen, Q.; Liu, P.; Li, X.; et al. Soil autotrophic-to-heterotrophic-respiration ratio and its controlling factors across several terrestrial biomes: A global synthesis. CATENA 2024, 242, 108118. [Google Scholar] [CrossRef]
- Ciais, P.; Yao, Y.; Gasser, T.; Baccini, A.; Wang, Y.; Lauerwald, R.; Peng, S.; Bastos, A.; Li, W.; Raymond, P.A.; et al. Empirical estimates of regional carbon budgets imply reduced global soil heterotrophic respiration. Natl. Sci. Rev. 2021, 8, nwaa145. [Google Scholar] [CrossRef]
- Gomez-Casanovas, N.; Matamala, R.; Cook, D.R.; Gonzalez-Meler, M.A. Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration with abiotic factors in prairie grasslands. Glob. Change Biol. 2012, 18, 2532–2545. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J. Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Glob. Change Biol. 2007, 13, 123–131. [Google Scholar] [CrossRef]
- Bhupinderpal-Singh; Nordgren, A.; Ottosson Löfvenius, M.; Högberg, M.N.; Mellander, P.-E.; Högberg, P. Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: Extending observations beyond the first year. Plant Cell Environ. 2003, 26, 1287–1296. [Google Scholar] [CrossRef]
- Konings, A.G.; Bloom, A.A.; Liu, J.; Parazoo, N.C.; Schimel, D.S.; Bowman, K.W. Global satellite-driven estimates of heterotrophic respiration. Biogeosciences 2019, 16, 2269–2284. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, Measuring, and Modeling Soil Respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Lynch, C.; Hartin, C.; Chen, M.; Bond-Lamberty, B. Causes of Uncertainty in Observed and Projected Heterotrophic Respiration from Earth System Models. Biogeosci. Discuss. 2017, 1–28, preprint. [Google Scholar] [CrossRef]
- Huang, Y.; Guenet, B.; Wang, Y.L.; Ciais, P. Global Simulation and Evaluation of Soil Organic Matter and Microbial Carbon and Nitrogen Stocks Using the Microbial Decomposition Model ORCHIMIC v2.0. Glob. Biogeochem. Cycles 2021, 35, e2020GB006836. [Google Scholar] [CrossRef]
- Tao, X.; Yang, Z.; Feng, J.; Jian, S.; Yang, Y.; Bates, C.T.; Wang, G.; Guo, X.; Ning, D.; Kempher, M.L.; et al. Experimental Warming Accelerates Positive Soil Priming in a Temperate Grassland Ecosystem. Nat. Commun. 2024, 15, 1178. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, J.; Liu, H.; Wang, W. Soil Respiration and Its Autotrophic and Heterotrophic Components in Response to Nitrogen Addition among Different Degraded Temperate Grasslands. Soil Biol. Biochem. 2018, 124, 255–265. [Google Scholar] [CrossRef]
- Dong, R.; Peng, Q.; He, Y.; Sun, X.; Qi, Y.; Dong, Y.; Li, Z.; Guo, Y. Diurnal Variation Characteristics of Soil Respiration and Heterotrophic Respiration in Freeze-Thaw Period of Temperate Grassland and Its Response to Water and Nitrogen Addition. Chin. J. Soil Sci. 2021, 52, 1129–1139. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol 2017, 2, 17105. [Google Scholar] [CrossRef]
- He, L.; Xu, X. Mapping Soil Microbial Residence Time at the Global Scale. Glob. Change Biol. 2021, 27, 6484–6497. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Zhuang, Q.; Harden, J.W.; McGuire, A.D.; Liu, Y.; Wang, G.; Gu, L. Incorporating microbial dormancy dynamics into soil decomposition models to improve quantification of soil carbon dynamics of northern temperate forests. J. Geophys. Res. Biogeosci. 2015, 120, 2596–2611. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Z.; Ma, Z.; Barron-Gafford, G.A.; Scott, R.L.; Niu, G. A microbial-explicit soil organic carbon decomposition model (MESDM): Development and testing at a semiarid grassland Site. J. Adv. Model Earth Syst. 2022, 14, e2021MS002485. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active Microorganisms in Soil: Critical Review of Estimation Criteria and Approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Smith, J.L.; Paul, E.A. The Significance of Soil Microbial Biomass Estimations. In Soil Biochemistry; Routledge: London, UK, 1990. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Zhu, E.; Cao, Z.; Jia, J.; Liu, C.; Zhang, Z.; Wang, H.; Dai, G.; He, J.; Feng, X. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Change Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef]
- Ghezzehei, T.A.; Sulman, B.; Arnold, C.L.; Bogie, N.A.; Berhe, A.A. On the role of soil water retention characteristic on aerobic microbial respiration. Biogeosciences 2019, 16, 1187–1209. [Google Scholar] [CrossRef]
- Wu, J.; Pang, H.; Zhuo, Y.; Wang, L.; Wang, F.; Xu, Z.; Wu, S.; Yang, J.; Wen, L. Effects of vegetation degradation on carbon and nitrogen distribution of roots-soil system in temperate typical steppe. Acta Ecol. Sin. 2018, 38, 5340–5350. [Google Scholar] [CrossRef]
- Zhang, H.; Goll, D.S.; Manzoni, S.; Ciais, P.; Guenet, B.; Huang, Y. Modeling the Effects of Litter Stoichiometry and Soil Mineral N Availability on Soil Organic Matter Formation Using CENTURY-CUE (v1.0). Geosci. Model Dev. 2018, 11, 4779–4796. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-Km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Pastorello, G.; Trotta, C.; Canfora, E.; Chu, H.; Christianson, D.; Cheah, Y.-W.; Poindexter, C.; Chen, J.; Elbashandy, A.; Humphrey, M.; et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 2020, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Poggio, L.; de Sousa, L.M.; Batjes, N.H.; Heuvelink, G.B.M.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing Soil Information for the Globe with Quantified Spatial Uncertainty. Soil 2021, 7, 217–240. [Google Scholar] [CrossRef]
- Huang, H.; Calabrese, S.; Rodriguez-Iturbe, I. Variability of ecosystem carbon source from microbial respiration is controlled by rainfall dynamics. Proc. Natl. Acad. Sci. USA 2021, 118, e2115283118. [Google Scholar] [CrossRef] [PubMed]
- Lasslop, G.; Reichstein, M.; Papale, D.; Richardson, A.D.; Arneth, A.; Barr, A.; Stoy, P.; Wohlfahrt, G. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob. Change Biol. 2010, 16, 187–208. [Google Scholar] [CrossRef]
- Running, S.; Mu, Q.; Zhao, M. MODIS/Terra Gross Primary Productivity 8-Day L4 Global 500m SIN Grid V006; USGS: Reston, VA, USA, 2015. [Google Scholar] [CrossRef]
- Running, S.; Zhao, M. MOD17A3H MODIS/Terra Net Primary Production Yearly L4 Global 500m SIN Grid V006; USGS: Reston, VA, USA, 2019. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, G.; Yang, Y.; Mao, T.; Chen, X. Non-growing season soil CO2 flux and its contribution to annual soil CO2 emissions in two typical grasslands in the permafrost region of the Qinghai-Tibet Plateau. Eur. J. Soil Biol. 2015, 71, 45–52. [Google Scholar] [CrossRef]
- Gu, H.; Qiao, Y.; Xi, Z.; Rossi, S.; Smith, N.G.; Liu, J.; Chen, L. Warming-induced increase in carbon uptake is linked to earlier spring phenology in temperate and boreal forests. Nat. Commun. 2022, 13, 3698. [Google Scholar] [CrossRef]
- Copernicus Climate Change Service. ERA5-Land Post-Processed Daily-Statistics from 1950 to Present. 2024. Available online: https://cds.climate.copernicus.eu/datasets/derived-era5-land-daily-statistics?tab=overview (accessed on 17 May 2025).
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. Xgboost: Extreme Gradient Boosting, 1.7.11.1; CRAN: Vienna, Austria, 2025. [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 1 May 2025).
- Bahn, M.; Rodeghiero, M.; Anderson-Dunn, M.; Dore, S.; Gimeno, C.; Drösler, M.; Williams, M.; Ammann, C.; Berninger, F.; Flechard, C.; et al. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems 2008, 11, 1352–1367. [Google Scholar] [CrossRef]
- Wan, S.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Change Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Jiang, H.; Guo, X. Converging Patterns of Heterotrophic Respiration Between Growing and Non-Growing Seasons in Northern Temperate Grasslands. Plants 2025, 14, 2590. https://doi.org/10.3390/plants14162590
Liu C, Jiang H, Guo X. Converging Patterns of Heterotrophic Respiration Between Growing and Non-Growing Seasons in Northern Temperate Grasslands. Plants. 2025; 14(16):2590. https://doi.org/10.3390/plants14162590
Chicago/Turabian StyleLiu, Caiqin, Honglei Jiang, and Xiali Guo. 2025. "Converging Patterns of Heterotrophic Respiration Between Growing and Non-Growing Seasons in Northern Temperate Grasslands" Plants 14, no. 16: 2590. https://doi.org/10.3390/plants14162590
APA StyleLiu, C., Jiang, H., & Guo, X. (2025). Converging Patterns of Heterotrophic Respiration Between Growing and Non-Growing Seasons in Northern Temperate Grasslands. Plants, 14(16), 2590. https://doi.org/10.3390/plants14162590





