Physiological, Biochemical, and Molecular Mechanisms of Resistance of Poacynum hendersonii to Melampsora apocyni
Abstract
1. Introduction
2. Results
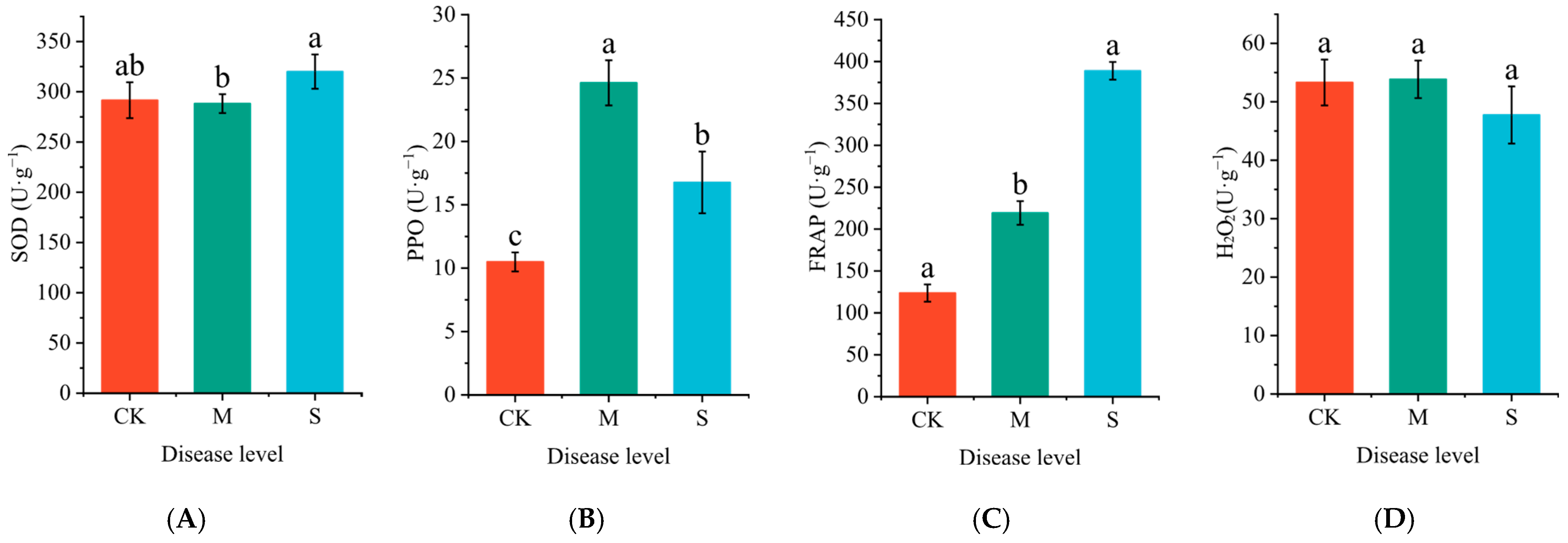
2.1. The Effect of Rust Disease on the Antioxidant Activity in Leaves of P. hendersonii
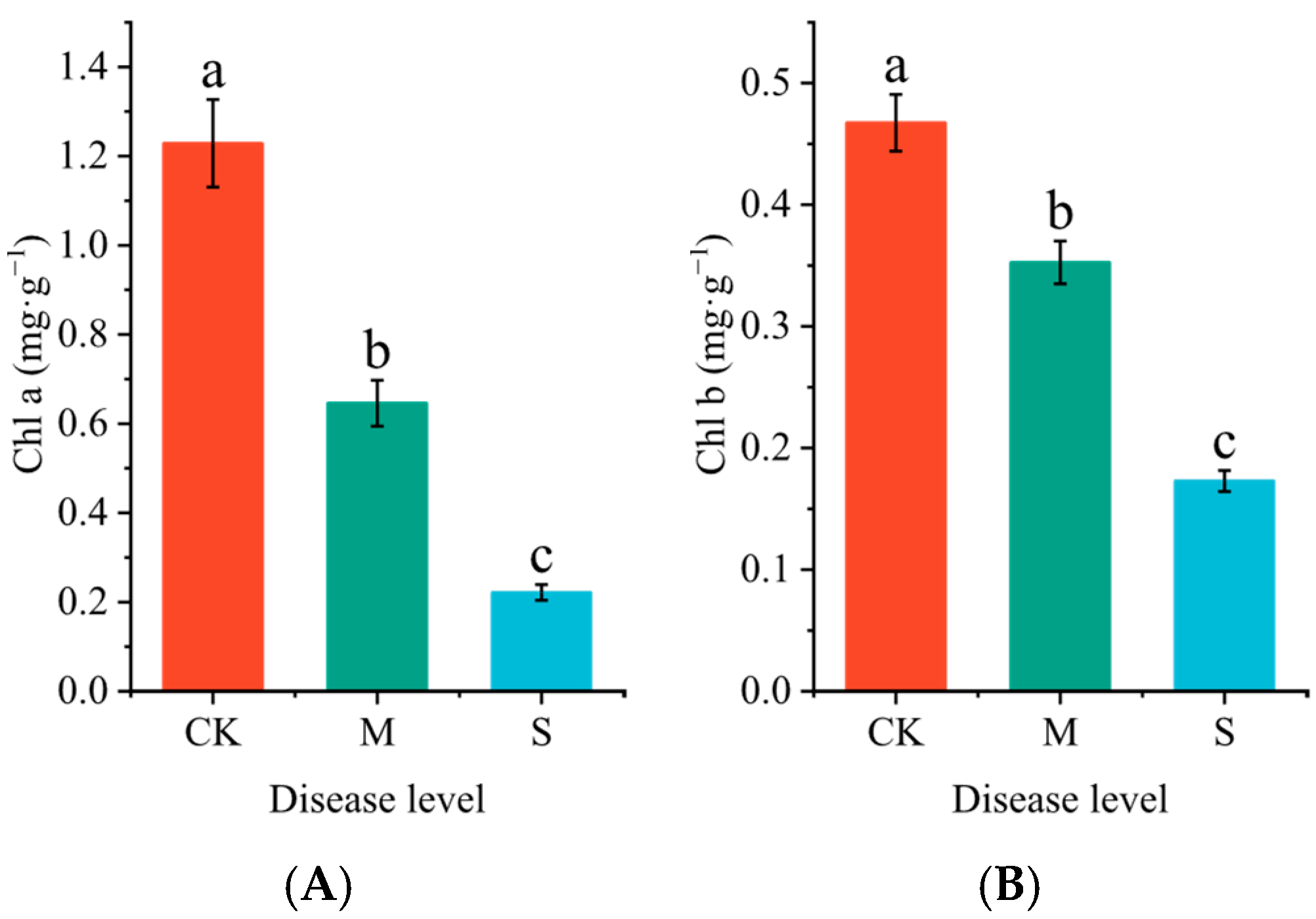
2.2. The Effect of Rust Disease on Chlorophyll Content in Leaves of P. hendersonii
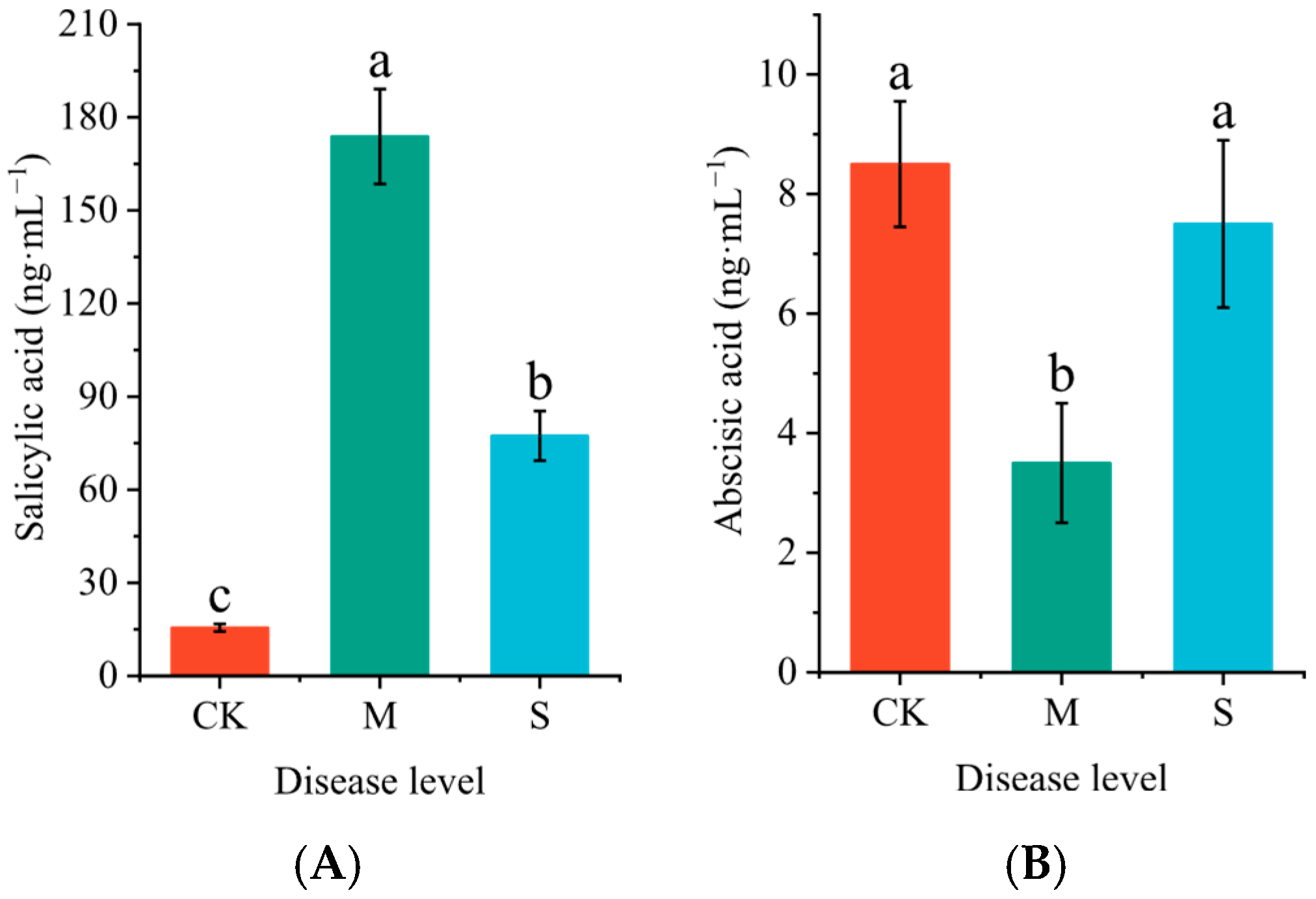
2.3. The Effect of Rust Disease on Hormone Content in Leaves of P. hendersonii
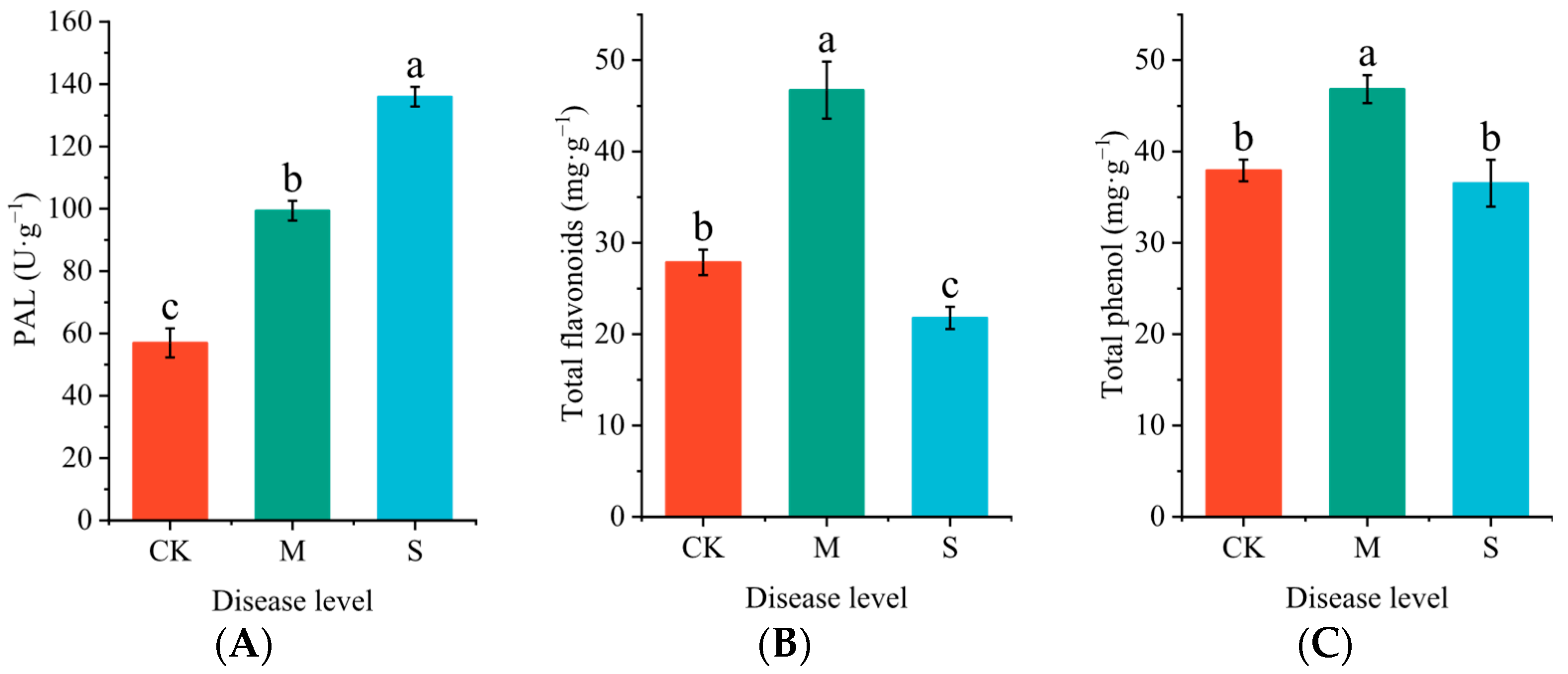
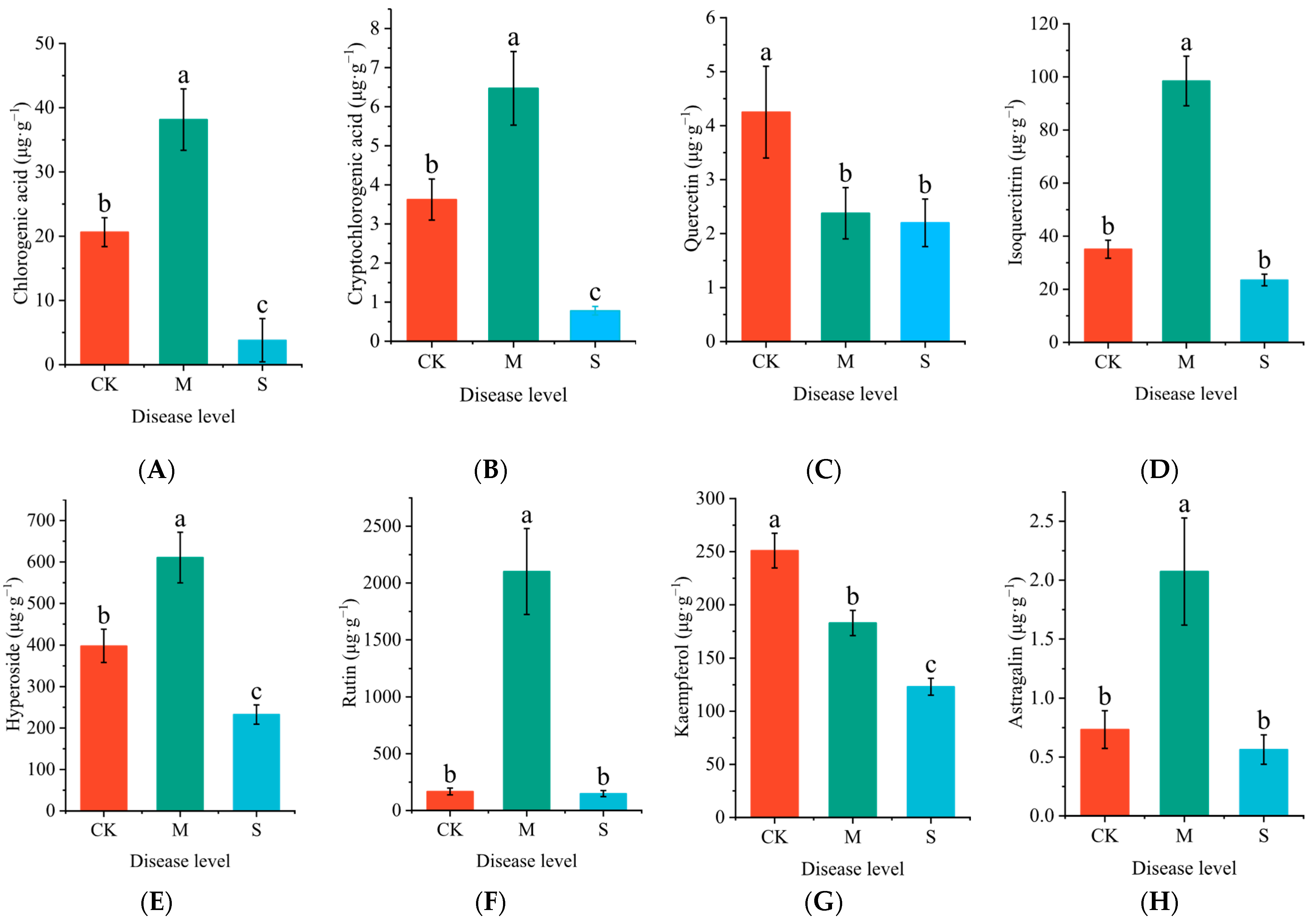
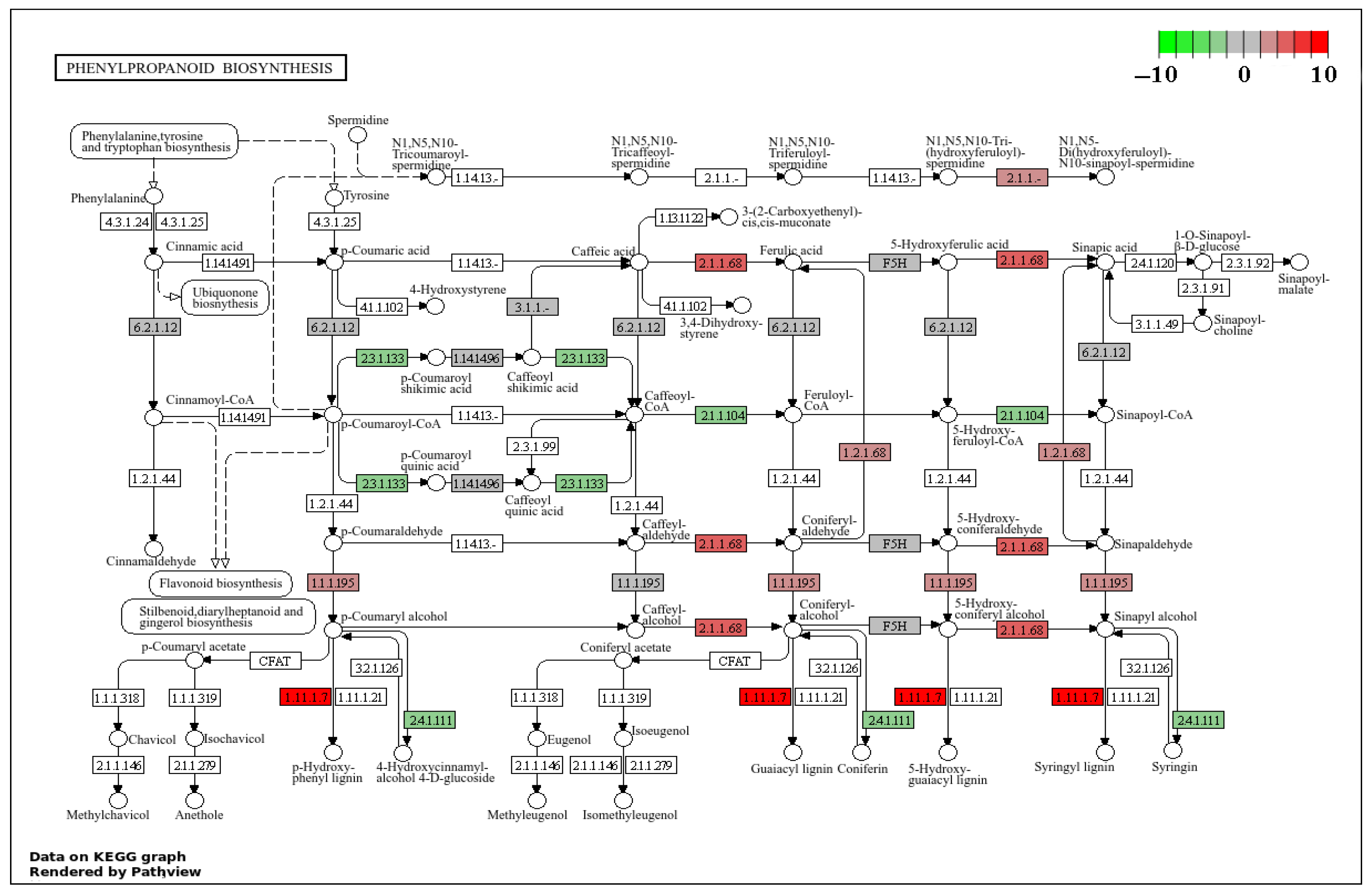
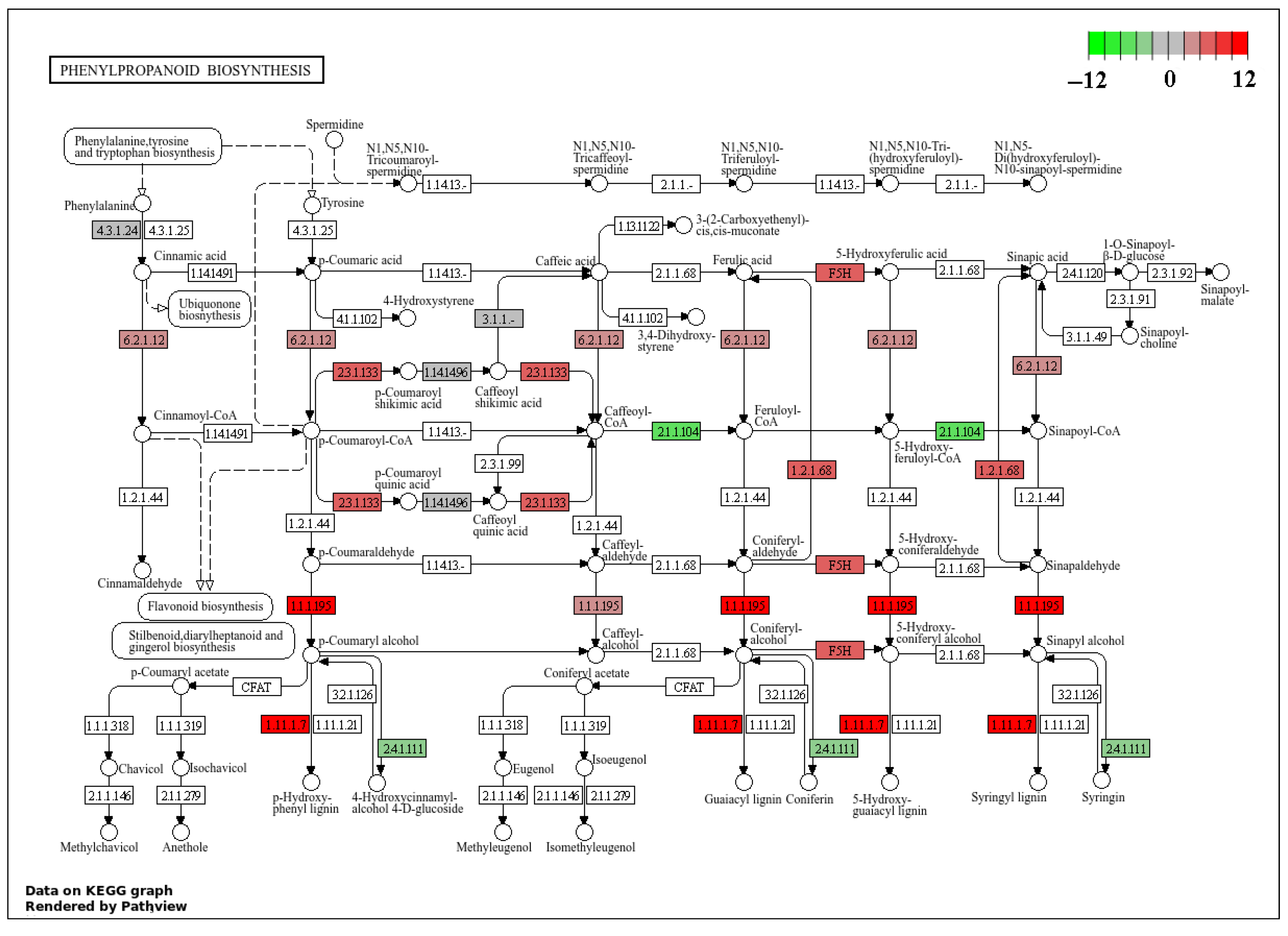
2.4. Effects of Rust Disease on Secondary Metabolites in the Leaves of P. hendersonii
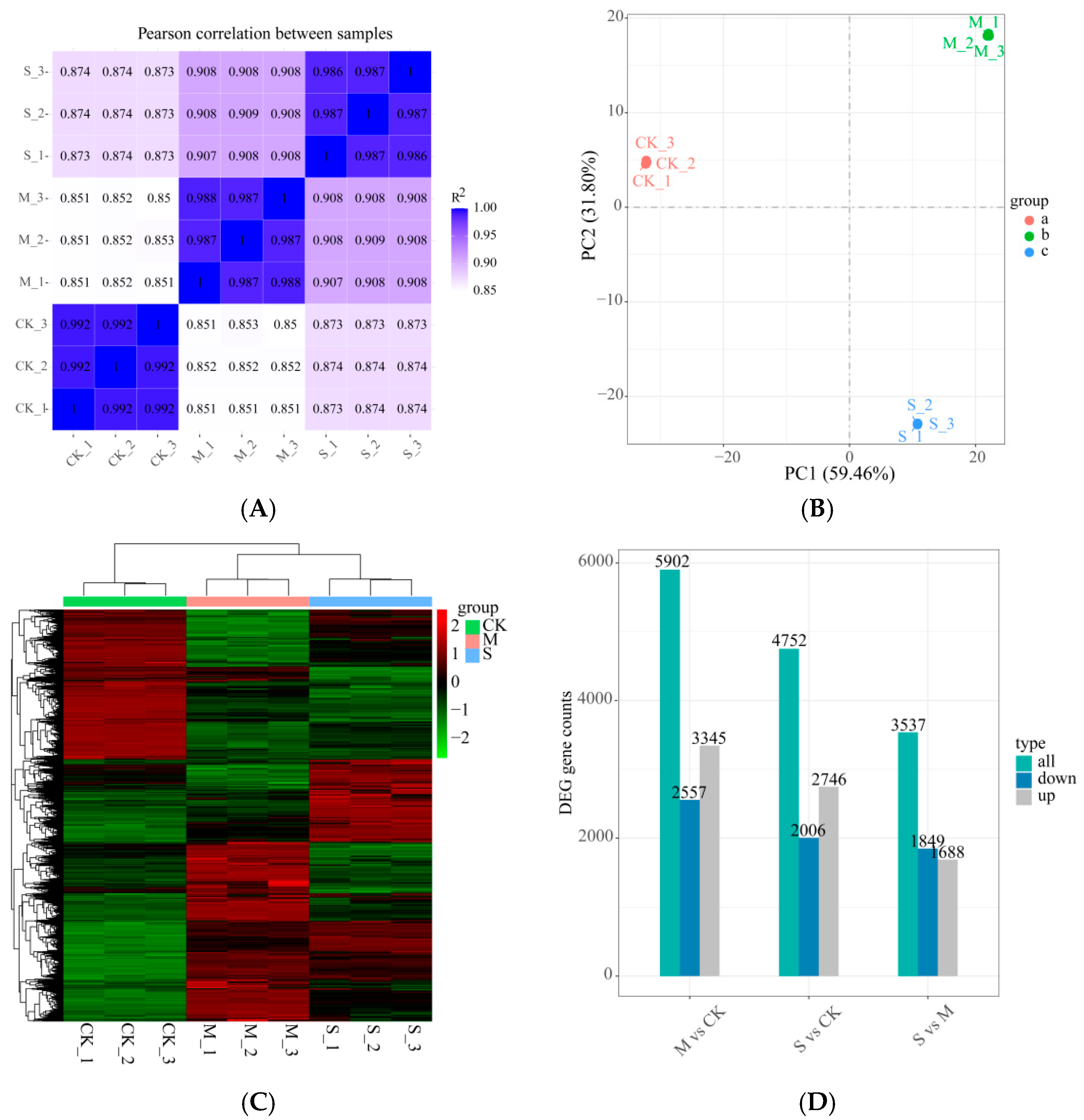
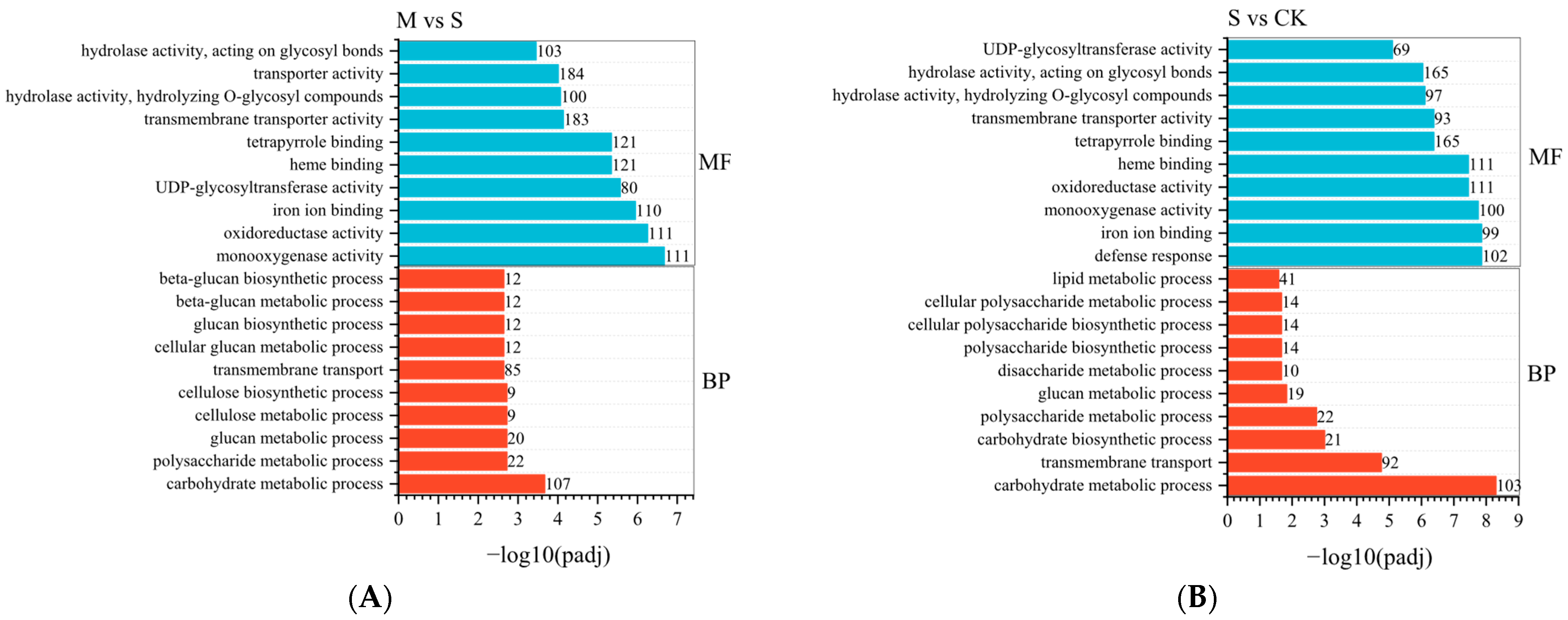
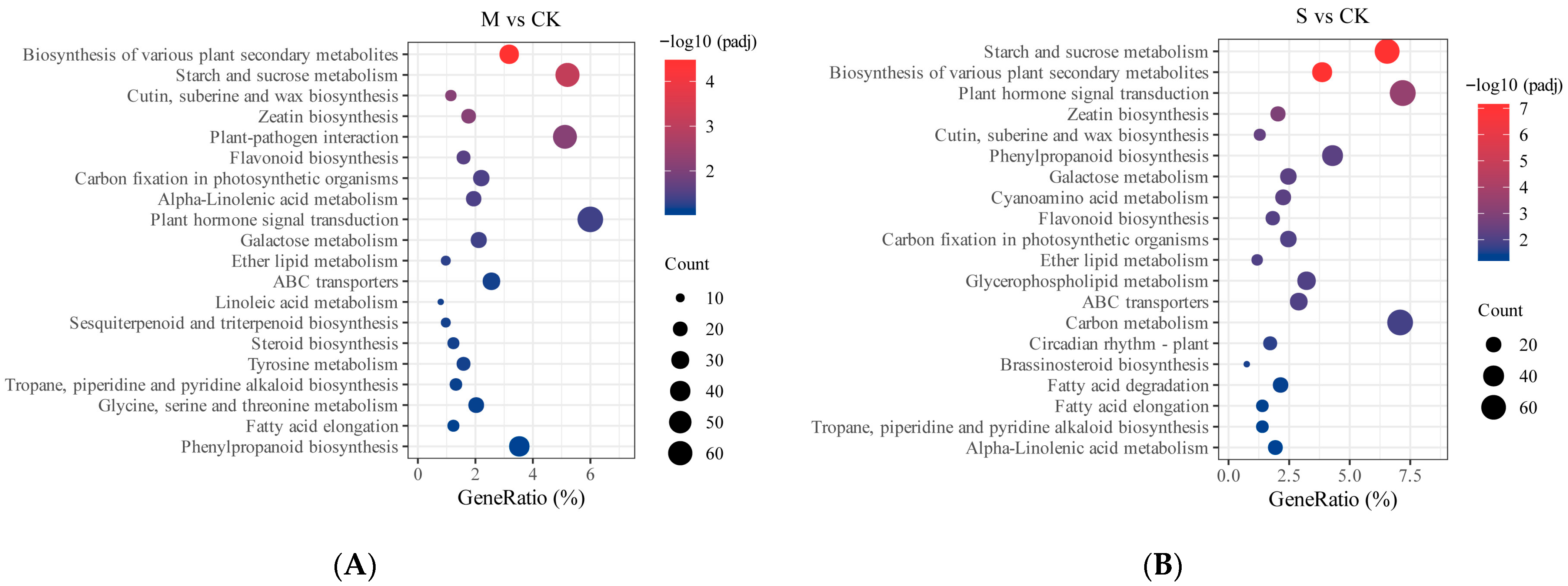
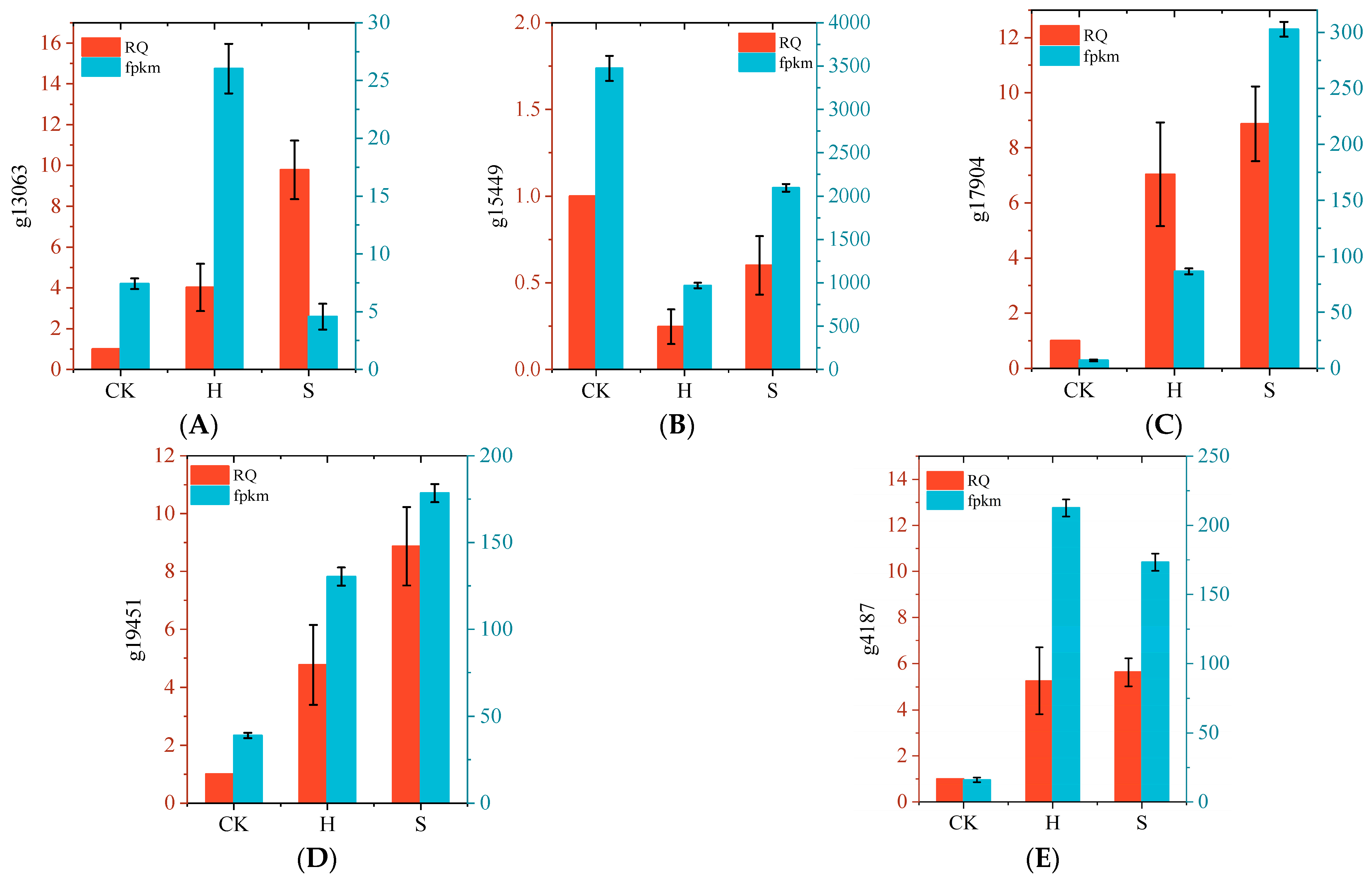
2.5. Transcriptome Analysis
3. Discussion
4. Materials and Methods
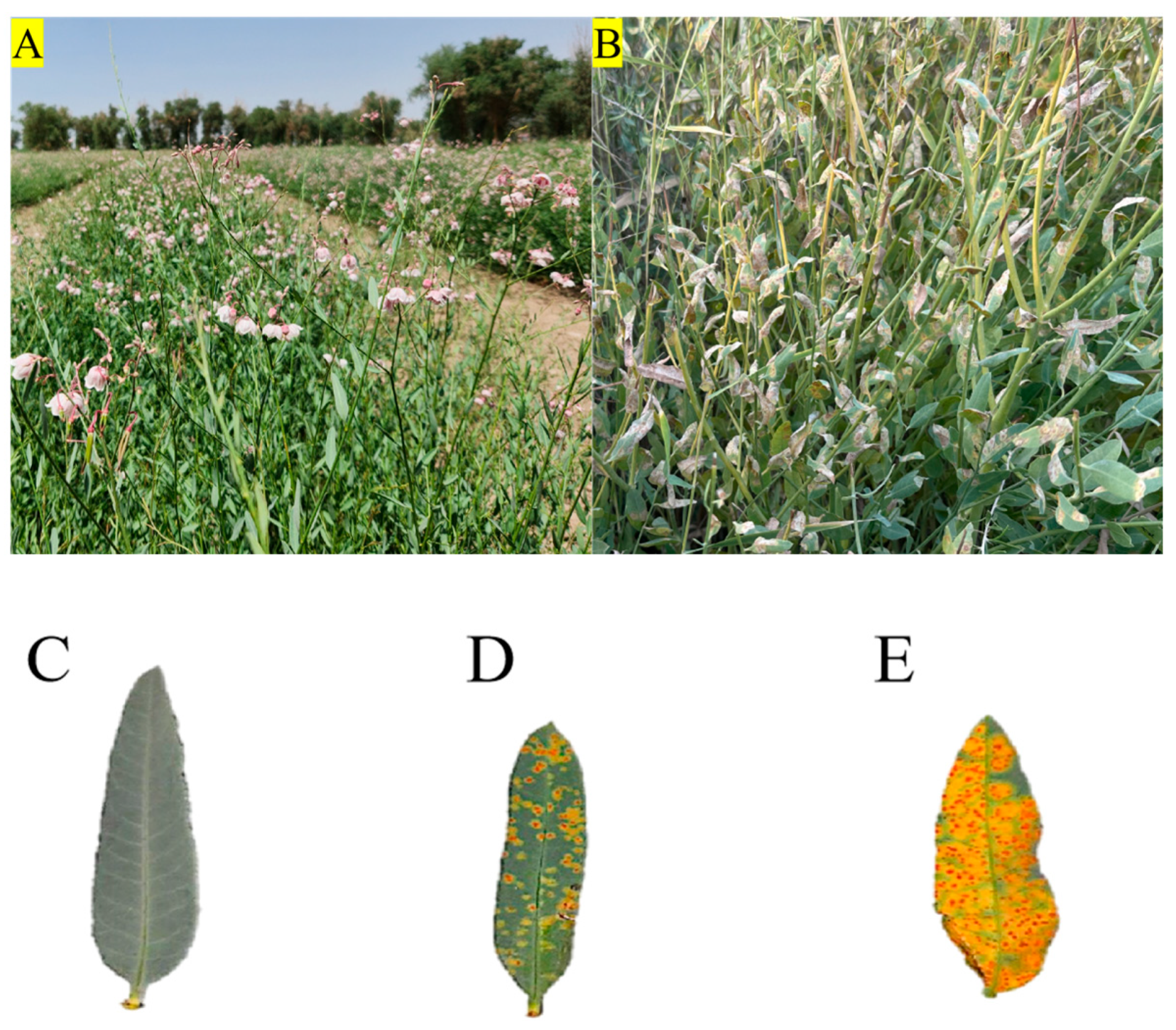
4.1. Experimental Materials
4.2. Experimental Methods
4.2.1. Determination of Antioxidant Activity and Defense Enzyme Activity
4.2.2. Determination of Chlorophyll Content
4.2.3. Determination of Hormone Content
4.2.4. Determination of Secondary Metabolites
- The total flavonoid content in the leaves was determined using a spectrophotometric method [49], where 50.0 mg of rutin standard (Solebao Biotechnology Co., Ltd., Beijing, China) was dissolved in 50 mL of 60% ethanol solution, with solubilization assisted by a water bath at 40 °C. After cooling to room temperature, the solution was made up to volume. Aliquots of 0, 1, 2, 3, 4, and 5 mL of the rutin standard solution were transferred into volumetric flasks and diluted to 5 mL with distilled water. Subsequently, 0.3 mL of 5% NaNO2 solution was added, mixed, and allowed to stand for 6 min. Then, 0.3 mL of 10% Al(NO3)3 solution was added, mixed, and allowed to stand for another 6 min. Finally, 4.0 mL of 4% NaOH solution was added, and the volume was adjusted to 10 mL with distilled water. The mixture was then mixed thoroughly and allowed to stand in the dark for 20 min. The absorbance (x) of these solutions was measured at a wavelength of 510 nm using a UV–visible spectrophotometer. A regression equation for rutin concentration (y, mg·mL−1) as the dependent variable and absorbance as the independent variable was established: y = 8.0597 x + 0.0092 (R2 = 0.9997).
- Healthy and diseased leaves were dried at 60 °C until a constant weight was achieved, then thoroughly ground and passed through an 80-mesh sieve. Then, 0.400 g of leaf powder was transferred to a centrifuge tube, and 10 mL of 60% ethanol was added for ultrasonic extraction (KQ5200B, Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, China) for 60 min. Subsequently, the mixture was centrifuged at 12,000 rpm for 10 min at 25 °C, and the supernatant was collected and diluted to a final volume of 10 mL for subsequent use. An aliquot of 1.0 mL of the supernatant was taken, and NaNO2, Al(NO3)3, and NaOH solutions were added sequentially, followed by dilution to a final volume of 10 mL in the dark for 20 min. The absorbance at 510 nm was measured, and the total flavonoid content was calculated based on the regression equation of rutin, expressed as rutin equivalent (mg·g−1). Each treatment was repeated three times, and the average value was calculated.
- The total phenolic content in the leaves was determined using the Folin–Ciocalteu method [50], where 50.0 mg of gallic acid standard (Soleibao Biotechnology Co., Ltd., Beijing, China) with a purity of ≥98% was weighed and dissolved in 50 mL of methanol to obtain a stock solution of 1 mg·mL−1. Subsequently, 0, 1, 2, 3, 4, and 5 mL of the stock solution were transferred into 10 mL volumetric flasks and diluted with methanol to obtain gallic acid standard solutions with concentrations of 0, 10, 20, 30, 40, and 50 mg·mL−1, respectively. The absorbance (x) of these solutions was measured at a wavelength of 760 nm using a UV-Vis spectrophotometer, and a regression equation was established with gallic acid concentration (y, mg·mL) as the dependent variable: y = 4.8332x − 0.045 (R2 = 0.9994).
- Weigh 0.10 g of powdered healthy leaves, mildly infected leaves, and severely infected leaves, and add 10 mL of a 5.5% methanol–hydrochloric acid solution. Then, perform ultrasonic extraction at 80 °C for 120 min, followed by centrifugation at 5,000 rpm for 20 min at 25 °C to collect the supernatant. Take 0.1 mL of the supernatant, add 5.0 mL of methanol and 0.1 mL of Folin–Ciocalteu reagent (Solaibao Biotechnology Co., Ltd., Beijing, China), mix thoroughly, and allow it to stand for 5 min. Next, add 0.2 mL of 20% Na2CO3 solution and allow it to stand in the dark for 30 min, followed by centrifugation at 12,000 rpm for 10 min. Take 1.0 mL of the supernatant and measure its absorbance at a wavelength of 760 nm. The total phenolic content is calculated using the regression equation of gallic acid, expressed as gallic acid equivalents (mg·g−1). Each sample solution is measured in triplicate, and the averages are calculated.
- Weigh 5.0 mg of standards including chlorogenic acid, cryptochlorogenic acid, quercetin, kaempferol, vitexin, hyperoside, isorhamnetin, and rutin (purity ≥ 98%) and dissolve them in methanol to a final volume of 5 mL, preparing a stock solution at a concentration of 1.0 mg·mL−1. The stock solution is then sequentially diluted to prepare standard solutions at concentrations of 1, 10, 100, 250, 500, 1000, 5000, and 10,000 ng·mL−1. The UPLC-MS/MS method is employed to measure the peak areas (y) of these eight compounds in the mixed solutions, establishing a regression equation with mass concentration (x, ng·mL) as the independent variable (Table 4). Weigh 0.500 g of powdered healthy and diseased leaves, add 5 mL of 60% (v/v) ethanol, and perform ultrasonic extraction for 120 min. The mixture is then centrifuged at 12,000 rpm for 20 min at room temperature. The supernatant is filtered through a 0.22 µm PTFE filter membrane, and 100 µL of the supernatant is transferred to a brown LC injection vial for UPLC-ESI-MS/MS analysis. The content of these eight substances is calculated based on the aforementioned regression equation, and the process is repeated three times to obtain the average value.
- Mass spectrometry conditions were established using an electrospray ionization source (ESI), with content determination conducted via multiple reaction monitoring mode (MRM). The desolvation gas temperature was maintained at 450 °C, while the ion source temperature was set to 150 °C. The desolvation gas flow rate was adjusted to 800 L·h−1, and the cone gas flow rate was specified at 150 L·h−1. The capillary voltage was configured to 3000 V (Table 4).
- The chromatographic conditions employed in this study include a mobile phase composed of phase A (0.1% formic acid aqueous solution) and phase B (acetonitrile). The analysis was conducted using a Waters ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm, 1.7 μm) at a flow rate of 0.3 mL·min−1 and an injection volume of 1 μL. The column temperature was maintained at 40 °C, as detailed in Table 4.
4.2.5. Transcriptome Sequencing and Analysis
4.2.6. Real-Time Quantitative Reverse Transcription PCR Analysis
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, C.; Zhang, W.; Peng, X.; Gu, G.; Chen, M.; Tang, Z. Development of randomly amplified polymorphic DNA-sequence characterized amplified region marker for identification of Apocynum venetum LINN. from A. pictum SCHRENK. Biol. Pharm. Bull. 2010, 33, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Zhou, J.H.; Zhang, C.S.; Li, Y.Q. Review of Current Research and Utilization Status of Apocynumvenetum Germplasm in China. Chin. Bull. Bot. 2018, 53, 382–390. [Google Scholar]
- Zhang, L.; Li, H.Q.; Yi, H.Y. Botanical characteristics, distribution and application of Lopnur Kender resources in Aksu prefecture. Xinjiang Agric. Sci. 2003, 40, 172–174. [Google Scholar]
- Zhang, G.L.; Qian, X.S.; Gu, G.P. Apocynum venetum L. Resources in Shanxi, Hubei, Henan and Shandong provinces and its cultivation techniques. Chin. Wild Plant 2005, 25, 26–27+50. [Google Scholar]
- Mei, Q.; Liu, Q.; Liang, Q.; Wan, S.; Chen, X.; Tang, Z.; Peng, X.; Jin, S. Curret Status and Suggertions for Development and Utilization of Apocynum venetum L. Eval. Anal. Drug-Use Hosp. China 2023, 23, 1404–1408. [Google Scholar] [CrossRef]
- Lv, J.; Gong, J.X.; Xu, X.X.; Li, Y.G. Research progress in the extraction and purification methods of Apocynum venetum L. Flavonoids. Knitt. Ind. 2024, 81–86. [Google Scholar]
- Xie, W.; Jiang, Z.; Wang, J.; Zhang, X.; Melzig, M.F. Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem. Biol. Interact. 2016, 246, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yokozawa, T.; Hattori, M.; Kadota, S.; Namba, T. Effects of aqueous extracts of Apocynum venetum leaves on spontaneously hypertensive, renal hypertensive and NaCl-fed-hypertensive rats. J. Ethnopharmacol. 2000, 72, 53–59. [Google Scholar] [CrossRef]
- Klemfuss, H.; Schrauzer, G.N. Effects of nutritional lithium deficiency on behavior in rats. Biol. Trace Elem. Res. 1995, 48, 131–139. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar] [CrossRef]
- Grundmann, O.; Nakajima, J.; Kamata, K.; Seo, S.; Butterweck, V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine 2009, 16, 295–302. [Google Scholar] [CrossRef]
- Abubakar, A.S.; Ahmad, B.; Ahmad, N.; Liu, L.; Liu, B.; Qu, Y.; Chen, J.; Chen, P.; Zhao, H.; Chen, J.; et al. Physicochemical evaluation, structural characterization, in vitro and in vivo bioactivities of water-soluble polysaccharides from Luobuma (Apocynum L.) tea. Food Chem. 2024, 460, 140453. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wei, D.; Guo, S.Y.; Chen, F.; Du, N.S. Studies on chemical constituents of Poacynum hendersonii. Nat. Prod. Res. Dev. 2006, 18, 954–957. [Google Scholar] [CrossRef]
- Mingyue, Z.; Fengxian, T.; Wenchao, C.; Yidong, L.; Chunhui, S. Effect of brewing condition on the quality of Apocynum venetum tea. J. Food Process. Preserv. 2022, 46, e17198. [Google Scholar] [CrossRef]
- Ma, M.; Hong, C.L.; An, S.Q.; Li, B. Seasonal, spatial, and interspecific variation in quercetin in Apocynum venetum and Poacynum hendersonii, Chinese traditional herbal teas. J. Agric. Food Chem. 2003, 51, 2390–2393. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kashiwada, Y.; Hattori, M.; Chung, H.Y. Study on the components of luobuma with peroxynitrite-scavenging activity. Biol. Pharm. Bull. 2002, 25, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Cui, S.S.; Yu, H.Q.; Jiang, L. Research progress on properties and degumming of Apocynum venetum L. J. Cellul. Sci. Technol. 2025, 33, 66–70. [Google Scholar] [CrossRef]
- Li, H.B.; Li, Y.H. The king of wild fiber-Apocyrmm vertetura L. Shandong Text Econ. 2006, 4, 80–81. [Google Scholar]
- Wang, L.; Jiang, L.; Zhao, Z.-Y.; Tian, C.-Y. Lithium content of some teas and their infusions consumed in China. Food Sci. Biotechnol. 2013, 23, 323–325. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Tanveer, M.; Tian, C. Lithium biofortification of medicinal tea Apocynum venetum. Sci. Rep. 2019, 9, 8182. [Google Scholar] [CrossRef]
- Li, T.; Feng, R.Q.; Zhang, Z.X.; Zhao, Y.F.; Lan, Y.R.; Malik, K.; Wang, L.; Liu, L.; White, J.; Li, C.J. Balancing quality and productivity of with N and P fertilizer management under drip irrigation in arid conditions of Northwest China. Ind. Crops Prod. 2023, 201, 116884. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, X.; Zhao, Z.; Zhang, K.; Tanveer, M.; Wang, L.; Huang, J.; Tian, C.; Wang, L. Luobuma (Apocynum)–Cash crops for saline lands. Ind. Crops Prod. 2021, 173, 114146. [Google Scholar] [CrossRef]
- Gao, P.; BiaoNan, Z.; Na, W.Y.; Liu, Q.T.; Meng, F.J.; Duan, T.Y. Identification of the pathogen causing rust disease of traditional chinese herb Apocynum venetum L. J. Plant Prot. 2017, 44, 129–136. [Google Scholar] [CrossRef]
- Gao, P.; Duan, T.Y.; Nan, Z.B.; Christensen, M.J.; Liu, Q.T.; Meng, F.J.; Huang, J.F. The influence of irrigation frequency on the occurrence of rust disease and determination of the optimum irrigation regime in organic production. Agric. Water Manag. 2018, 205, 81–89. [Google Scholar] [CrossRef]
- Bethenod, O.; Le Corre, A.; Huber, L.; Sache, I. Modelling the impact of brown rust on wheat crop photosynthesis after flowering. Agric. For. Meteorol. 2005, 131, 41–53. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Boeckx, T.; Winters, A.L.; Webb, K.J.; Kingston-Smith, A.H. Polyphenol oxidase in leaves: Is there any significance to the chloroplastic localization? J. Exp. Bot. 2015, 66, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Yi Chou, E.; Schuetz, M.; Hoffmann, N.; Watanabe, Y.; Sibout, R.; Samuels, A.L. Distribution, mobility, and anchoring of lignin-related oxidative enzymes in Arabidopsis secondary cell walls. J. Exp. Bot. 2018, 69, 1849–1859. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Omara, R.I.; Mostafa, Y.S.; Alamri, S.A.; Hashem, M.; Alrumman, S.A.; Ahmad, A.A. Mechanism of Wheat Leaf Rust Control Using Chitosan Nanoparticles and Salicylic Acid. J. Fungi 2022, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Tsai, C.J.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic acid activates poplar defense against the biotrophic rust fungus Melampsora larici-populina via increased biosynthesis of catechin and proanthocyanidins. New Phytol. 2019, 221, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Li, Z.; Waadt, R.; Schroeder, J.I. SnapShot: Abscisic Acid Signaling. Cell 2017, 171, 1708–1708.e0. [Google Scholar] [CrossRef]
- Pagan, I.; Garcia-Arenal, F. Tolerance of Plants to Pathogens: A Unifying View. Annu. Rev. Phytopathol. 2020, 58, 77–96. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Jia, K.; Chu, Z.; Xu, S.; Tran, L.S.P.; Guo, J.; Li, W.; Li, K. Role of abscisic acid-mediated stomatal closure in responses to pathogens in plants. Physiol. Plant. 2024, 176, e14135. [Google Scholar] [CrossRef]
- Monte, I. Jasmonates and salicylic acid: Evolution of defense hormones in land plants. Curr. Opin. Plant Biol. 2023, 76, 102470. [Google Scholar] [CrossRef]
- Han, S.; Xu, X.; Yuan, H.; Li, S.; Lin, T.; Liu, Y.; Li, S.; Zhu, T. Integrated Transcriptome and Metabolome Analysis Reveals the Molecular Mechanism of Rust Resistance in Resistant (Youkang) and Susceptive (Tengjiao) Zanthoxylum armatum Cultivars. Int. J. Mol. Sci. 2023, 24, 14761. [Google Scholar] [CrossRef]
- Li, B.; Ni, Z.Y.; Wang, J.; Lv, M.; Fan, L. Advances on Key Enzyme Gene (COMT) Involved in Lignin Biosynthesis. Mol. Plant Breed 2010, 8, 117–124. [Google Scholar]
- Jin, S.Y.; Lu, M.Z.; Gao, J. Research progress of lignin biosynthesis with genetic engineering technology and its application prospect in bamboo improvement. J. Anhui Agric. Sci. 2008; 20, 8497–8499+8548. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Men, X.; Wu, F.; Zhang, Q.; Li, W.; Sun, L.; Xing, S. Transcriptome and metabolome analyses of lignin biosynthesis mechanism of Platycladus orientalis. PeerJ 2022, 10, e14172. [Google Scholar] [CrossRef]
- Tetreault, H.M.; Gries, T.; Palmer, N.A.; Funnell-Harris, D.L.; Sato, S.; Ge, Z.; Sarath, G.; Sattler, S.E. Overexpression of ferulate 5-hydroxylase increases syringyl units in Sorghum bicolor. Plant Mol. Biol. 2020, 103, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Ruegger, M.; Meyer, K.; Cusumano, J.C.; Chapple, C. Regulation of ferulate-5-hydroxylase expression in Arabidopsis in the context of sinapate ester biosynthesis. Plant Physiol. 1999, 119, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.G.; Muchero, W. Regulation of Lignin Biosynthesis and Its Role in Growth-Defense Tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L.; Han, C.; Huang, T.; Ren, S.; Wang, X.; Xing, Q.; Liu, F. Chlorogenic acid inhibits Pseudomonas toxin pyocyanin and activates mitochondrial UPR to protect host against pathogen infection. Sci. Rep. 2025, 15, 5508. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Technol. 2018, 54, 1697–1705. [Google Scholar] [CrossRef]
- Qi, S.; He, B.; Wang, H.; Duan, Y.; Wang, L.; Gao, Y.; Guo, M. A Muti-Substrate Flavonol O-glucosyltransferases from Safflower. Molecules 2023, 28, 7613. [Google Scholar] [CrossRef]
- Wei, X.; Ya, S.G. Effect of different extraction methods on chlorophyll content. J. Langfang Norm. Univ. 2022, 22, 56–59. [Google Scholar]
- Shao, D.; Gao, G.; Abubakar, A.S.; Hazaisi, H.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Wang, Y.; Chen, Y.; et al. Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis. Molecules 2022, 27, 7343. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Xie, W.; Bai, B.; Wang, Y. Chromosome-scale genome assembly of Apocynum pictum, a drought-tolerant medicinal plant from the Tarim Basin. G3 Genes Genomes Genet. 2024, 14, jkae237. [Google Scholar] [CrossRef] [PubMed]
| Sample | Raw (Reads) | Raw_Bases (G) | Clean (Reads) | Clean_Bases (G) | Error_Rate (%) | Q20 (%) | Q30 (%) | GC_pct (%) |
|---|---|---|---|---|---|---|---|---|
| CK_1 | 21,213,008 | 6.4 | 20,764,146 | 6.2 | 0.03 | 97.75 | 93.57 | 43.85 |
| CK_2 | 22,013,756 | 6.6 | 21,448,738 | 6.4 | 0.03 | 97.67 | 93.43 | 43.84 |
| CK_3 | 21,793,993 | 6.5 | 21,114,531 | 6.3 | 0.03 | 97.67 | 93.39 | 43.87 |
| M_1 | 20,722,380 | 6.2 | 20,285,020 | 6.1 | 0.02 | 98.1 | 94.45 | 44.32 |
| M_2 | 22,825,204 | 6.8 | 22,153,576 | 6.6 | 0.02 | 98.04 | 94.29 | 44.44 |
| M_3 | 20,829,249 | 6.2 | 20,386,203 | 6.1 | 0.03 | 97.94 | 94.04 | 44.32 |
| S_1 | 19,765,517 | 5.9 | 19,295,848 | 5.8 | 0.02 | 98.15 | 94.53 | 45.06 |
| S_2 | 20,893,270 | 6.3 | 20,258,248 | 6.1 | 0.02 | 98 | 94.18 | 45.15 |
| S_3 | 19,844,042 | 6 | 19,259,470 | 5.8 | 0.02 | 98.15 | 94.55 | 45.11 |
| KEGGID | Description | p Value | padj | Up | Down |
|---|---|---|---|---|---|
| ath00999 | Biosynthesis of various plant secondary metabolites | 0.000 | 0.000 | 21 | 15 |
| ath00500 | Starch and sucrose metabolism | 0.000 | 0.001 | 34 | 25 |
| ath00073 | Cutin, suberine and wax biosynthesis | 0.000 | 0.008 | 9 | 4 |
| ath00908 | Zeatin biosynthesis | 0.000 | 0.008 | 10 | 10 |
| ath04626 | Plant-pathogen interaction | 0.000 | 0.008 | 42 | 16 |
| ath00941 | Flavonoid biosynthesis | 0.001 | 0.026 | 3 | 15 |
| ath00710 | Carbon fixation in photosynthetic organisms | 0.002 | 0.034 | 4 | 21 |
| ath00592 | alpha-Linolenic acid metabolism | 0.002 | 0.034 | 14 | 8 |
| ath04075 | Plant hormone signal transduction | 0.003 | 0.043 | 38 | 30 |
| ath00052 | Galactose metabolism | 0.003 | 0.043 | 18 | 6 |
| ath00565 | Ether lipid metabolism | 0.005 | 0.053 | 9 | 2 |
| ath02010 | ABC transporters | 0.007 | 0.066 | 17 | 12 |
| ath00591 | Linoleic acid metabolism | 0.008 | 0.066 | 8 | 1 |
| ath00909 | Sesquiterpenoid and triterpenoid biosynthesis | 0.008 | 0.066 | 4 | 7 |
| ath00100 | Steroid biosynthesis | 0.008 | 0.066 | 3 | 11 |
| ath00350 | Tyrosine metabolism | 0.008 | 0.066 | 8 | 10 |
| ath00960 | Tropane, piperidine and pyridine alkaloid biosynthesis | 0.009 | 0.069 | 5 | 10 |
| ath00260 | Glycine, serine and threonine metabolism | 0.010 | 0.071 | 8 | 15 |
| ath00062 | Fatty acid elongation | 0.012 | 0.080 | 4 | 10 |
| ath00940 | Phenylpropanoid biosynthesis | 0.015 | 0.096 | 23 | 17 |
| KEGGID | Description | p Value | padj | Up | Down |
|---|---|---|---|---|---|
| ath00500 | Starch and sucrose metabolism | 0.000 | 0.000 | 28 | 33 |
| ath00999 | Biosynthesis of various plant secondary metabolites | 0.000 | 0.000 | 22 | 14 |
| ath04075 | Plant hormone signal transduction | 0.000 | 0.000 | 35 | 32 |
| ath00908 | Zeatin biosynthesis | 0.000 | 0.001 | 9 | 10 |
| ath00073 | Cutin, suberine and wax biosynthesis | 0.000 | 0.004 | 9 | 3 |
| ath00940 | Phenylpropanoid biosynthesis | 0.000 | 0.006 | 25 | 15 |
| ath00052 | Galactose metabolism | 0.000 | 0.006 | 15 | 8 |
| ath00460 | Cyanoamino acid metabolism | 0.000 | 0.006 | 11 | 10 |
| ath00941 | Flavonoid biosynthesis | 0.001 | 0.008 | 4 | 13 |
| ath00710 | Carbon fixation in photosynthetic organisms | 0.001 | 0.009 | 7 | 16 |
| ath00565 | Ether lipid metabolism | 0.001 | 0.009 | 9 | 2 |
| ath00564 | Glycerophospholipid metabolism | 0.001 | 0.009 | 21 | 9 |
| ath02010 | ABC transporters | 0.001 | 0.009 | 17 | 10 |
| ath01200 | Carbon metabolism | 0.002 | 0.013 | 23 | 43 |
| ath04712 | Circadian rhythm—plant | 0.003 | 0.022 | 3 | 13 |
| ath00905 | Brassinosteroid biosynthesis | 0.004 | 0.031 | 7 | 0 |
| ath00071 | Fatty acid degradation | 0.005 | 0.037 | 12 | 8 |
| ath00062 | Fatty acid elongation | 0.006 | 0.042 | 6 | 7 |
| ath00960 | Tropane, piperidine and pyridine alkaloid biosynthesis | 0.009 | 0.056 | 6 | 7 |
| ath00592 | alpha-Linolenic acid metabolism | 0.010 | 0.062 | 11 | 7 |
| Secondary Metabolite | Parent Ion (m·z−1) | Daughter Ion (m·z−1) | Ionization Mode | Voltage (V) | Collisional Energy (eV) | Retention Time (min) | Regression Equation | R2 | Linear over (ng·mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| Chlorogenic Acid | 352.9 | 190.9 * | − | 40 | 22 | 2.57 | Y = 194.3 * X − 11.5 | 0.9996 | 0.9–5043.2 |
| 84.9 | 40 | 36 | |||||||
| Cryptochlorogenic Acid | 256.9 | 173.0 * | + | 40 | 25 | 2.75 | Y = 79.0 * X − 176.3 | 0.9992 | 11.9–5056.3 |
| 190.9 | 40 | 22 | |||||||
| Quercetin | 301.1 | 150.9 * | − | 4 | 24 | 7.29 | Y = 353.3 * X + 8370.9 | 0.9991 | 1.3–5035.6 |
| 178.9 | 4 | 18 | |||||||
| Kaempferol | 287.0 | 152.9 * | + | 48 | 32 | 7.43 | Y = 1620.1 * X + 50674.8 | 0.9994 | 1.1–4983.1 |
| 121.0 | 48 | 30 | |||||||
| Astragalin | 446.9 | 254.9 | − | 18 | 38 | 6.87 | Y = 501.2 * X + 666.2 | 0.9999 | 10.1–5000.3 |
| 284.0 * | 18 | 24 | |||||||
| Hyperoside | 463.1 | 300.2 * | − | 8 | 26 | 5.98 | Y = 253.9 * X − 1964.7 | 0.9990 | 12.5–5069.6 |
| 270.9 | 8 | 40 | |||||||
| Isoquercitrin | 463.0 | 300.2 * | − | 66 | 28 | 6.30 | Y = 277.1 * X − 977.4 | 0.9992 | 11.5–5060.1 |
| 270.9 | 66 | 40 | |||||||
| Rutin | 609.2 | 300.2 * | − | 8 | 36 | 5.94 | Y = 111.2 * X − 183.2 | 0.9993 | 11.6–5067.5 |
| 255.0 | 8 | 52 |
| Amplification Region | Primer Name | Primer Sequence |
|---|---|---|
| g15449 | g15449-F | GTGATATGTGCTCTAAGGATCTGG |
| g15449-R | CTATGCCACTTCCAGCTATTATTT | |
| g19451 | g19451-F | GTTTCTTCAAAATCTATGCTGCTT |
| g19451-R | TTCTTCCCAAAGGAATTTCATAAT | |
| g4187 | g4187-F | GTTAGGGTAGGGGATAAAGTAGGT |
| g4187-R | CACCAAGTTTTACTAAGGCTTCCT | |
| g13063 | g13063-F | TGGGTGTTGTGGTGGAGTTA |
| g13063-R | TTCACCTTGCTACAGTCGGT | |
| g17904 | g17904-F | AAGTCCGCAGTAGAGAGAGTGTGT |
| g17904-R | TTTCTTGTTTGAGCTAAAGCAGTG | |
| Actin2 | Actin2-F | TGCTGGATTCTGGTGATGGT |
| Actin2-R | AATTTCCCGCTCTGCTGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Shang, E.; Ma, M. Physiological, Biochemical, and Molecular Mechanisms of Resistance of Poacynum hendersonii to Melampsora apocyni. Plants 2025, 14, 2589. https://doi.org/10.3390/plants14162589
Gu J, Shang E, Ma M. Physiological, Biochemical, and Molecular Mechanisms of Resistance of Poacynum hendersonii to Melampsora apocyni. Plants. 2025; 14(16):2589. https://doi.org/10.3390/plants14162589
Chicago/Turabian StyleGu, Junjun, Endong Shang, and Miao Ma. 2025. "Physiological, Biochemical, and Molecular Mechanisms of Resistance of Poacynum hendersonii to Melampsora apocyni" Plants 14, no. 16: 2589. https://doi.org/10.3390/plants14162589
APA StyleGu, J., Shang, E., & Ma, M. (2025). Physiological, Biochemical, and Molecular Mechanisms of Resistance of Poacynum hendersonii to Melampsora apocyni. Plants, 14(16), 2589. https://doi.org/10.3390/plants14162589





