Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Medium Preparation
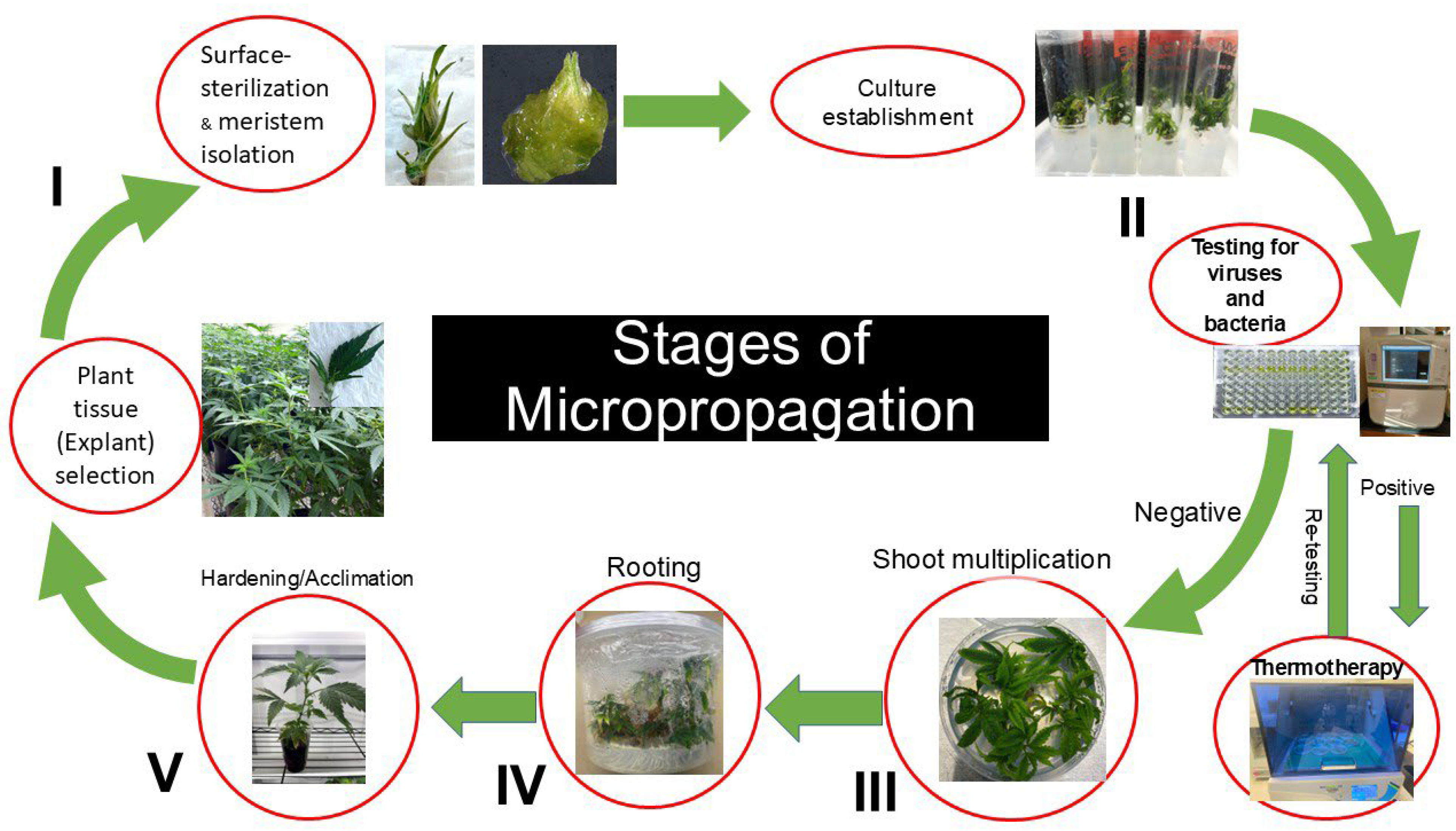
2.3. Culture Initiation
2.4. Influence of Meta-Topolin on Shoot Proliferation
2.5. Screening Cultures for Endophytic Contamination and Growth Abnormalities
2.6. Influence of Plant Growth Regulators and Photoperiod on Root Production
2.7. Plant Acclimatization
2.8. Statistical Analyses
3. Results and Discussion
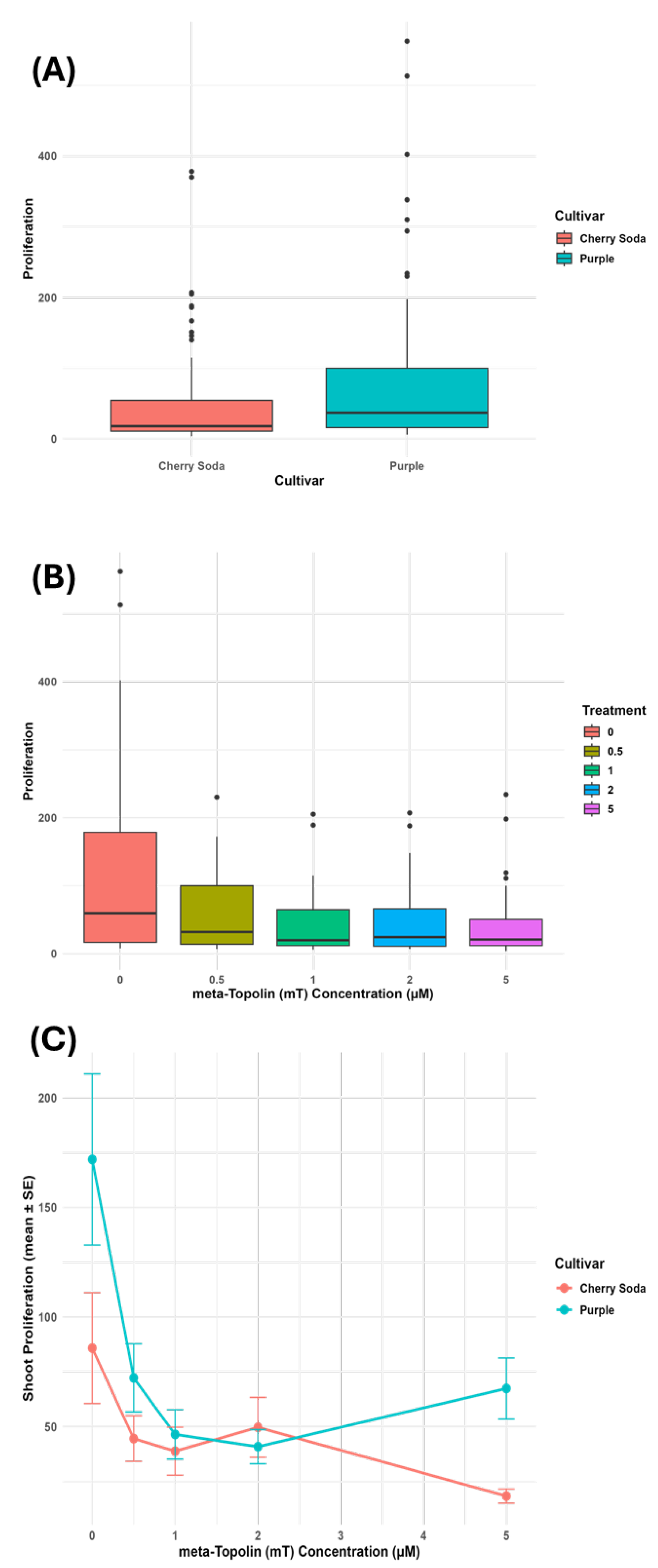
3.1. Effect of mT Concentration on Shoot Proliferation
3.2. Endophytic Contamination and Growth Abnormalities in Shoot Cultures
3.3. Effect of Auxin Concentration and Combination on Rooting
3.4. Plant Acclimatization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The Past, Present, and Future of Cannabis sativa Tissue Culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Small, E.; Cronquist, A. A Practical and Natural Taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis: Evolution and Ethnobotany. Plant Ecol. Evol. 2013, 147, 149. [Google Scholar]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis Domestication, Breeding History, Present-Day Genetic Diversity, and Future Prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent Advances in Cannabis sativa Genomics Research. New Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef]
- Johnson, R. Hemp as an Agricultural Commodity. Congressional Research Service. 2014. Available online: https://apps.dtic.mil/sti/pdfs/ADA599368.pdf (accessed on 12 August 2025).
- Thomas, B.F.; Elsohly, M.A. The Botany of Cannabis sativa L. In The Analytical Chemistry of Cannabis; Thomas, B.F., Elsohly, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Hammond, C.T.; Mahlberg, P.G. Morphology of Glandular Hairs of Cannabis sativa from Scanning Electron Microscopy. Am. J. Bot. 1973, 60, 524–528. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.M.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative Propagation of Cannabis by Stem Cuttings: Effects of Leaf Number, Cutting Position, Rooting Hormone, and Leaf Tip Removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Chiginsky, J.; Langemeier, K.; MacWilliams, J.; Albrecht, T.; Cranshaw, W.; Fulladolsa, A.C.; Kapuscinski, M.; Stenglein, M.; Nachappa, P. First Insights into the Virus and Viroid Communities in Hemp (Cannabis sativa). Front. Agron. 2021, 3, 778433. [Google Scholar] [CrossRef]
- Punja, Z.K. Emerging Diseases of Cannabis sativa and Sustainable Management. Pest Manag. Sci. 2021, 77, 3857–3870. [Google Scholar] [CrossRef] [PubMed]
- Adkar-Purushothama, C.R.; Sano, T.; Perreault, J.P. Hop Latent Viroid: A Hidden Threat to the Cannabis Industry. Viruses 2023, 15, 681. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.E. Micropropagation. Axillary bud multiplication. In Plant Cell and Tissue Culture. Methods in Molecular Biology; Pollard, J.W., Walker, J.M., Eds.; Humana Press: Totowa, NJ, USA, 1990; Volume 6, pp. 93–103. [Google Scholar]
- Grout, B.W.W. Meristem-Tip Culture for Propagation and Virus Elimination. In Plant Cell Culture Protocols; Hall, R.D., Ed.; Humana Press: Totowa, NJ, USA, 1999; pp. 115–125. [Google Scholar]
- Holmes, J.E.; Lung, S.; Collyer, D.; Punja, Z.K. Variables Affecting Shoot Growth and Plantlet Recovery in Tissue Cultures of Drug-Type Cannabis sativa L. Front. Plant Sci. 2021, 12, 732344. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Adelberg, J. Physical Factors Increased Quantity and Quality of Micropropagated Shoots of Cannabis sativa L. in a Repeated Harvest System with Ex Vitro Rooting. Vitr. Cell. Dev. Biol. Plant. 2021, 57, 923–931. [Google Scholar] [CrossRef]
- Feeney, M.; Punja, Z.K. Tissue Culture and Agrobacterium-Mediated Transformation of Hemp (Cannabis sativa L.). Vitr. Cell. Dev. Biol. Plant 2003, 39, 578–585. [Google Scholar] [CrossRef]
- Piunno, K.; Golenia, G.; Boudko, E.A.; Downey, K.; Jones, A.M.P. Regeneration of Shoots from Immature and Mature Inflorescences of Cannabis sativa. Can. J. Plant Sci. 2019, 99, 556–559. [Google Scholar] [CrossRef]
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Development of a Direct In Vitro Plant Regeneration Protocol from Cannabis sativa L. Seedling Explants. Front. Plant Sci. 2020, 11, 645. [Google Scholar] [CrossRef]
- Moher, M.; Jones, M.; Zheng, Y. Photoperiodic Response of In Vitro Cannabis sativa Plants. HortScience 2021, 56, 108–113. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.A.; Elsohly, M.A. Thidiazuron-Induced High-Frequency Direct Shoot Organogenesis of Cannabis sativa L. Vitr. Cell. Dev. Biol. Plant 2009, 45, 12–19. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; Elsohly, M.A. In Vitro Mass Propagation of Cannabis sativa L.: A Protocol Refinement Using Novel Aromatic Cytokinin meta-Topolin and the Assessment of Eco-Physiological, Biochemical, and Genetic Fidelity of Micropropagated Plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Mestinšek-Mubi, S.; Svetik, S.; Flajsman, M.; Murovec, J. In Vitro Culture and Genetic Analysis of Two High-CBD Medicinal Cannabis (Cannabis sativa L.) Breeding Lines. Genetika 2020, 52, 925–941. [Google Scholar] [CrossRef]
- Page, S.R.G.; Monthony, A.S.; Jones, A.M.P. DKW Basal Salts Improve Micropropagation and Callogenesis Compared with MS Basal Salts in Multiple Commercial Cultivars of Cannabis sativa. Botany 2021, 99, 269–279. [Google Scholar] [CrossRef]
- Driver, J.; Kuniyuki, A.H. In Vitro Propagation of Paradox Walnut Rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Codesido, V.; Myer, S.; Casano, S. Influence of Media Composition and Genotype for Successful Cannabis sativa L. Introduction. Acta Hortic. 2020, 1285, 75–80. [Google Scholar] [CrossRef]
- Lubell-Brand, J.D.; Kurtz, L.E.; Brand, M.H. An In Vitro—Ex Vitro Micropropagation System for Hemp. HortTechnology 2021, 31, 199–207. [Google Scholar] [CrossRef]
- Rico, S.; Garrido, J.; Sanchez, C.; Ferrerio-Vera, C.; Codesido, V.; Vidal, N. A Temporary Immersion System to Improve Cannabis sativa Micropropagation. Front. Plant Sci. 2022, 13, 895971. [Google Scholar] [CrossRef]
- Stephen, C.; Zayas, V.A.; Galic, A. Micropropagation of Hemp (Cannabis sativa L.). HortScience 2022, 58, 307–316. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Elsohly, M.A. Propagation of Cannabis for Clinical Research: An Approach Towards a Modern Herbal Medicinal Products Development. Front. Plant Sci. 2020, 11, 958. [Google Scholar] [CrossRef]
- Smykalova, I.; Vrbova, M.; Cveckova, M.; Plackova, L.; Zukauskaite, A.; Zatloukal, M.; Hrdlicka, J.; Pliholova, L.; Dolezal, K.; Griga, M. The Effects of Synthetic Cytokinin Derivatives and Endogenous Cytokinins on the In Vitro Growth Response of Hemp (Cannabis sativa L.) Explants. Plant Cell Tiss. Org. Cult. 2019, 139, 381–394. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Dolezal, K.; Finnie, J.F.; Van Staden, J. Topolins: A Panacea to Plant Tissue Culture Challenges. Plant Cell Tiss. Org. Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Elayaraja, D.; Subramaniam, K.; Vasudevan, V.; Sathish, S.; Kasthurirengan, S.; Ganapathy, A.; Manickavasagam, M. Meta-Topolin (mT) Enhances the In Vitro Regeneration Frequency of Sesamum indicum L. Biocatal. Agric. Biotechnol. 2019, 21, 101320. [Google Scholar]
- Valero-Aracama, C.; Kane, M.E.; Wilson, S.B.; Philman, N.L. Substitution of Benzyladenine with meta-Topolin During Shoot Multiplication Increases Acclimatization of Difficult- and Easy-to-Acclimatize Sea Oats (Uniola paniculata L.) Genotypes. Plant Growth Regul. 2010, 60, 43–49. [Google Scholar] [CrossRef]
- Cassells, A.C. Detection and Elimination of Microbial Endophytes and Prevention of Contamination in Plant Tissue Culture. In Plant Tissue Culture, Development, and Biotechnology; Gray, D.J., Trigiano, R.N., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 223–238. [Google Scholar]
- Thomas, P.; Prakash, C.S. Sanitizing Long-Term Micropropagated Grapes from Covert and Endophytic Bacteria and Preliminary Field Testing of Plants After 8 Years In Vitro. Vitr. Cell. Dev. Biol. Plant 2004, 40, 603–607. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Araujo de Oliveira, A.M.; Henk, A.D. Minimizing the Deleterious Effects of Endophytes in Plant Shoot Tip Cryopreservation. Appl. Plant Sci. 2022, 10, e11489. [Google Scholar] [CrossRef]
- Gray, D.J.; Benton, C.M. In Vitro Micropropagation and Plant Establishment of Muscadine Grape Cultivars (Vitis rotundifolia). Plant Cell Tiss. Org. Cult. 1991, 27, 7–14. [Google Scholar] [CrossRef]
- Punja, Z.K.; Scott, C. Organically Grown Cannabis (Cannabis sativa L.) Plants Contain a Diverse Range of Culturable Epiphytic and Endophytic Fungi in Inflorescences and Stem Tissues. Botany 2023, 101, 255–269. [Google Scholar] [CrossRef]
- Grout, B.W.W. Meristem-Tip Culture. In Plant Cell and Tissue Culture; Pollard, J.W., Walker, J.M., Eds.; Humana Press: Totowa, NJ, USA, 1990; pp. 81–90. [Google Scholar]
- Duta-Cornescu, G.; Constantin, N.; Pojoga, D.M.; Nicuta, D.; Simon-Gruita, A. Somaclonal Variation—Advantage or Disadvantage in Micropropagation of Medicinal Plants. Int. J. Mol. Sci. 2023, 24, 838. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, S.H.; Chung, M.Y.; Park, S.K.; Kim, C.K. In Vitro Propagation Method for the Production of Morphologically and Genetically Stable Plants of Different Strawberry Cultivars. BMC Plant Methods 2019, 15, 36. [Google Scholar] [CrossRef]
- Adamek, K.; Grainger, C.; Jones, A.M.P.; Torkamaneh, D. Genotype by Sequencing (GBS) Reveals Greater Somatic Mutations than Single Sequence Repeats (SSRs) in Micropropagated Cannabis Plants. Vitr. Cell. Dev. Biol. Plant 2023, 59, 757–766. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A. Influence of Polyamines on Hyperhydricity Reversion and Its Associated Mechanism During Micropropagation of China Pink (Dianthus chinensis L.). Physiol. Mol. Biol. Plants 2020, 26, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Žd’árská, M.; Zatloukalová, P.; Benítez, M.; Šedo, O.; Potěšil, D.; Novák, O.; Svačinová, J.; Pešek, B.; Malbeck, J.; Vašíčková, J.; et al. Proteome Analysis in Arabidopsis Reveals Shoot- and Root-Specific Targets of Cytokinin Action and Differential Regulation of Hormonal Homeostasis. Plant Physiol. 2013, 161, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; Van Staden, J. Influence of Gelling Agent and Cytokinins on the Control of Hyperhydricity in Aloe polyphylla. Plant Cell Tiss. Org. Cult. 2011, 104, 13–21. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, F.; Kong, X.; Tian, J.; Wu, Z. Effect of Multiple Factors on Hyperhydricity of Allium sativum L. Sci. Hortic. 2017, 217, 285–296. [Google Scholar] [CrossRef]
- Wróbel, T.; Dreger, M.; Wieglus, K.; Slomski, R. Modified Nodal Cuttings and Shoot Tips Protocol for Rapid Propagation of Cannabis sativa L. J. Nat. Fibers 2019, 19, 536–545. [Google Scholar] [CrossRef]
- Rajput, R.; Kumar, K. A Review on Cannabis sativa: Its Compounds and Their Effects. Int. J. Pharm. Sci. Rev. Res. 2018, 53, 59–63. [Google Scholar]
- Ivanova, M.; Novák, O.; Strnad, M.; Van Staden, J. Endogenous cytokinins in shoots of Aloe polyphylla cultured in vitro in relation to hyperhydricity, exogenous cytokinins and gelling agents. Plant Growth Regul. 2006, 50, 219–230. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L. Critical Roles of the Activation of Ethylene Pathway Genes Mediated by DNA Demethylation in Arabidopsis Hyperhydricity. Plant Genome 2022, 15, e20202. [Google Scholar] [CrossRef]
- van den Dries, N.; Giannì, S.; Czerednik, A.; Krens, F.A.; de Klerk, G.J.M. Flooding of the Apoplast Is a Key Factor in the Development of Hyperhydricity. J. Exp. Bot. 2013, 64, 5221–5230. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L.; Xin, X.; Liang, Y. Reversion of Hyperhydricity in Dianthus chinensis L. Plantlets by AgNO3 and Its Associated Mechanism During In Vitro Culture. Plant Sci. 2017, 254, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gielis, J.; Goetghebeur, P.; Debergh, P. Morphological and Biochemical Aspects of Flowering in Bamboo—The Development of Model Systems. In The Bamboos; Chapman, G.P., Ed.; Linnean Society: London, UK, 1997; pp. 179–186. [Google Scholar]
- Ramanayake, S.S.S.D.; Wanniarachchi, A.V.R.; Tennakoon, T.M.A. Axillary Shoot Proliferation and In Vitro Flowering in an Adult Giant Bamboo, Dendrocalamus giganteus Wall. Ex Munro. Vitr. Cell. Dev. Biol. Plant. 2001, 37, 667–671. [Google Scholar] [CrossRef]
- Cardoso, J.C. Silver Nitrate Enhances In Vitro Development and Quality of Shoots in Anthurium andraeanum. Sci. Hortic. 2019, 253, 358–363. [Google Scholar] [CrossRef]
- Wang, R.; He, L.S.; Xia, B.; Tong, J.F.; Li, N.; Peng, F. A Micropropagation System for Cloning of Hemp (Cannabis sativa L.) by Shoot Tip Culture. Pak. J. Bot. 2009, 41, 603–608. [Google Scholar]
- Ioannidis, K.; Tomprou, I.; Mitsis, V. An Alternative In Vitro Propagation Protocol of Cannabis sativa L. Presenting Efficient Rooting for Commercial Production. Plants 2022, 11, 1333. [Google Scholar] [CrossRef]
- Adams, T.K.; Masondo, N.A.; Malatsi, P.; Makunga, N.P. Cannabis sativa: From Therapeutic Uses to Micropropagation and Beyond. Plants 2021, 10, 2078. [Google Scholar] [CrossRef]
- Kurtz, L.E.; Borbas, L.N.; Brand, M.H.; Lubell-Brand, J.D. Ex Vitro Rooting of Cannabis sativa Micro-cuttings and Their Performance Compared to Retip and Stem Cuttings. HortScience 2022, 57, 1576–1579. [Google Scholar] [CrossRef]
- Quambusch, M.; Gruß, S.; Pscherer, T.; Winkelmann, T.; Bartsch, M. Improved In Vitro Rooting of Prunus avium microshoots Using a Dark Treatment and an Auxin Pulse. Sci. Hortic. 2017, 220, 52–56. [Google Scholar] [CrossRef]
- Monteuuis, O.; Bon, M.C. Influence of Auxins and Darkness on In Vitro Rooting of Micropropagated Shoots from Mature and Juvenile Acacia mangium. Plant Cell Tiss. Org. Cult. 2000, 63, 173–177. [Google Scholar] [CrossRef]
- Klopotek, Y.; Haensch, K.T.; Hause, B.; Hajirezaei, M.R.; Druege, U. Dark Exposure of Petunia Cuttings Strongly Improves Adventitious Root Formation and Enhances Carbohydrate Availability During Rooting in Light. J. Plant Physiol. 2010, 167, 547–554. [Google Scholar] [CrossRef]
- Halliday, K.J.; Martinez-Garcia, J.F.; Josse, E.M. Integration of Light and Auxin Signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, a001586. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimation of Tissue Culture Plantlets: From Laboratory to Land. Biotechnol. Lett. 2010, 32, 1199–1305. [Google Scholar] [CrossRef]




| Meta-Topolin Level (μM) | Transfer Period | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| 0.5 | 13.00 a | 28.40 b | 93.60 b | 174.50 ab |
| 1.0 | 11.00 a | 25.00 b | 69.80 b | 102.67 b |
| 2.0 | 9.60 a | 23.80 b | 44.80 b | 85.40 b |
| 5.0 | 13.60 a | 29.60 b | 96.60 b | 130.00 b |
| 0 | 14.40 a | 54.80 a | 248.00 a | 370.20 a |
| Meta-Topolin Level (μM) | Transfer Period | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| 0.5 | 11.20 a | 13.40 b | 43.40 b | 110.40 b |
| 1.0 | 11.80 a | 12.20 b | 45.00 b | 86.40 c |
| 2.0 | 11.60 a | 17.00 a | 46.40 b | 124.00 b |
| 5.0 | 9.20 b | 8.20 c | 21.00 c | 39.25 d |
| 0 | 14.00 a | 22.00 a | 96.80 a | 210.60 a |
| Source | Df | Sum Sq | Mean Sq | F Value | Pr (>F) | Significance |
|---|---|---|---|---|---|---|
| Cultivar | 1 | 52,643 | 52,643 | 8.163 | 0.00476 | ** |
| Treatment | 4 | 216,582 | 54,145 | 8.396 | 3.01 × 10−6 | *** |
| Cultivar × Treatment | 4 | 54,478 | 13,619 | 2.112 | 0.08100 | . |
| Residuals | 186 | 1,199,450 | 6449 |
| Treatment | Rooting Percentage (%) | |
|---|---|---|
| Light | Dark | |
| IAA 4.0 μM | 40.00 b | 13.33 b |
| IAA 6.0 μM | 33.33 b | 20.00 b |
| IBA 4.0 μM | 33.33 b | 20.00 b |
| IBA 6.0 μM | 46.67 b | 20.00 b |
| IBA 6.0 μM + IAA 1.0 μM | 26.67 b | 13.33 b |
| IBA 6.0 μM + NAA1.0 μM | 46.67 b | 66.67 a |
| NAA 2.0 μM | 53.33 a | 20.00 b |
| NAA 4.0 μM | 53.33 a | 66.67 a |
| Control | 26.67 b | 20.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, G.A.; Jackson, C.L.; Timoteo, A., Jr.; Sardaru, P.; Foland, M.H.; Natarajan, P.; Dhekney, S.A. Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation. Plants 2025, 14, 2586. https://doi.org/10.3390/plants14162586
Johnson GA, Jackson CL, Timoteo A Jr., Sardaru P, Foland MH, Natarajan P, Dhekney SA. Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation. Plants. 2025; 14(16):2586. https://doi.org/10.3390/plants14162586
Chicago/Turabian StyleJohnson, Gabrielle A., Carissa L. Jackson, Antonio Timoteo, Jr., Papaiah Sardaru, Michael H. Foland, Purushothaman Natarajan, and Sadanand A. Dhekney. 2025. "Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation" Plants 14, no. 16: 2586. https://doi.org/10.3390/plants14162586
APA StyleJohnson, G. A., Jackson, C. L., Timoteo, A., Jr., Sardaru, P., Foland, M. H., Natarajan, P., & Dhekney, S. A. (2025). Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation. Plants, 14(16), 2586. https://doi.org/10.3390/plants14162586





