Drought Resistance Evaluation of Camellia oleifera var. “Xianglin 210” Grafted onto Different Rootstocks
Abstract
1. Introduction
2. Results and Analysis
2.1. Effects of Different Soil Water Content Levels on Photosynthetic Parameters of C. oleifera
2.2. Physiological Responses to Soil Water Gradients in C. oleifera Graft Combinations
2.3. Comprehensive Evaluation by Membership Function Analysis
2.4. Effect of Different Soil Water Contents on the Expression of Relevant Genes
3. Discussion
3.1. Photosynthetic Parameters
3.2. Physiological and Biochemical Responses
3.3. Gene Expression
4. Materials and Methods
4.1. Experimental Materials and Treatments
4.2. Measurement of Photosynthetic Parameters
4.3. Determination of Physiological and Biochemical Indicators
4.4. Membership Function Analysis
4.5. RNA Extraction and Real-Time Quantitative PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Shi, J.; Liu, K.; Wang, Y.; Xu, Y.; Liu, Y. Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans. Food Sci. Hum. Wellness 2023, 12, 1391–1401. [Google Scholar] [CrossRef]
- Qin, P.; Shen, J.; Wei, J.; Chen, Y. A critical review of the bioactive ingredients and biological functions of camellia oleifera oil. Curr. Res. Food Sci. 2024, 8, 100753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, F.; Ouyang, Z.; Tu, N.; Xu, W.; Wang, X.; Miao, H.; Li, X.; Tian, Y. Impacts of reforestation approaches on runoff control in the hilly red soil region of Southern China. J. Hydrol. 2008, 356, 174–184. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, A.; Nguyen, V.; Bisht, A.; Almqvist, C.; De Veylder, L.; Carlsbecker, A.; Melnyk, C.W. A conserved graft formation process in Norway spruce and Arabidopsis identifies the PAT gene family as central regulators of wound healing. Nat. Plants 2024, 10, 53–65. [Google Scholar] [CrossRef]
- Ren, K.; Liu, J.; Zou, Z.; Tao, W.; Zuo, J.; Zhou, W.; Hu, D. Effects of different rootstocks on leaf anatomical structure and physiological characteristics of 5-year-old bud-seedling grafted Camellia oleifera. J. Cent. South Univ. For. Technol. 2025, 45, 98–107. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Y.; Wang, Z.; Liu, J.; Zhang, L.; Zhang, W.; Hu, D. Temporal and spatial distribution dynamics of fine roots in young Camellia oleifera trees with different scion-rootstock combinations. J. Cent. South Univ. For. Technol. 2022, 42, 57–64. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, K.; Ling, Z.; Ye, H. Study on waterlogging tolerance of grafted seedlings of Camellia oleifera ‘Cenruan 3’. Guangxi For. Sci. 2015, 44, 279–282. [Google Scholar] [CrossRef]
- Ge, X.; Zhong, Q.; Tan, X.; Wang, J.; Cao, L.; Zhou, Y.; Zou, Y.; Yuan, Y.; Wan, X.; Yan, C.; et al. Integrated multi-omics analysis to elucidate the role of shikimic acid and phenethylamine in the effect of scions on rootstocks of Camellia oleifera. Ind. Crops Prod. 2023, 203, 117222. [Google Scholar] [CrossRef]
- Long, W.; Huang, G.; Yao, X.; Lv, L.; Yu, C.; Wang, K. Untargeted metabolism approach reveals difference of varieties of bud and relation among characteristics of grafting seedlings in Camellia oleifera. Front. Plant Sci. 2022, 13, 1024353. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Chen, Y.; Deng, X.; Wang, R.; Lai, H. Study on drought resistance characteristics of ‘Xianglin 210’ Camellia oleifera. J. Cent. South Univ. For. Technol. 2024, 44, 37–48. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhang, Z.; Wang, R.; Chen, Y. Integration of mRNA and miRNA analysis reveals the differentially regulatory network in two different Camellia oleifera cultivars under drought stress. Front. Plant Sci. 2022, 13, 1001357. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Rosenfield, G.R.; Cleves, P.A.; Krediet, C.J.; Paul, M.R.; Clowez, S.; Grossman, A.R.; Pringle, J.R. Photosynthesis and other factors affecting the establishment and maintenance of cnidarian–dinoflagellate symbiosis. Philos. Trans. R. Soc. B 2024, 379, 1901. [Google Scholar] [CrossRef] [PubMed]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Songsri, P. Photosynthetic and physiological responses to drought of Jerusalem artichoke genotypes differing in drought resistance. Agric. Water Manag. 2022, 259, 107252. [Google Scholar] [CrossRef]
- Dong, B.; Wei, X.; Lan, L.; Huang, Y.; Xue, L.; Lai, J.; Lu, J. Effects of drought stress on photosynthetic characteristics of four Camellia oleifera varieties. Nonwood For. Res. 2018, 36, 9–15. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, Q.; Cao, L.; Guo, H.; Yan, C.; Yuan, T.; Luo, S. Effects of drought stress on photosynthesis of Camellia oleifera mature forest. Nonwood For. Res. 2017, 35, 72–79. [Google Scholar] [CrossRef]
- Dong, B.; Wu, B.; Hong, W.; Li, X.; Li, Z.; Xue, L.; Huang, Y. Transcriptome analysis of the tea oil camellia (Camellia oleifera) reveals candidate drought stress genes. PLoS ONE 2017, 12, e0181835. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.; Chen, Y.; Xu, Y.; Tang, W.; Chen, L.; Li, Z. Physiological and biochemical effects of exogenous calcium on Camellia oleifera Abel under drought stress. Forests 2023, 14, 2082. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of trees from drought depends on belowground sink control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef] [PubMed]
- Langaroudi, I.K.; Piri, S.; Chaeikar, S.S.; Salehi, B. Evaluating drought stress tolerance in different Camellia sinensis L. cultivars and effect of melatonin on strengthening antioxidant system. Sci. Hortic. 2023, 307, 111517. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Wang, D.; Dong, Y.; Li, J.; Lu, F.; Tao, W.; Guo, Y.; Xiang, W.; Wen, M.; et al. Trade-off strategies between drought resistance and growth rate of dominant tree species in karst forests within heterogeneous habitats. Sci. Rep. 2025, 15, 26381. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, W.; Zhang, Q.; Xu, Z.; Zhu, Z.; Duan, F.; Wu, R. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol. Biochem. 2011, 49, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Surówka, E.; Potocka, I.; Dziurka, M.; Wróbel-Marek, J.; Kurczyńska, E.; Żur, I.; Maksymowicz, A.; Gajewska, E.; Miszalski, Z. Tocopherols mutual balance is a key player for maintaining Arabidopsis thaliana growth under salt stress. Plant Physiol. Biochem. 2020, 156, 369–383. [Google Scholar] [CrossRef]
- Guo, P.R.; Wu, L.L.; Wang, Y.; Liu, D.; Li, J.A. Effects of Drought Stress on the Morphological Structure and Flower Organ Physiological Characteristics of Camellia oleifera Flower Buds. Plants 2023, 12, 2585. [Google Scholar] [CrossRef]
- Park, H.; Hong, T.; An, G.; Park, J.; Song, G.; Lim, W. Bifenox induces hepatotoxicity and vascular toxicity in zebrafish embryos via ROS production and alterations in signaling pathways. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 281, 109918. [Google Scholar] [CrossRef]
- Deep, S.N.; Seelig, S.; Paul, S.; Poddar, R. Homocysteine-induced sustained GluN2A NMDA receptor stimulation leads to mitochondrial ROS generation and neurotoxicity. J. Biol. Chem. 2024, 300, 107253. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, H.; Lou, C.; Wang, W.; Cai, T.; Lin, Z.; Jiang, L.; Lin, S.; Xue, X.; Pan, X. Asiatic acid prevents glucocorticoid-induced femoral head osteonecrosis via PI3K/AKT pathway. Int. Immunopharmacol. 2024, 130, 111758. [Google Scholar] [CrossRef]
- Jiahui, Y.; Chengcheng, Z.; Shihui, N.; Wei, L.; Jiahui, Y.; Chengcheng, Z.; Shihui, N.; Wei, L. Identification of SAUR gene family in Pinus tabuliformis and analysis on its expression patterns under drought stress. J. Beijing For. Univ. 2024, 46, 57–67. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Antioxidant defences in olive trees during drought stress: Changes in activity of some antioxidant enzymes. Funct. Plant Biol. 2005, 32, 45–53. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Mano, K.; Suganami, M.; Kondo, E.; Suzuki, Y.; Makino, A. Effects of overexpression of the Rubisco small subunit gene under the control of the Rubisco activase promoter on Rubisco contents of rice leaves at different positions. Soil Sci. Plant Nutr. 2020, 66, 569–578. [Google Scholar] [CrossRef]
- Kachare, S.; Tiwari, S.; Tripathi, N. Expression of DREB1, RBCL, PIP, SGR genes and morpho-physiological changes under water stress in soybean. J. Plant Biochem. Biotechnol. 2023, 32, 338–355. [Google Scholar] [CrossRef]
- Liang, T.; Yu, S.; Pan, Y.; Wang, J.; Kay, S.A. The interplay between the circadian clock and abiotic stress responses mediated by ABF3 and CCA1/LHY. Proc. Natl. Acad. Sci. USA 2024, 121, e2316825121. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Hu, Q.; Yang, X.; Zhao, Y.; Lin, Y.; Yuan, J.; Gu, J.; Li, Y.; He, J.; et al. PSEUDO-RESPONSE REGULATOR 3b and transcription factor ABF3 modulate abscisic acid-dependent drought stress response in soybean. Plant Physiol. 2024, 195, 3053–3071. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Shih, C.F.; Yang, C.H. Expression of An Antisense Brassica oleracea GIGANTEA (BoGI) Gene in Transgenic Broccoli Causes Delayed Flowering, Leaf Senescence, and Post-Harvest Yellowing Retardation. Plant Mol. Biol. Rep. 2015, 33, 1499–1509. [Google Scholar] [CrossRef]
- Yang, X.; Huang, S.; Li, W.; Li, Z.; Xu, Z.; Zhou, W.; Meng, X.; Xie, Y.; Wang, S.; Jin, L.; et al. Identification of biological rhythms related GIGANTEA genes in tomato and functional analysis under heat stress. Plant Stress 2025, 15, 100736. [Google Scholar] [CrossRef]
- Chen, H.; Yue, Y.; Ren, M.; Quan, Y.; Gao, R. Cloning and expression analysis of CmGATA8 gene and its promoter in Chrysanthemum mongolicum. Mol. Plant Breed. 2021, 19, 1804–1810. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yina, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Shi, G.; Liu, G.; Liu, H.; Xu, N.; Yang, Q.; Song, Z.; Ye, W.; Wang, L. WRKY Transcriptional Factor IlWRKY70 from Iris laevigata Enhances Drought and Salinity Tolerances in Nicotiana tabacum. Int. J. Mol. Sci. 2023, 24, 16174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.Y.; Márquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Zhang, Z.; Liu, X.; Hsu, C.C.; Du, Y.; Sang, T.; Zhu, C.; Wang, Y.; Satheesh, V.; et al. A RAF-SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nat. Commun. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Soma, F.; Takahashi, F.; Suzuki, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nat. Commun. 2020, 11, 1373. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Xu, C.; Wang, P.; Sun, W.; Yin, T.; Zhuge, Q. Heterologous overexpression of the Arabidopsis SnRK2. 8 gene enhances drought and salt tolerance in Populus × euramericana cv ‘Nanlin895’. Plant Biotechnol. Rep. 2019, 13, 245–261. [Google Scholar] [CrossRef]
- Xie, X.; Lin, M.; Xiao, G.; Wang, Q.; Li, Z. Identification and Characterization of the AREB/ABF Gene Family in Three Orchid Species and Functional Analysis of DcaABI5 in Arabidopsis. Plants 2024, 13, 774. [Google Scholar] [CrossRef]
- Li, R.; Song, Y.; Wang, X.; Zheng, C.; Liu, B.; Zhang, H.; Ke, J.; Wu, X.; Wu, L.; Yang, R.; et al. OsNAC5 orchestrates OsABI5 to fine-tune cold tolerance in rice. J. Integr. Plant Biol. 2024, 66, 660–682. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Q.; Zhang, Y.; Niu, M.; Yu, X.; Lian, C.; Liu, C.; Wang, H.L.; Yin, W.; Xia, X. Increased abscisic acid sensitivity and drought tolerance of Arabidopsis by overexpression of poplar abscisic acid receptors. Plant Cell Tissue Organ Cult. 2022, 148, 231–245. [Google Scholar] [CrossRef]
- Santosh Kumar, V.V.; Yadav, S.K.; Verma, R.K.; Shrivastava, S.; Ghimire, O.; Pushkar, S.; Rao, M.V.; Senthil Kumar, T.; Chinnusamy, V. The abscisic acid receptor OsPYL6 confers drought tolerance to indica rice through dehydration avoidance and tolerance mechanisms. J. Exp. Bot. 2021, 72, 1411–1431. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, T.; She, Z.; Huang, S.; Wang, L.; Aslam, M.; Qin, R.; Wang, X.; Qin, Y.; Niu, X. Small Auxin Up RNA (SAUR) gene family identification and functional genes exploration during the floral organ and fruit developmental stages in pineapple (Ananas comosus L.) and its response to salinity and drought stresses. Int. J. Biol. Macromol. 2023, 237, 124061. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, B.; Yang, D.; Wang, S.; Yu, L.; Zhan, H.; Li, J. An optimal genomic DNA extraction method for shoots of four Dendrocalamus species based on membership function analysis. BioTechniques 2023, 76, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Min, X.; Su, Z. Comprehensive evaluation of drought resistance and screening of drought resistance indicators for geographical populations of Tamarix taklamakanensis. J. Xinjiang Norm. Univ. (Nat. Sci. Ed.) 2023, 42, 49–56. [Google Scholar] [CrossRef]
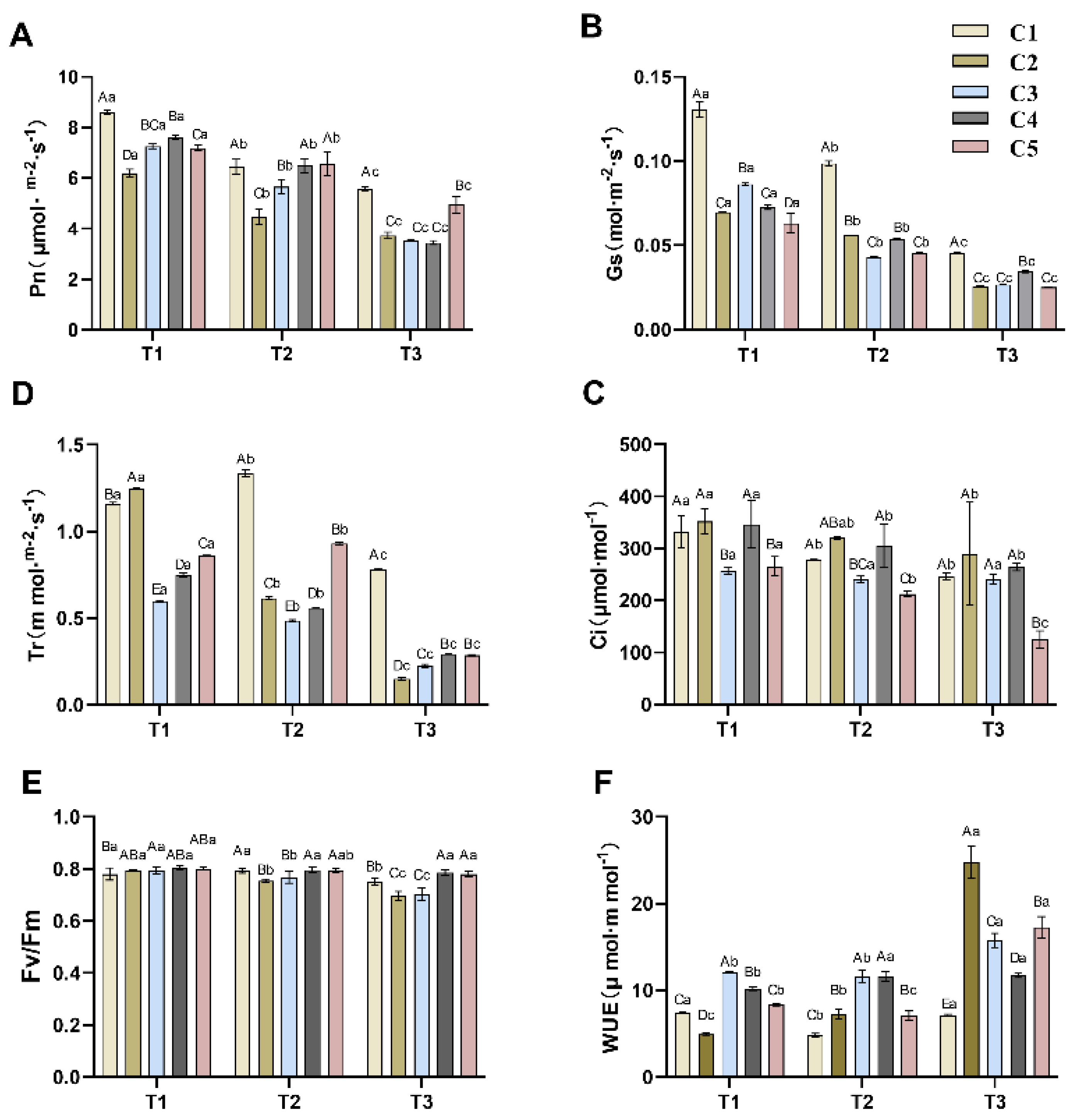
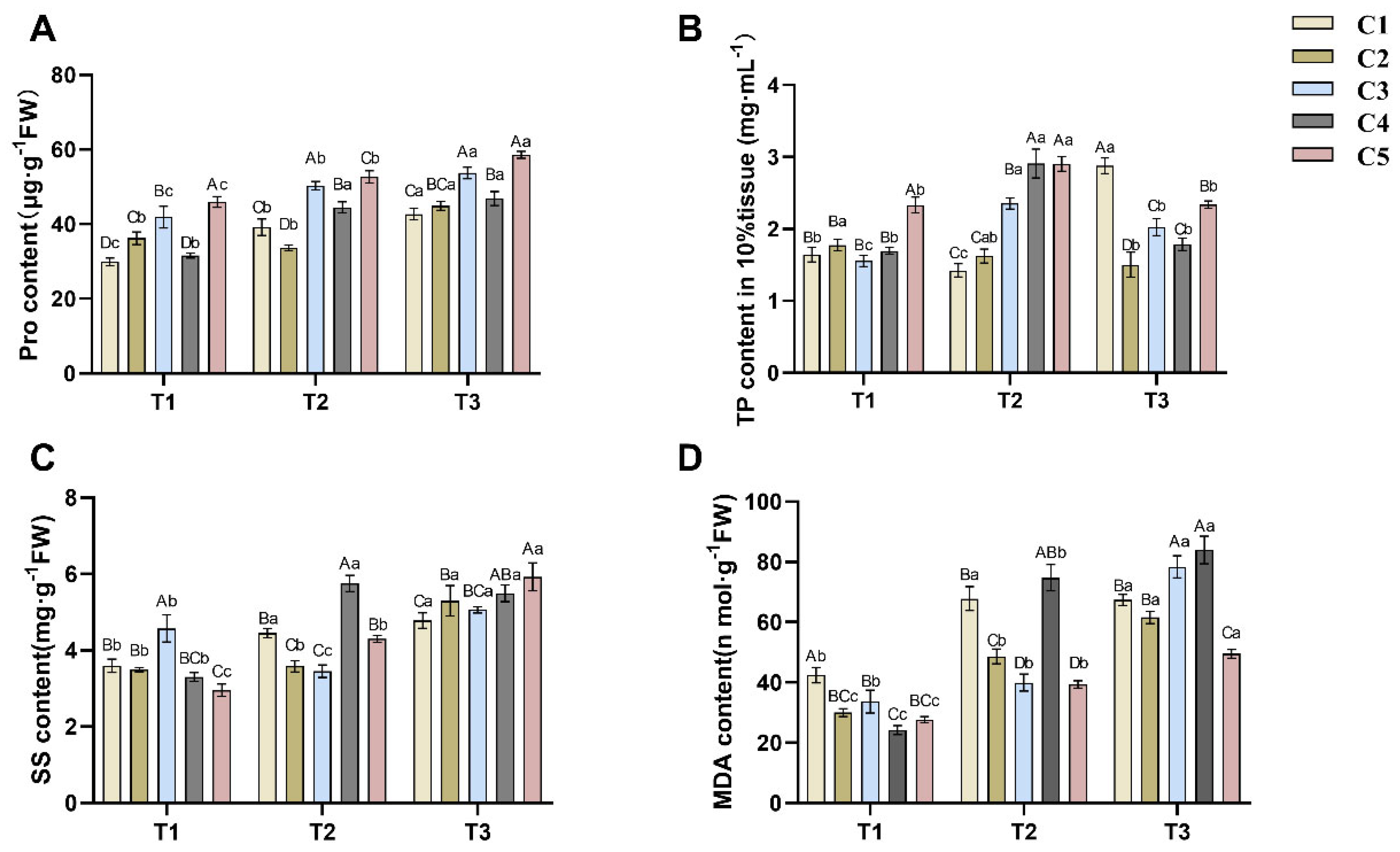

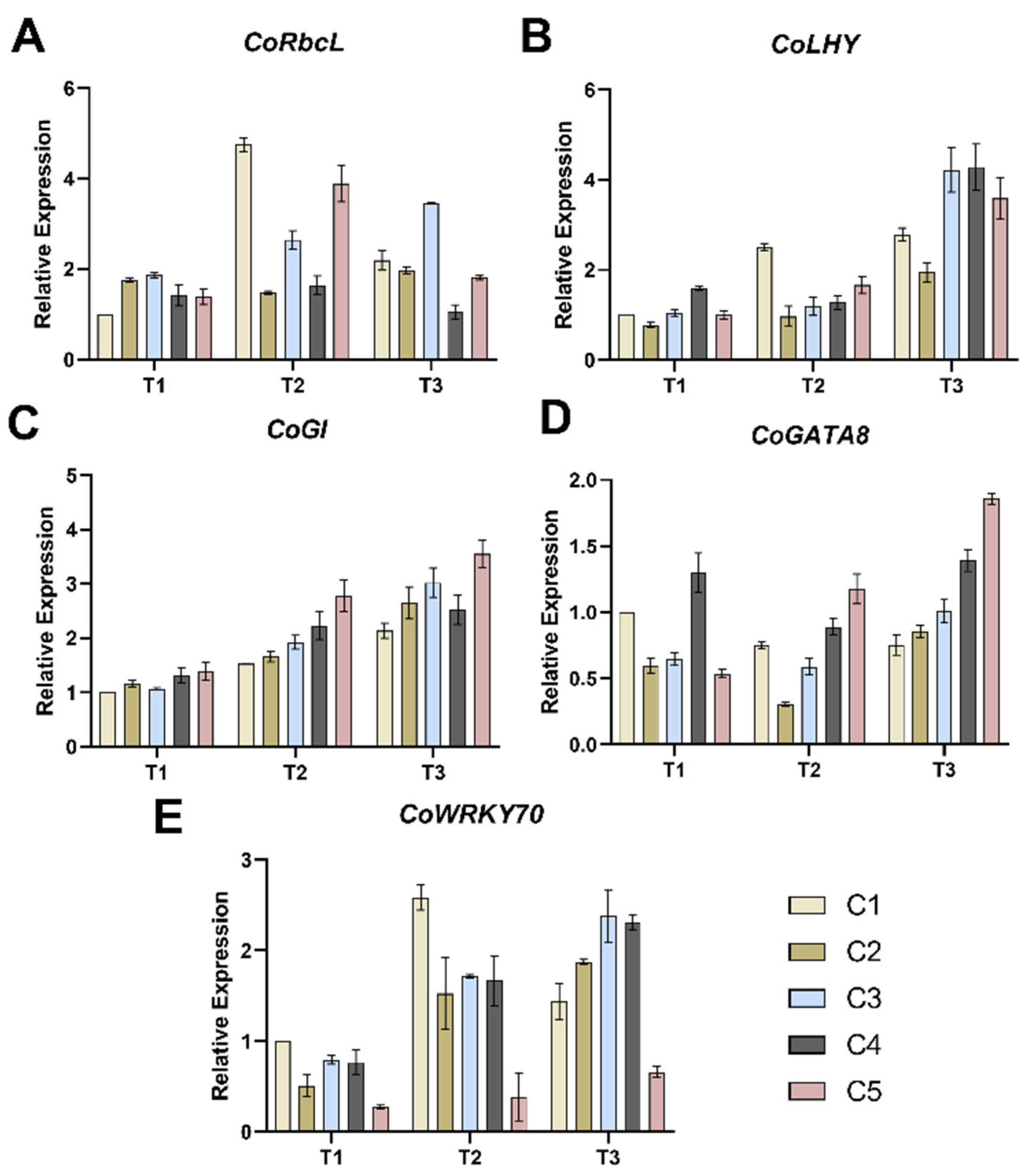
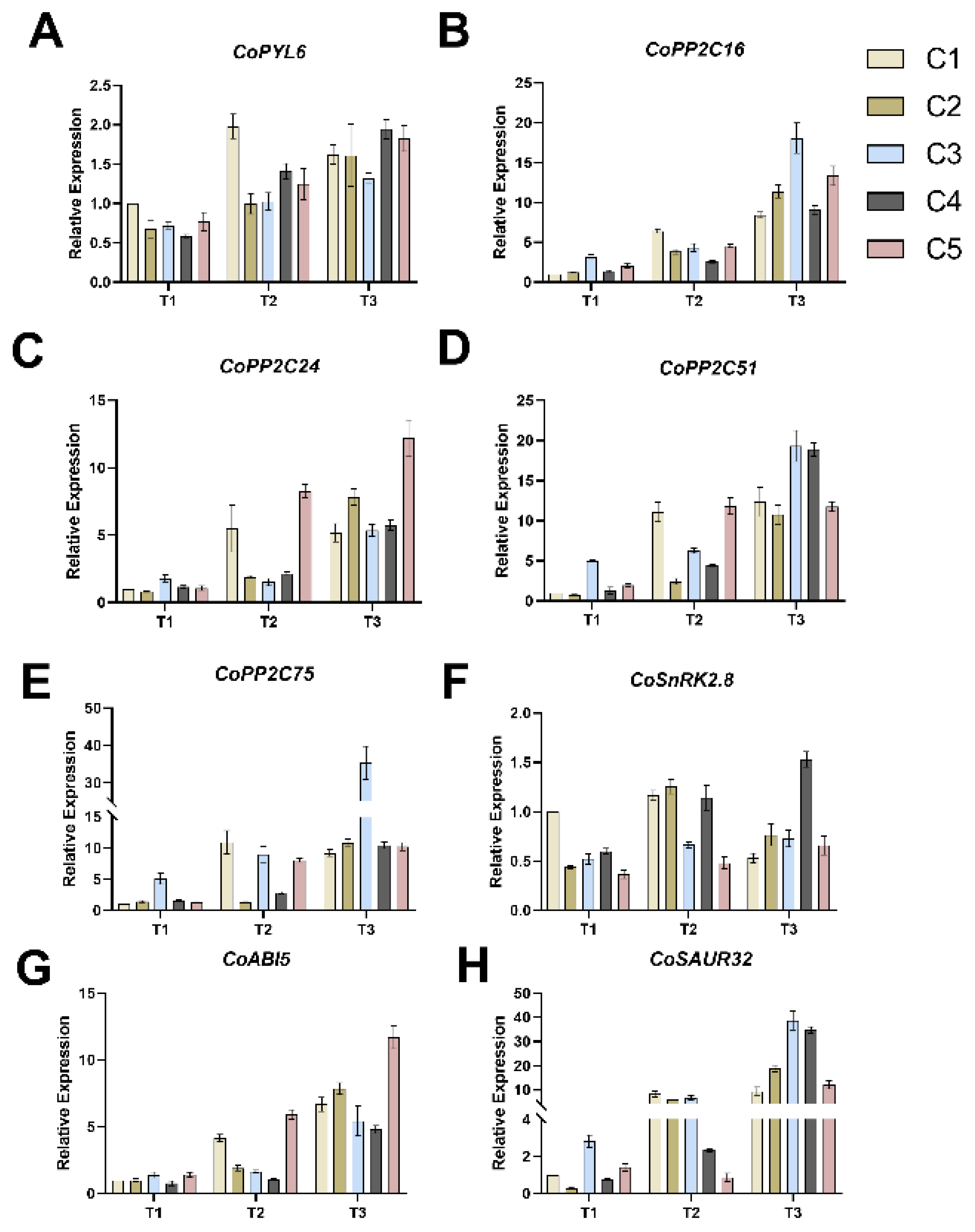
| Group | Membership Function Value | D | Rank | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pn | Gs | Ci | Tr | Fv/Fm | WUE | Pro | TP | SS | MDA | SOD | POD | CAT | GSH | |||
| C1 | 1.00 | 1.00 | 0.73 | 0.00 | 0.39 | 0.00 | 0.00 | 1.00 | 0.00 | 0.48 | 1.00 | 0.72 | 0.24 | 0.00 | 0.47 | 3 |
| C2 | 0.13 | 0.02 | 1.00 | 0.00 | 1.00 | 1.00 | 0.14 | 0.00 | 0.45 | 0.65 | 0.68 | 0.52 | 0.36 | 1.00 | 0.50 | 2 |
| C3 | 0.04 | 0.07 | 0.70 | 0.12 | 0.92 | 0.49 | 0.70 | 0.38 | 0.25 | 0.16 | 0.00 | 0.64 | 0.00 | 0.81 | 0.38 | 4 |
| C4 | 0.00 | 0.45 | 0.84 | 0.23 | 0.00 | 0.26 | 0.26 | 0.21 | 0.62 | 0.00 | 0.67 | 0.00 | 0.15 | 0.59 | 0.31 | 5 |
| C5 | 0.70 | 0.00 | 0.00 | 0.21 | 0.06 | 0.57 | 1.00 | 0.61 | 1.00 | 1.00 | 0.98 | 1.00 | 1.00 | 0.69 | 0.63 | 1 |
| Code | Rootstock | Rootstock Source | Scion | Treatment |
|---|---|---|---|---|
| C1 | C. yuhsienensis | Changsha, Hunan Province; mother tree originated from seedling (12-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T1 (plants subjected to water withholding for 6 days) |
| C1 | C. yuhsienensis | Changsha, Hunan Province; mother tree originated from seedling (12-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T2 (plants subjected to water withholding for 8 days) |
| C1 | C. yuhsienensis | Changsha, Hunan Province; mother tree originated from seedling (12-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T3 (plants subjected to water withholding for 10 days) |
| C2 | C. oleifera (Guangxi Superior Germplasm) | Nanning, Guangxi Zhuang Autonomous Region; mother tree originated from seedling (10-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T1 (plants subjected to water withholding for 6 days) |
| C2 | C. oleifera (Guangxi Superior Germplasm) | Nanning, Guangxi Zhuang Autonomous Region; mother tree originated from seedling (10-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T2 (plants subjected to water withholding for 8 days) |
| C2 | C. oleifera (Guangxi Superior Germplasm) | Nanning, Guangxi Zhuang Autonomous Region; mother tree originated from seedling (10-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T3 (plants subjected to water withholding for 10 days) |
| C3 | C. oleifera (Hunan Superior Germplasm) | Changsha, Hunan Province; mother tree originated from tissue-cultured plant (12-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T1 (plants subjected to water withholding for 6 days) |
| C3 | C. oleifera (Hunan Superior Germplasm) | Changsha, Hunan Province; mother tree originated from tissue-cultured plant (12-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T2 (plants subjected to water withholding for 8 days) |
| C3 | C. oleifera (Hunan Superior Germplasm) | Changsha, Hunan Province; mother tree originated from tissue-cultured plant (12-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T3 (plants subjected to water withholding for 10 days) |
| C4 | C. oleifera ‘Xianglin1’ | Changsha, Hunan Province; mother tree originated from grafted plant (18-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T1 (plants subjected to water withholding for 6 days) |
| C4 | C. oleifera ‘Xianglin1’ | Changsha, Hunan Province; mother tree originated from grafted plant (18-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T2 (plants subjected to water withholding for 8 days) |
| C4 | C. oleifera ‘Xianglin1’ | Changsha, Hunan Province; mother tree originated from grafted plant (18-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T3 (plants subjected to water withholding for 10 days) |
| C5 | C. oleifera ‘Xianglin27’ | Changsha, Hunan Province; mother tree originated from grafted plant (18-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T1 (plants subjected to water withholding for 6 days) |
| C5 | C. oleifera ‘Xianglin27’ | Changsha, Hunan Province; mother tree originated from grafted plant (18-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T2 (plants subjected to water withholding for 8 days) |
| C5 | C. oleifera ‘Xianglin27’ | Changsha, Hunan Province; mother tree originated from grafted plant (18-year-old) | Camellia oleifera “Xianglin 210” (12-year-old) | T3 (plants subjected to water withholding for 10 days) |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| CoGAPDH | CAGGTCGAGCATCTTTGATTCC | CCACCAACTTAACAAAGAAATCATTC |
| CoRbcL | TGGCATCCAAGTTGAAAGAG | ACGCATAAATGGTTGGGAGT |
| CoLHY | GAGGCGAAGCAGAGCAAGG | CACACATCCACACGACAAGTTTC |
| CoGI | GTTGGTGTGGTGGAGTGATGG | CCGTTGTTGGAGGAGGAAGC |
| CoGATA8 | GTAGCAGCAGCAGCAGTTC | TCGGTGATGGAAGAGGTTGG |
| CoWRKY70 | GATGAGTTCTTGTTGTGATGAGTC | GCACCTAAAGTAGCACCTTGG |
| CoPYL6 | ACTCTTCAATCATCACTGTCCATCC | CTGCTCCAACAAATGTCTACAAAGG |
| CoPP2C16 | CTACGGTGGCAGTGAATAGTG | CTTCCATCTCTGACCTCTTTCC |
| CoPP2C24 | TCTGATACTGGCGAGCGATG | CCACCACAACCACACTTACG |
| CoPP2C51 | AGAAGCCTGATAGAGAAGATGAAC | ATCCTCGTCACTCCTTGTCG |
| CoPP2C75 | GCTATTGACTGCTCTGGTTGTTC | TTGCGGTTTCGTGTTCTATTCG |
| CoSnRK2.8 | TTCGGCTACTCAAAGTCATCAG | CACCAACCAACATCACATATAAGG |
| CoABI5 | GATGACACTGGAGGAGTTCTTG | TTGTTCTCTGGTATCCGATTAGC |
| CoSAUR32 | CCACCACCACCACCATCAC | CTTCTCATCAGCATCGTCTACAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhang, Y.; Xun, C.; Yang, D.; Zhang, Z.; Ma, Y.; Wei, X.; Wan, Z.; Wang, X.; Zhang, Y.; et al. Drought Resistance Evaluation of Camellia oleifera var. “Xianglin 210” Grafted onto Different Rootstocks. Plants 2025, 14, 2568. https://doi.org/10.3390/plants14162568
He Z, Zhang Y, Xun C, Yang D, Zhang Z, Ma Y, Wei X, Wan Z, Wang X, Zhang Y, et al. Drought Resistance Evaluation of Camellia oleifera var. “Xianglin 210” Grafted onto Different Rootstocks. Plants. 2025; 14(16):2568. https://doi.org/10.3390/plants14162568
Chicago/Turabian StyleHe, Zhilong, Ying Zhang, Chengfeng Xun, Dayu Yang, Zhen Zhang, Yushen Ma, Xin Wei, Zhentao Wan, Xiangnan Wang, Yufeng Zhang, and et al. 2025. "Drought Resistance Evaluation of Camellia oleifera var. “Xianglin 210” Grafted onto Different Rootstocks" Plants 14, no. 16: 2568. https://doi.org/10.3390/plants14162568
APA StyleHe, Z., Zhang, Y., Xun, C., Yang, D., Zhang, Z., Ma, Y., Wei, X., Wan, Z., Wang, X., Zhang, Y., Chen, Y., & Wang, R. (2025). Drought Resistance Evaluation of Camellia oleifera var. “Xianglin 210” Grafted onto Different Rootstocks. Plants, 14(16), 2568. https://doi.org/10.3390/plants14162568






