Karrikins Regulate the Redox Balance and Sugar Metabolism of Postharvest Kiwifruit (Actinidia deliciosa)
Abstract
1. Introduction
2. Results
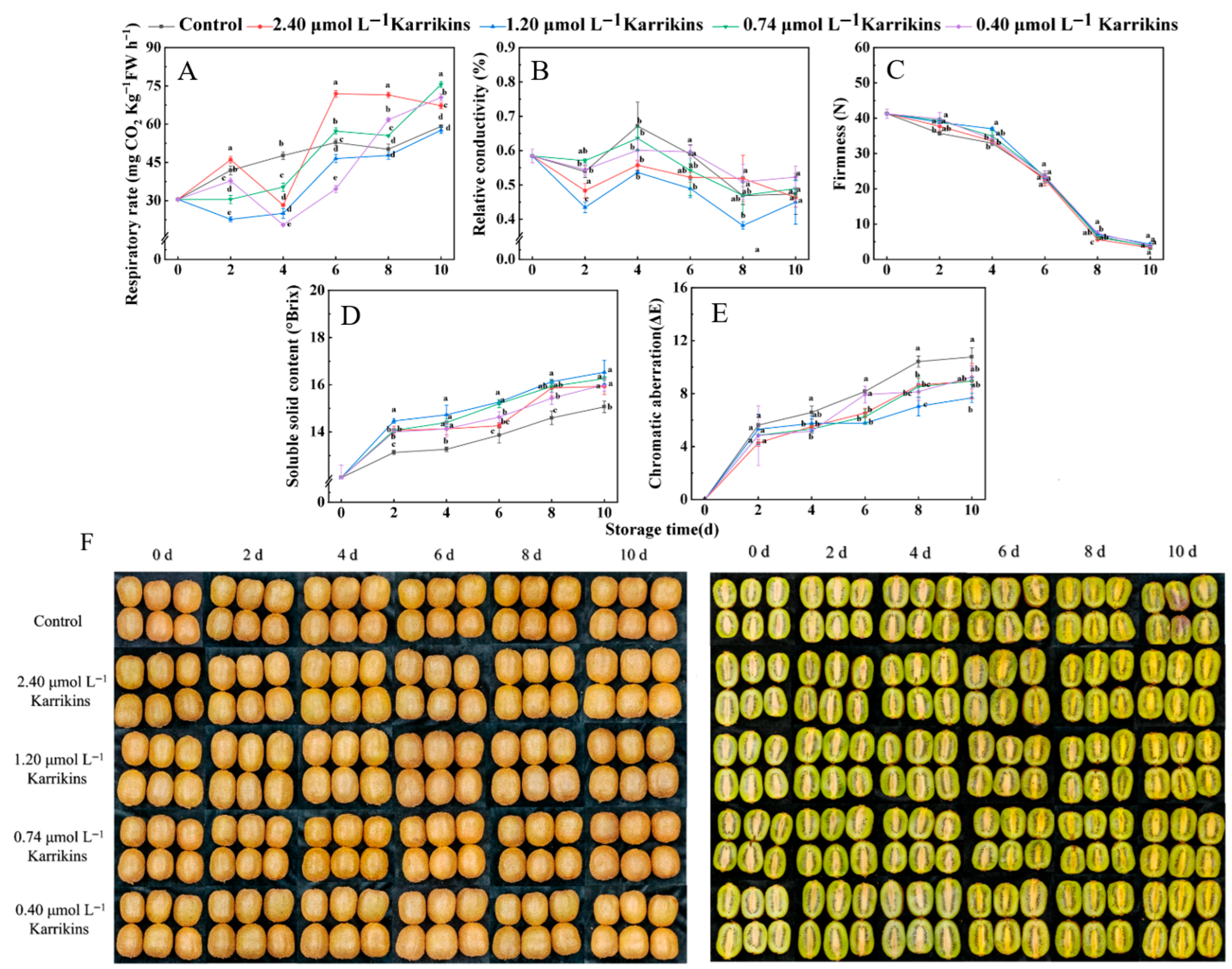
2.1. Karrikins Extended Storage and Maintained Kiwifruit Quality
2.2. Karrikins Decreased ROS Concentrations and Regulated Antioxidative Enzyme Activities
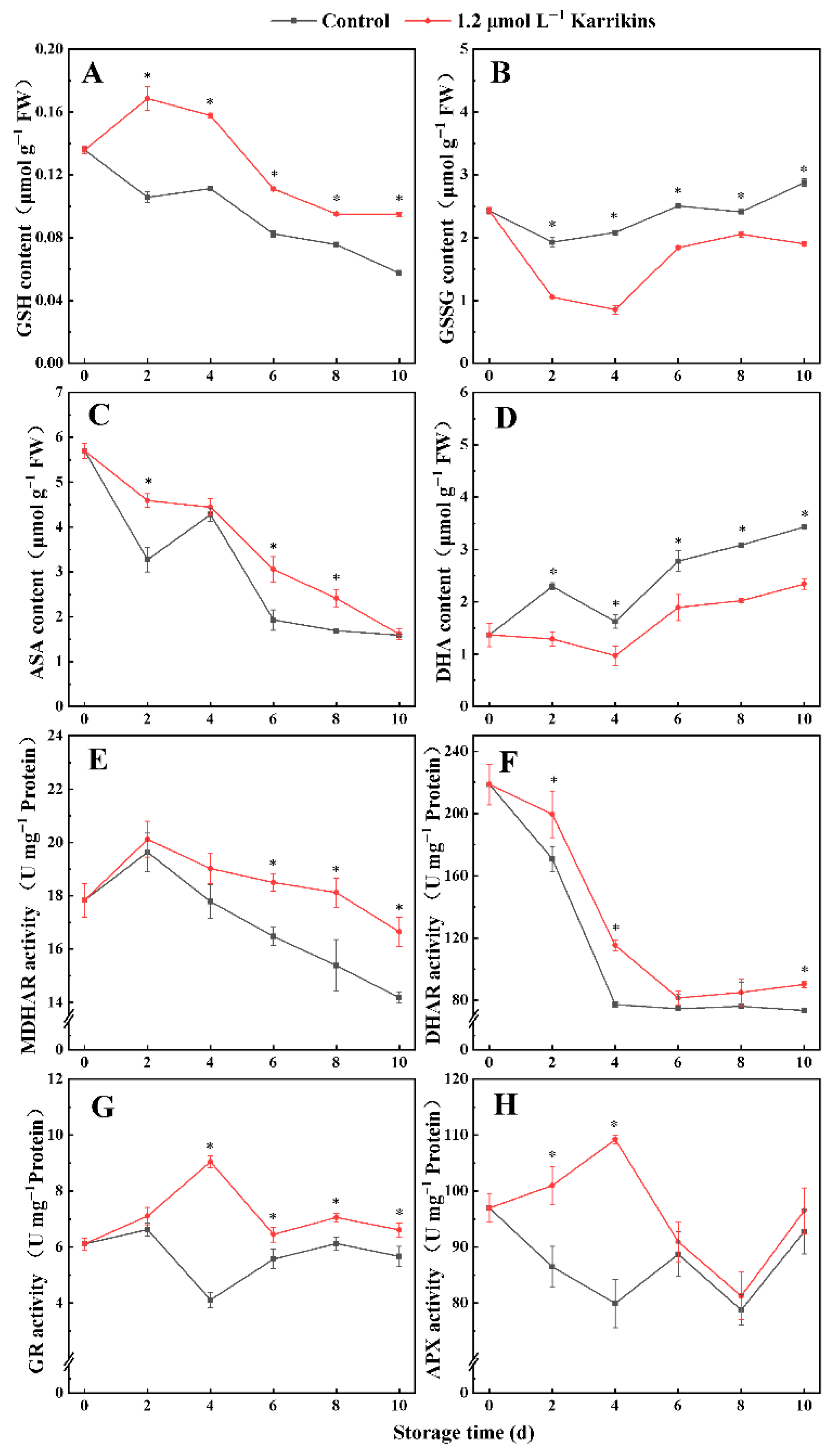
2.3. Karrikins Promoted the AsA-GSH Cycle in Kiwifruit
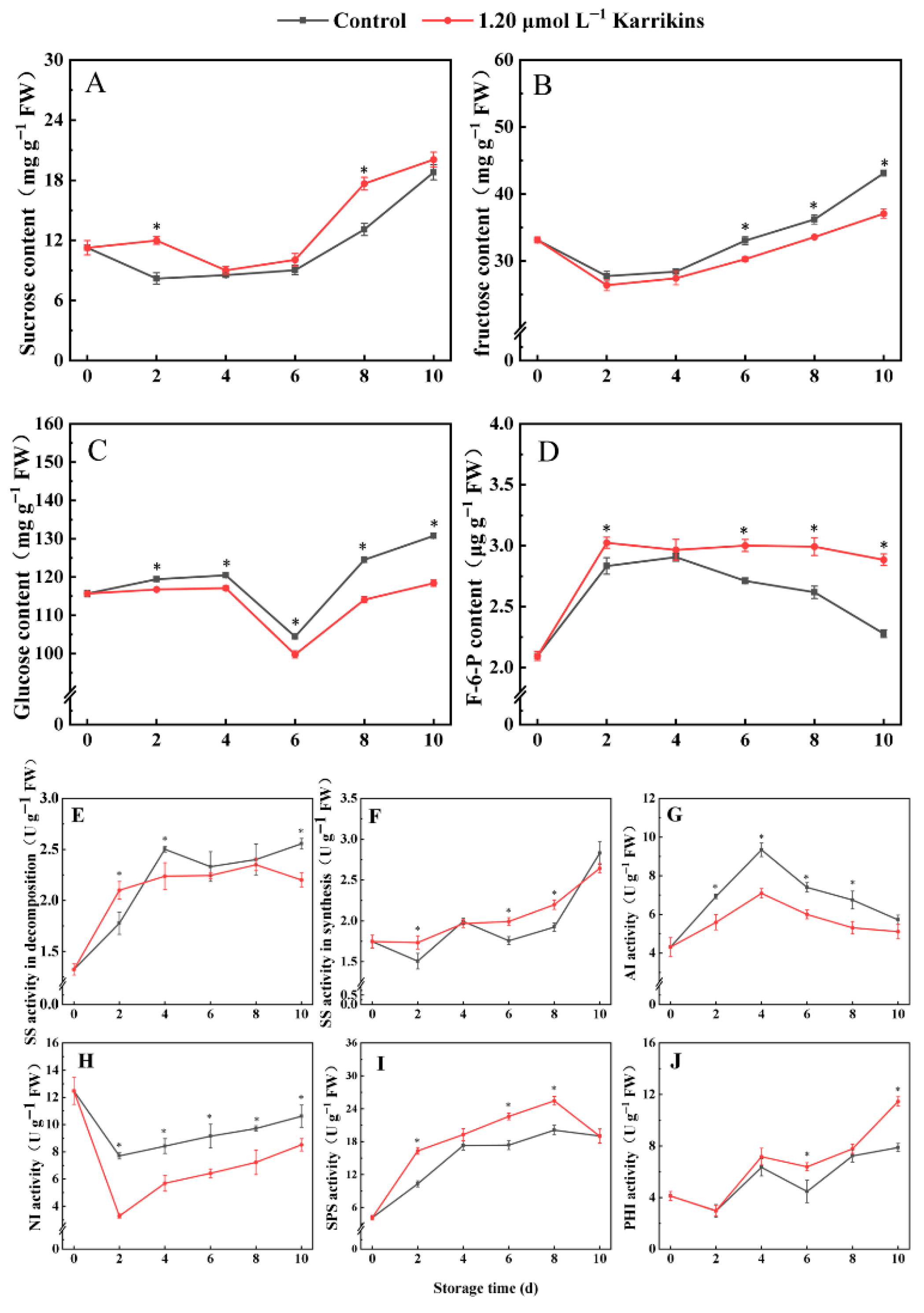
2.4. Karrikins Modulated Soluble Sugars Concentrations and Enzyme Activities Related to Sugar Metabolism
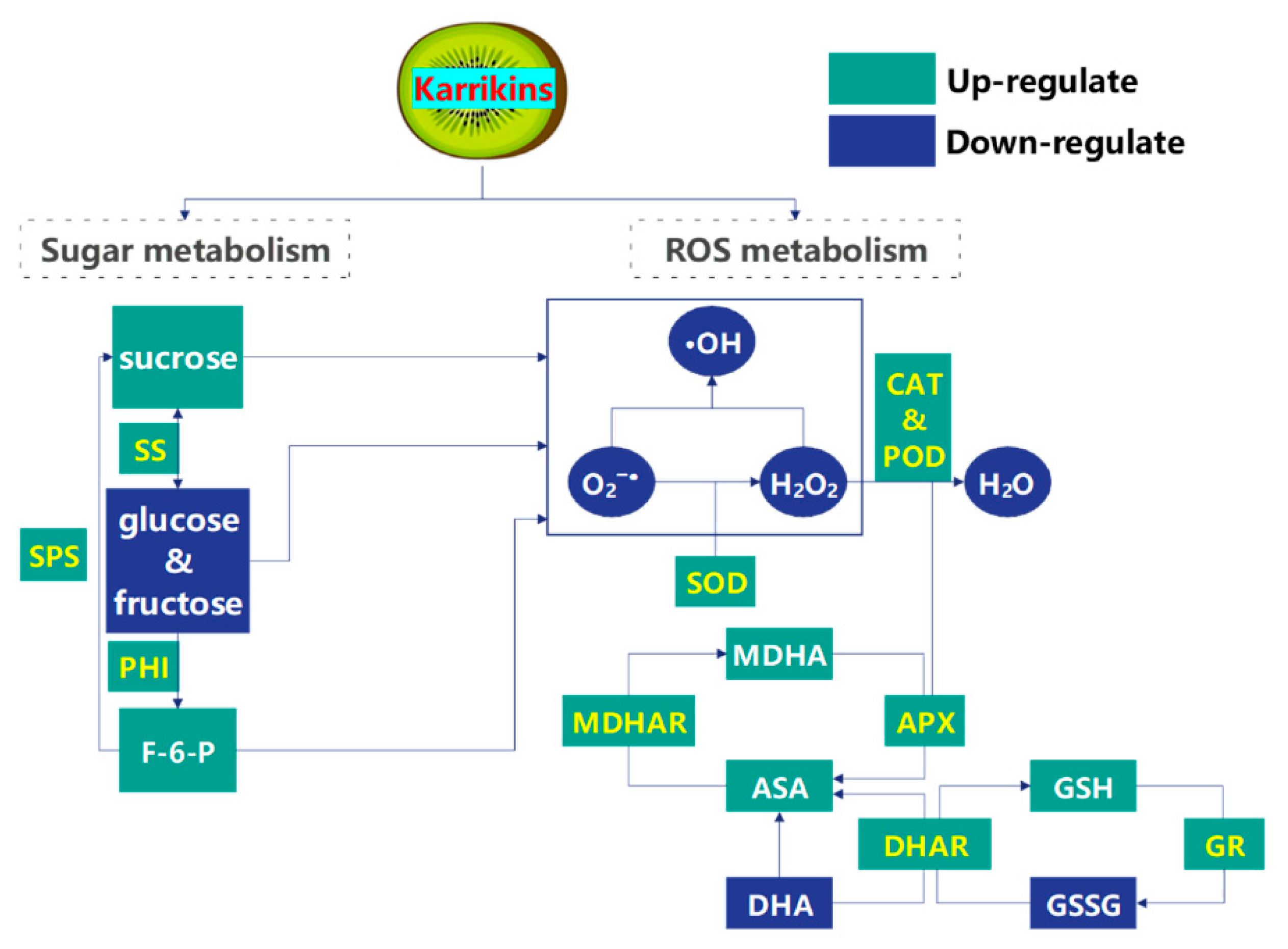
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Fruit Quality
4.3. Measurement of ROS Concentration and Antioxidative Enzyme Activities
4.4. Measurement of Sugar Concentrations and Enzyme Activities in Sugar Metabolism
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salzano, A.M.; Renzone, G.; Sobolev, A.P.; Carbone, V.; Petriccione, M.; Capitani, D.; Vitale, M.; Novi, G.; Zambrano, N.; Pasquariello, M.S.; et al. Unveiling kiwifruit metabolite and protein changes in the course of postharvest cold storage. Front. Plant Sci. 2019, 10, 71. [Google Scholar] [CrossRef]
- Han, Y.; Heyes, J.; Glowacz, M.; Nicholson, S.; Jeffery, P.; East, A. The responses of ‘Hayward’ kiwifruit to ethylene during regular and controlled atmosphere storage. N. Z. J. Crop Hortic. Sci. 2024, 52, 265–279. [Google Scholar] [CrossRef]
- Niu, Y.; Ye, L.; Wang, Y.; Shi, Y.; Liu, Y.; Luo, A. Improvement of storage quality of ‘Hayward’ kiwifruit by MeJA combined with SA treatment through activation of phenylpropane metabolism. Sci. Hortic. 2023, 321, 112354. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Xu, C.; Jiang, Z.; Liu, J.; Sui, Y.; Zhang, H. Control of postharvest blue and gray mold in kiwifruit by Wickerhamomyces anomalus and its mechanism of antifungal activity. Postharvest Biol. Technol. 2023, 201, 112345. [Google Scholar] [CrossRef]
- Chai, J.; Wang, Y.; Liu, Y.; Yong, K.; Liu, Z. 1-MCP extends the shelf life of ready-to-eat ‘Hayward’ and ‘Qihong’ kiwifruit stored at room temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, L.; Zhou, J. Effects of different nitric oxide application on quality of kiwifruit during 20 °C storage. Int. J. Food Sci. Technol. 2010, 45, 245–251. [Google Scholar] [CrossRef]
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci. Hortic. 2022, 296, 110876. [Google Scholar] [CrossRef]
- Yang, S.; He, M.; Li, D.; Shi, J.; Peng, L.; Jinjing, L. Antifungal activity of 40 plant essential oil components against Diaporthe fusicola from postharvest kiwifruits and their possible action mode. Ind. Crops Prod. 2023, 194, 116102. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Leng, J.; Sui, Y.; Jiang, M.; Wisniewski, M.; Liu, J.; Wang, Q. Eco-friendly management of postharvest fungal decays in kiwifruit. Crit. Rev. Food Sci. Nutr. 2022, 62, 8307–8318. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, D.-T.; Ali, M.; Liu, Y.; Zhuang, Q.-G.; Wadood, S.A.; Liao, Q.-H.; Liu, H.-Y.; Gan, R.-Y. Innovative postharvest strategies for maintaining the quality of kiwifruit during storage: An updated review. Food Front. 2024, 5, 1933–1950. [Google Scholar] [CrossRef]
- Kanayama, Y. Sugar metabolism and fruit development in the tomato. Hortic. J. 2017, 86, 417–425. [Google Scholar] [CrossRef]
- Rossiter, K.L.; Young, H.; Walker, S.B.; Miller, M.; Dawson, D.M. The effects of sugars and acids on consumer acceptability of kiwifruit. J. Sens. Stud. 2000, 15, 241–250. [Google Scholar] [CrossRef]
- Titeli, V.S.; Michailidis, M.; Tanou, G.; Molassiotis, A. Physiological and metabolic traits linked to kiwifruit quality. Horticulturae 2023, 9, 915. [Google Scholar] [CrossRef]
- Liao, G.; Li, Y.; Wang, H.; Liu, Q.; Zhong, M.; Jia, D.; Huang, C.; Xu, X. Genome-wide identification and expression profiling analysis of sucrose synthase (SUS) and sucrose phosphate synthase (SPS) genes family in Actinidia chinensis and A. eriantha. BMC Plant Biol. 2022, 22, 215. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Zhen, X.-H.; Zhou, Y.-J.; Wang, Y.-L.; Hou, J.-Y.; Wang, X.; Li, R.-M.; Liu, J.; Hu, X.-W.; Geng, M.-T.; et al. MeNINV1: An alkaline/neutral invertase gene of Manihot esculenta, enhanced sucrose catabolism and promoted plant vegetative growth in transgenic Arabidopsis. Plants 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, H.; Lei, D.; Zhao, B.; Zhou, X.; Yao, W.; Fan, J.; Lin, Y.; Chen, Q.; Wang, Y.; et al. Exogenous melatonin maintains postharvest quality in kiwiberry fruit by regulating sugar metabolism during cold storage. LWT 2023, 174, 114385. [Google Scholar] [CrossRef]
- Corpas, F.J.; Freschi, L.; Palma, J.M. ROS metabolism and ripening of fleshy fruits. Adv. Bot. Res. 2023, 105, 205–238. [Google Scholar]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Y.; Jiang, Y.; Zhang, Z. Advances in control technologies and mechanisms to treat peel browning in postharvest fruit. Sci. Hortic. 2023, 311, 111798. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, T.; Qin, G.; Li, B.; Tian, S. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Nair, J.; Pošta, M.; Papenfus, H.; Munro, O.; Beier, P.; Van Staden, J. Synthesis, X-ray structure determination and germination studies on some smoke-derived karrikins. South Afr. J. Bot. 2014, 91, 53–57. [Google Scholar] [CrossRef]
- Choi, J.; Lee, T.; Cho, J.; Servante, E.K.; Pucker, B.; Summers, W.; Bowden, S.; Rahimi, M.; An, K.; An, G. The negative regulator SMAX1 controls mycorrhizal symbiosis and strigolactone biosynthesis in rice. Nat. Commun. 2020, 11, 2114. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, H.; Qiu, Y.; Wang, D.; Xia, Y.; Zhang, Y.; Pei, M.; Zhao, Y.; Xu, X.; Zhang, H. The Multifaceted Impact of Karrikin Signaling in Plants. Int. J. Mol. Sci. 2025, 26, 2775. [Google Scholar] [CrossRef]
- Kamran, M.; Melville, K.T.; Waters, M.T. Karrikin signalling: Impacts on plant development and abiotic stress tolerance. J. Exp. Bot. 2023, 75, 1174–1186. [Google Scholar] [CrossRef]
- Shah, F.A.; Wei, X.; Wang, Q.; Liu, W.; Wang, D.; Yao, Y.; Hu, H.; Chen, X.; Huang, S.; Hou, J.; et al. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. Front. Plant Sci. 2020, 11, 216. [Google Scholar] [CrossRef]
- Liu, M.; Shan, Q.; Ding, E.; Gu, T.; Gong, B. Karrikin increases tomato cold tolerance via strigolactone and the abscisic acid signaling network. Plant Sci. 2023, 332, 111720. [Google Scholar] [CrossRef]
- Baldrianová, J.; Černý, M.; Novák, J.; Jedelský, P.L.; Divíšková, E.; Brzobohatý, B. Arabidopsis proteome responses to the smoke-derived growth regulator karrikin. J. Proteom. 2015, 120, 7–20. [Google Scholar] [CrossRef]
- Sami, A.; Rehman, S.; Tanvir, M.A.; Zhou, X.Y.; Zhu, Z.H.; Zhou, K. Assessment of the germination potential of Brassica oleracea seeds treated with karrikin 1 and cyanide, which modify the ethylene biosynthetic pathway. J. Plant Growth Regul. 2021, 40, 1257–1269. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C. Karrikin perception and signalling. New Phytol. 2023, 237, 1525–1541. [Google Scholar] [CrossRef]
- Wang, Q.; Smith, S.M.; Huang, J. Origins of strigolactone and karrikin signaling in plants. Trends Plant Sci. 2022, 27, 450–459. [Google Scholar] [CrossRef]
- Li, L.; Gupta, A.; Zhu, C.; Xu, K.; Watanabe, Y.; Tanaka, M.; Seki, M.; Mochida, K.; Kanno, Y.; Seo, M.; et al. Strigolactone and karrikin receptors regulate phytohormone biosynthetic and catabolic processes. Plant Cell Rep. 2025, 44, 60. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, R.; Suo, J.; Ding, Y.; Tan, J.; Zhu, Q.; Ma, Y. Understanding quality differences between kiwifruit varieties during softening. Food Chem. 2024, 430, 136983. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, W.; Shi, J.; Zhang, W.; Shen, Y.; Du, H.; Wu, S. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J. Sci. Food Agric. 2014, 94, 2699–2704. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Guillén, F.; Ilea, M.I.M.; Martínez-Romero, D.; Lorente-Mento, J.M.; Valverde, J.M. Comparative effect of melatonin and 1-methylcyclopropene postharvest applications for extending ‘Hayward’ kiwifruit storage life. Agriculture 2023, 13, 806. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, L.; Liu, M.; Zhou, J. Effect of nitric oxide on reactive oxygen species and antioxidant enzymes in kiwifruit during storage. J. Sci. Food Agric. 2008, 88, 2324–2331. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Y.; Ye, L.; Shi, Y.; Luo, A. Ozone treatment modulates reactive oxygen species levels in kiwifruit through the antioxidant system: Insights from transcriptomic analysis. J. Plant Physiol. 2023, 291, 154135. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ren, X.; Meng, L.; Zheng, R.; Li, D.; Kong, Q. Exogenous phytosulfokine α (PSKα) alleviates chilling injury of kiwifruit by regulating Ca2+ and protein kinase-mediated reactive oxygen species metabolism. Foods 2023, 12, 4196. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, X.; Su, J.; Lu, Z.; Li, Y.; Gao, H. Delayed senescence of kiwifruit by p-coumaric acid pretreatment during storage at 20 °C: Toward regulating the ascorbate–glutathione cycle and phenolic anabolism. Sci. Hortic. 2021, 280, 109913. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Xiao, X.; Cao, S.; Chen, W.; Yang, Z.; Shi, L. Effect of 1-MCP on the regulation processes involved in ascorbate metabolism in kiwifruit. Postharvest Biol. Technol. 2021, 179, 111563. [Google Scholar] [CrossRef]
- Ahmad, A.; Shahzadi, I.; Mubeen, S.; Yasin, N.A.; Akram, W.; Khan, W.U.; Wu, T. Karrikinolide alleviates BDE-28, heat and Cd stressors in Brassica alboglabra by correlating and modulating biochemical attributes, antioxidative machinery and osmoregulators. Ecotoxicol. Environ. Saf. 2021, 213, 112047. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Yasin, N.A. Seed priming with karrikinolide improves growth and physiochemical features of Coriandrum sativum under cadmium stress. Environ. Adv. 2021, 5, 100082. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Nishikawa, F.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Bot. 2005, 56, 65–72. [Google Scholar] [CrossRef]
- Huang, L.; Yu, L.-J.; Zhang, X.; Fan, B.; Wang, F.-Z.; Dai, Y.-S.; Qi, H.; Zhou, Y.; Xie, L.-J.; Xiao, S. Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis. Autophagy 2019, 15, 407–422. [Google Scholar] [CrossRef]
- Bogdanović, J.; Mojović, M.; Milosavić, N.; Mitrović, A.; Vučinić, Ž.; Spasojević, I. Role of fructose in the adaptation of plants to cold-induced oxidative stress. Eur. Biophys. J. 2008, 37, 1241–1246. [Google Scholar] [CrossRef]
- Hu, W.; She, J.; Fu, Z.; Yang, B.; Zhang, H.; Jiang, D. Highly efficient and tunable visible-light-catalytic synthesis of 2,5-diformylfuran using HBr and molecular oxygen. RSC Adv. 2021, 11, 23365–23373. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Huang, H.; Wang, B.; Xiao, L.-P.; Song, G. Integration of enzymatic and heterogeneous catalysis for one-pot production of fructose from glucose. ChemSusChem 2018, 11, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kępczyński, J.; Kępczyńska, E. Plant-derived smoke and karrikin 1 in seed priming and seed biotechnology. Plants 2023, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Hrdlička, J.; Gucký, T.; Novák, O.; Kulkarni, M.; Gupta, S.; van Staden, J.; Doležal, K. Quantification of karrikins in smoke water using ultra-high performance liquid chromatography–tandem mass spectrometry. Plant Methods 2019, 15, 81. [Google Scholar] [CrossRef]
- Huan, C.; Zhang, J.; Jia, Y.; Li, S.e.; Jiang, T.; Shen, S.; Zheng, X. Effect of 1-methylcyclopropene treatment on quality, volatile production and ethanol metabolism in kiwifruit during storage at room temperature. Sci. Hortic. 2020, 265, 109266. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, Z.; He, H.; Shi, L.; Zhu, X.; Cui, K. Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022, 387, 132921. [Google Scholar] [CrossRef] [PubMed]
- Magri, A.; Cice, D.; Capriolo, G.; Petriccione, M. Effects of ascorbic acid and melatonin treatments on antioxidant system in fresh-cut avocado fruits during cold storage. Food Bioprocess Technol. 2022, 15, 2468–2482. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2020, 336, 127618. [Google Scholar] [CrossRef]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Sci. Hortic. 2023, 321, 112314. [Google Scholar] [CrossRef]
- Jing, G.; Huang, H.; Yang, B.; Li, J.; Zheng, X.; Jiang, Y. Effect of pyrogallol on the physiology and biochemistry of litchi fruit during storage. BMC Chem. 2013, 7, 9. [Google Scholar] [CrossRef]
- Maehly, A.C. The assay of catalases and peroxidases. Methods Biochem. Anal. 1955, 2, 764–775. [Google Scholar]
- Rocha, A.M.C.N.; Morais, A.M.M.B. Characterization of polyphenoloxidase (PPO) extracted from ‘Jonagored’ apple. Food Control. 2001, 12, 85–90. [Google Scholar] [CrossRef]
- Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol. Technol. 2006, 40, 116–122. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, D.; Chen, C.; Zhu, S.; Gao, J. Regulation of ascorbate-glutathione cycle in peaches via nitric oxide treatment during cold storage. Sci. Hortic. 2019, 247, 400–406. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liang, L.; Jiang, Y.; Chen, J. Fibroin delays chilling injury of postharvest banana fruit via enhanced antioxidant capability during cold storage. Metabolites 2019, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Chotikakham, S.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Exogenous methyl salicylate alleviates senescent spotting by enhancing the activity of antioxidative ascorbate-glutathione cycle in harvested ‘Sucrier’ bananas. Sci. Hortic. 2020, 267, 109324. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC-ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef]
- Barker, J.; Jakes, R.; Solomos, T.; Younis, M.E.; Isherwood, F.A. Studies in the respiratory and carbohydrate metabolism of plant tissues. J. Exp. Bot. 1964, 15, 284–296. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhou, J.; Zhu, S.H. Effects of exogenous nitric oxide on contents of soluble sugars and related enzyme activities in ‘Feicheng’ peach fruit. J. Sci. Food Agric. 2011, 91, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Hizukuri, S.; Takeda, Y.; Nikuni, Z. Glucose-6-phosphate isomerase from peas. Methods Enzymol. 1975, 41, 388–392. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; Li, H.; Zhu, S.; Huang, D.; Li, C. Karrikins Regulate the Redox Balance and Sugar Metabolism of Postharvest Kiwifruit (Actinidia deliciosa). Plants 2025, 14, 2567. https://doi.org/10.3390/plants14162567
Shao M, Li H, Zhu S, Huang D, Li C. Karrikins Regulate the Redox Balance and Sugar Metabolism of Postharvest Kiwifruit (Actinidia deliciosa). Plants. 2025; 14(16):2567. https://doi.org/10.3390/plants14162567
Chicago/Turabian StyleShao, Mingxia, Hongli Li, Shuhua Zhu, Dandan Huang, and Chengkun Li. 2025. "Karrikins Regulate the Redox Balance and Sugar Metabolism of Postharvest Kiwifruit (Actinidia deliciosa)" Plants 14, no. 16: 2567. https://doi.org/10.3390/plants14162567
APA StyleShao, M., Li, H., Zhu, S., Huang, D., & Li, C. (2025). Karrikins Regulate the Redox Balance and Sugar Metabolism of Postharvest Kiwifruit (Actinidia deliciosa). Plants, 14(16), 2567. https://doi.org/10.3390/plants14162567





