Biobased Postharvest Treatment Using Eucalyptus Essential Oils in Edible Coatings to Inhibit Colletotrichum acutatum and Prolong Strawberry Shelf Life
Abstract
1. Introduction
2. Results
2.1. In Vitro Antifungal Activity of the EOs Against Colletotrichum acutatum Isolated from Strawberries
2.2. Morphological Effect of Essential Oils from Eucalyptus staigeriana and Eucalyptus urograndis on the Fungus
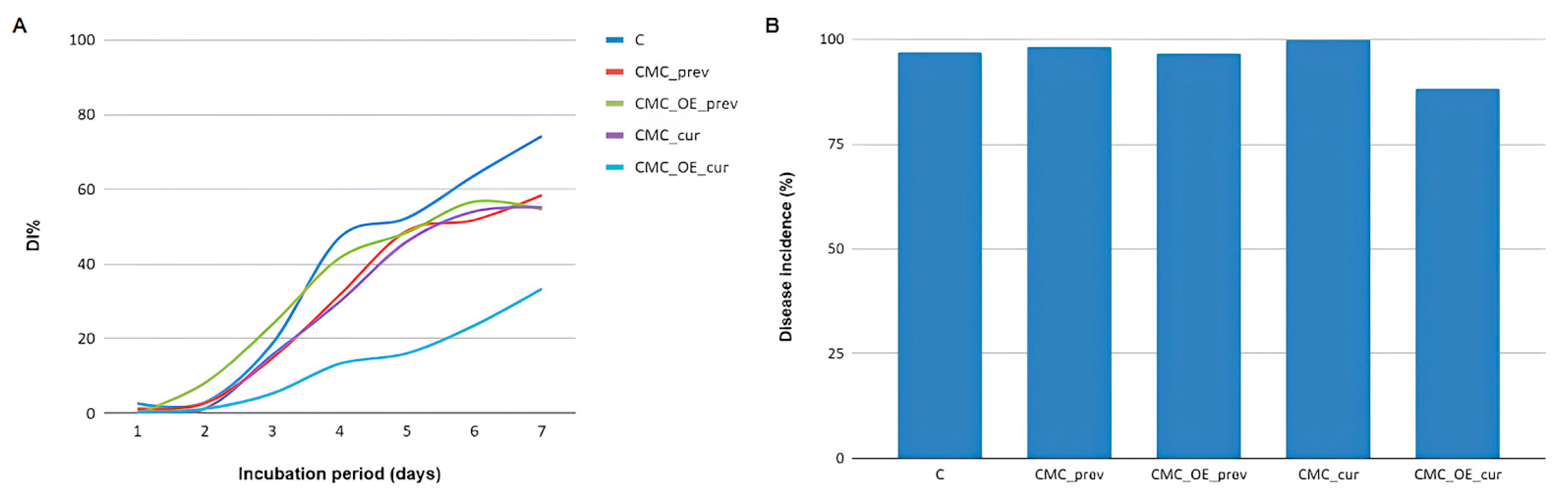
2.3. In Vivo Antifungal Activity of Essential Oils Incorporated into a Carboxymethylcellulose Edible Coating Against Colletotrichum acutatum
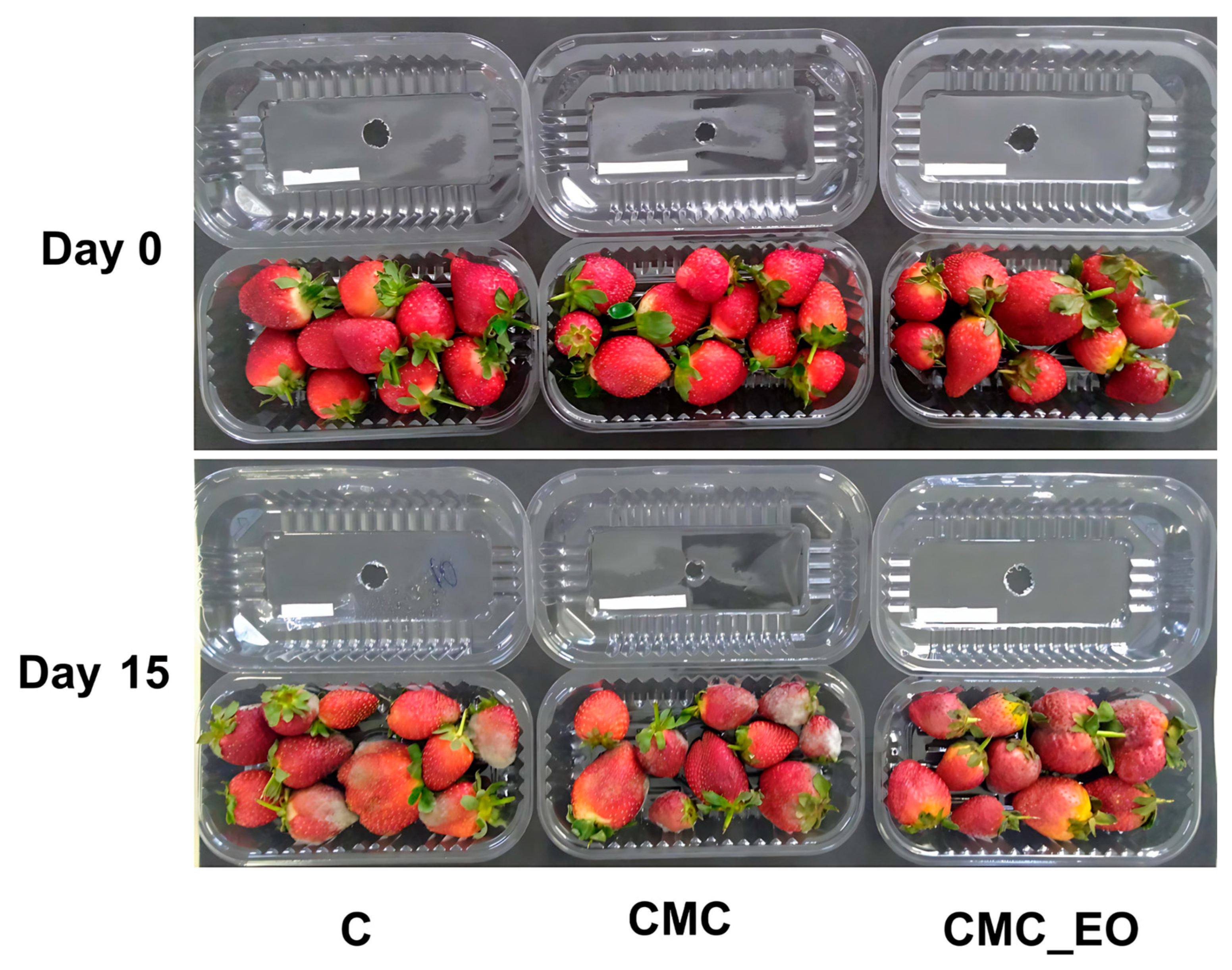
2.4. Postharvest and Sensory Parameters of Strawberries Treated with CMC Incorporated with EO of Eucalyptus staigeriana
3. Discussion
4. Materials and Methods
4.1. Isolation, PCR-Based Identification of Colletotrichum acutatum, and Plant Materials
4.2. In Vitro Antifungal Activity of Essential Oils Against C.acutatum Isolated from Strawberries
4.3. Morphological Effect of Essential Oils from Eucalyptus staigeriana and Eucalyptus urograndis on the Fungus
4.4. In Vivo Antifungal Activity of Essential Oils Incorporated into a Carboxymethylcellulose Edible Coating Against Colletotrichum acutatum
4.4.1. Preparation of the Edible Coating
4.4.2. In Vivo Antifungal Activity and Disease Evaluation
4.5. Evaluation of the Physicochemical and Sensory Quality of Strawberries Treated with CMC with Eucalyptus staigeriana EO
4.5.1. Physicochemical Parameters and Postharvest Quality
4.5.2. Sensory Analysis: Difference from Control Test
4.6. Statistical Analysis of the Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomas-Grau, R.H.; Hael-Conrad, V.; Requena-Serra, F.J.; Perato, S.M.; Caro, M.D.P.; Salazar SMDíaz-Ricci, J.C. Biological Control of Strawberry Grey Mold Disease Caused by Botrytis Cinerea Mediated by Colletotrichum Acutatum Extracts. BioControl 2020, 65, 461–473. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Liu, H.; Du, Y.; Jiao, W.; Sun, F.; Fu, M. Rhizopus Stolonifer and Related Control Strategies in Postharvest Fruit: A Review. Heliyon 2024, 10, e29522. [Google Scholar] [CrossRef] [PubMed]
- Paim Fraga, G.; Berlitz, F.; Bender, R.J.; Souza Da Silva, L.; Oliveira, I. Pesticide Residues in Strawberries Cultivated in the State of Rio Grande Do Sul, Brazil. Ciênc. Rural. 2022, 53, 20220153. [Google Scholar] [CrossRef]
- Vischetti, C.; Feliziani, E.; Landi, L.; De Bernardi, A.; Marini, E.; Romanazzi, G. Effectiveness of Four Synthetic Fungicides in the Control of Post-Harvest Gray Mold of Strawberry and Analyses of Residues on Fruit. Agronomy 2023, 14, 65. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Wisniewski, M.; Droby, S.; Yu, L.; An, F.; Leng, Y.; Wang, C.; Li, X.; He, M.; et al. Current and Future Trends in the Biocontrol of Postharvest Diseases. Crit. Rev. Food Sci. Nutr. 2024, 64, 5672–5684. [Google Scholar] [CrossRef]
- La Bella, E.; Riolo, M.; Luz, C.; Baglieri, A.; Puglisi, I.; Meca, G.; Olga Cacciola, S. Fermentates of Consortia of Lactic Acid Bacteria and a Cyanobacterium Are Effective against Toxigenic Fungi Contaminating Agricultural Produces. Biol. Control 2024, 191, 105478. [Google Scholar] [CrossRef]
- Rahman, F.U.; Zhu, Q.; Wu, Z.; Li, X.; Chen, W.; Xiong, T.; Zhu, X. Current Insights into the Biocontrol and Biotechnological Approaches for Postharvest Disease Management of Botrytis Cinerea. Postharvest Biol. Technol. 2024, 216, 113055. [Google Scholar] [CrossRef]
- Chen, X.; Hu, X.; Zhang, J. Properties of an Active Film Based on Chitosan/Silk Fibroin Loaded with an Essential Oil Microemulsion and Its Application in Preservation of Strawberries. Food Packag. Shelf Life 2024, 43, 101270. [Google Scholar] [CrossRef]
- Goldbeck, J.C.; do Nascimento, J.E.; Jacob, R.G.; Fiorentini, Â.M.; da Silva, W.P. Bioactivity of Essential Oils from Eucalyptus Globulus and Eucalyptus Urograndis against Planktonic Cells and Biofilms of Streptococcus Mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- de Souza, E.J.D.; Kringel, D.H.; de Lima Costa, I.H.; dos Santos Hackbart, H.C.; Cantillano, R.F.F.; Ueno, B.; Dias, A.R.G.; Zavareze, E.d.R. Antifungal Potential of Essential Oils from Different Botanical Sources against Penicillium Digitatum: Chemical Composition and Antifungal Mechanisms of Action by Direct Contact and Volatile. Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Moussa, H.H.; Sara, B.; Benhalima, H.; Benaliouche, F.; Sbartai, I.; Sbartai, H. Chemical Characterization of Eucalyptus (Eucalyptus Globulus) Leaf Essential Oil and Evaluation of Its Antifungal, Antibacterial and Antioxidant Activities. Cell. Mol. Biol. 2024, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. Application of Carboxymethyl Cellulose and Chitosan Coatings Containing Mentha Spicata Essential Oil in Fresh Strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef]
- Hassan, H.S.; El-Hefny, M.; Ghoneim, I.M.; Abd El-Lahot, M.S.R.; Akrami, M.; Al-Huqail, A.A.; Ali, H.M.; Abd-Elkader, D.Y. Assessing the Use of Aloe Vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings 2022, 12, 489. [Google Scholar] [CrossRef]
- Esteves, F.M.; da Silva Ecker, A.B. Avaliação da Atividade Antimicrobiana in Vitro Do Óleo Essencial de Eucalyptus Urograndis Em Cepas Padrão de Bacilos Gram Negativos. Rev. Uningá 2020, 57, 11–23. [Google Scholar] [CrossRef]
- da Silva, P.P.M.; de Oliveira, J.; dos Mares Biazotto, A.; Parisi, M.M.; da Glória, E.M.; Spoto, M.H.F. Essential Oils from Eucalyptus staigeriana, F. Muell. Ex Bailey and Eucalyptus urograndis W. Hill Ex Maiden Associated to Carboxymethylcellulose Coating for the Control of Botrytis cinerea Pers. Fr. and Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. in Strawberries. Ind. Crops Prod. 2020, 156, 112884. [Google Scholar] [CrossRef]
- Simko, I.; Piepho, H.-P. The Area Under the Disease Progress Stairs: Calculation, Advantage, and Application. Phytopathology 2012, 102, 381–389. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Essential Oil and Antifungal Therapy. In Recent Trends in Antifungal Agents and Antifungal Therapy; Springer: New Delhi, India, 2016; pp. 29–74. [Google Scholar]
- Martins, G.A.; Bicas, J.L. Antifungal Activity of Essential Oils of Tea Tree, Oregano, Thyme, and Cinnamon, and Their Components. Braz. J. Food Technol. 2024, 27, e2023071. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Habibi Najafi, M.B.; Farhoosh, R. Synergistic Effects of Some Essential Oils against Fungal Spoilage on Pear Fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Tejeswini, M.G.; Sowmya, H.V.; Swarnalatha, S.P.; Negi, P.S. Antifungal Activity of Essential Oils and Their Combinations in in Vitro and in Vivo Conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 564–570. [Google Scholar] [CrossRef]
- de SouzaI, E.L., II; de Oliveira Lima, E.; de Luna Freire, K.R.; de Sousa, C.P. Inhibitory Action of Some Essential Oils and Phytochemicals on the Growth of Various Moulds Isolated from Foods. Braz. Arch. Biol. Technol. 2005, 48, 245–250. [Google Scholar] [CrossRef]
- Pedrotti, C.; Marcon, Â.R.; Laguna Echeverrigaray, S.; da Silva Ribeiro, R.T.; Schwambach, J. Essential Oil as Sustainable Alternative for Diseases Management of Grapes in Postharvest and in Vineyard and Its Influence on Wine. J. Environ. Sci. Health B 2020, 56, 73–81. [Google Scholar] [CrossRef]
- Pedrotti, C.; Trentin, T.R.; Cavião, H.C.; Vilasboa, J.; Scariot, F.J.; Echeverrigaray, S.; Piemolini-Barreto, L.T.; Schwambach, J. Eucalyptus Staigeriana Essential Oil in the Control of Postharvest Fungal Rots and on the Sensory Analysis of Grapes. Pesqui. Agropecu. Bras. 2022, 57, e02782. [Google Scholar] [CrossRef]
- Pedrotti, C.; Franzoi, C.; Rosa, M.T.S.; Trentin, T.R.; Vilasboa, J.; Scariot, F.J.; Echeverrigaray, S.L.; Schwambach, J. Antifungal Activity of Essential Oil from Eucalyptus Staigeriana against Alternaria Alternata Causing of Leaf Spot and Black Rot in Table Grapes. An. Acad. Bras. Ciênc. 2022, 94, e20200394. [Google Scholar] [CrossRef] [PubMed]
- Teker, T.; Sefer, Ö.; Gazdağlı, A.; Yörük, E.; Varol, G.İ.; Albayrak, G. α-Thujone Exhibits an Antifungal Activity against F. Graminearum by Inducing Oxidative Stress, Apoptosis, Epigenetics Alterations and Reduced Toxin Synthesis. Eur. J. Plant Pathol. 2021, 160, 611–622. [Google Scholar] [CrossRef]
- Balta, I.; Brinzan, L.; Stratakos, A.C.; Linton, M.; Kelly, C.; Pinkerton LCorcionivoschi, N. Geraniol and Linalool Loaded Nanoemulsions and Their Antimicrobial Activity. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2017, 74, 157. [Google Scholar] [CrossRef]
- Narnoliya, L.K.; Jadaun, J.S.; Singh, S.P. The Phytochemical Composition, Biological Effects and Biotechnological Approaches to the Production of High-Value Essential Oil from Geranium. In Essential Oil Research; Malik, S., Ed.; Springer International Publishing: Cham, Germany, 2019; pp. 327–352. [Google Scholar]
- Borges, D.J.V.; Souza, R.A.C.; de Oliveira, A.; de Sousa, R.M.F.; Venâncio, H.; Demetrio, G.R.; Ambrogi, B.G.; Santos, J.C. Green Lacewing Chrysoperla Externa Is Attracted to Volatile Organic Compounds and Essential Oils Extracted from Eucalyptus Urograndis Leaves. Plants 2024, 13, 2192. [Google Scholar] [CrossRef]
- Maronde, D.N.; Venancio, A.N.; Lopes, R.P.; de Souza, G.R.; Parreira, L.A.; Menini, L. Influence of Light on the Composition of Eucalyptus Essential Oil (Eucalyptus Urograndis). Braz. J. Dev. 2020, 6, 98082–98090. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, X.; Liu, L.; Xing, R.; Song, X.; Zou, Y.; Li, L.; Wan, H.; Jia, R.; et al. Study on the Anti-Biofilm Mechanism of 1,8-Cineole against Fusarium Solani Species Complex. Front. Pharmacol. 2022, 13, 1010593. [Google Scholar] [CrossRef] [PubMed]
- Konuk, H.B.; Ergüden, B. Investigation of Antifungal Activity Mechanisms of Alpha-Pinene, Eugenol, and Limonene. J. Adv. VetBio Sci. Tech. 2022, 7, 385–390. [Google Scholar] [CrossRef]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida Spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Nguyen-Ngoc, H.; Tran-Trung, H.; Nguyen, D.-K.; Thi Nguyen, L.-T. Limonene and Eucalyptol Rich Essential Oils with Their Antimicrobial Activity from the Leaves and Rhizomes of Conamomum vietnamense N.S. Lý & T.S. Hoang (Zingiberaceae). Pharmacia 2023, 70, 91–96. [Google Scholar] [CrossRef]
- de Oliveira Moraes, C.R.; Venancio, A.N.; Camara, M.P.S.; Bento, C.d.S.; Parreira, L.A.; Santos, M.F.C.; Menini, L. Citrus Essential Oils in the Control of the Anthracnose-Causing Fungus Colletotrichum okinawense in Papaya Fruits. Int. J. Plant Biol. 2025, 16, 50. [Google Scholar] [CrossRef]
- White, L.A.S.; Blank, A.F.; Gagliardi, P.R.; Arrigoni-Blank, M.d.F.; Nizio, D.A.d.C.; Sampaio, T.S.; Alves, M.F.; Almeida-Pereira, C.S. In Vitro Antifungal Activity of Myrcia Ovata Essential Oils and Their Major Compounds against Pathogens of Citrus, Sweet Potato, and Coconut. Biosci. J. 2019, 35, 1695–1707. [Google Scholar] [CrossRef]
- He, J.; Wu, D.; Zhang, Q.; Chen, H.; Li, H.; Han, Q.; Lai, X.; Wang, H.; Wu, Y.; Yuan, J.; et al. Efficacy and Mechanism of Cinnamon Essential Oil on Inhibition of Colletotrichum acutatum Isolated From ‘Hongyang’ Kiwifruit. Front. Microbiol 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum Acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef]
- Pineda, R.; Vizcaíno, S.; García, C.M.; Gil, J.H.; Durango, D.L. Antifungal Activity of Extracts, Essential Oil and Constituents from Petroselinum Crispum against Colletotrichum Acutatum. Rev. Fac. Nac. De Agron. Medellín 2018, 71, 8563–8572. [Google Scholar] [CrossRef]
- Oliveira, J.; Gloria, E.M.; Parisi, M.C.M.; Baggio, J.S.; Silva, P.P.M.; Ambrosio, C.M.S.; Spoto, M.H.F. Antifungal Activity of Essential Oils Associated with Carboxymethylcellulose against Colletotrichum Acutatum in Strawberries. Sci. Hortic. 2019, 243, 261–267. [Google Scholar] [CrossRef]
- Rashid, T.S.; Awla, H.K.; Sijam, K. Antifungal Effects of Rhus coriaria L. Fruit Extracts against Tomato Anthracnose Caused by Colletotrichum acutatum. Ind. Crops Prod. 2018, 113, 391–397. [Google Scholar] [CrossRef]
- Fukuyama, C.W.T.; Brexó, R.P.; Duarte, L.G.R.; Martins, M.E.M.; Astolfo, M.E.A.; Osti, Y.G.P.; Pedrino, I.C.; Santos, H.V.; de Oliveira Filho, J.G.; Procopio, F.R.; et al. Impact of Essential Oils Composition and Exposure Methods on Fungal Growth and Morphology: Insights for Postharvest Management. Future Postharvest Food 2025, 2, 159–173. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A.; Mukherjee, A. Nanoencapsulation-Based Edible Coating of Essential Oils as a Novel Green Strategy Against Fungal Spoilage, Mycotoxin Contamination, and Quality Deterioration of Stored Fruits: An Overview. Front. Microbiol. 2021, 12, 768414. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal Activity of Plant Essential Oils Against Botrytis Cinerea, Penicillium Italicum and Penicillium Digitatum. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 86. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Pedrino, I.C.; Osti, Y.G.P.; Fukuyama, C.W.T.; Nogueira, P.H.B.; de A. Astolfo, M.E.; da M. Martins, M.E.; Junior, S.B.; Ferreira, M.D. Nigrospora sp. in Post-Harvest Papayas: Efficacy of Essential Oils in Antifungal Inhibition. Technol. Hortic. 2025, 5, e009. [Google Scholar] [CrossRef]
- Moura, V.S.; Olandin, L.D.; Mariano, B.S.; Rodrigues, J.; Devite, F.T.; Arantes, A.C.C.; Queiroga, C.L.; Sartoratto, A.; de Azevedo, F.A.; Bastianel, M. Antifungal Activity of Citrus Essential Oil in Controlling Sour Rot in Tahiti Acid Lime Fruits. Plants 2024, 13, 3075. [Google Scholar] [CrossRef]
- Mohd Israfi, N.A.; Mohd Ali, M.I.A.; Manickam, S.; Sun, X.; Goh, B.H.; Tang, S.Y.; Ismail, N.; Abdull Razis, A.F.; Ch’ng, S.E.; Chan, K.W. Essential Oils and Plant Extracts for Tropical Fruits Protection: From Farm to Table. Front. Plant Sci. 2022, 13, 999270. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.-H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elkader, D.Y.; Salem, M.Z.M.; Komeil, D.A.; Al-Huqail, A.A.; Ali, H.M.; Salah, A.H.; Akrami, M.; Hassan, H.S. Post-Harvest Enhancing and Botrytis Cinerea Control of Strawberry Fruits Using Low Cost and Eco-Friendly Natural Oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Adeogun, O.O.; Oluwa, O.K.; Nejo, A.O.; Salami, S.O.; Egwu, P.C.; Adekunle, A.A. Assessment of Eucalyptus Oil Integrated with Carboxymethyl Cellulose (CMC) for Controlling Postharvest Soft Rot in Citrus Sinensis Fruits Induced by Colletotrichum Gloeosporioides and Aspergillus Niger. Bull. Natl. Res. Cent. 2025, 49, 17. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.R.; Tzortzakis, N. Application of Rosemary and Eucalyptus Essential Oils and Their Main Component on the Preservation of Apple and Pear Fruits. Horticulturae 2021, 7, 479. [Google Scholar] [CrossRef]
- Kumar Tyagi, A.; Bukvicki, D.; Gottardi, D.; Tabanelli, G.; Montanari, C.; Malik, A.; Guerzoni, M.E. Eucalyptus Essential Oil as a Natural Food Preservative: In Vivo and In Vitro Antiyeast Potential. BioMed Res. Int. 2014, 2014, 969143. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; de Aguiar Andrade, E.H.; de Oliveira, M.S. Recent Trends in the Application of Essential Oils: The next Generation of Food Preservation and Food Packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Giello, M.; Miele, N.A.; Volpe, S.; Di Monaco, R.; Cavella, S.; Villani, F.; Torrieri, E. Design of an Active Edible Coating: Antimicrobial, Physical, and Sensory Properties of Sodium Caseinate-Guar Gum Blends Enriched in Essential Oils. Food Packag. Shelf Life 2024, 46, 101395. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.; da Cruz Silva, G.; de Aguiar, A.C.; Cipriano, L.; de Azeredo, H.M.C.; Bogusz Junior, S.; Ferreira, M.D. Chemical Composition and Antifungal Activity of Essential Oils and Their Combinations against Botrytis cinerea in Strawberries. J. Food Meas. Charact. 2021, 15, 1815–1825. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M. Boosting Antifungal Effect of Essential Oils Using Combination Approach as an Efficient Strategy to Control Postharvest Spoilage and Preserving the Jujube Fruit Quality. Postharvest Biol. Technol. 2020, 164, 111159. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Das, P.; Rustagi, S.; Sharma, M.; Sridhar, N.; Hazarika, T.K.; Rana, P.; Nayak, P.K.; Sridhar, K. Essential Oil-Nanoemulsion Based Edible Coating: Innovative Sustainable Preservation Method for Fresh/Fresh-Cut Fruits and Vegetables. Food Chem. 2024, 460, 140545. [Google Scholar] [CrossRef]
- Eckert, J.W.; Ogawa, J.M. The Chemical Control of Postharvest Diseases: Deciduous, Berries, Vegetables and Root/Tuber Crops. Annu. Rev. Phytopathol. 1988, 26, 433–469. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Sangchote, S.; Chinsirikul, W.; Sane, A.; Chonhenchob, V. Antifungal Activity of Plant-Derived Compounds and Their Synergism against Major Postharvest Pathogens of Longan Fruit in Vitro. Int. J. Food Microbiol. 2018, 271, 8–14. [Google Scholar] [CrossRef]
- Escanferla, M.E.; Moraes, S.R.G.; Salaroli, R.B.; Massola, N.S. Prepenetration Stages of Guignardia Psidii in Guava: Effects of Temperature, Wetness Duration and Fruit Age. J. Phytopathol. 2009, 157, 618–624. [Google Scholar] [CrossRef]
- Cia, P.; Benato, E.A.; Pascholati, S.F.; Garcia, E.O. Quitosana No Controle Pós-Colheita Da Podridão Mole Em Caqui “Rama Forte”. Bragantia 2010, 69, 745–752. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Wall, M.M.; Nishijima, K.A.; Keith, L.M.; Nagao, M.A. Influence of Packaging on Quality Retention of Longans (Dimocarpus Longan) Under Constant and Fluctuating Posth arvest Temperatures. HortScience 2011, 46, 917–923. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan–Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- AOAC Association of Official Agricultural. Chemists Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2015. [Google Scholar]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Civille, G.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429195143. [Google Scholar]
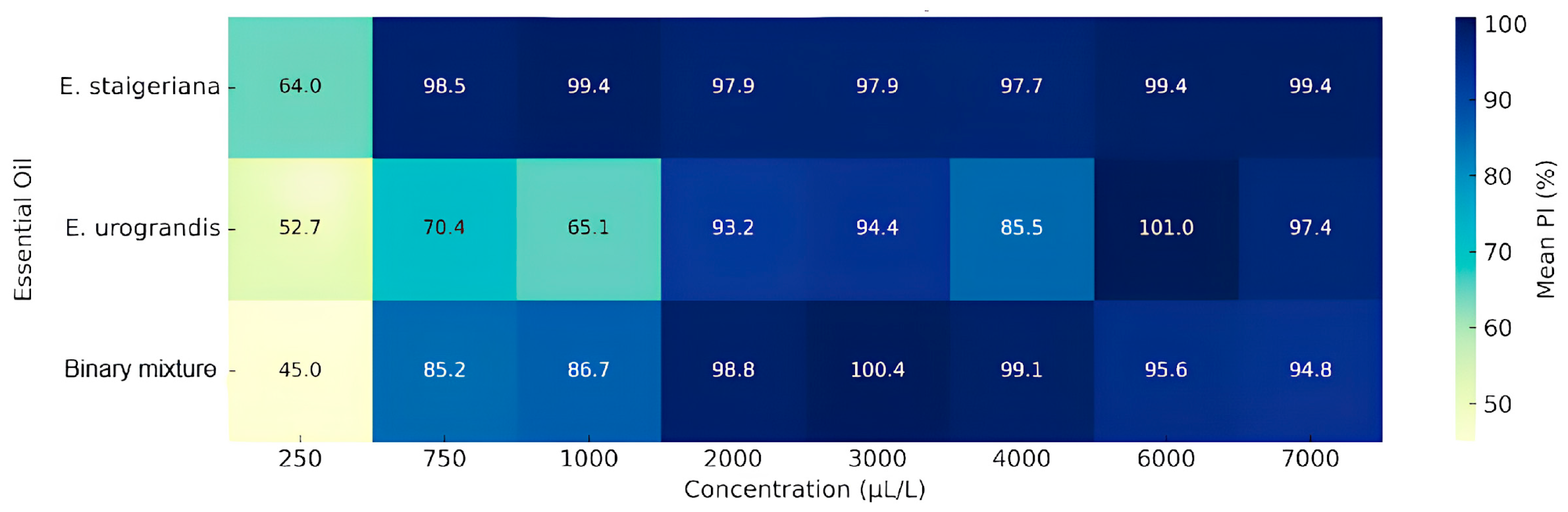


| EO or Binary Mixture | Concentration (µL L−1) | PI (%) | MIC (µL L−1) | MFC (µL L−1) |
|---|---|---|---|---|
| Eucalyptus staigeriana | 250 | 63.99 ± 8.49 B | >750 | >2000 |
| 750 | 98.53 ± 2.33 A | |||
| 1000 | 99.44 ± 0.26 A | |||
| 2000 | 97.92 ± 0.94 A | |||
| 3000 | 98.48 ± 0.92 A | |||
| 4000 | 97.72 ± 1.46 A | |||
| 6000 | 99.40 ± 1.18 A | |||
| 7000 | 97.93 ± 1.51 A | |||
| Eucalyptus urograndis | 250 | 52.71 ± 8.51 C | >6000 | >7000 |
| 750 | 70.44 ± 2.65 BC | |||
| 1000 | 65.08 ± 9.21 C | |||
| 2000 | 93.21 ± 4.76 A | |||
| 3000 | 94.41 ± 6.85 A | |||
| 4000 | 85.50 ± 5.25 AB | |||
| 6000 | 100.0 ± 1.52 A | |||
| 7000 | 97.41 ± 4.29 A | |||
| Binary mixture | 250 | 44.95 ± 4.52 C | >2000 | >4000 |
| 750 | 85.19 ± 2.92 B | |||
| 1000 | 86.68 ± 10.13 B | |||
| 2000 | 98.79 ± 1.47 AB | |||
| 3000 | 100.0 ± 2.25 A | |||
| 4000 | 99.10 ± 0.91 AB | |||
| 6000 | 95.58 ± 2.95 AB | |||
| 7000 | 94.80 ± 1.27 AB |
| EO or Binary Mixture | EC50 (µL L−1) | Confidence Intervals (95%) |
|---|---|---|
| Eucalyptus staigeriana | 185.49 | 155.48–214.46 |
| Eucalyptus urograndis | 337.01 | 281.80–392.65 |
| Binary mixture | 355.62 | 314.70–396.31 |
| Treatments | AUDPC * |
|---|---|
| C | 206.91 ± 41.46 AB |
| CMC_prev | 182.14 ± 14.46 AB |
| CMC_EO_prev | 223.13 ± 49.02 A |
| CMC_cur | 168.49 ± 20.39 B |
| CMC_EO_cur | 78.45 ± 11.59 C |
| Storage (Days) | ||||||
|---|---|---|---|---|---|---|
| Treatments | 0 | 3 | 6 | 9 | 12 | 15 |
| Weight Loss (%) | ||||||
| C | 0.00 ± 0.00 | 1.66 ± 0.75 | 3.07 ± 1.29 | 4.21 ± 1.70 | 5.73 ± 2.38 | 7.14 ± 2.24 |
| CMC | 0.00 ± 0.00 | 2.53 ± 0.68 | 5.56 ± 0.65 | 8.24 ± 1.30 | 11.07 ± 1.66 | 12.89 ± 1.59 |
| CMC_EO | 0.00 ± 0.00 | 2.62 ± 0.33 | 4.04 ± 0.82 | 5.74 ± 1.32 | 7.64 ±1.60 | 9.79 ±1.74 |
| Index of Diseases in the Pericarp | ||||||
| C | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.44 ± 3.14 | 16.67 ± 5.44 | 43.33 ± 7.20 | 63.64 ± 4.29 |
| CMC | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.44 ± 1.57 | 16.67 ± 2.72 | 44.44 ± 6.29 | 67.68 ± 5.63 |
| CMC_EO | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.44 ± 4.16 | 31.11 ± 17.50 | 55.35 ± 17.68 |
| Fungal Spoilage (%) | ||||||
| C | 0.00 ± 0.00 | 0.00 ± 0.00 | 16.36 ± 5.14 | 45.15 ± 12.25 | 81.90 ± 8.60 | 90.61 ± 0.43 |
| CMC | 0.00 ± 0.00 | 0.00 ± 0.00 | 10.00 ± 8.16 | 44.85 ± 10.66 | 65.15 ± 10.71 | 90.61 ± 7.44 |
| CMC_EO | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 12.42 ± 11.26 | 56.67 ± 26.25 | 83.64 ± 9.65 |
| Luminosity (L) | ||||||
| C | 36.75 ± 1.12 | 36.25 ± 0.35 | 36.10 ± 0.32 | 32.58 ± 0.60 | 34.80 ± 0.32 | 33.85 ± 0.98 |
| CMC | 35.83 ± 0.64 | 36.18 ± 1.16 | 36.11 ± 1.41 | 31.57 ± 1.34 | 34.19 ± 0.46 | 32.37 ± 1.64 |
| CMC_EO | 35.95 ± 0.54 | 35.10 ± 0.29 | 35.30 ± 0.09 | 33.12 ± 1.24 | 35.61 ± 0.67 | 32.60 ± 1.00 |
| Hue Angle (º) | ||||||
| C | 35.89 ± 1.79 | 36.33 ± 0.87 | 35.75 ± 0.33 | 38.07 ± 1.67 | 38.59 ± 1.11 | 38.24 ± 1.64 |
| CMC | 36.52 ± 0.66 | 39.36 ± 2.21 | 39.94 ± 2.51 | 38.59 ± 2.49 | 37.48 ±0.46 | 38.62 ± 1.18 |
| CMC_EO | 35.97 ± 1.14 | 33.49 ± 0.27 | 33.51 ± 0.33 | 34.26 ± 1.81 | 36.17 ± 0.65 | 38.36 ± 2.47 |
| Chromaticity | ||||||
| C | 40.06 ± 1.33 | 41.42 ± 0.23 | 41.40 ± 0.29 | 41.33 ± 0.66 | 43.24 ± 0.36 | 43.69 ± 1.50 |
| CMC | 42.67 ± 0.79 | 44.19 ± 0.64 | 44.07 ± 0.76 | 39.02 ± 0.18 | 41.48 ± 1.18 | 41.63 ± 2.35 |
| CMC_EO | 39.77 ± 0.47 | 36.64 ± 1.05 | 37.34 ± 0.44 | 35.86 ± 0.94 | 35.56 ± 1.31 | 36.80 ± 2.00 |
| Firmness (N) | ||||||
| C | 1.14 ± 0.19 | 1.38 ± 0.08 | 1.63 ± 0.24 | 1.57 ± 0.13 | 1.63 ± 0.05 | 1.46 ± 0.33 |
| CMC | 1.33 ± 0.13 | 1.50 ±0.16 | 1.43 ± 0.17 | 1.68 ± 0.16 | 1.37 ± 0.21 | 1.56 ± 0.05 |
| CMC_EO | 0.79 ± 0.14 | 0.68 ± 0.05 | 1.06 ± 0.15 | 0.96 ± 0.19 | 1.68 ± 0.46 | 1.40 ± 0.26 |
| pH | ||||||
| C | 3.69 ± 0.07 | 3.70 ± 0.04 | 3.63 ± 0.04 | 3.80 ± 0.05 | 3.67 ± 0.04 | 3.64 ± 0.07 |
| CMC | 3.73 ± 0.08 | 3.65 ± 0.04 | 3.77 ± 0.03 | 3.80 ± 0.05 | 3.83 ± 0.08 | 3.65 ± 0.02 |
| CMC_EO | 3.79 ± 0.05 | 3.72 ± 0.07 | 3.82 ± 0.03 | 3.87 ± 0.04 | 3.83 ± 0.03 | 3.84 ± 0.06 |
| Total Soluble Solids (Brix) | ||||||
| C | 8.22 ± 0.50 | 8.23 ± 0.14 | 7.82 ± 0.59 | 8.77 ± 0.69 | 8.20 ± 0.46 | 7.32 ± 0.18 |
| CMC | 8.37 ± 0.31 | 8.37 ± 0.10 | 8.20 ± 0.23 | 8.50 ± 0.23 | 8.68 ± 0.47 | 7.82 ± 0.66 |
| CMC_EO | 8.28 ± 0.05 | 8.12 ± 0.88 | 8.27 ± 1.09 | 7.92 ± 0.36 | 9.15 ± 1.76 | 7.78 ± 0.97 |
| Titratable Acidity (mg citric acid g−1 fruit) | ||||||
| C | 0.72 ± 0.03 | 0.63 ± 0.03 | 0.63 ± 0.02 | 0.74 ± 0.08 | 0.75 ± 0.02 | 0.72 ± 0.00 |
| CMC | 0.74 ± 0.02 | 0.62 ± 0.03 | 0.65 ± 0.00 | 0.67 ± 0.01 | 0.73 ± 0.03 | 0.76 ± 0.06 |
| CMC_EO | 0.74 ± 0.02 | 0.65 ± 0.00 | 0.66 ± 0.01 | 0.60 ± 0.04 | 0.68 ± 0.06 | 0.84 ± 0.07 |
| Ratio | ||||||
| C | 11.49 ± 1.21 | 13.15 ± 0.73 | 12.44 ± 0.89 | 11.95 ± 0.86 | 10.94 ± 0.27 | 10.20 ± 0.25 |
| CMC | 11.30 ± 0.53 | 13.56 ± 0.55 | 12.66 ± 0.43 | 12.65 ± 0.46 | 11.90 ± 0.53 | 10.32 ± 0.99 |
| CMC_EO | 12.53 ± 0.46 | 12.49 ± 1.37 | 12.48 ± 1.73 | 13.12 ± 0.28 | 13.40 ± 1.64 | 9.24 ± 0.46 |
| Total Monomeric Anthocyanins (mg cyanidin 3-glucoside equivalent 100 g−1 fruit) | ||||||
| C | 12.87 ± 0.26 | 20.21 ± 3.19 | 18.23 ± 3.36 | 21.94 ± 5.51 | 22.35 ± 1.76 | 16.55 ± 3.37 |
| CMC | 13.24 ± 0.02 | 16.98 ± 2.20 | 18.82 ± 1.70 | 20.85 ± 1.86 | 25.56 ± 2.64 | 17.69 ± 2.37 |
| CMC_EO | 14.11 ± 1.47 | 16.02 ± 3.83 | 17.32 ± 2.60 | 17.10 ± 2.44 | 12.78 ± 3.31 | 14.33 ± 0.52 |
| Total Phenolic Compounds (mg gallic acid equivalents g−1 fruit) | ||||||
| C | 0.356 ± 0.009 | 0.451 ± 0.044 | 0.452 ± 0.042 | 0.442 ± 0.015 | 0.528 ± 0.017 | 0.428 ± 0.007 |
| CMC | 0.412 ± 0.061 | 0.419 ± 0.015 | 0.424 ± 0.005 | 0.509 ± 0.024 | 0.554 ± 0.060 | 0.454 ± 0.055 |
| CMC_EO | 0.347 ± 0.025 | 0.368 ± 0.006 | 0.419 ± 0.010 | 0.435 ± 0.020 | 0.415 ± 0.028 | 0.530 ± 0.094 |
| Sensory Attributes | ||||
|---|---|---|---|---|
| Treatments | Characteristic Color | Characteristic Aroma | Appearance | Overall Difference |
| C | 1.88 | 3.00 | 3.13 | 2.85 |
| CMC | 3.08 * | 4.90 * | 4.05 n.s. | 4.28 * |
| CMC_EO | 5.55 * | 7.53 * | 6.38 * | 6.95 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, P.P.M.; de Toledo, N.M.V.; de Oliveira, J.; da Gloria, E.M.; Maximo, F.B.; Spoto, M.H.F. Biobased Postharvest Treatment Using Eucalyptus Essential Oils in Edible Coatings to Inhibit Colletotrichum acutatum and Prolong Strawberry Shelf Life. Plants 2025, 14, 2565. https://doi.org/10.3390/plants14162565
da Silva PPM, de Toledo NMV, de Oliveira J, da Gloria EM, Maximo FB, Spoto MHF. Biobased Postharvest Treatment Using Eucalyptus Essential Oils in Edible Coatings to Inhibit Colletotrichum acutatum and Prolong Strawberry Shelf Life. Plants. 2025; 14(16):2565. https://doi.org/10.3390/plants14162565
Chicago/Turabian Styleda Silva, Paula Porrelli Moreira, Nataly Maria Viva de Toledo, Jacqueline de Oliveira, Eduardo Micotti da Gloria, Fabiane Barco Maximo, and Marta Helena Fillet Spoto. 2025. "Biobased Postharvest Treatment Using Eucalyptus Essential Oils in Edible Coatings to Inhibit Colletotrichum acutatum and Prolong Strawberry Shelf Life" Plants 14, no. 16: 2565. https://doi.org/10.3390/plants14162565
APA Styleda Silva, P. P. M., de Toledo, N. M. V., de Oliveira, J., da Gloria, E. M., Maximo, F. B., & Spoto, M. H. F. (2025). Biobased Postharvest Treatment Using Eucalyptus Essential Oils in Edible Coatings to Inhibit Colletotrichum acutatum and Prolong Strawberry Shelf Life. Plants, 14(16), 2565. https://doi.org/10.3390/plants14162565








