Analysis of Insect Resistance and Ploidy in Hybrid Progeny of Transgenic BtCry1Ac Triploid Poplar 741
Abstract
1. Introduction
2. Results
2.1. Comparative Analysis of Pollen Storage Temperatures and Paternal Line Viability
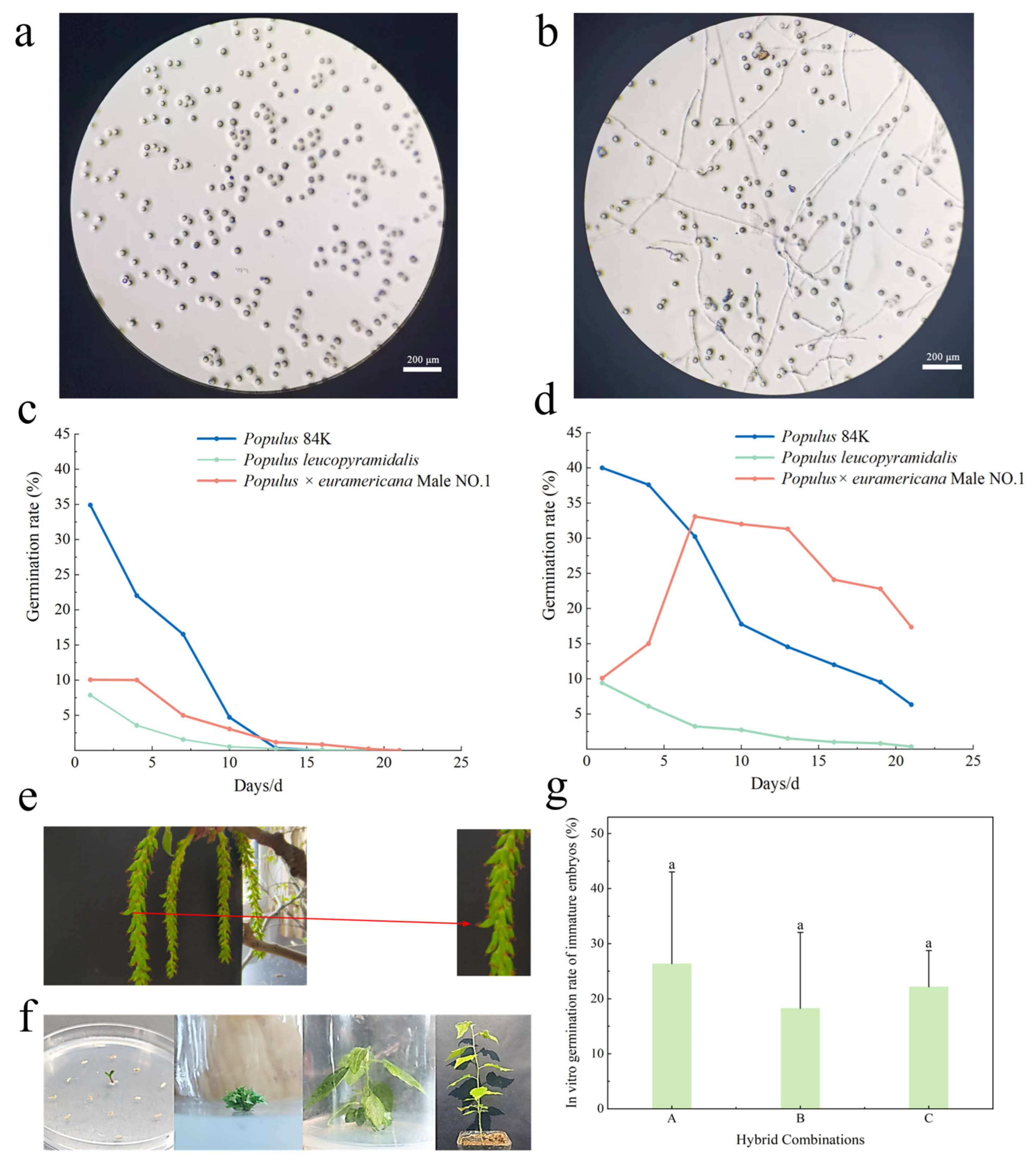
2.2. Artificial Pollination, Hybridization, and Hybrid Embryo Rescue
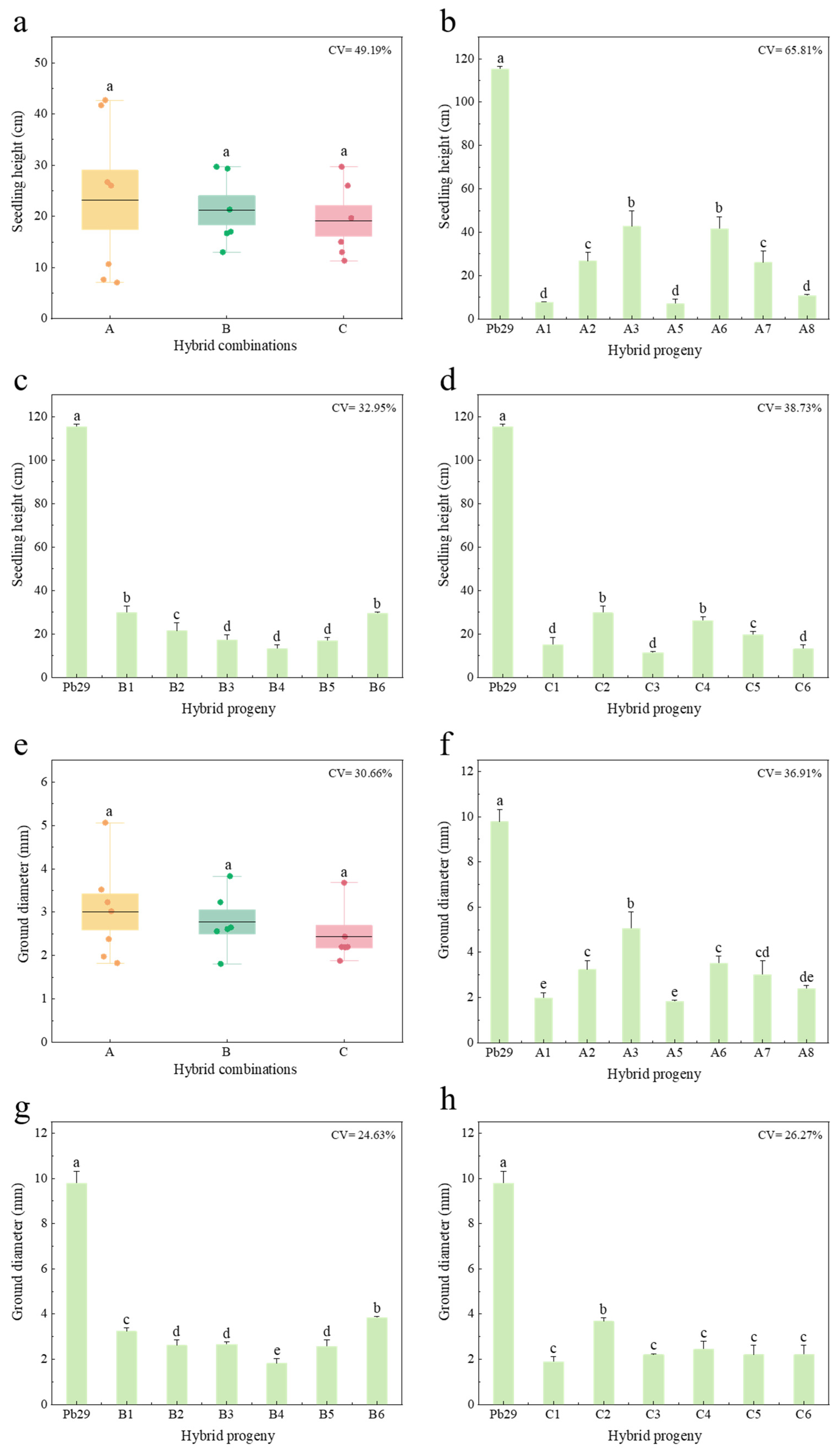
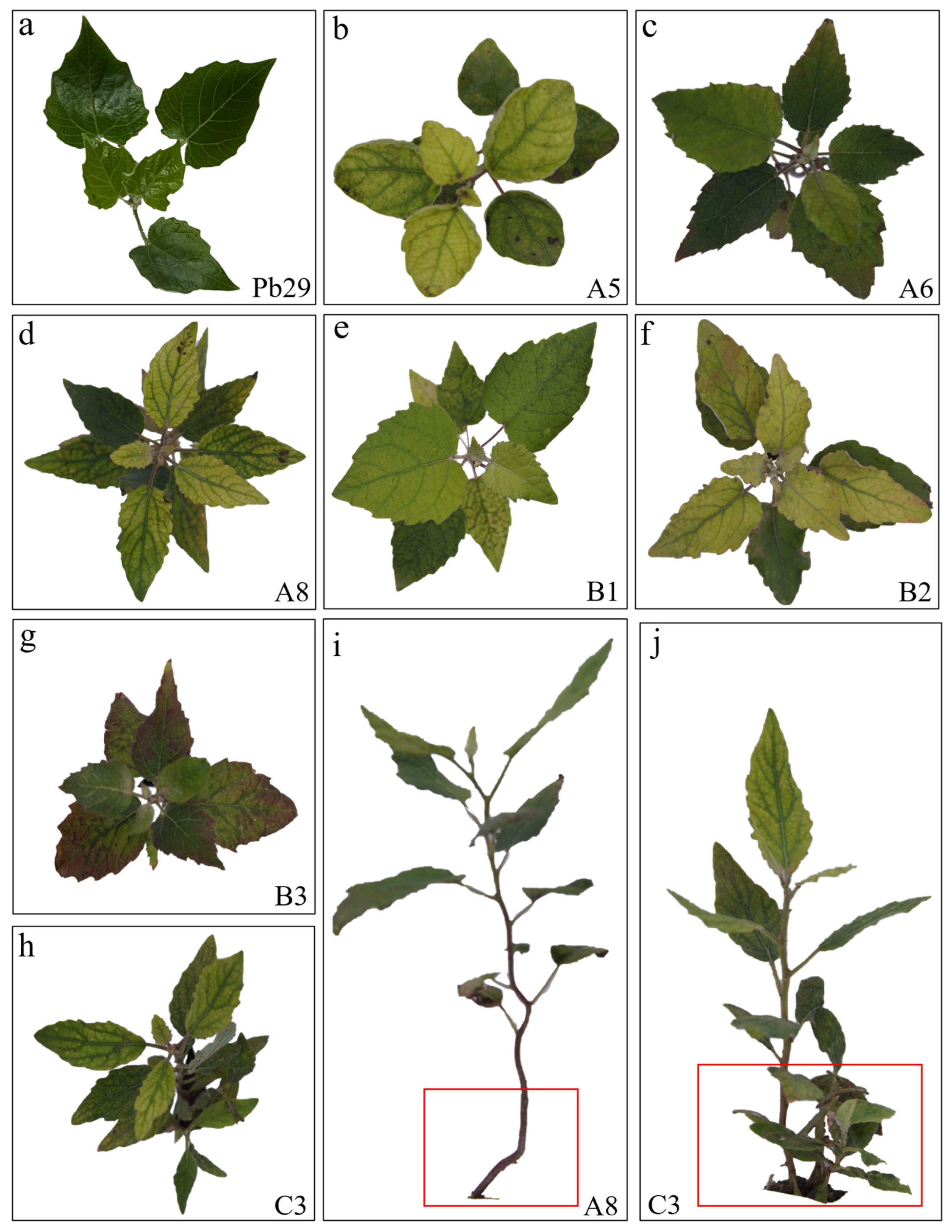
2.3. Growth Analysis of Progeny from Different Hybrid Combinations
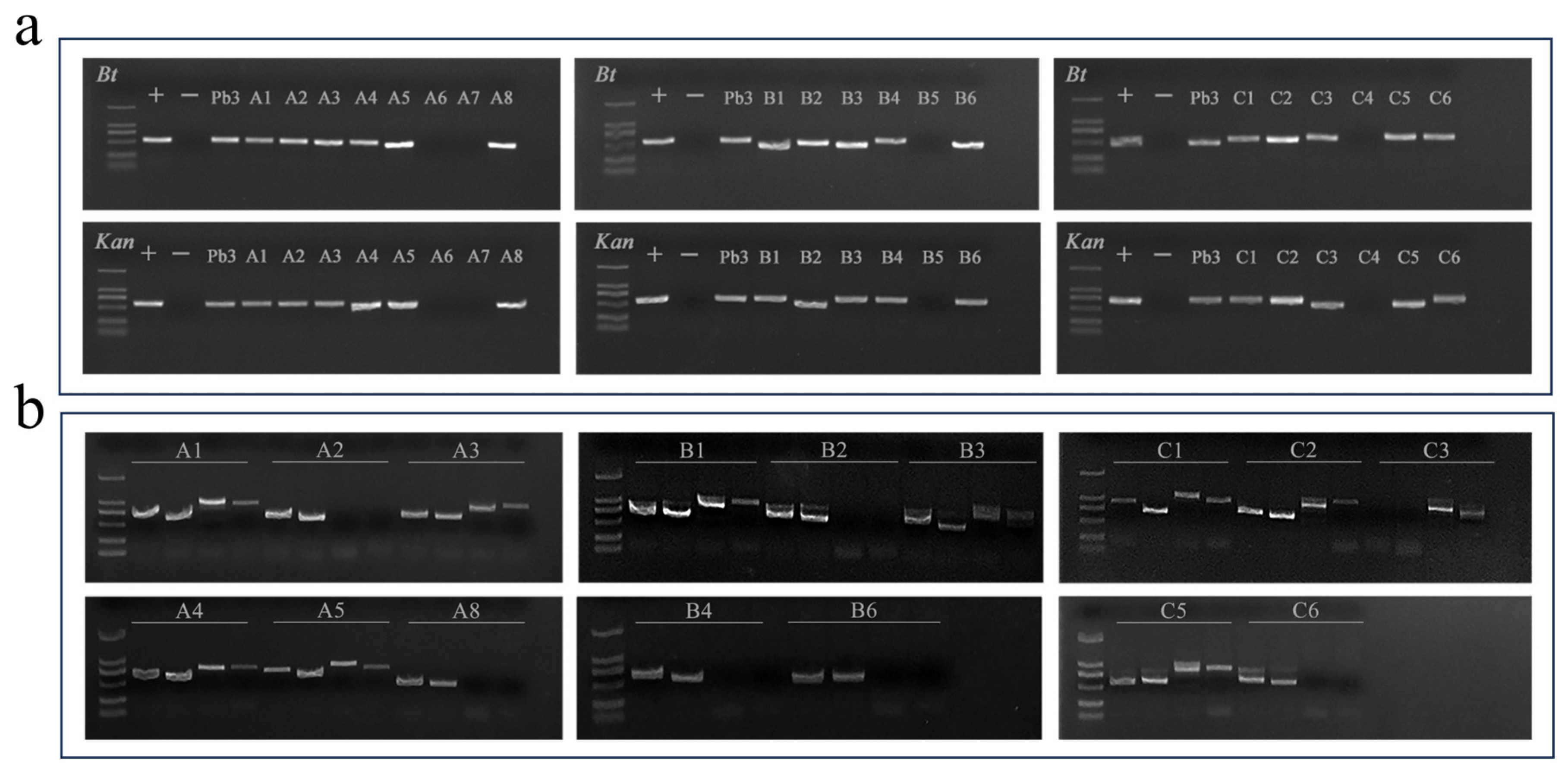
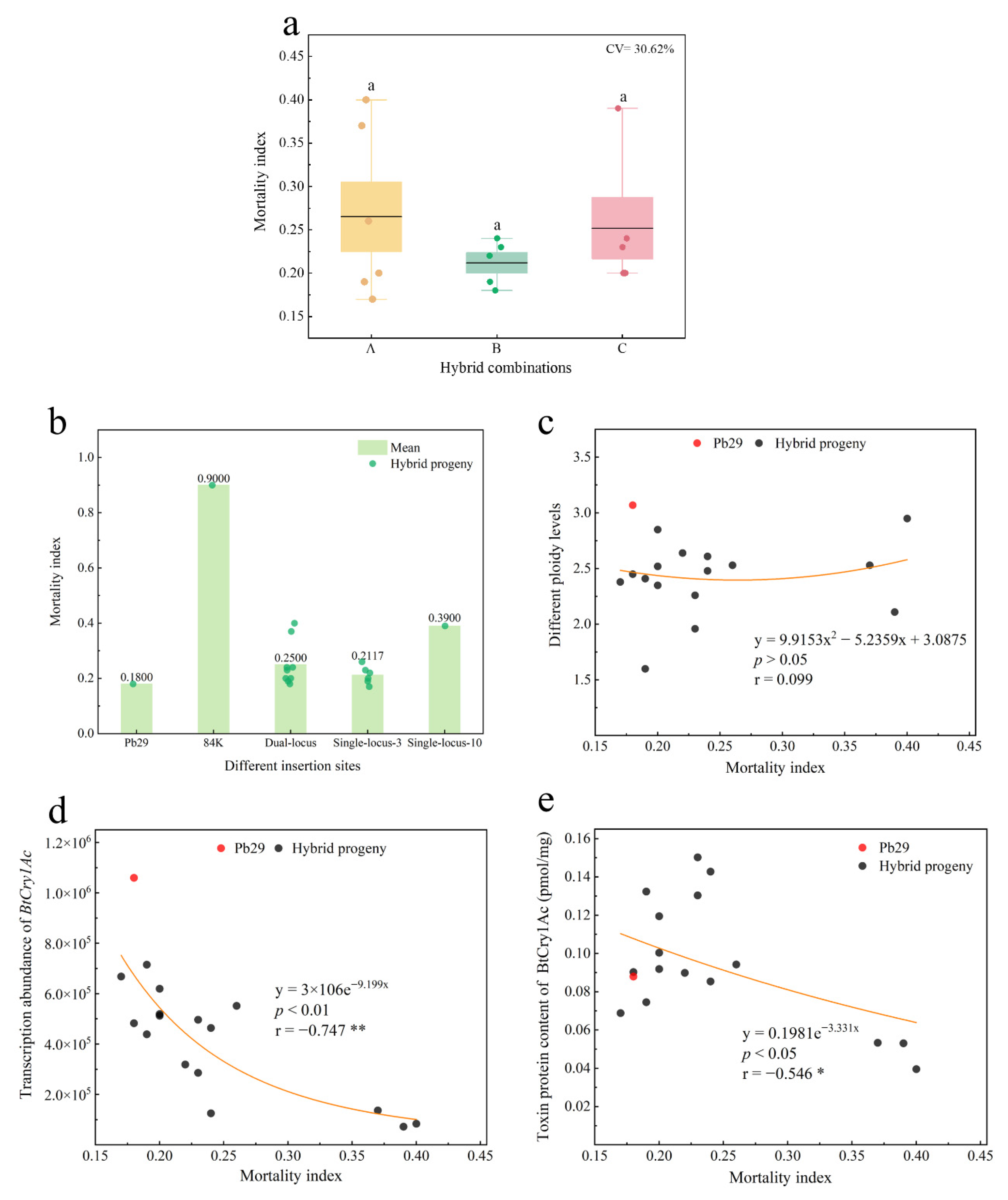
2.4. Detection of Foreign Genes and Insertion Sites in Hybrid Progeny
2.5. Real-Time Fluorescence Quantitative PCR (qPCR) Detection of Progeny Carrying the BtCry1Ac Gene
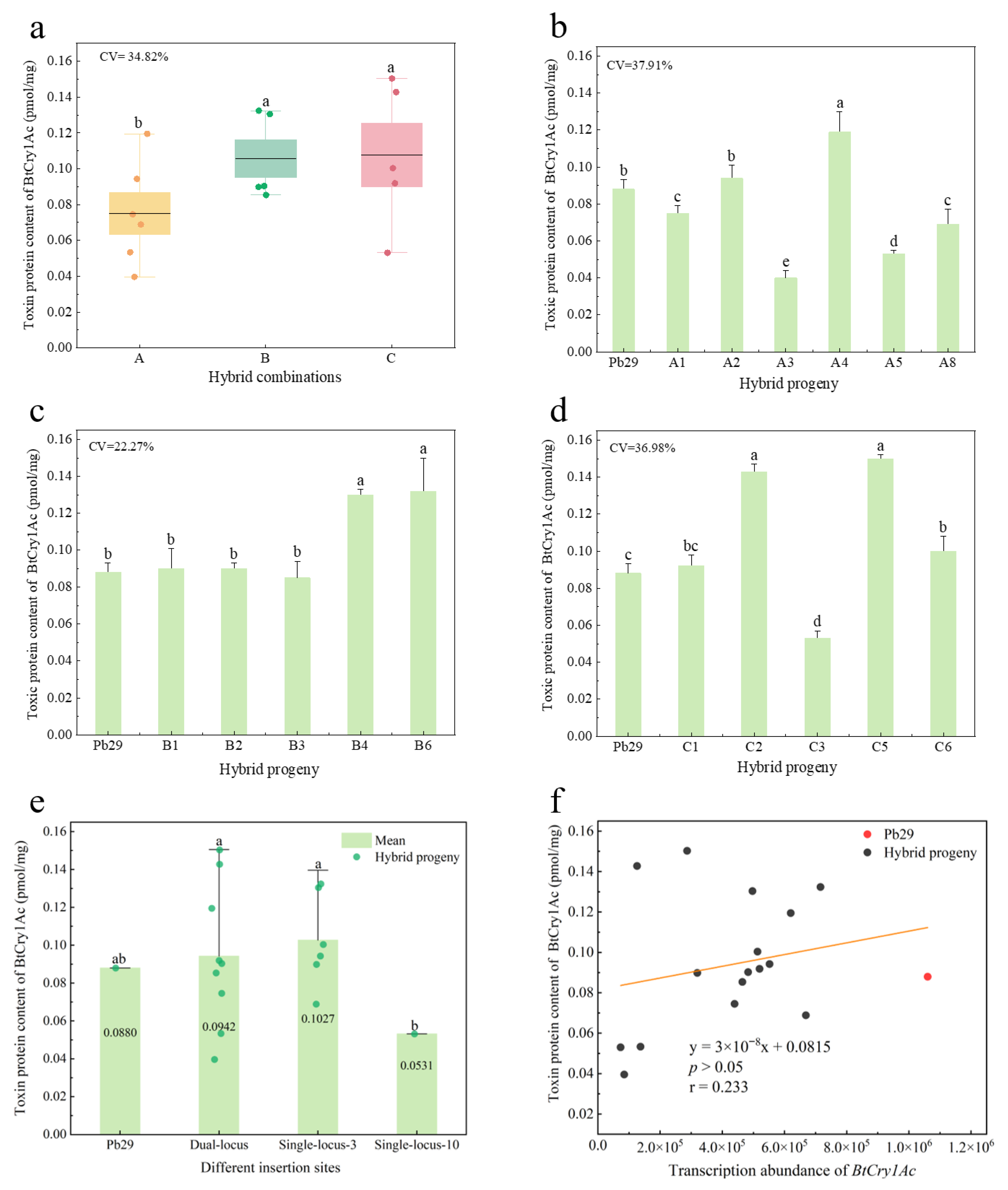
2.6. Detection of Toxin Protein Content in Progeny Carrying the Bt Gene
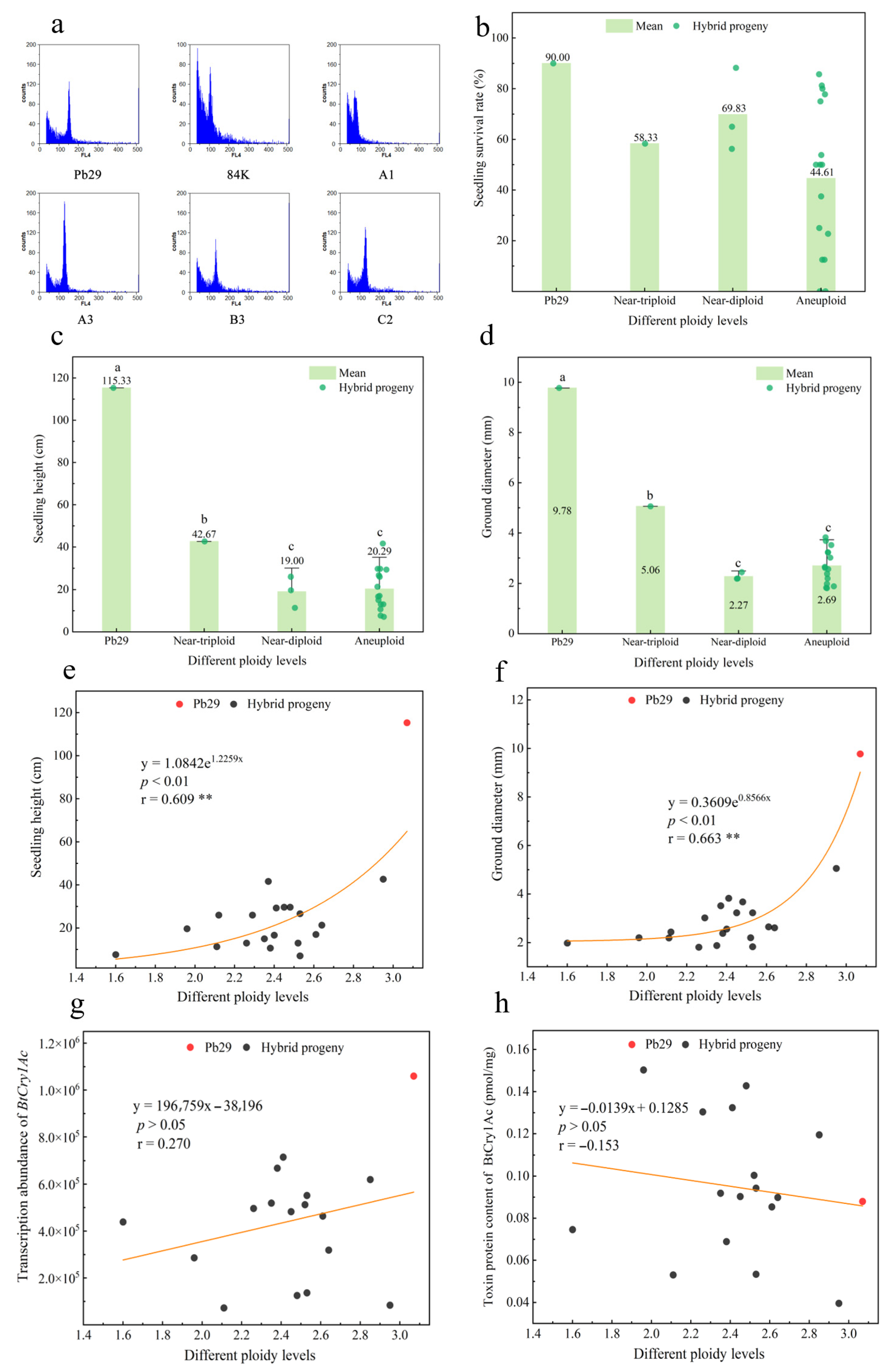
2.7. Ploidy Detection in Hybrid Progeny
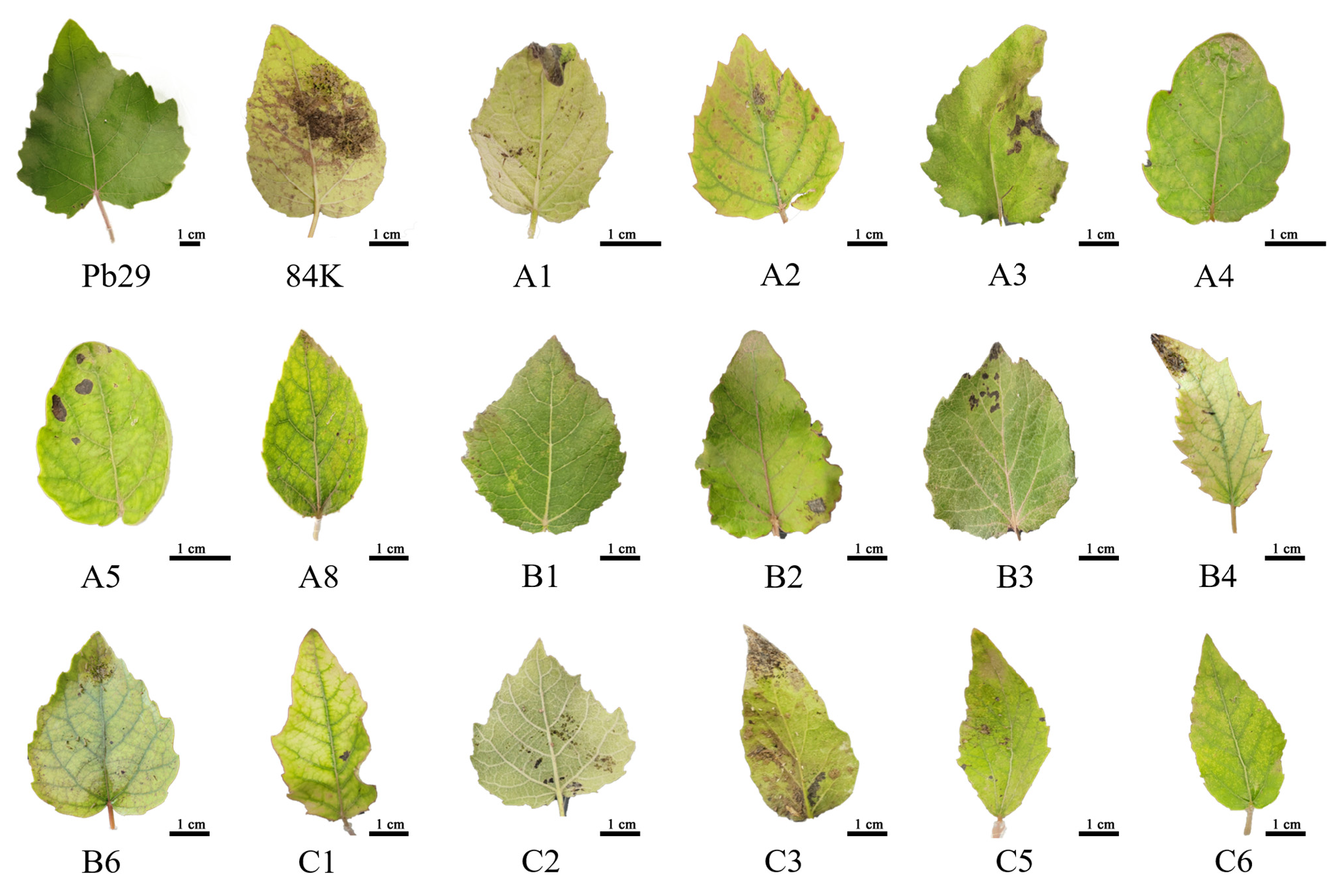
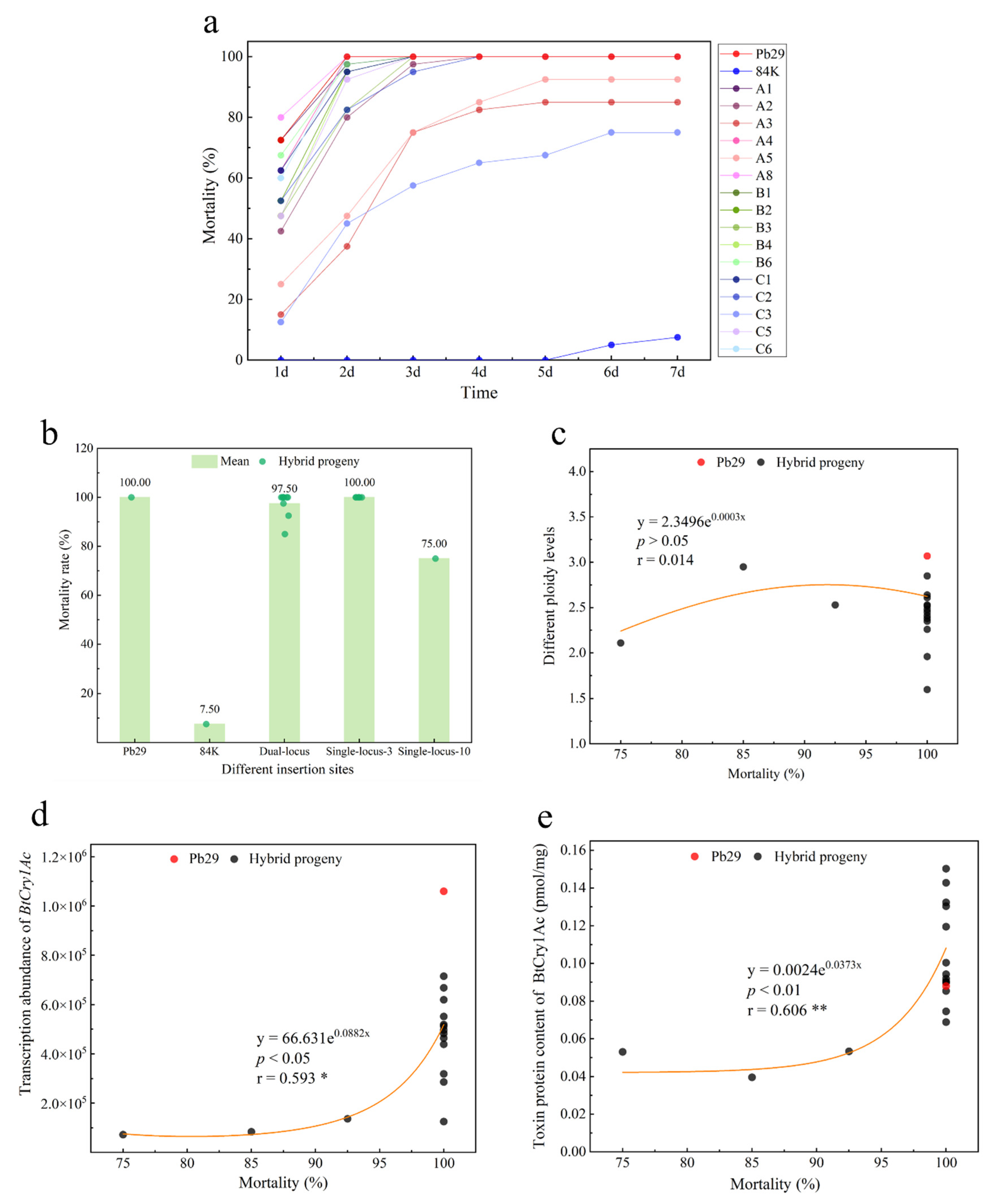
2.8. Insect Resistance Detection in Progeny Carrying the BtCry1Ac Gene
3. Discussion
4. Materials and Methods
4.1. Plant and Insect Materials
4.2. Paternal Pollen Viability and Hybridization Methods
4.3. Embryo Rescue and Hybrid Progeny Propagation
4.4. Measurement of Seedling Survival Rate and Growth Traits in Hybrid Progeny
4.5. PCR Detection of the BtCry1Ac Gene
4.6. Detection of Foreign Gene Insertion Sites in Hybrid Progeny
4.7. qPCR Detection of the BtCry1Ac Gene
4.8. ELISA Detection of Bt Toxin Protein in Hybrid Progeny
4.9. Ploidy Verification of Hybrid Progeny
4.10. Insect-Feeding Assay of Hybrid Progeny
4.11. Data Processing and Software
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noack, F.; Engist, D.; Gantois, J.; Gaur, V.; Hyjazie, B.F.; Larsen, A.; M’Gonigle, L.K.; Missirian, A.; Qaim, M.; Sargent, R.D.; et al. Environmental impacts of genetically modified crops. Science 2024, 385, eado9340. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Brenizer, B.D.; Kropf, A.L.; McCulloch, J.B.; Radosevich, D.L.; Shrestha, R.B.; Smith, E.M.; St. Clair, C.R. Sequential evolution of resistance by western corn rootworm to multiple Bacillus thuringiensis traits in transgenic maize. Proc. Natl. Acad. Sci. USA 2025, 122, e2422337122. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-C.; Zheng, J.-B.; Yu, H.-M.; Liang, H.Y.; Wang, J.M. Studies of transgenic hybrid poplar 741 carrying two insect-resistant genes. J. Integr. Plant Biol. 2000, 42, 263. [Google Scholar]
- Wang, Y.; Zhou, X.; Zhang, F.; Zhang, L.; Yang, P.; Maimaitiniyazi, R. Effects of Pb (II) and Zn (II) Contamination on Adsorption, Desorption and Degradation of Cry1Ac Toxin Identical to Bt Transgenic Poplar in Black Soil. Toxics 2023, 11, 89. [Google Scholar] [CrossRef]
- Huo, X.M. Construction of Plant Expression Vector for Insect-Resistant Genes, Genetic Transformation and Expression Studies. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2009. [Google Scholar]
- Wang, G.Y.; Yang, M.S.; Huo, X.M.; Wang, Y.; Li, S. Genetic Transformation of Dual Bt Genes into Poplar 741 and Insect Resistance of Transgenic Lines. Sci. Silvae Sin. 2012, 48, 42–49. [Google Scholar]
- Ren, Y.; Zhou, X.; Dong, Y.; Zhang, J.; Wang, J.; Yang, M. Exogenous Gene Expression and Insect Resistance in Dual Bt Toxin Populus × euramericana ‘Neva’ Transgenic Plants. Front. Plant Sci. 2021, 12, 660226. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Dong, Y.; Li, Y.; Huang, Y.; Ren, M.; Yang, M.; Wang, J. Dynamic monitoring of the impact of insect-resistant transgenic poplar field stands on arthropod communities. For. Ecol. Manag. 2022, 505, 119921. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Wang, S.; Yang, K.; Long, L.; Jiang, M.; Wang, J.; Yang, M. Analyses of multicopy insertions and unintended responses to drought stress in dual Bt gene transgenic poplar. Ind. Crops Prod. 2025, 223, 120247. [Google Scholar] [CrossRef]
- Shao, Z.; Huang, L.; Zhang, Y.; Qiang, S.; Song, X. Transgene was silenced in hybrids between transgenic herbicide-resistant crops and their wild relatives utilizing alien chromosomes. Plants 2022, 11, 3187. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, Z.; Huang, H.; Tang, Z.; Wei, W.; Stewart, C.N.; Liu, Y. Inheritance and ecological effects of exogenous genes from transgenic Brassica napus to Brassica juncea hybrids. Plant Sci. 2024, 349, 112245. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Yang, X.; Lu, B.R. Increased Longevity and Dormancy of Soil-Buried Seeds from Advanced Crop–Wild Rice Hybrids Overexpressing the EPSPS Transgene. Biology 2021, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, L.; Fang, Z.; Shen, W.; Dai, Y.; Jia, R.; Liang, J.; Liu, B. Fitness changes in wild soybean caused by gene flow from genetically modified soybean. BMC Plant Biol. 2023, 23, 424. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, Y.; Lei, C.; Xia, Q.; Wu, D.; He, Q.; Jing, D.; Guo, Q.; Liang, G.; Dang, J. A female fertile triploid loquat line produces fruits with less seed and aneuploid germplasm. Sci. Hortic. 2023, 319, 112141. [Google Scholar] [CrossRef]
- Yasuda, K.; Yahata, M.; Sato, M.; Sudo, M.; Tominaga, A.; Kunitake, H. Identification of Parental Genome Construction and Inherited Morphological Characteristics in Triploid and Aneuploid Intergeneric Hybrids from a Diploid—Diploid Cross between Citrus and Fortunella. Agronomy 2021, 11, 1988. [Google Scholar] [CrossRef]
- Wang, P.; Yang, S.; Chen, M.; Liu, Y.; He, Q.; Sun, H.; Wu, D.; Xiang, S.; Jing, D.; Wang, S.; et al. Karyotype variation patterns and phenotypic responses of hybrid progenies of triploid loquat (Eriobotrya japonica) provide new insight into aneuploid germplasm innovation. Hortic. Res. 2025, 5, 5. [Google Scholar] [CrossRef]
- Singh, S.K.; Sahi, A.N.; Singhal, S.; Ahlawat, A.K.; Mandal, P.K.; Makharia, G.; Mahendru-Singh, A. Molecular Marker Assisted Development of Chromosome Aneuploid Lines of Durum Wheat Lacking Gli-A1 or Gli-A2 Loci Harbouring Immunogenic Epitopes for Celiac Disease. J. Cereal Sci. 2025, 122, 104124. [Google Scholar] [CrossRef]
- Jin, S.B.; Kim, M.J.; Choi, C.W.; Park, S.M.; Yun, S.H. Anther culture-derived haploids of Citrus aurantium L. (sour orange) and genetic verification of haploid-derived regenerated plants. Plants 2022, 11, 3022. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Chen, J.J.; Cao, Y.M.; Duan, J.-X.; Cai, X.-D. Induction of tetraploids in ‘Red Flash’ caladium using colchicine and oryzalin: Morphological, cytological, photosynthetic and chilling tolerance analysis. Sci. Hortic. 2020, 272, 109524. [Google Scholar] [CrossRef]
- Sevilleno, S.S.; Ha, M.K.T.T.; Park, H.W.; Cabahug-Braza, R.A.; Hwang, Y.-J. Effects of storage temperature and duration on the pollen viability of Phalaenopsis ‘KS Little Gem’. Hortic. Environ. Biotechnol. 2025, 66, 245–252. [Google Scholar] [CrossRef]
- Chen, X.; Dong, Y.; Huang, Y.; Fan, J.; Yang, M.; Zhang, J. Whole-genome resequencing using next-generation and Nanopore sequencing for molecular characterization of T-DNA integration in transgenic poplar 741. BMC Genom. 2021, 22, 329. [Google Scholar] [CrossRef]
- Touchell, D.H.; Lynch, N.; Shekasteband, R.; Dickey, A.N.; Chinn, M.C.; Whitfield, M.; Ranney, T.G. Biomass yields, reproductive fertility, compositional analysis, and genetic diversity of newly developed triploid giant miscanthus hybrids. GCB Bioenergy 2024, 16, e13174. [Google Scholar] [CrossRef]
- Yang, M.; Gao, B.; Wang, J.; Liang, H.; Du, K. Analysis of Basic Characteristics of Transgenic Double Insect-resistant Poplar 741. For. Sci. 2005, 41, 7. [Google Scholar]
- Nakano, A.; Mii, M.; Hoshino, Y. Endosperm culture-based allotriploid hybrid production from an interspecific cross of Haemanthus spp.: New insights into polyploidization and hybridization. BMC Plant Biol. 2025, 25, 158. [Google Scholar] [CrossRef] [PubMed]
- Doyac, Y.; Boztepe, Z.; Kandilli, G.G.; Atak, A. Embryo recovery(rescue) studies in different Vitis species. BMC Plant Biol. 2024, 24, 822. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, Q.; Luo, Y.; Zhu, P.; Guan, P.; Zhang, J. Study on influencing factors of embryo rescue and germplasm innovation in seedless grape. Plant Cell Tissue Organ Cult. 2024, 157, 24. [Google Scholar] [CrossRef]
- Kojima, S.; Cimini, D. Aneuploidy and gene expression: Is there dosage compensation? Epigenomics 2019, 11, 1827–1837. [Google Scholar] [CrossRef]
- Wang, J.; Huo, B.; Liu, W.; Li, D.; Liao, L. Abnormal meiosis in an intersectional allotriploid of Poulus L. and segregation of ploidy levels in 2x × 3x progeny. PLoS ONE 2017, 12, e181767. [Google Scholar]
- Luo, Y.; Chen, W.; Pan, Y.; Ge, L.; Wu, C.; Wang, J.; Liu, M.; Yan, F. Comparison and genetic variation analysis of important fruit traits in jujube F1 hybrids by different male parents. Agronomy 2024, 14, 459. [Google Scholar] [CrossRef]
- Xia, H.X.; Li, Q.; Cushman, S.A.; Yuan, W.-J.; Li, Y. Expression dosage effects of a small number of genes after the artificial doubling of weeping forsythia. Plant Physiol. Biochem. 2023, 202, 10. [Google Scholar] [CrossRef]
- Xiong, S.; Wu, L.; Chen, Y.; Shi, X.; Wang, Y. Multi-omics analysis reveals the regulatory mechanism of branching development in Quercus fabri. J. Proteom. 2025, 14, 29. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, K.; Wang, B.; Wu, L.; Xiong, Z. Transgressive expression and dosage effect of A09 chromosome genes and their homoeologous genes influence the flowering time of resynthesized allopolyploid Brassica napus. BMC Plant Biol. 2025, 25, 226. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Yang, T.; Chao, H.; Ma, X.; Wu, J.; Yang, Q.; Ren, G.; Song, L.; Wang, Q.; Qi, L.; et al. Genomic insights into drought adaptation of the forage shrub Caragana korshinskii (Fabaceae) widely planted in drylands. Plant J. 2025, 121, e17255. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Carabelli, M.; Sassi, M. The Ins and Outs of Homeodomain-Leucine Zipper/Hormone Networks in the Regulation of Plant Development. Int. J. Mol. Sci. 2024, 25, 5657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhao, C.; Gao, H.; Gu, Z.; Wu, Y.; Wen, S.; Chen, N.; An, Y.; Huang, L.; Lu, Y.; et al. SUPERMAN Targets PHRAGMOPLAST ORIENTING KINESIN to Negatively Regulate Leaf Cell Division in Poplar. Plant Cell Environ. 2024, 48, 634–648. [Google Scholar] [CrossRef]
- Du, J.; Shao, C.; Wang, D.; Feng, Z.; Cui, C.; Li, R.; Jewaria, P.K.; Wang, X.; Xiao, J.; Wang, X. Deletion of the PtrDJ1C Gene Leads to Increased Branching in Poplar. Plant Physiol. Biochem. 2025, 223, 109789. [Google Scholar] [CrossRef]
- Castle, W.E. Mendel’s Law of Heredity. Science 1903, 18, 396–406. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, S.; Wang, H.; Feng, Z.; Cui, C.; Li, R.; Jewaria, P.K.; Wang, X.; Xiao, J.; Wang, X. Innovations in Transgene Integration Analysis: A Comprehensive Review of Enrichment and Sequencing Strategies in Biotechnology. ACS Appl. Mater. Interfaces 2025, 17, 2716–2735. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Ku, H.; Huangfu, B.; Wang, K.; Du, C.; Zhang, M. Contiguous identity between entire coding regions of transgenic and native genes rather than special regions is essential for a strong co-suppression. Plant Sci. 2024, 341, 11. [Google Scholar] [CrossRef]
- Clark, A.; Bissinger, P.; Bullock, D.; Damak, S.; Wallace, R.; Whitelaw, C.B.; Yull, F. Chromosomal position effects and the modulation of transgene expression. Reprod. Fertil. Dev. 1994, 6, 589–598. [Google Scholar] [CrossRef]
- Castro, B.M.D.C.E.; Martinez, L.C.; Barbosa, S.G.; Serrão, J.E.; Wilcken, C.F.; Soares, M.A.; Silva, A.A.; Carvalho, A.G.; Zanuncio, J.C. Toxicity and cytopathology mediated by Bacillus thuringiensis in the midgut of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 6667. [Google Scholar] [CrossRef]
- Yang, M.; Lang, H.; Gao, B.; Wang, J.; Zheng, J. Insecticidal Activity and Transgene Expression Stability of Transgenic Hybrid Poplar Clone 741 Carrying two Insect-Resistant Genes. Silvae Genet. 2003, 52, 5–6. [Google Scholar]
- Huang, Y.; Zhen, Z.; Cui, Z.; Liu, J.; Wang, S.; Yang, M.; Wu, J. Growth and arthropod community characteristics of transgenic poplar 741 in an experimental forest. Ind. Crops Prod. 2021, 162, 113284. [Google Scholar] [CrossRef]
- Pellicer, J.; Leitch, I.J. The application of flow cytometry for estimating genome size and ploidy level in plants. Methods Mol. Biol. 2014, 1115, 279–307. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Cai, H.; Zhao, R.; Wang, C.; Zhang, J.; Yang, M.; Wang, J. Analysis of Insect Resistance and Ploidy in Hybrid Progeny of Transgenic BtCry1Ac Triploid Poplar 741. Plants 2025, 14, 2563. https://doi.org/10.3390/plants14162563
Zhou Y, Cai H, Zhao R, Wang C, Zhang J, Yang M, Wang J. Analysis of Insect Resistance and Ploidy in Hybrid Progeny of Transgenic BtCry1Ac Triploid Poplar 741. Plants. 2025; 14(16):2563. https://doi.org/10.3390/plants14162563
Chicago/Turabian StyleZhou, Yan, Hongyu Cai, Renjie Zhao, Chunyu Wang, Jun Zhang, Minsheng Yang, and Jinmao Wang. 2025. "Analysis of Insect Resistance and Ploidy in Hybrid Progeny of Transgenic BtCry1Ac Triploid Poplar 741" Plants 14, no. 16: 2563. https://doi.org/10.3390/plants14162563
APA StyleZhou, Y., Cai, H., Zhao, R., Wang, C., Zhang, J., Yang, M., & Wang, J. (2025). Analysis of Insect Resistance and Ploidy in Hybrid Progeny of Transgenic BtCry1Ac Triploid Poplar 741. Plants, 14(16), 2563. https://doi.org/10.3390/plants14162563




