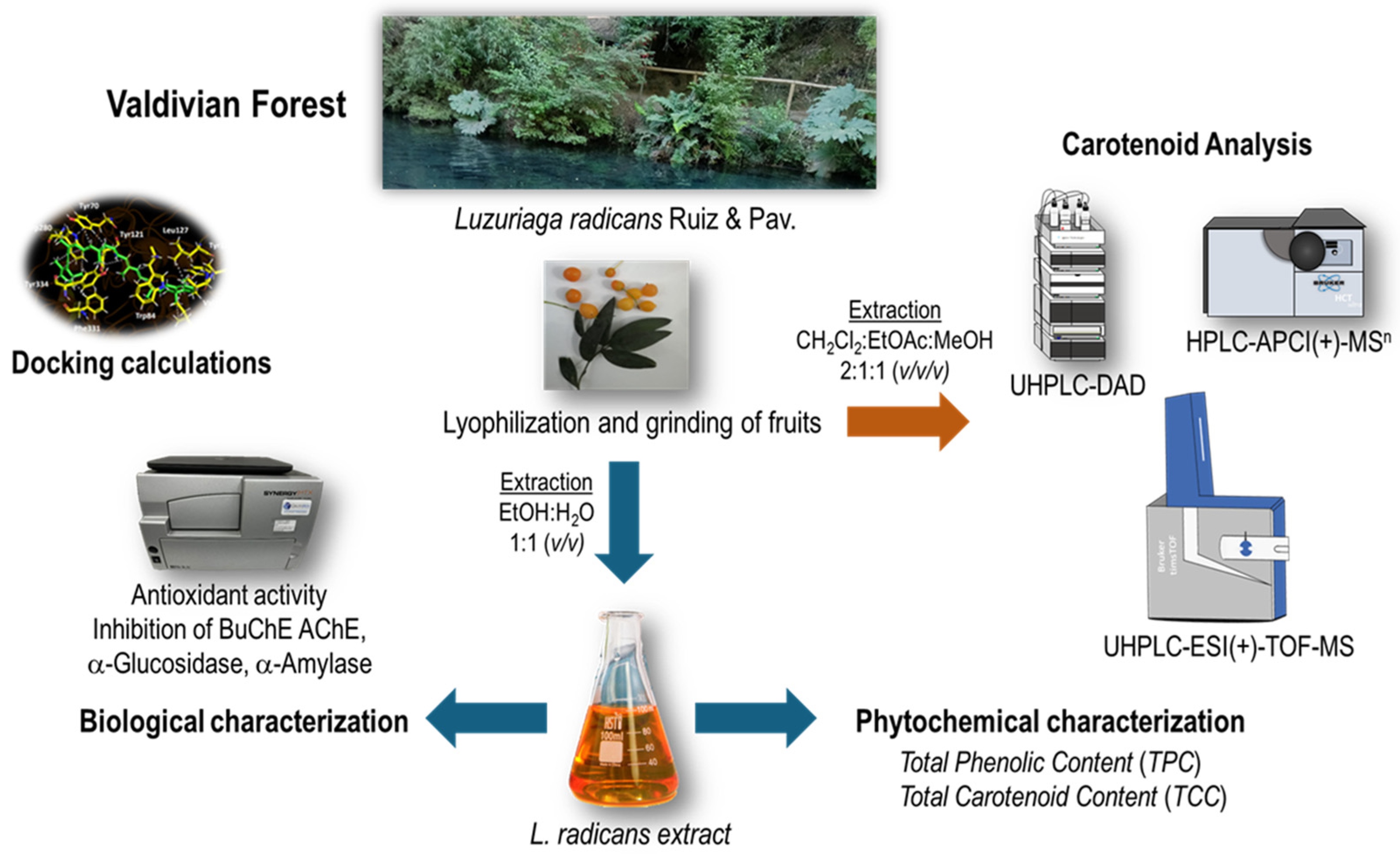
Berries from Luzuriaga radicans Ruiz & Pav.: A Southern Chile Climbing Shrub as a Source of Antioxidants Against Chronic Diseases
Abstract
1. Introduction
2. Results and Discussion
2.1. Metals and Proximate Composition
2.2. Antioxidant Activity and Content of Phenolics and Carotenes
2.3. Enzyme Inhibitory Properties
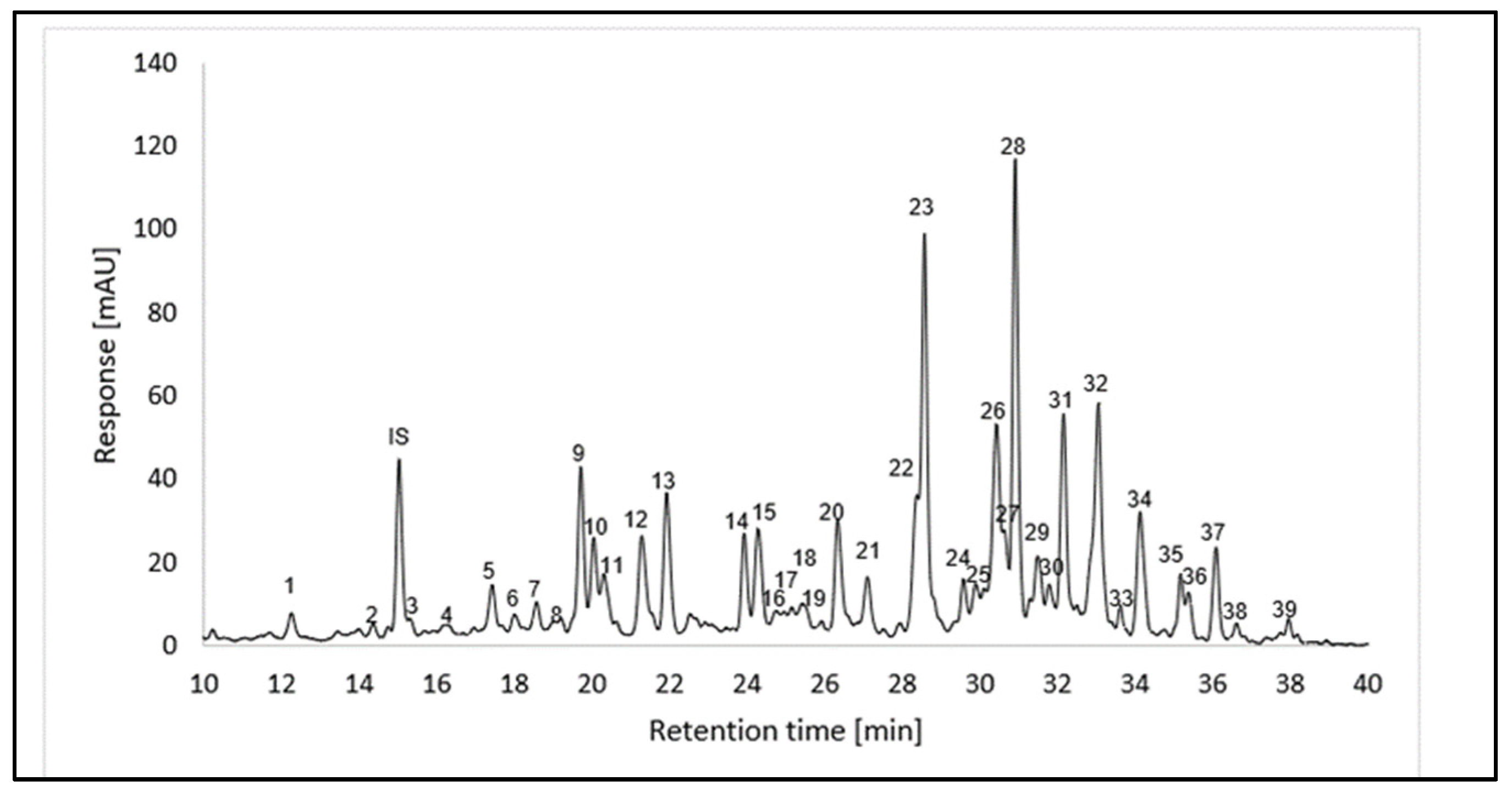
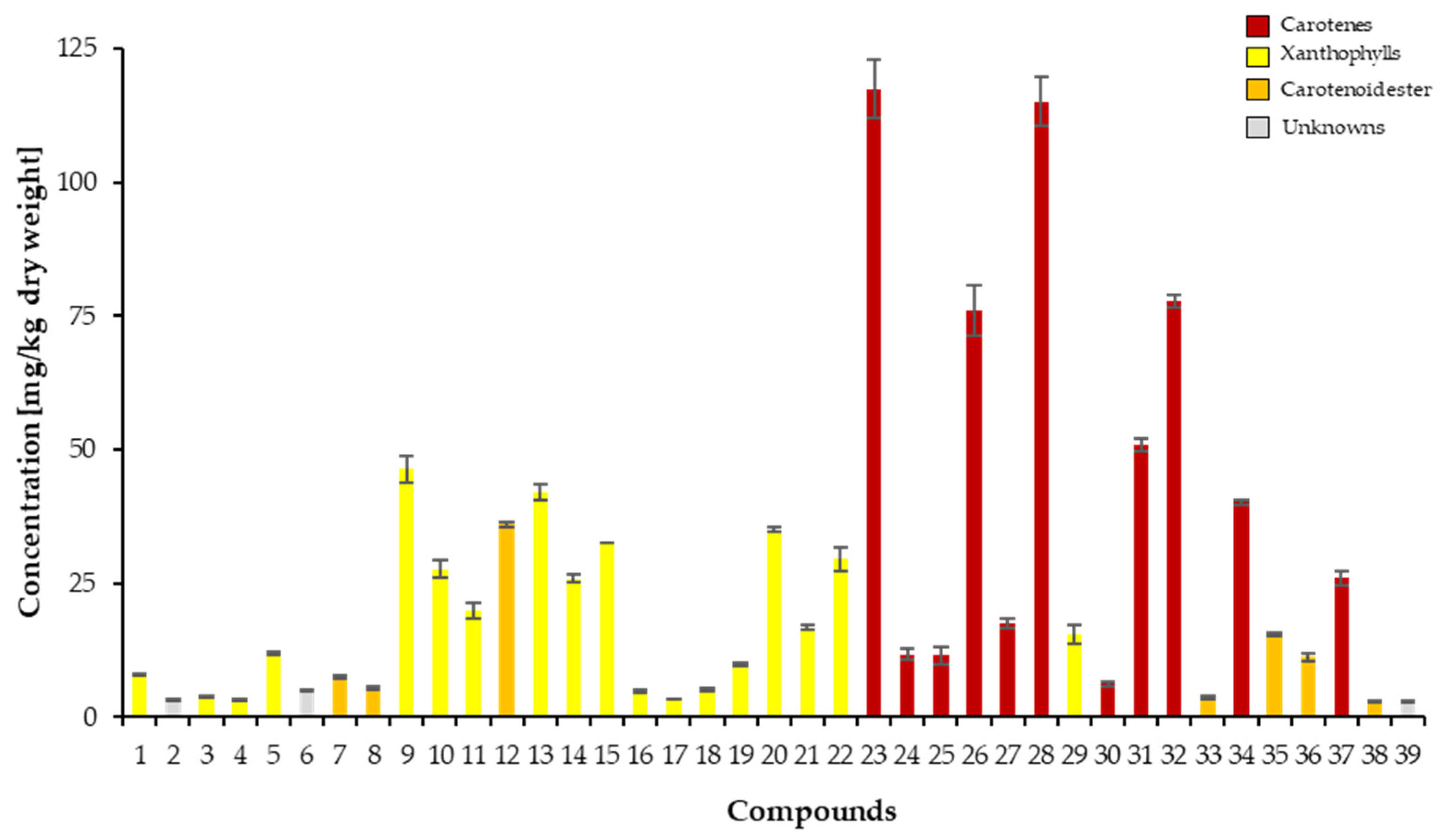
2.4. Analysis of the Carotenoid Profile
2.4.1. Chromatographic Analysis of Carotenoids
2.4.2. Qualitative Analysis of Carotenoids
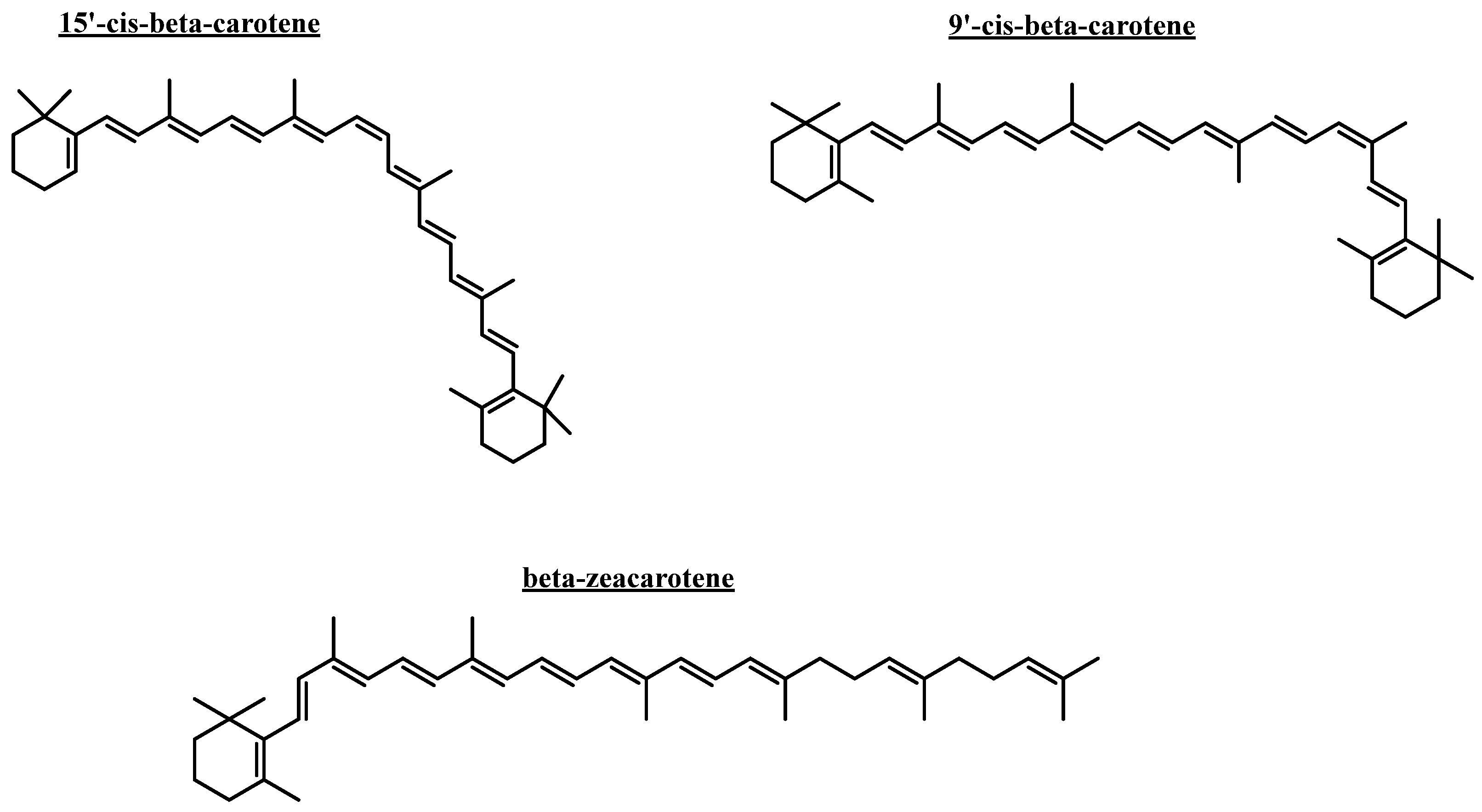
2.5. Docking Simulations
2.5.1. Acetylcholinesterase (TcAChE) Docking Results
2.5.2. Butyrylcholinesterase (hBuChE) Docking Results
3. Materials and Methods
3.1. Chemicals, Reagents, and Materials
3.2. Plant Material
3.3. Berry Extract Preparation
3.4. Chemical Contents
3.4.1. Determination of Proximate Composition
3.4.2. Total Polyphenol and Carotene Quantification
3.4.3. Ultra High-Performance Liquid Chromatography (UHPLC) Diode Array Detector (DAD) Analysis for the Quantification of Carotenoids
3.4.4. HPLC-APCI(+)-MSn Analysis for the Characterization of Carotenoids
3.4.5. UHPLC-TOF-MS Analysis for the Characterization of Carotenoids
3.5. Antioxidant Activity
3.5.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5.3. DPPH Scavenging Activity
3.5.4. ABTS Scavenging Activity
3.6. Enzymatic Inhibitory Activity
3.6.1. Acetylcholinesterase and Butyrylcholinesterase Inhibition Assays
3.6.2. α-Glucosidase Inhibition Assay
3.6.3. α-Amylase Inhibition Assay
3.7. Docking Calculations
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fuentes-Castillo, T.; Hernández, H.J.; Pliscoff, P. Hotspots and ecoregion vulnerability driven by climate change velocity in Southern South America. Reg. Environ. Chang. 2020, 20, 27. [Google Scholar] [CrossRef]
- Tecklin, D.; DellaSala, D.A.; Luebert, F.; Pliscoff, P. Valdivian Temperate Rainforests of Chile and Argentina. In Temperate and Boreal Rainforests of the World: Ecology and Conservation; Island Press/Center for Resource Economics: Washington DC, USA, 2011; pp. 132–153. [Google Scholar]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef]
- Ramos, L.C.; Palacios, J.; Barrientos, R.E.; Gómez, J.; Castagnini, J.M.; Barba, F.J.; Tapia, A.; Paredes, A.; Cifuentes, F.; Simirgiotis, M.J. UHPLC-MS Phenolic Fingerprinting, Aorta Endothelium Relaxation Effect, Antioxidant, and Enzyme Inhibition Activities of Azara dentata Ruiz & Pav Berries. Foods 2023, 12, 643. [Google Scholar] [CrossRef]
- Hopfstock, P.; Romero-Parra, J.; Winterhalter, P.; Gök, R.; Simirgiotis, M. In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile. Plants 2024, 13, 2756. [Google Scholar] [CrossRef] [PubMed]
- Jara-Seguel, P.; Zúniga, C.; Romero-Mieres, M.; Palma-Rojas, C.; von Brand, E.L. Karyotype study in Luzuriaga radicans (Liliales: Luzuriagaceae). Biologia 2010, 65, 813–816. [Google Scholar] [CrossRef]
- Shi, S.; Li, K.; Peng, J.; Li, J.; Luo, L.; Liu, M.; Chen, Y.; Xiang, Z.; Xiong, P.; Liu, L.; et al. Chemical characterization of extracts of leaves of Kadsua coccinea (Lem.) A.C. Sm. by UHPLC-Q-Exactive Orbitrap Mass spectrometry and assessment of their antioxidant and anti-inflammatory activities. Biomed. Pharmacother. 2022, 149, 112828. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Piovesana, S.; Aita, S.E.; Cavaliere, C.; Felletti, S.; Laganà, A.; Montone, C.M.; Vargas-de-la-Cruz, C.; Capriotti, A.L. Detailed investigation of the composition and transformations of phenolic compounds in fresh and fermented Vaccinium floribundum berry extracts by high-resolution mass spectrometry and bioinformatics. Phytochem. Anal. 2022, 33, 507–516. [Google Scholar] [CrossRef]
- Mohamed Yunus, S.N.; Abas, F.; Jaafar, A.H.; Azizan, A.; Zolkeflee, N.K.Z.; Abd Ghafar, S.Z. Antioxidant and α-glucosidase inhibitory activities of eight neglected fruit extracts and UHPLC-MS/MS profile of the active extracts. Food Sci. Biotechnol. 2021, 30, 195–208. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, H.; Zhang, Y.; Liu, Y.; Zheng, X.; Xu, J.; Ye, J.; Deng, X. Carotenoid extraction, detection, and analysis in citrus. Methods Enzymol. 2022, 670, 179–212. [Google Scholar]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Chen, J.; Shi, Y.; Cai, J.; Yang, J.; Wu, Y. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int. J. Cancer 2014, 135, 1444–1453. [Google Scholar] [CrossRef]
- Gan, R.Y.; Kuang, L.; Xu, X.R.; Zhang, Y.A.; Xia, E.Q.; Song, F.L.; Li, H.B. Screening of natural antioxidants from traditional Chinese medicinal plants associated with treatment of rheumatic disease. Molecules 2010, 15, 5988–5997. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Bazmandegan, G.; Sureda, A.; Sobarzo-Sanchez, E.; Yousefi-Manesh, H.; Shirooie, S. The Protective Roles and Molecular Mechanisms of Troxerutin (Vitamin P4) for the Treatment of Chronic Diseases: A Mechanistic Review. Curr. Neuropharmacol. 2021, 19, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Tkacz, K.; Nowicka, P.; Wojdyło, A. Investigating in vitro anticholinergic potential (anti-AChE and anti-BuChE) of Chaenomeles leaves extracts and its phytochemicals including chlorophylls, carotenoids and minerals. Sci. Rep. 2024, 14, 23132. [Google Scholar] [CrossRef] [PubMed]
- Charles, D.; Mgina, C. Proximate composition of Vitex doniana and Saba comorensis fruits. Sci. Rep. 2023, 13, 20553. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, P.N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci. Nutr. 2017, 5, 1029–1036. [Google Scholar] [CrossRef]
- Abdi Bellau, M.L.; Chiurato, M.A.; Maietti, A.; Fantin, G.; Tedeschi, P.; Marchetti, N.; Tacchini, M.; Sacchetti, G.; Guerrini, A. Nutrients and Main Secondary Metabolites Characterizing Extracts and Essential Oil from Fruits of Ammodaucus leucotrichus Coss. & Dur. (Western Sahara). Molecules 2022, 27, 5013. [Google Scholar] [CrossRef]
- Kalili, A.; El Ouafi, R.; Aboukhalaf, A.; Naciri, K.; Tbatou, M.; Essaih, S.; Belahyan, A.; Belahsen, R. Chemical composition and antioxidant activity of extracts from Moroccan fresh fava beans pods (Vicia faba L.). Rocz. Państwowego Zakładu Hig. 2022, 73, 79–86. [Google Scholar] [CrossRef]
- Ilić, T.; Đuričić, I.; Kodranov, I.; Ušjak, L.; Kolašinac, S.; Milenković, M.; Marčetić, M.; Božić, D.D.; B Vidović, B. Nutritional Value, Phytochemical Composition and Biological Activities of Lycium barbarum L. fruits from Serbia. Plant Foods Hum. Nutr. 2024, 79, 662–668. [Google Scholar] [CrossRef]
- Katunzi-Kilewela, A.; Rweyemamu, L.M.; Kaale, L.D.; Kibazohi, O.; Fortunatus, R.M. Proximate composition, pasting and functional properties of composite flour blends from cassava and chia seeds flour. Food Sci. Technol. Int. 2023, 29, 217–227. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Merino-Zegarra, C.; Riquelme-Penaherrera, M.; Nonato-Ramirez, L.; Delgado-Wong, H.; Pertino, M.W.; Parra, C.; Simirgiotis, M.J. Antihyperlipidemic and antioxidant capacities, nutritional analysis and uhplc-pda-ms characterization of cocona fruits (Solanum sessiliflorum dunal) from the peruvian amazon. Antioxidants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Jara-Pinuer, N.; Ley-Martínez, J.S.; Velarde, S.H.; Pereira, I.; Sánchez, M.; Gómez-Serranillos, M.P.; Sasso, F.C.; Simirgiotis, M.; et al. Antioxidant and Antidiabetic Potential of the Antarctic Lichen Gondwania regalis Ethanolic Extract: Metabolomic Profile and In Vitro and In Silico Evaluation. Antioxidants 2025, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Conta, A.; Simirgiotis, M.J.; Martínez Chamás, J.; Isla, M.I.; Zampini, I.C. Extraction of Bioactive Compounds from Larrea cuneifolia Cav. Using Natural Deep Eutectic Solvents: A Contribution to the Plant Green Extract Validation of Its Pharmacological Potential. Plants 2025, 14, 1016. [Google Scholar] [CrossRef] [PubMed]
- Inta Portal Antioxidantes. Available online: https://portalantioxidantes.com/orac-base-de-datos-actividad-antioxidante-y-contenido-de-polifenoles-totales-en-frutas/ (accessed on 3 July 2025).
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Van De Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; Van De Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. α-Glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rossignol, D.A. Treatments for biomedical abnormalities associated with autism spectrum disorder. Front. Pediatr. 2014, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Rolinski, M.; Fox, C.; Maidment, I.; Mcshane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. 2012, 2012, CD006504. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Kuniga, T.; Nakajima, N.; Yoshida, T. Quantification of carotenoids in citrus fruit by LC-MS and comparison of patterns of seasonal changes for carotenoids among citrus varieties. J. Agric. Food Chem. 2007, 55, 2356–2368. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of New Carotenoid Esters in Mango and Citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef] [PubMed]
- Lux, P.E.; Carle, R.; Zacarías, L.; Rodrigo, M.J.; Schweiggert, R.M.; Steingass, C.B. Genuine Carotenoid Profiles in Sweet Orange [Citrus sinensis (L.) Osbeck cv. Navel] Peel and Pulp at Different Maturity Stages. J. Agric. Food Chem. 2019, 67, 13164–13175. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef]
- Wen, X.; Hempel, J.; Schweiggert, R.M.; Ni, Y.; Carle, R. Carotenoids and Carotenoid Esters of Red and Yellow Physalis (Physalis alkekengi L. and P. pubescens L.) Fruits and Calyces. J. Agric. Food Chem. 2017, 65, 6140–6151. [Google Scholar] [CrossRef]
- Kiokias, S.; Gordon, M.H. Antioxidant properties of carotenoids in vitro and in vivo. Food Rev. Int. 2004, 20, 99–121. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef]
- Böhm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox equivalent antioxidant capacity of different geometrical isomers of alpha-carotene, beta-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. In Nutrition and Health; Birkhäuser-Verlag: Berlin, Germany, 2009; Volume 5. [Google Scholar]
- Guerra, F.; Peñaloza, P.; Vidal, A.; Cautín, R.; Castro, M. Seed Maturity and Its In Vitro Initiation of Chilean Endemic Geophyte Alstroemeria pelegrina L. Horticulturae 2022, 8, 464. [Google Scholar] [CrossRef]
- Aros, D.; Barraza, P.; Peña-Neira, Á.; Mitsi, C.; Pertuzé, R. Seed Characterization and Evaluation of Pre-Germinative Barriers in the Genus Alstroemeria (Alstroemeriaceae). Seeds 2023, 2, 474–495. [Google Scholar] [CrossRef]
- Guerra, F.; Cautín, R.; Castro, M. In Vitro Micropropagation of the Vulnerable Chilean Endemic Alstroemeria pelegrina L. Horticulturae 2024, 10, 674. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Chen, M.; Yang, L.; Zhang, Y. Molecular and Metabolic Insights into Anthocyanin Biosynthesis for Spot Formation on Lilium leichtlinii var. maximowiczii Flower Petals. Int. J. Mol. Sci. 2023, 24, 1844. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E.; Nagy, V.; Menchaca, D.; Deli, J.; Agócs, A. Changes in the Carotenoids of Zamia dressleri Leaves during Development. Plants 2024, 13, 1251. [Google Scholar] [CrossRef]
- Aurori, M.; Niculae, M.; Hanganu, D.; Pall, E.; Cenariu, M.; Vodnar, D.C.; Fiţ, N.; Andrei, S. The Antioxidant, Antibacterial and Cell-Protective Properties of Bioactive Compounds Extracted from Rowanberry (Sorbus aucuparia L.) Fruits In Vitro. Plants 2024, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Bhuker, A.; Malik, A.; Punia, H.; McGill, C.; Sofkova-Bobcheva, S.; Mor, V.S.; Singh, N.; Ahmad, A.; Mansoor, S. Probing the Phytochemical Composition and Antioxidant Activity of Moringa oleifera under Ideal Germination Conditions. Plants 2023, 12, 3010. [Google Scholar] [CrossRef]
- Baenas, N.; Ruales, J.; Moreno, D.A.; Barrio, D.A.; Stinco, C.M.; Martínez-Cifuentes, G.; Meléndez-Martínez, A.J.; García-Ruiz, A. Characterization of Andean Blueberry in Bioactive Compounds, Evaluation of Biological Properties, and In Vitro Bioaccessibility. Foods 2020, 9, 1483. [Google Scholar] [CrossRef]
- Kovarik, Z.; Radić, Z.; Berman, H.A.; Simeon-Rudolf, V.; Reiner, E.; Taylor, P. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem. J. 2003, 373, 33–40. [Google Scholar] [CrossRef]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R.; Perry, N.S.L.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alz-heimer’s disease and other cognitive disorders. Phyther. Res. 2003, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Release, S. Maestro, version 11.8, References—Scientific Research Publishing; Schrodinger, LLC: New York, NY, USA, 2018. [Google Scholar]
- Giuffrida, D.; Cacciola, F.; Mapelli-Brahm, P.; Stinco, C.M.; Dugo, P.; Oteri, M.; Mondello, L.; Meléndez-Martínez, A.J. Free carotenoids and carotenoids esters composition in Spanish orange and mandarin juices from diverse varieties. Food Chem. 2019, 300, 125139. [Google Scholar] [CrossRef]
- Latimer, G. Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Vargas-Arana, G.; Merino-Zegarra, C.; del-Castillo, Á.M.R.; Quispe, C.; Viveros-Valdez, E.; Simirgiotis, M.J. Antioxidant, Antiproliferative and Anti-Enzymatic Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Ungurahui Palm Fruits (Oenocarpus bataua Mart) from the Peruvian Amazon. Antioxidants 2022, 11, 1598. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model Seeking for parameter-free double-hybrid functionals: The PBE0-DH model Accurate excitation energies from time-dependent density functional theory: Assessing the PBE0. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Ratner, M.A.; Cheeseman, J.R. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Greenblatt, H.M.; Kryger, G.; Lewis, T.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 Å resolution. FEBS Lett. 1999, 463, 321–326. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyrylcholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Silman, I.; Harel, M.; Axelsen, P.; Raves, M.; Sussman, J. Three-dimensional structures of acetylcholinesterase and of its complexes with anticholinesterase agents. Biochem. Soc. Trans. 1994, 22, 745–749. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef]
- Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.H.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase inhibition activity, alkaloid profiling and molecular docking of chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- PyMOL. Available online: https://www.pymol.org/ (accessed on 24 October 2024).


| Proximate Composition | Mineral Content | ||
|---|---|---|---|
| Humidity | 12.2 ± 0.42 p | Ca | 3.35 ± 0.3 g |
| Ashes | 5.62 ± 0.16 o | Mg | 6.82 ± 0.2 h |
| Total lipids | 0.03 ± 0.001 c | Fe | 5.37 ± 0.3 i |
| Crude protein | 6.34 ± 0.2 d | Zn | 3.36± 0.1 g |
| Crude fiber | 10.45 ± 0.17 e | Mn | 1.22 ± 0.1 j |
| Carbohydrates | 65.5 ± 9.2 f | Cu | 0.67 ± 0.1 k |
| K | 43.26 ± 0.3 l | ||
| Na | 0.92 ± 0.1 m | ||
| Sample | DPPH a | ABTS a | ORAC b | FRAP b | TPC c | TCC d | AChE e | BuChE e | α-Glucosidase e | α-Amylase e |
|---|---|---|---|---|---|---|---|---|---|---|
| Extract | 6.65 ± 0.5 | 9.95 ± 0.05 | 108.9 ± 4.07 | 47.8 ± 0.01 | 9.33 ± 0.01 | 79.0 ± 0.3 | 6.904 ± 0.42 | 18.38 ± 0.48 | >1000 | >1000 |
| Gallic acid | 4.32 ± 0.5 | 16.7± 0.05 | - | - | - | - | - | - | - | - |
| Acarbose | - | - | - | - | - | - | - | - | 138.9 ± 0.01 | 10.04 ± 0.02 |
| Galantamine | - | - | - | - | - | - | 0.402 ± 0.02 e | 5.33 ± 0.01 | - | - |
| Quercetin | 12.23 ± 0.8 | 15.72 ± 0.05 | - | - | - | - | - | - | - | - |
| Peak a | Compounds | Rt [min] b | Molecular Formula | λmax [nm] c | III/II [%] | APCI(+)-MSn | ESI(+)-TOF-MS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | m/z [M+H]+ | MSn Fragmentation m/z | Detected Mass m/z | Theor. Mass m/z | Mass Error [ppm] | |||||
| 1 | (all-E)-lutein and (all-E)-zeaxanthin | 12.24 | C40H56O2 | 424 | 448 | 476 | 50 | 569.3 | 551.3 [M+H-18]+ | |||
| 2 | Unknown 1 | 14.34 | - | 450 | - | - | 591.3 | |||||
| IS | β-Apo-8′-carotenal | 15.01 | C30H40O | 464 | - | 417.2 | 399.1 [M+H-18]+, 325.1, 293.1 | 417.3150 | 417.3152 | 0.4 | ||
| 3 | Ni d, xanthophyll_MW e 568 | 15.30 | C40H56O2 | 422 | 440 | 466 | nd f | 569.3 | 551.3 [M+H-18]+; 429.2 | |||
| 4 | ni, xanthophyll_MW 552 g ni, xanthophyll_MW 568 | 16.30 | C40H56O C40H56O2 | 426 | 452 | 484 | nd | 553.3 569.3 | 535.3 [M+H-18]+; 495.2 551.3 [M+H-18]+; 429.2 | |||
| 5 | ni, xanthophyll_MW 568 | 17.42 | C40H56O2 | 416 | 442 | 466 | 25 | 569.3 | 551.2 [M+H-18]+; 483.3 | |||
| 6 | ni, xanthophyll_MW 600 caprate | 18.02 | C52H79O5 | 398 | 424 | 446 | 83 | 755.4 | 737.4 [M+H-18]+; 645.4; 583.3 [M+H-172]+ | |||
| 7 | ni, xanthophyll_MW 600 caprate | 18.57 | C52H79O5 | 424 | 448 | 478 | 24 | 755.4 | 737.4 [M+H-18]+; 645.4; 583.3 [M+H-172]+ | |||
| 8 | ni, xanthophyll_MW 600 laurate | 19.02 | C52H79O5 | 418 | 442 | 468 | 20 | 783.5 | 765.5 [M+H-18]+; 583.3 [M+H-200]+; 565.3 [M+H-18-200]+ | |||
| 9 | ni, xanthophyll_MW 568 | 19.72 | C40H56O2 | 296 436 | 460 | 490 | 58 | 569.3 | 551.3 [M+H-18]+; 483.2 | |||
| 10 | (all-E)-β-cryptoxanthin ni, xanthophyll_MW 600 laurate | 20.04 | C40H56O C54H83O5 | 422 | 444 | 474 | 42 | 553.3 783.5 | 535.3 [M+H-18]+ 765.4 [M+H-18]+; 583.2 [M+H-200]+; 565.3 [M+H-18-200]+ | |||
| 11 | ni, xanthophyll_MW 552 | 20.31 | C40H56O | 430 | 454 | 484 | nd | 553.3 | 535.3 [M+H-18]+ | 552.4318 | 552.4326 | 1.5 |
| 12 | (all-E)-violaxanthin laurate ni, xanthophyll_MW 552 | 21.23 | C52H79O5 C40H56O2 | 425 | 448 | 478 | 5.0 | 783.4 553.3 | 765.5 [M+H-18]+; 673.3; 583.2 [M+H-200]+; 565.2 [M+H-18-200]+; 535.2 [M+H-18]+ | 553.4394 | 553.4404 | 1.8 |
| 13 | ni, xanthophyll_MW 552 xanthophyll_MW 568 myristate palmitate | 21.92 | C40H56O C70H113O4 | 420 | 440 | 468 | nd | 553.3 1017.6 | 535.3 [M+H-18]+ 999.6 [M+H-18]+ | 553.4393 | 553.4404 | 2.1 |
| ms1 | (all-E)-violaxanthin myristate | C54H83O5 | 811.7 | 793.7 [M+H-18]+; 583.2 [M+H-228]+; 565.3 [M+H-18-228]+ | ||||||||
| 14 | ni, xanthophyll_MW 552 | 23.93 | C40H56O | 418 | 442 | 466 | 42 | 553.3 | 535.3 [M+H-18]+; 471.2; 429.2 | 553.4395 | 553.4404 | 1.6 |
| 15 | ni, xanthophyll_MW 552 | 24.28 | C40H56O | 416 | 438 | 464 | 44 | 553.3 | 535.3 [M+H-18]+; 471.2; 429.2 | 553.4392 | 553.4404 | 2.2 |
| 16 | ni, xanthophyll_MW 552 | 24.78 | C40H56O | - | 460 | 488 | nd | 553.3 | 535.3 [M+H-18]+; 493.2 | |||
| 17 | ni, xanthophyll_MW 552 | 24.96 | C40H56O | 436 | 454 | 486 | nd | 553.3 | 535.3 [M+H-18]+; 493.2 | |||
| 18 | ni, xanthophyll_MW 552 | 25.15 | C40H56O | - | 454 | 486 | 40 | 553.3 | 535.3 [M+H-18]+; 493.2 | |||
| 19 | ni, xanthophyll_MW 552 | 25.44 | C40H56O | - | 448 | 488 | nd | 553.3 | 535.3 [M+H-18]+; 493.2 | |||
| ms2 | (all-E)-violaxanthin palmitate | C56H87O5 | 839.8 | |||||||||
| 20 | ni, xanthophyll_MW 552 | 26.34 | C40H56O | 296 438 | 460 | 490 | 46 | 553.4 | 535.3 [M+H-18]+; 429.2; 385.2 | 553.4396 | 553.4404 | 1.4 |
| 21 | ni, xanthophyll_MW 552 | 27.10 | C40H56O | 436 | 460 | 488 | nd | 553.4 | 535.3 [M+H-18]+; 461.3; 413.2 | |||
| ms3 | (all-E)-antheraxanthin myristate | C54H82O4 | 795.7 | 795.6291 | 795.6286 | −0.6 | ||||||
| 22 | ni, xanthophyll_MW 552 | C40H56O | 362 440 | 468 | 498 | 55 | 553.3 | 535.3; 467.2 [M+H-18]+; 413.2 | 553.4399 | 553.4404 | 0.9 | |
| 23 | (15-Z)-β-carotene phytoene phytofluene isomer 1 | 28.56 | C40H56 C40H64 C40H62 | 418 274 332 | 440 286 348 | 468 298 367 | nd | 537.4 545.5 543.5 | 455.1; 413.2 463.2 [M+H-82]+ 461.5 [M+H-82]+; 337.3 [M-205]+ | 543.4902 536.4367 | 543.4924 536.4377 | 4.1 1.9 |
| ms4 | ζ-carotene isomer 1 | C40H60 | 541.4 | 459.2 [M+H-82]+; 417.2 | ||||||||
| 24 | β-zeacarotene phytofluene isomer 2 | 29.56 | C40H58 C40H62 | 410 332 | 432 348 | 460 367 | nd | 539.3 543.5 | 457.2 5 [M+H-82]+; 389.2 | 538.4528 | 538.4533 | 0.9 |
| 25 | ni, carotene_MW 536 | 29.89 | 416 | 438 | 460 | nd | 537.3 | 455.1; 413.2 | 536.4368 | 536.4377 | 1.6 | |
| 26 | 13-cis-β-carotene | 30.42 | C40H56 | 338 422 | 444 | 472 | 34 | 537.3 | 444.3 [M+H-92]+; 347.2 | 536.4378 | 536.4377 | −0.3 |
| 27 | ζ-carotene isomer 2 ni, xanthophyll_MW 600 caprate laurate | 30.58 | C40H60 C62H96O6 | 380 | 402 | 426 | nd | 541.4 937.6 | 459.2 [M+H-82]+; 391.2 919.6 [M+H-18]+; 765.5; 737.4 [M+H-200]+; 547.3 [M+H-18-200]+ | 540.4684 | 540.4690 | 1.0 |
| 28 | (all-E)-β-carotene ζ-carotene isomer 3 | 30.90 | C40H56 C40H60 | 428 | 452 | 476 | 14 | 537.3 541.3 | 444 [M+H-92]+; 413; 399; 347; 279 | 536.4382 | 536.4377 | −1.0 |
| 29 | ni, xanthophyll_MW 552 | 31.48 | C40H56O | 294 440 | 466 | 502 | nd | 553.3 | 535.3 [M+H-18]+; 413.2 | |||
| 30 | ni, carotene_MW 536 | 31.76 | C40H56 | 430 | 458 | 484 | 30 | 537.3 | ||||
| 31 | (9-Z)-β-carotene (all-E)-violaxanthin dilaurate | 32.15 | C40H56 C64H100O6 | 422 | 448 | 472 | 88 | 537.3 965.7 | 455.2; 413.1 947.7 [M+H-18]+; 765.7 [M+H-200]+, 747.7 [M+H-18-200]+, 565.4 [M+H-200-200]+ | 536.4375 | 536.4377 | 0.2 |
| 32 | γ-carotene (all-E)-antheraxanthin-dilaurate | 33.04 | C40H56 C64H100O5 | 436 | 462 | 492 | 47 | 537.3 949.7 | 455.2; 413.2 931.8 [M+H-18]+; 669.4; 599.3 | 536.4375 | 536.4377 | 0.3 |
| 33 | (all-E)-violaxanthin-laurate myristate ni, xanthophyll_MW 568 dilaurate | 33.60 | C66H104O6 C64H100O4 | 424 | 454 | 478 | 40 | 993.7 933.7 | 975.7 [M+H-18]+; 793 [M+H-200]+; 765 [M+H-18-200]+ 916.7 [M+H-18]+; 733.5 [M+H-200]+ | |||
| 34 | (Z)-Lycopene | 34.12 | C40H56 | 442 | 468 | 498 | 71 | 537.3 | 413.2 | 536.4377 | 536.4377 | −0.2 |
| 35 | (all-E)-β-cryptoxanthin laurate | 35.16 | C52H78O2 | 422 | 449 | 476 | 24 | 735.5 | 718.6 [M+H-18]+; 535.3 [M+H-200]+; 443.2 [M+H-92-200]+ | |||
| 36 | ni, xanthophyll_MW 552 laurate | 35.37 | C52H78O2 | 448 | 444 | 472 | 110 | 735.5 | 718.6 [M+H-18]+; 535.3 [M+H-200]+; 443.2 [M+H-92-200]+ | |||
| 37 | (all-E)-lycopene ni, xanthophyll_MW 568 dimyristate | 36.09 | C40H56 C68H108O4 | 448 | 474 | 504 | 71 | 537.4 989.7 | 455.2; 413.2 933.7 | 536.4372 | 536.4377 | 0.8 |
| 38 | (all-E)-β-cryptoxanthin palmitate | 36.60 | C56H86O2 | 424 | 452 | 478 | 88 | 791.6 | 535.3 [M+H-256]+ | |||
| 39 | Unknown 2 | 37.95 | 424 | 454 | 478 | 88 | ||||||
| Compound | Binding Energy (kcal/mol) Acetylcholinesterase | Binding Energy (kcal/mol) Butyrylcholinesterase |
|---|---|---|
| 9′-cis-β-carotene | −9.780 | −9.815 |
| 15′-cis-β-carotene | −11.356 | −8.353 |
| β-zeacarotene | - | −7.948 |
| Galantamine | −12.989 | −7.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scharf, S.; Romero-Parra, J.; Winterhalter, P.; Torres-Benítez, A.; Gök, R.; Simirgiotis, M.J. Berries from Luzuriaga radicans Ruiz & Pav.: A Southern Chile Climbing Shrub as a Source of Antioxidants Against Chronic Diseases. Plants 2025, 14, 2555. https://doi.org/10.3390/plants14162555
Scharf S, Romero-Parra J, Winterhalter P, Torres-Benítez A, Gök R, Simirgiotis MJ. Berries from Luzuriaga radicans Ruiz & Pav.: A Southern Chile Climbing Shrub as a Source of Antioxidants Against Chronic Diseases. Plants. 2025; 14(16):2555. https://doi.org/10.3390/plants14162555
Chicago/Turabian StyleScharf, Sebastian, Javier Romero-Parra, Peter Winterhalter, Alfredo Torres-Benítez, Recep Gök, and Mario J. Simirgiotis. 2025. "Berries from Luzuriaga radicans Ruiz & Pav.: A Southern Chile Climbing Shrub as a Source of Antioxidants Against Chronic Diseases" Plants 14, no. 16: 2555. https://doi.org/10.3390/plants14162555
APA StyleScharf, S., Romero-Parra, J., Winterhalter, P., Torres-Benítez, A., Gök, R., & Simirgiotis, M. J. (2025). Berries from Luzuriaga radicans Ruiz & Pav.: A Southern Chile Climbing Shrub as a Source of Antioxidants Against Chronic Diseases. Plants, 14(16), 2555. https://doi.org/10.3390/plants14162555







