The Feedback of Stress Phytohormones in Avena sativa (L.) on Soil Multi-Contamination
Abstract
1. Introduction
2. Results
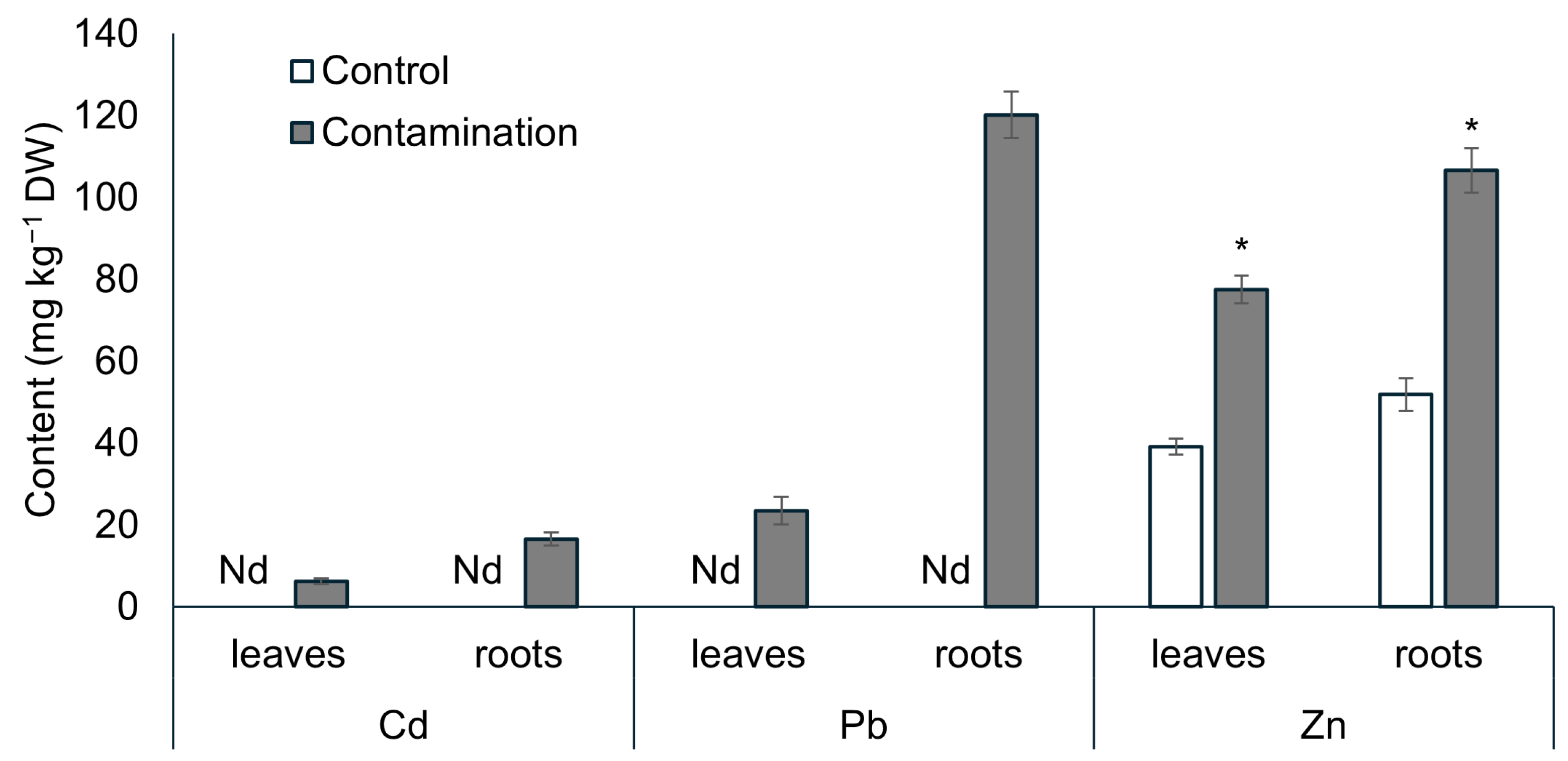
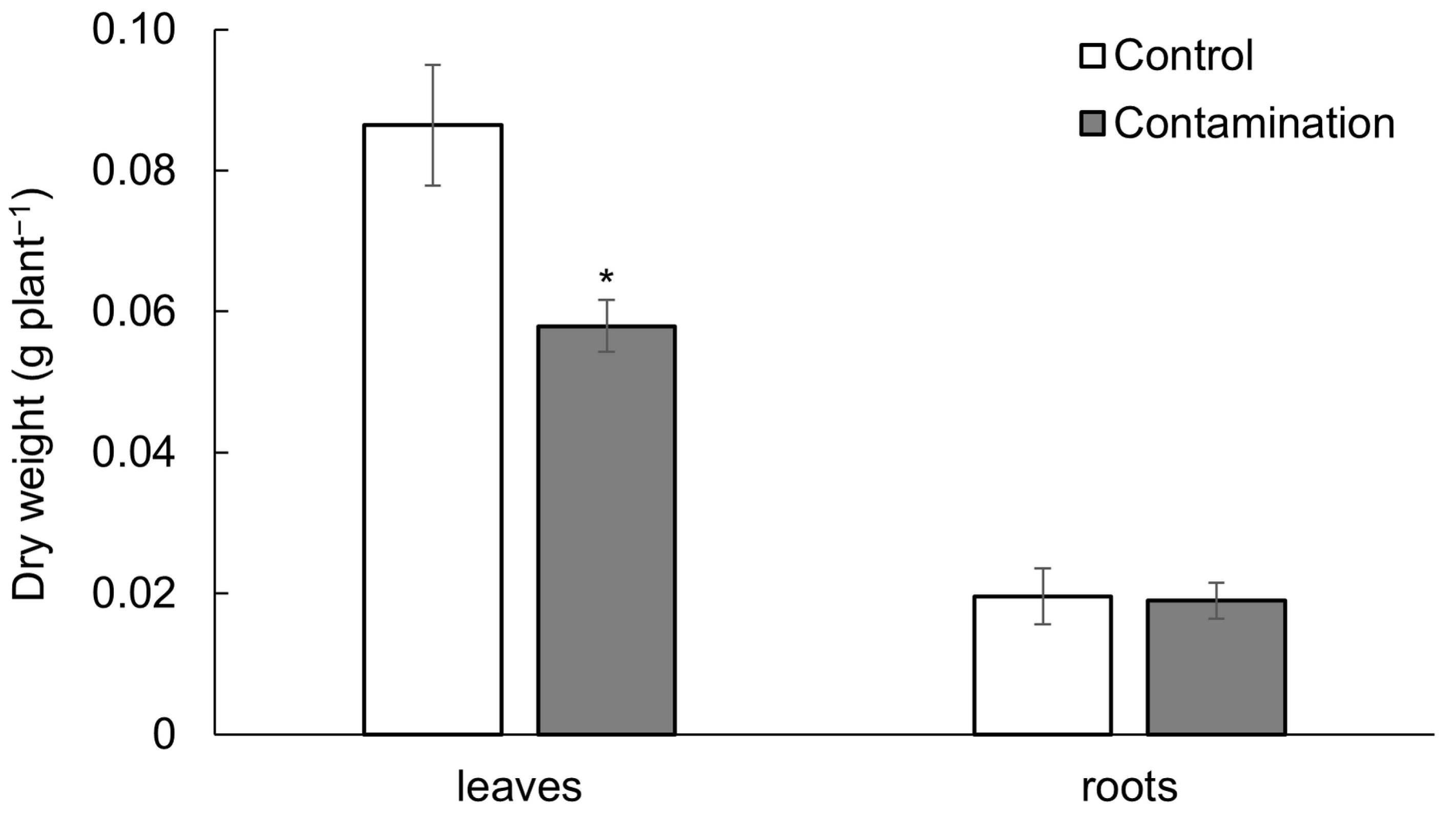
2.1. Uptake of Cd, Pb, and Zn and Growth of Oat
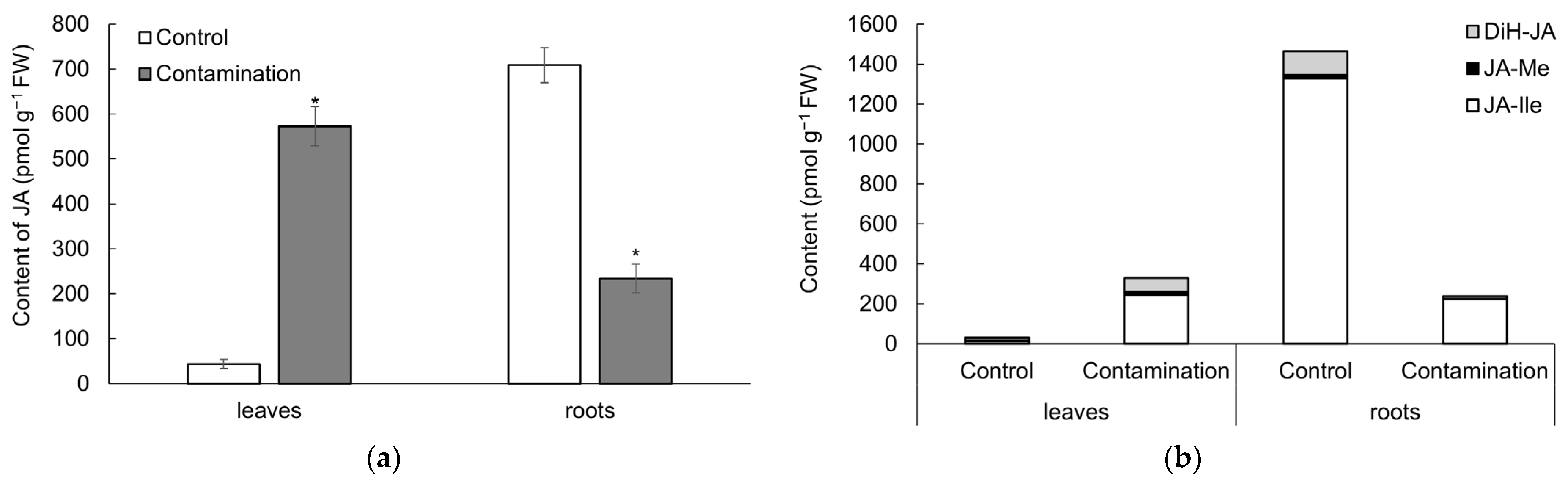
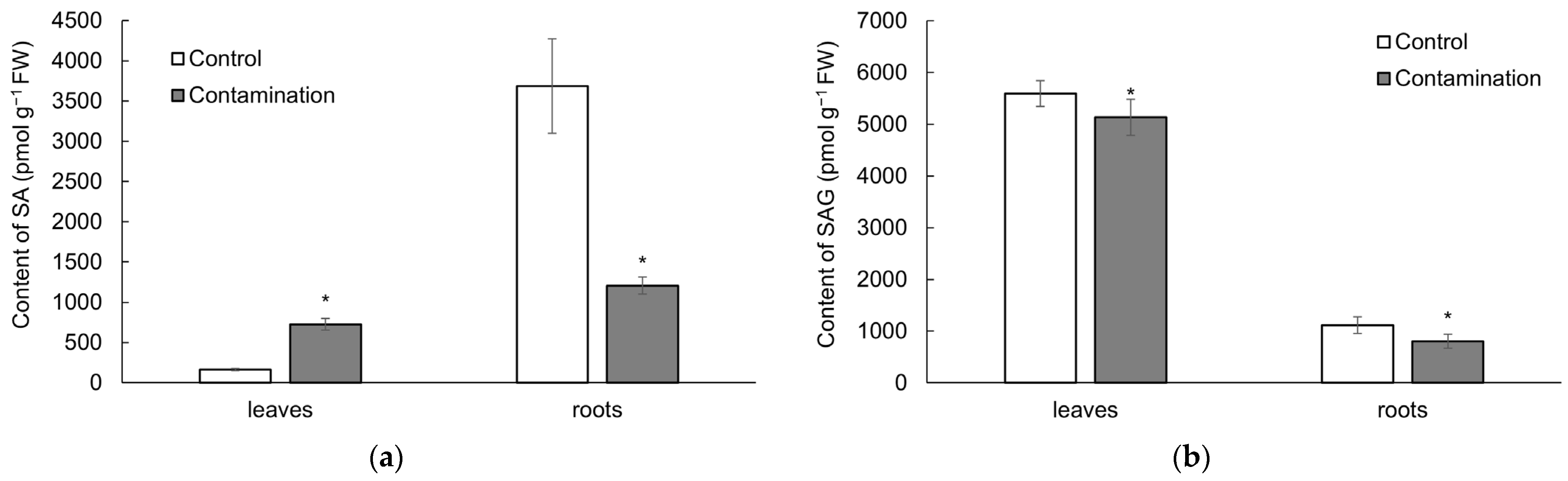
2.2. Stress Phytohormones of Oat
2.2.1. Jasmonates
2.2.2. Salicylic Acid
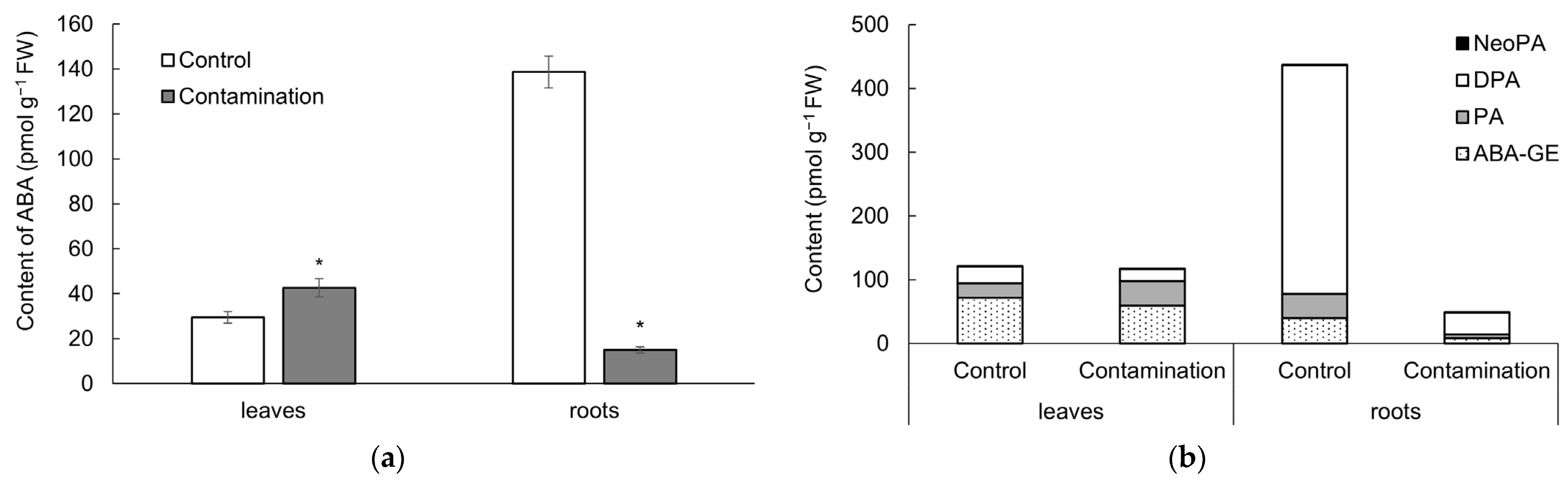
2.2.3. Abscisic Acid
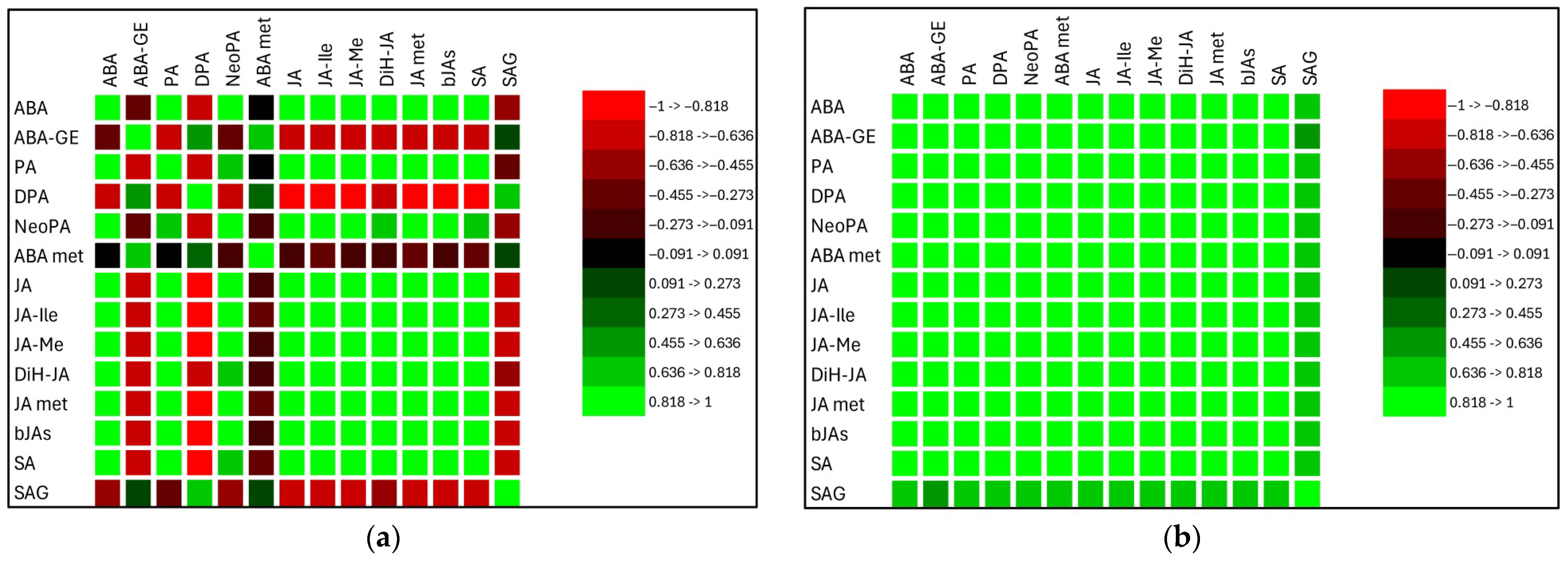
2.2.4. Relationships Between Stress Phytohormones
3. Discussion
3.1. Cd, Pb, and Zn Accumulation and Its Influence on the Growth of Oat
3.2. Stress Phytohormones of Oat Under Cd, Pb, and Zn Influence
4. Materials and Methods
4.1. Plant Growing Experiment
4.1.1. Soils
4.1.2. Plants
4.2. Chemical Analysis
4.2.1. Determination of Toxic Elements
4.2.2. Determination of Phytohormones
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.-H.; Hu, Q.; Zhao, F.-J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Wang, H.; Yu, S.; Sun, L.; Wang, Y.; Wu, H.; Wang, X. Pollution assessment and health risk of metals in surface soil near a Pb–Zn mine, northeast China. Front. Environ. Sci. 2025, 13, 1585272. [Google Scholar] [CrossRef]
- Vaněk, A.; Ettler, V.; Grygar, T.; Borůvka, L.; Šebek, O.; Drábek, O. Combined chemical and mineralogical evidence for heavy metal binding in mining- and smelting-affected alluvial soils. Pedosphere 2008, 18, 464–478. [Google Scholar] [CrossRef]
- Hajam, A.H.; Ali, M.S.; Singh, S.K.; Bashri, G. Understanding cytokinin: Biosynthesis, signal transduction, growth regulation, and phytohormonal crosstalk under heavy metal stress. Environ. Exp. Bot. 2024, 228, 106025. [Google Scholar] [CrossRef]
- Zemanová, V.; Lhotská, M.; Novák, M.; Hnilička, F.; Popov, M.; Pavlíková, D. Multicontamination toxicity evaluation in the model plant Lactuca sativa L. Plants 2024, 13, 1356. [Google Scholar] [CrossRef]
- Pavlíková, D.; Zemanová, V.; Pavlík, M.; Lhotská, M.; Kubeš, J.; Novák, M.; Dobrev, P.I.; Motyka, V. Phytohormone and amino acid changes in cherry radish as metabolic adaptive response to arsenic single and multi-contamination. Biomolecules 2025, 15, 390. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta 2015, 241, 1435–1451. [Google Scholar] [CrossRef]
- Ronzana, M.; Piacentinia, D.; Fattorinia, L.; Della Roverea, F.; Eicheb, E.; Riemannc, M.; Altamuraa, M.M.; Falasca, G. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding HMs responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Jikumaru, Y.; Kamiya, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J. 2017, 90, 17–36. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal. Behav. 2020, 15, 1777372. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.M.; Hatamleh, A.A.; et al. Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L.). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef] [PubMed]
- Torun, H.; Cetin, B.; Stojnic, S.; Petrík, P. Salicylic acid alleviates the effects of cadmium and drought stress by regulating water status, ions, and antioxidant defense in Pterocarya fraxinifolia. Front. Plant Sci. 2024, 14, 1339201. [Google Scholar] [CrossRef]
- Li, Q.; Guan, C.; Zhao, Y.; Duan, X.; Yang, Z.; Zhu, J. Salicylic acid alleviates Zn-induced inhibition of growth via enhancing antioxidant system and glutathione metabolism in alfalfa. Ecotoxicol. Environ. Saf. 2023, 265, 115500. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Li, Y.; Hussain, S.; Hussain, B.; Khan, W.-U.-D.; Riaz, L.; Ashraf, M.N.; Khaliq, M.A.; Du, Z.; Cheng, H. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Bilal, S.; Saad Jan, S.; Shahid, M.; Asaf, S.; Khan, A.L.; Lubna; Al-Rawahi, A.; Lee, I.-J.; AL-Harrasi, A. Novel insights into exogenous phytohormones: Central regulators in the modulation of physiological, biochemical, and molecular responses in rice under metal(loid) stress. Metabolites 2023, 13, 1036. [Google Scholar] [CrossRef]
- Ndecky, S.; Malherbe, L.; Villette, C.; Chalvon, V.; Meusnier, I.; Beltran-Valencia, D.; Baumberger, N.; Riemann, M.; Kroj, T.; Champion, A.; et al. Rice JASMONIC ACID OXIDASES control resting jasmonate metabolism to promote growth and repress basal immune responses. Plant Physiol. 2025, 198, kiaf161. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, W.; Tong, T.; Chen, G.; Zeng, F.; Jang, S.; Gao, W.; Li, Z.; Mak, M.; Deng, F.; et al. Molecular interaction and evolution of jasmonate signaling with transport and detoxification of heavy metals and metalloids in plants. Front. Plant Sci. 2021, 12, 665842. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Deng, F.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, L.; Chen, Z.-H. Evolution of Abscisic Acid Signaling for Stress Responses to Toxic Metals and Metalloids. Front. Plant Sci. 2020, 11, 909. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Huang, W.; Zhang, D.; Wu, J.; Li, B.; Li, M.; Liu, L.; Yan, M. Abscisic-acid-regulated responses to alleviate cadmium toxicity in plants. Plants 2023, 12, 1023. [Google Scholar] [CrossRef]
- Leng, Y.; Li, Y.; Ma, Y.-H.; He, L.-F.; Li, S.-W. Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ. Sci. Pollut. Res. 2020, 28, 6030–6043. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Orroño, D.I.; Schindler, V.; Lavado, R.S. Heavy metal availability in Pelargonium hortorum rhizosphere: Interactions, uptake and plant accumulation. J. Plant Nutr. 2012, 35, 1374–1386. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; El Mzibri, M.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 175. [Google Scholar] [CrossRef]
- Jatav, P.K.; Verma, R.; Kothari, S.L.; Jain, R.; Kachhwaha, S. Relative morpho-physiological responses of millets and oats against lead toxicity. Environ. Exp. Bot. 2021, 192, 104658. [Google Scholar] [CrossRef]
- Piršelová, B.; Galuščáková, Ľ.; Lengyelová, L.; Kubová, V.; Matúšová, R.; Bojnanská, K.; Havrlentová, M. Phytoremediation potential of oat (Avena sativa L.) in soils contaminated with cadmium. Agron. Res. 2024, 22, 227–237. [Google Scholar]
- Dogan, M.; Karatas, M.; Aasim, M. Cadmium and lead bioaccumulation potentials of an aquatic macrophyte Ceratophyllum demersum L.: A laboratory study. Ecotoxicol. Environ. Saf. 2018, 148, 431–440. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Qu, J.Y.; Zhao, M.; Zhao, J.; Wang, W.C.; Huang, J.J.; Peng, J.S.; Xiao, Y.H.; Han, Y.L.; Peng, Y.; et al. Nitrogen utilization in response to cadmium and abscisic acid in rice. Plant Soil 2025. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Rehman, M.Z.U.; Rizwan, M.; Ali, S.; Sabir, M.; Sohail, M.I. Contrasting effects of organic and inorganic amendments on reducing lead toxicity in wheat. Bull. Environ. Contam. Toxicol. 2017, 99, 642–647. [Google Scholar] [CrossRef]
- Howladar, S.M.; Al-Robai, S.A.; Al-Zahrani, F.S.; Howladar, M.M.; Aldhebiani, A.Y. Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotoxicol. Environ. Saf. 2018, 159, 143–152. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Favas, P.J.C.; Pratas, J.; Varun, M.; Paul, M.S. Metal(loid) induced toxicity and defense mechanisms in Spinacia oleracea L. Ecological hazard and prospects for phytoremediation. Ecotox. Environ. Saf. 2019, 183, 109570. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Novák, M.; Zemanová, V.; Černý, J.; Pavlíková, D. Roots of Lupinus angustifolius L. and enzyme activities in soil contaminated by toxic elements. Plant Soil Environ. 2024, 70, 552–561. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Dobrev, P.I.; Motyka, V.; Pavlík, M. Endogenous phytohormone profiles in Pteris fern species differing in arsenic accumulating ability. Environ. Exp. Bot. 2019, 166, 103822. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Cui, X.; Wang, K.; Wang, Y.; He, Y. Phytohormones regulate the abiotic stress: An overview of physiological, biochemical, and molecular responses in horticultural crops. Front. Plant Sci. 2023, 13, 1095363. [Google Scholar] [CrossRef]
- Dar, T.A.; Moinuddin Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic acid in plant abiotic stress tolerance and interaction with abscisic acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Li, X.; Liu, X.; Fang, L.; Zeng, R.; Wang, Q.; Song, Y.; Chen, D. Jasmonic acid enhances rice cadmium tolerance by suppressing cadmium uptake and translocation. Plants 2025, 14, 1068. [Google Scholar] [CrossRef]
- Yang, J.B.; Wang, H.Y.; Huang, J.; Shan, C.J.; Yan, J.; Zhong, C.W.; Hu, D.; Zhang, Q.; Shen, R.F.; Zhu, X.F.; et al. Jasmonic acid improves cadmium tolerance in rice (Oryza sativa) by reducing the production of nitric oxide. Ecotox. Environ. Saf. 2025, 290, 117722. [Google Scholar] [CrossRef]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Z. Evolution of jasmonate biosynthesis and signaling mechanisms. J. Exp. Bot. 2017, 68, 1323–1331. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.H. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonate signaling in plant stress responses and development—Active and inactive compounds. N. Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M. Salicylic acid accumulation as a result of Cu, Zn, Cd and Pb interactions in common reed (Phragmites australis) growing in natural ecosystems. Acta Physiol. Plant. 2017, 39, 182. [Google Scholar] [CrossRef]
- Freeman, J.L.; Persans, M.W.; Nieman, K.; Albrecht, C.; Peer, W.; Pickering, I.J.; Salt, D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 2004, 16, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, T.; Peng, X.; Yan, M.; Tang, X. Up-regulation of chloroplastic antioxidant capacity is involved in alleviation of nickel toxicity of Zea mays L. by exogenous salicylic acid. Ecotoxicol. Environ. Saf. 2009, 72, 1354–1362. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotox. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Khalil, R.; Haroun, S.; Bassyoini, F.; Nagah, A.; Yusuf, M. Salicylic acid in combination with kinetin or calcium ameliorates HMs stress in Phaseolus vulgaris plant. J. Agric. Food Res. 2021, 5, 100182. [Google Scholar]
- Pál, M.; Szalai, G.; Horváth, E.; Janda, T.; Páldi, E. Effect of salicylic acid during heavy metal stress. Acta Biol. Szeged. 2002, 46, 119–120. [Google Scholar]
- Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Jumali, S.S.; Said, I.M.; Ismail, I.; Zainal, Z. Genes induced by high concentration of salicylic acid in Mitragyna speciosa. Aust. J. Crop. Sci. 2011, 5, 296–303. [Google Scholar]
- Mishra, A.K.; Baek, K.-H. Salicylic acid biosynthesis and metabolism: A divergent pathway for plants and bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, H.; Song, Q.; Jing, L.; Yu, H.; Li, R.; Zhang, P.; Liu, F.; Li, W.; Sun, L.; et al. Physiological and molecular bases of the boron deficiency response in tomatoes. Hortic. Res. 2023, 10, uhad229. [Google Scholar] [CrossRef]
- Qiu, G.; Han, Z.; Wang, Q.; Wang, T.; Sun, Z.; Yu, Y.; Han, X.; Yu, H. Toxicity effects of nanoplastics on soybean (Glycine max L.): Mechanisms and transcriptomic analysis. Chemosphere 2023, 313, 137571. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxic. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Lim, J.; Lim, C.W.; Lee, S.C. Core components of abscisic acid signaling and their post-translational modification. Front. Plant Sci. 2022, 13, 895698. [Google Scholar] [CrossRef]
- Long, H.; Zheng, Z.; Zhang, Y.; Xing, P.; Wan, X.; Zheng, Y.; Li, L. An abscisic acid (ABA) homeostasis regulated by its production, catabolism and transport in peanut leaves in response to drought stress. PLoS ONE 2019, 14, e0213963. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid sgnaling and abiotic stress tolerance in plants. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Wei, P.; Guo, S.; Wu, J. A briefly overview of the research progress for the abscisic acid analogues. Front. Chem. 2022, 10, 967404. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Czech Ministry of the Environment. Public Notice No. 153/2016 for the Management of Soil Protection; Czech Ministry of the Environment: Prague, Czech Republic, 2016.
- Šichorová, K.; Tlustoš, P.; Száková, J.; Kořínek, K.; Balík, J. Horizontal and vertical variability of heavy metals in the soil of a polluted area. Plant Soil Environ. 2004, 50, 525–534. [Google Scholar] [CrossRef]
- Pavlíková, D.; Zemanová, V.; Pavlík, M. Health risk and quality assessment of vegetables cultivated on soils from a heavily polluted old mining area. Toxics 2023, 11, 583. [Google Scholar] [CrossRef]
- Přerostová, S.; Dobrev, P.I.; Knirsch, V.; Jarošová, J.; Gaudinová, A.; Zupková, B.; Prášil, I.T.; Janda, T.; Brzobohatý, B.; Skalák, J. Light quality and intensity modulate cold acclimation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 2736. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemanová, V.; Pavlík, M.; Novák, M.; Pavlíková, D. The Feedback of Stress Phytohormones in Avena sativa (L.) on Soil Multi-Contamination. Plants 2025, 14, 2554. https://doi.org/10.3390/plants14162554
Zemanová V, Pavlík M, Novák M, Pavlíková D. The Feedback of Stress Phytohormones in Avena sativa (L.) on Soil Multi-Contamination. Plants. 2025; 14(16):2554. https://doi.org/10.3390/plants14162554
Chicago/Turabian StyleZemanová, Veronika, Milan Pavlík, Milan Novák, and Daniela Pavlíková. 2025. "The Feedback of Stress Phytohormones in Avena sativa (L.) on Soil Multi-Contamination" Plants 14, no. 16: 2554. https://doi.org/10.3390/plants14162554
APA StyleZemanová, V., Pavlík, M., Novák, M., & Pavlíková, D. (2025). The Feedback of Stress Phytohormones in Avena sativa (L.) on Soil Multi-Contamination. Plants, 14(16), 2554. https://doi.org/10.3390/plants14162554





