Shallot virus X p42 Protein Expressed in Concert with Virus Movement Proteins Is a Suppressor of Two Plant Antiviral Defense Mechanisms
Abstract
1. Introduction
2. Results
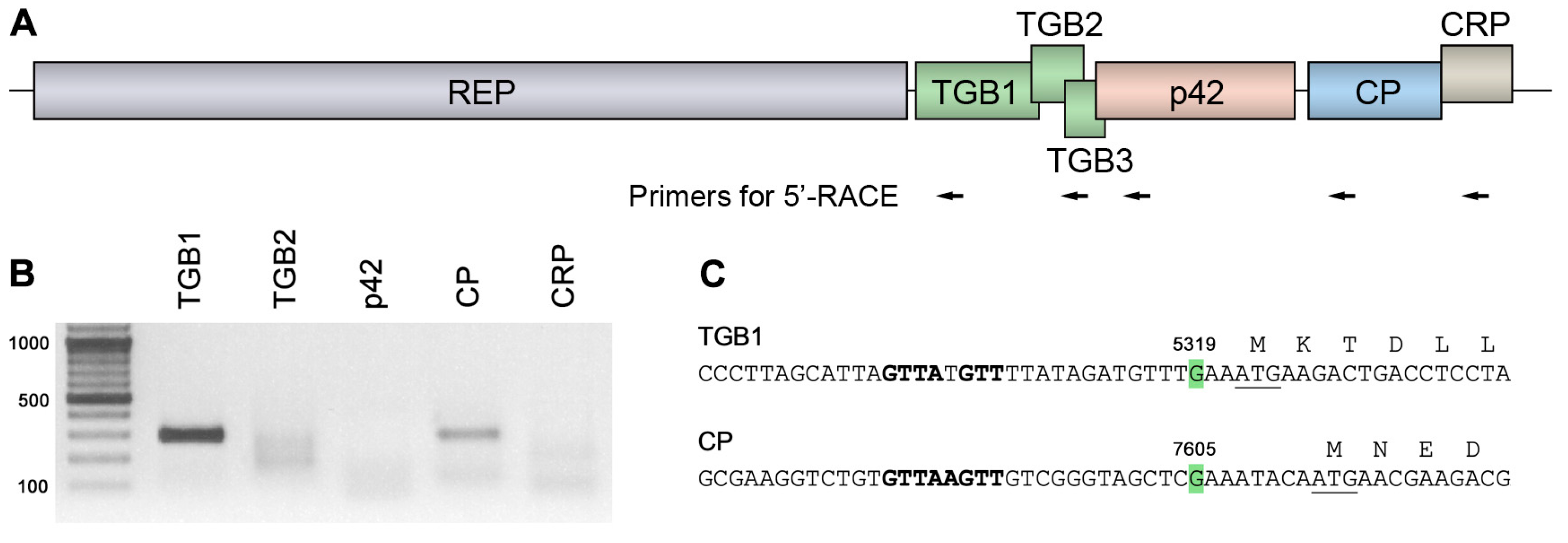
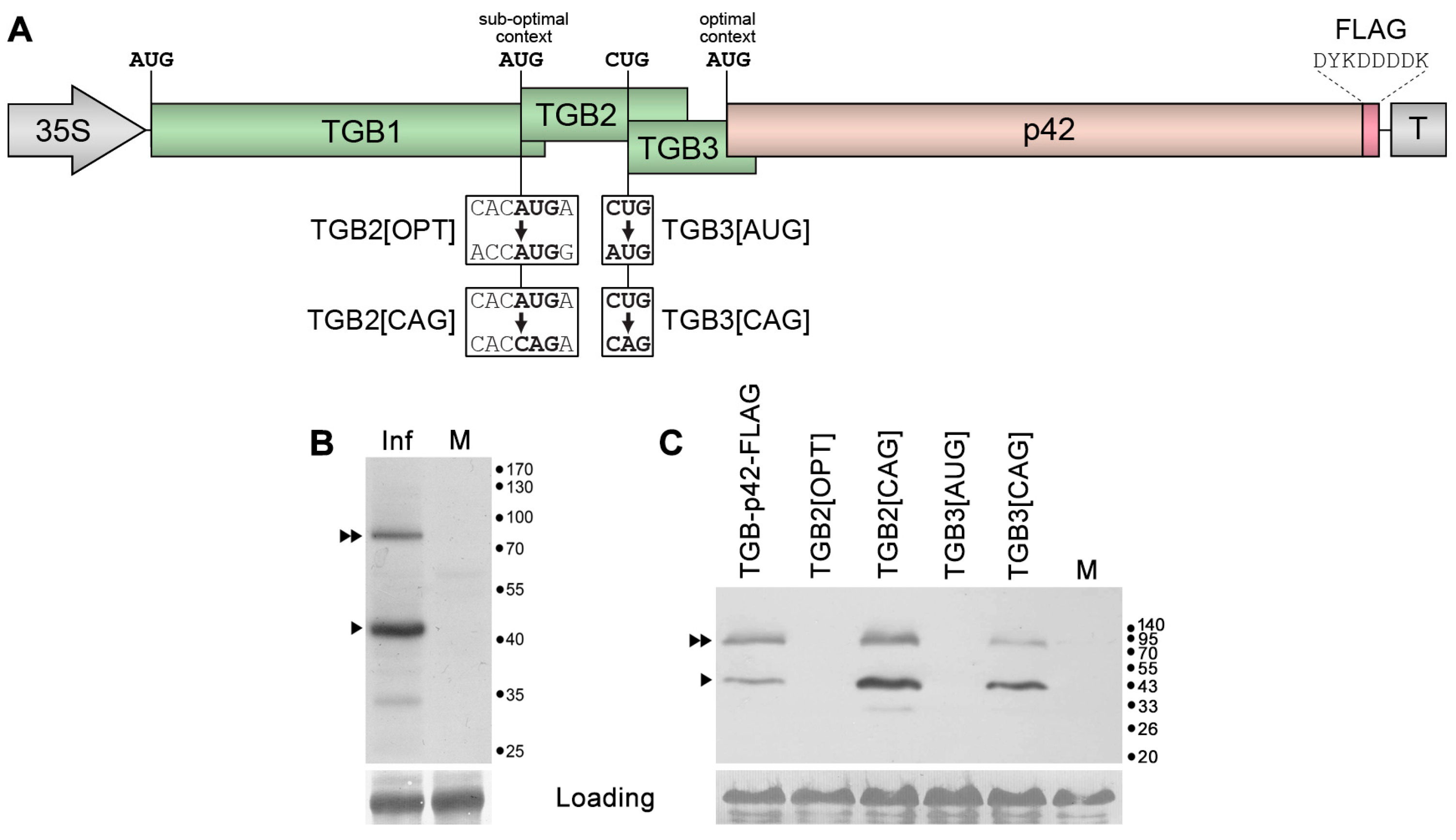
2.1. Expression of ShVX p42 Gene
2.2. Possible Mechanism of p42 Expression in Other Allexiviruses
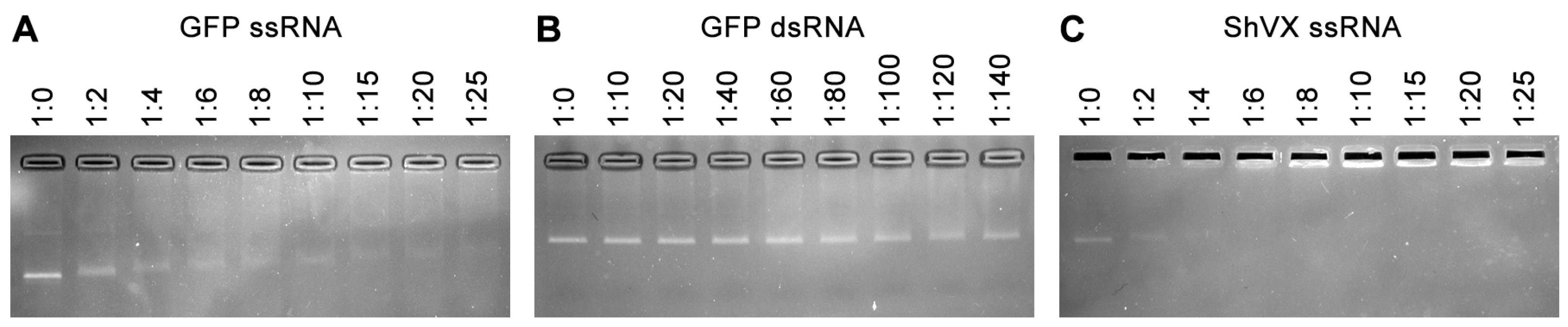
2.3. RNA Binding Properties of p42
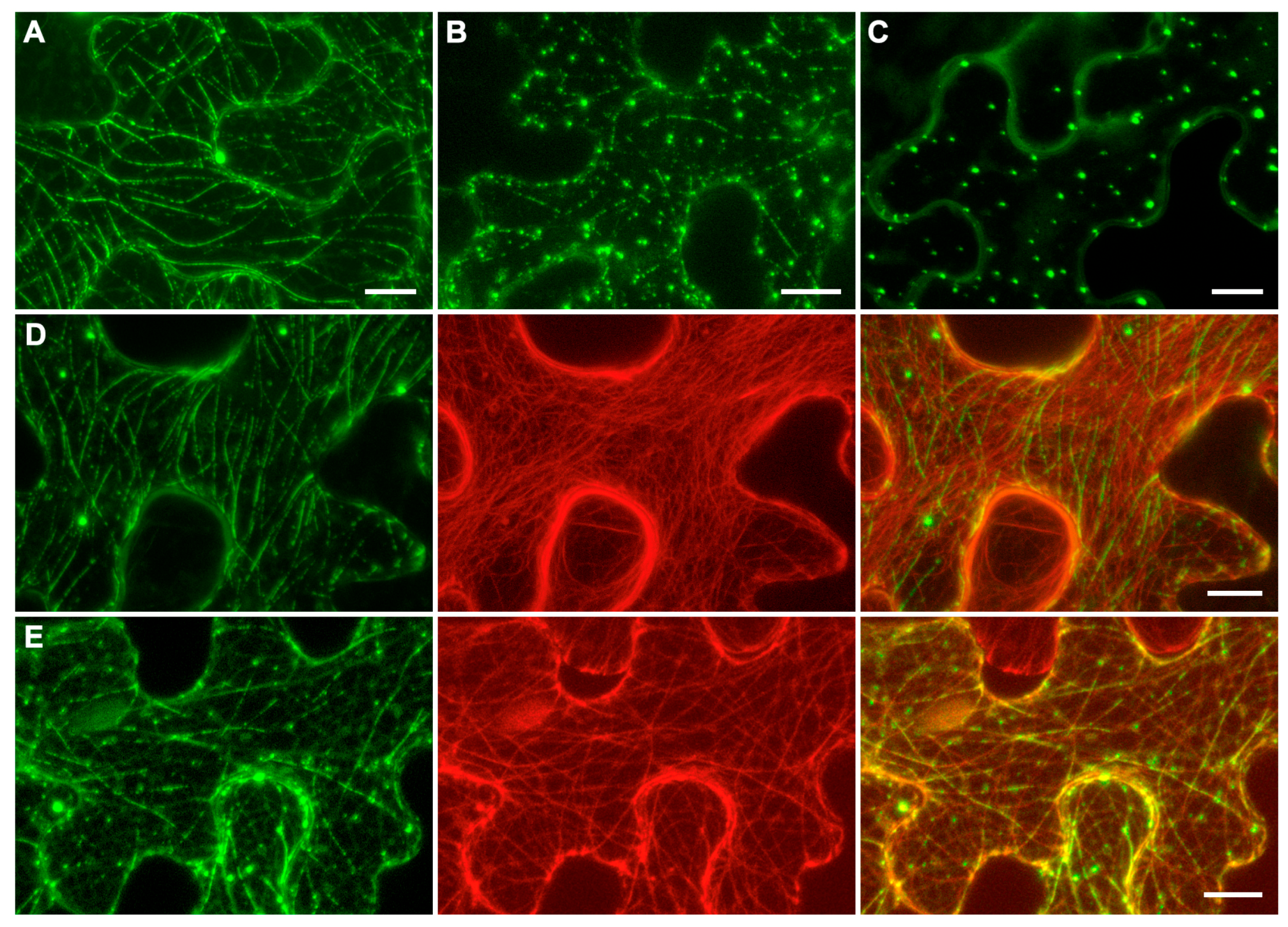
2.4. Subcellular Localization of p42
2.5. Analysis of p42 Ability to Suppress RNA Silencing
2.6. p42 Suppresses Nonsense-Mediated RNA Decay
3. Discussion
4. Materials and Methods
4.1. Primers
4.2. Recombinant Constructs
4.3. 5′-RACE
4.4. Protein Expression and Purification
4.5. Gel-Shift Experiments
4.6. Plant Material
4.7. Plant Agroinfiltration
4.8. Western Blot Analysis
4.9. Quantitative PCR
4.10. Microscopy and Visualization of Fluorescence
4.11. TCV-GFP Assay
4.12. Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origin of Viruses: Primordial Replicators Recruiting Capsids from Hosts. Nat. Rev. Microbiol. 2019, 17, 449–458. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V. Evolution and Taxonomy of Positive-Strand RNA Viruses: Implications of Comparative Analysis of Amino Acid Sequences. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 375–430. [Google Scholar] [CrossRef] [PubMed]
- Agranovsky, A.A. Structure and Expression of Large (+)RNA Genomes of Viruses of Higher Eukaryotes. Biochemistry 2021, 86, 248–256. [Google Scholar] [CrossRef]
- Lucas, W.J. Plant Viral Movement Proteins: Agents for Cell-to-Cell Trafficking of Viral Genomes. Virology 2006, 344, 169–184. [Google Scholar] [CrossRef]
- Heinlein, M. Plant Virus Replication and Movement. Virology 2015, 479–480, 657–671. [Google Scholar] [CrossRef]
- Heinlein, M. Plasmodesmata: Channels for Viruses on the Move. Methods Mol. Biol. 2015, 1217, 25–52. [Google Scholar] [CrossRef]
- Tee, E.E.; Faulkner, C. Plasmodesmata and Intercellular Molecular Traffic Control. New Phytol. 2024, 243, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Burgyán, J.; Havelda, Z. Viral Suppressors of RNA Silencing. Trends Plant Sci. 2011, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Pumplin, N.; Voinnet, O. RNA Silencing Suppression by Plant Pathogens: Defence, Counter-Defence and Counter-Counter-Defence. Nat. Rev. Microbiol. 2013, 11, 745–760. [Google Scholar] [CrossRef]
- Csorba, T.; Kontra, L.; Burgyán, J. Viral Silencing Suppressors: Tools Forged to Fine-Tune Host-Pathogen Coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef]
- Pantaleo, V.; Masuta, C. Diversity of Viral RNA Silencing Suppressors and Their Involvement in Virus-Specific Symptoms. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2022; Volume 113, pp. 1–23. ISBN 978-0-323-98992-3. [Google Scholar]
- Yelina, N.E.; Savenkov, E.I.; Solovyev, A.G.; Morozov, S.Y.; Valkonen, J.P.T. Long-Distance Movement, Virulence, and RNA Silencing Suppression Controlled by a Single Protein in Hordei- and Potyviruses: Complementary Functions between Virus Families. J. Virol. 2002, 76, 12981–12991. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.-H.; Ding, S.-W. The Suppressor of Transgene RNA Silencing Encoded by Cucumber mosaic virus Interferes with Salicylic Acid-Mediated Virus Resistance. Mol. Plant-Microbe Interact. 2001, 14, 715–724. [Google Scholar] [CrossRef]
- Ding, X.S.; Liu, J.; Cheng, N.-H.; Folimonov, A.; Hou, Y.-M.; Bao, Y.; Katagi, C.; Carter, S.A.; Nelson, R.S. The Tobacco mosaic virus 126-kDa Protein Associated with Virus Replication and Movement Suppresses RNA Silencing. Mol. Plant-Microbe Interact. 2004, 17, 583–592. [Google Scholar] [CrossRef]
- Kurihara, Y.; Inaba, N.; Kutsuna, N.; Takeda, A.; Tagami, Y.; Watanabe, Y. Binding of Tobamovirus Replication Protein with Small RNA Duplexes. J. Gen. Virol. 2007, 88, 2347–2352. [Google Scholar] [CrossRef]
- Csorba, T.; Bovi, A.; Dalmay, T.; Burgyán, J. The P122 Subunit of Tobacco mosaic virus Replicase is a Potent Silencing Suppressor and Compromises Both Small Interfering RNA- and microRNA-Mediated Pathways. J. Virol. 2007, 81, 11768–11780. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Lin, S.-S.; Hung, T.-H.; Li, T.-K.; Lin, N.-C.; Shen, T.-L. Multiple Domains of the Tobacco mosaic virus P126 Protein Can Independently Suppress Local and Systemic RNA Silencing. Mol. Plant-Microbe Interact. 2012, 25, 648–657. [Google Scholar] [CrossRef]
- Qu, F.; Ren, T.; Morris, T.J. The Coat Protein of Turnip crinkle virus Suppresses Posttranscriptional Gene Silencing at an Early Initiation Step. J. Virol. 2003, 77, 511–522. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Fu, Y.; Tang, W.; Zhao, P.; Ren, Y.; Liu, Z.; Wu, K.; Zhang, X. Turnip Crinkle Virus-encoded Suppressor of RNA Silencing Interacts with Arabidopsis SGS3 to Enhance Virus Infection. Mol. Plant Pathol. 2023, 24, 154–166. [Google Scholar] [CrossRef]
- Kanyuka, K.V.; Vishnichenko, V.K.; Levay, K.E.; Kondrikov, D.Y.; Ryabov, E.V.; Zavriev, S.K. Nucleotide Sequence of Shallot Virus X RNA Reveals a 5′-Proximal Cistron Closely Related to Those of Potexviruses and a Unique Arrangement of the 3′-Proximal Cistrons. J. Gen. Virol. 1992, 73 Pt 10, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Vishnichenko, V.K.; Konareva, T.N.; Zavriev, S.K. A New Filamentous virus in Shallot. Plant Pathol. 1993, 42, 121–126. [Google Scholar] [CrossRef]
- Katis, N.I.; Maliogka, V.I.; Dovas, C.I. Viruses of the Genus Allium in the Mediterranean Region. Adv. Virus Res. 2012, 84, 163–208. [Google Scholar] [CrossRef]
- Riabov, E.V.; Generozov, E.V.; Fetten, G.D.; Zavriev, S.K. Analysis of the 3′-terminal region of the tick-borne filament-like virus substantiates its affiliation with the shallot X-virus group. Mol. Biol. 1996, 30, 104–110. [Google Scholar]
- Úbeda, J.R.; Aranda, M.A.; Donaire, L. Alphaflexiviridae in Focus: Genomic Signatures, Conserved Elements and Viral-Driven Cellular Remodeling. Viruses 2025, 17, 611. [Google Scholar] [CrossRef]
- Morozov, S.Y.; Solovyev, A.G. Triple Gene Block: Modular Design of a Multifunctional Machine for Plant Virus Movement. J. Gen. Virol. 2003, 84, 1351–1366. [Google Scholar] [CrossRef]
- Verchot-Lubicz, J.; Torrance, L.; Solovyev, A.G.; Morozov, S.Y.; Jackson, A.O.; Gilmer, D. Varied Movement Strategies Employed by Triple Gene Block-Encoding Viruses. Mol. Plant-Microbe Interact. 2010, 23, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Tilsner, J.; Linnik, O.; Louveaux, M.; Roberts, I.M.; Chapman, S.N.; Oparka, K.J. Replication and Trafficking of a Plant Virus Are Coupled at the Entrances of Plasmodesmata. J. Cell Biol. 2013, 201, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, J.; Chai, M.; Wang, J.; Li, D.; Wang, A.; Cheng, X. The Potato Virus X TGBp2 Protein Plays Dual Functional Roles in Viral Replication and Movement. J. Virol. 2019, 93, e01635-18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, X.; Jiang, L.; Yang, X.; Chen, Y.; Song, X.; Lu, Y.; Peng, J.; Zheng, H.; Wu, Y.; et al. P15 Encoded by Garlic Virus X Is a Pathogenicity Factor and RNA Silencing Suppressor. J. Gen. Virol. 2018, 99, 1515–1521. [Google Scholar] [CrossRef]
- Kim, H.; Kawakubo, S.; Takahashi, H.; Masuta, C. Two Mutually Exclusive Evolutionary Scenarios for Allexiviruses That Overcome Host RNA Silencing and Autophagy by Regulating Viral CRP Expression. PLoS Pathog. 2023, 19, 1–30. [Google Scholar] [CrossRef]
- Arshava, N.V.; Kondareva, T.N.; Riabov, E.V.; Zavriev, S.K. 42K protein of shallot X virus is expressed in infected Allium species plants. Mol. Biol. 1995, 29, 192–198. [Google Scholar]
- Vishnichenko, V.K.; Stel’mashchuk, V.I.; Zavriev, S.K. The 42k Protein of the Shallot Virus X Participates in Formation of the Viral Particles. Mol. Biol. 2002, 36, 1080–1084. [Google Scholar] [CrossRef]
- Sztuba-Solińska, J.; Stollar, V.; Bujarski, J.J. Subgenomic Messenger RNAs: Mastering Regulation of (+)-Strand RNA Virus Life Cycle. Virology 2011, 412, 245–255. [Google Scholar] [CrossRef]
- Morozov, S.Y.; Miroshnichenko, N.A.; Solovyev, A.G.; Fedorkin, O.N.; Zelenina, D.A.; Lukasheva, L.I.; Karasev, A.V.; Dolja, V.V.; Atabekov, J.G. Expression Strategy of the Potato Virus X Triple Gene Block. J. Gen. Virol. 1991, 72 Pt 8, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Verchot, J.; Angell, S.M.; Baulcombe, D.C. In Vivo Translation of the Triple Gene Block of Potato Virus X Requires Two Subgenomic mRNAs. J. Virol. 1998, 72, 8316–8320. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Keima, T.; Hashimoto, M.; Hagiwara-Komoda, Y.; Hosoe, N.; Nishida, S.; Nijo, T.; Oshima, K.; Verchot, J.; Namba, S.; et al. Short 5′ Untranslated Region Enables Optimal Translation of Plant Virus Tricistronic RNA via Leaky Scanning. J. Virol. 2022, 96, e0214421. [Google Scholar] [CrossRef] [PubMed]
- Lezzhov, A.A.; Gushchin, V.A.; Lazareva, E.A.; Vishnichenko, V.K.; Morozov, S.Y.; Solovyev, A.G. Translation of the Shallot Virus X TGB3 Gene Depends on Non-AUG Initiation and Leaky Scanning. J. Gen. Virol. 2015, 96, 3159–3164. [Google Scholar] [CrossRef]
- Kozak, M. Comparison of Initiation of Protein Synthesis in Procaryotes, Eucaryotes, and Organelles. Microbiol. Rev. 1983, 47, 1–45. [Google Scholar] [CrossRef]
- Kozak, M. Context Effects and Inefficient Initiation at Non-AUG Codons in Eucaryotic Cell-Free Translation Systems. Mol. Cell. Biol. 1989, 9, 5073–5080. [Google Scholar] [CrossRef]
- Hopp, T.P.; Prickett, K.S.; Price, V.L.; Libby, R.T.; March, C.J.; Pat Cerretti, D.; Urdal, D.L.; Conlon, P.J. A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification. Nat. Biotechnol. 1988, 6, 1204–1210. [Google Scholar] [CrossRef]
- Kost, B.; Spielhofer, P.; Chua, N.H. A GFP-Mouse Talin Fusion Protein Labels Plant Actin Filaments in Vivo and Visualizes the Actin Cytoskeleton in Growing Pollen Tubes. Plant J. 1998, 16, 393–401. [Google Scholar] [CrossRef]
- Shemyakina, E.A.; Solovyev, A.G.; Leonova, O.G.; Popenko, V.I.; Schiemann, J.; Morozov, S.Y. The Role of Microtubule Association in Plasmodesmal Targeting of Potato Mop-Top Virus Movement Protein TGBp1. Open Virol. J. 2011, 5, 1–11. [Google Scholar] [CrossRef]
- Yelina, N.E.; Erokhina, T.N.; Lukhovitskaya, N.I.; Minina, E.A.; Schepetilnikov, M.V.; Lesemann, D.-E.; Schiemann, J.; Solovyev, A.G.; Morozov, S.Y. Localization of Poa semilatent virus Cysteine-Rich Protein in Peroxisomes Is Dispensable for Its Ability to Suppress RNA Silencing. J. Gen. Virol. 2005, 86, 479–489. [Google Scholar] [CrossRef]
- Vargason, J.M.; Szittya, G.; Burgyán, J.; Hall, T.M.T. Size Selective Recognition of siRNA by an RNA Silencing Suppressor. Cell 2003, 115, 799–811. [Google Scholar] [CrossRef]
- Ye, K.; Malinina, L.; Patel, D.J. Recognition of Small Interfering RNA by a Viral Suppressor of RNA Silencing. Nature 2003, 426, 874–878. [Google Scholar] [CrossRef]
- Ruiz, M.T.; Voinnet, O.; Baulcombe, D.C. Initiation and Maintenance of Virus-Induced Gene Silencing. Plant Cell 1998, 10, 937–946. [Google Scholar] [CrossRef][Green Version]
- Himber, C.; Dunoyer, P.; Moissiard, G.; Ritzenthaler, C.; Voinnet, O. Transitivity-Dependent and -Independent Cell-to-Cell Movement of RNA Silencing. EMBO J. 2003, 22, 4523–4533. [Google Scholar] [CrossRef]
- Senshu, H.; Yamaji, Y.; Minato, N.; Shiraishi, T.; Maejima, K.; Hashimoto, M.; Miura, C.; Neriya, Y.; Namba, S. A Dual Strategy for the Suppression of Host Antiviral Silencing: Two Distinct Suppressors for Viral Replication and Viral Movement Encoded by Potato Virus M. J. Virol. 2011, 85, 10269–10278. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Sit, T.L.; Qu, F.; Morris, T.J.; Kim, K.-H.; Lommel, S.A. A Versatile Assay for the Identification of RNA Silencing Suppressors Based on Complementation of Viral Movement. Mol. Plant-Microbe Interact. 2008, 21, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Sit, T.L.; Heinsohn, C.; George, C.G.; Kim, K.-H.; Lommel, S.A. The Red clover necrotic mosaic virus RNA-2 Encoded Movement Protein is a Second Suppressor of RNA Silencing. Virology 2008, 381, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, E.; Lezzhov, A.; Vassetzky, N.; Solovyev, A.; Morozov, S. Acquisition of Full-Length Viral Helicase Domains by Insect Retrotransposon-Encoded Polypeptides. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Kervestin, S.; Jacobson, A. NMD: A Multifaceted Response to Premature Translational Termination. Nat. Rev. Mol. Cell Biol. 2012, 13, 700–712. [Google Scholar] [CrossRef]
- Garcia, D.; Garcia, S.; Voinnet, O. Nonsense-Mediated Decay Serves as a General Viral Restriction Mechanism in Plants. Cell Host Microbe 2014, 16, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Kertész, S.; Kerényi, Z.; Mérai, Z.; Bartos, I.; Pálfy, T.; Barta, E.; Silhavy, D. Both Introns and Long 3′-UTRs Operate as Cis-Acting Elements to Trigger Nonsense-Mediated Decay in Plants. Nucleic Acids Res. 2006, 34, 6147–6157. [Google Scholar] [CrossRef] [PubMed]
- Arkhipov, A.V.V.; Gushchin, V.A.A.; Vishnichenko, V.K.K.; Solovyev, A.G.G. Accumulation of Changes in the Genome of Shallot Virus X Persisting in Vegetatively Reproduced Plants. Dokl. Biochem. Biophys. 2013, 452, 237–240. [Google Scholar] [CrossRef]
- Niehl, A.; Peña, E.J.; Amari, K.; Heinlein, M. Microtubules in Viral Replication and Transport. Plant J. 2013, 75, 290–308. [Google Scholar] [CrossRef]
- Vaucheret, H. Post-Transcriptional Small RNA Pathways in Plants: Mechanisms and Regulations. Genes Dev. 2006, 20, 759–771. [Google Scholar] [CrossRef]
- Ding, S.-W.; Voinnet, O. Antiviral Immunity Directed by Small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. RNA Quality Control as a Key to Suppressing RNA Silencing of Endogenous Genes in Plants. Mol. Plant 2016, 9, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomollon, S.; Baulcombe, D.C. Roles of RNA Silencing in Viral and Non-Viral Plant Immunity and in the Crosstalk between Disease Resistance Systems. Nat. Rev. Mol. Cell Biol. 2022, 23, 645–662. [Google Scholar] [CrossRef]
- Li, F.; Wang, A. RNA Decay Is an Antiviral Defense in Plants That Is Counteracted by Viral RNA Silencing Suppressors. PLOS Pathog. 2018, 14, e1007228. [Google Scholar] [CrossRef]
- Wu, K.; Xie, Q.; Liu, X.; Fu, Y.; Li, S.; Yu, X.; Li, W.; Zhao, P.; Ren, Y.; Ruan, M.; et al. Capsid Protein of Turnip crinkle virus Suppresses Antiviral RNA Decay by Degrading Arabidopsis Dcp1 via Ubiquitination Pathway. Plant J. 2025, 121, e70075. [Google Scholar] [CrossRef]
- Lukhovitskaya, N.; Ryabova, L.A. Cauliflower mosaic virus Transactivator Protein (TAV) Can Suppress Nonsense-Mediated Decay by Targeting VARICOSE, a Scaffold Protein of the Decapping Complex. Sci. Rep. 2019, 9, 7042. [Google Scholar] [CrossRef]
- May, J.P.; Johnson, P.Z.; Ilyas, M.; Gao, F.; Simon, A.E. The Multifunctional Long-Distance Movement Protein of Pea enation mosaic virus 2 Protects Viral and Host Transcripts from Nonsense-Mediated Decay. mBio 2020, 11, e00204-20. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Solovieva, A.D.; Chergintsev, D.A.; Solovyev, A.G.; Morozov, S.Y. Role of Plant Virus Movement Proteins in Suppression of Host RNAi Defense. Int. J. Mol. Sci. 2023, 24, 9049. [Google Scholar] [CrossRef]
- Bayne, E.H.; Rakitina, D.V.; Morozov, S.Y.; Baulcombe, D.C. Cell-to-Cell Movement of Potato Potexvirus X Is Dependent on Suppression of RNA Silencing. Plant J. 2005, 44, 471–482. [Google Scholar] [CrossRef]
- Lim, H.-S.; Vaira, A.M.; Reinsel, M.D.; Bae, H.; Bailey, B.A.; Domier, L.L.; Hammond, J. Pathogenicity of Alternanthera mosaic virus is Affected by Determinants in RNA-Dependent RNA Polymerase and by Reduced Efficacy of Silencing Suppression in a Movement-Competent TGB1. J. Gen. Virol. 2010, 91, 277–287. [Google Scholar] [CrossRef]
- Chiu, M.-H.; Chen, I.-H.; Baulcombe, D.C.; Tsai, C.-H. The Silencing Suppressor P25 of Potato Virus X Interacts with Argonaute1 and Mediates Its Degradation through the Proteasome Pathway. Mol. Plant Pathol. 2010, 11, 641–649. [Google Scholar] [CrossRef]
- Okano, Y.; Senshu, H.; Hashimoto, M.; Neriya, Y.; Netsu, O.; Minato, N.; Yoshida, T.; Maejima, K.; Oshima, K.; Komatsu, K.; et al. In Planta Recognition of a Double-Stranded RNA Synthesis Protein Complex by a Potexviral RNA Silencing Suppressor. Plant Cell 2014, 26, 2168–2183. [Google Scholar] [CrossRef]
- Morozov, S.Y.; Solovyev, A.G. Bioinformatic Analysis Predicts a Novel Genetic Module Related to Triple Gene and Binary Movement Blocks of Plant Viruses: Tetra-Cistron Movement Block. Biomolecules 2022, 12, 861. [Google Scholar] [CrossRef]
- Chergintsev, D.A.; Solovieva, A.D.; Atabekova, A.K.; Lezzhov, A.A.; Golyshev, S.A.; Morozov, S.Y.; Solovyev, A.G. Properties of Plant Virus Protein Encoded by the 5′-Proximal Gene of Tetra-Cistron Movement Block. Int. J. Mol. Sci. 2023, 24, 14144. [Google Scholar] [CrossRef]
- Makarova, S.S.; Solovyev, A.G.; Morozov, S.Y. RNA-Binding Properties of the Plant Protein Nt-4/1. Biochemistry 2014, 79, 717–726. [Google Scholar] [CrossRef]
- Rubino, L.; Russo, M. Molecular Analysis of the Pothos Latent Virus Genome. J. Gen. Virol. 1997, 78, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Mérai, Z.; Kerényi, Z.; Molnár, A.; Barta, E.; Válóczi, A.; Bisztray, G.; Havelda, Z.; Burgyán, J.; Silhavy, D. Aureusvirus P14 Is an Efficient RNA Silencing Suppressor That Binds Double-Stranded RNAs without Size Specificity. J. Virol. 2005, 79, 7217–7226. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, A.G.; Minina, E.A.; Makarova, S.S.; Erokhina, T.N.; Makarov, V.V.; Kaplan, I.B.; Kopertekh, L.; Schiemann, J.; Richert-Pöggeler, K.R.; Morozov, S.Y. Subcellular Localization and Self-Interaction of Plant-Specific Nt-4/1 Protein. Biochimie 2013, 95, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, E.A.; Lezzhov, A.A.; Komarova, T.V.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. A Novel Block of Plant Virus Movement Genes. Mol. Plant Pathol. 2017, 18, 611–624. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chergintsev, D.A.; Lezzhov, A.A.; Lazareva, E.A.; Atabekova, A.K.; Solovieva, A.D.; Morozov, S.Y.; Solovyev, A.G. Shallot virus X p42 Protein Expressed in Concert with Virus Movement Proteins Is a Suppressor of Two Plant Antiviral Defense Mechanisms. Plants 2025, 14, 2552. https://doi.org/10.3390/plants14162552
Chergintsev DA, Lezzhov AA, Lazareva EA, Atabekova AK, Solovieva AD, Morozov SY, Solovyev AG. Shallot virus X p42 Protein Expressed in Concert with Virus Movement Proteins Is a Suppressor of Two Plant Antiviral Defense Mechanisms. Plants. 2025; 14(16):2552. https://doi.org/10.3390/plants14162552
Chicago/Turabian StyleChergintsev, Denis A., Alexander A. Lezzhov, Ekaterina A. Lazareva, Anastasia K. Atabekova, Anna D. Solovieva, Sergey Y. Morozov, and Andrey G. Solovyev. 2025. "Shallot virus X p42 Protein Expressed in Concert with Virus Movement Proteins Is a Suppressor of Two Plant Antiviral Defense Mechanisms" Plants 14, no. 16: 2552. https://doi.org/10.3390/plants14162552
APA StyleChergintsev, D. A., Lezzhov, A. A., Lazareva, E. A., Atabekova, A. K., Solovieva, A. D., Morozov, S. Y., & Solovyev, A. G. (2025). Shallot virus X p42 Protein Expressed in Concert with Virus Movement Proteins Is a Suppressor of Two Plant Antiviral Defense Mechanisms. Plants, 14(16), 2552. https://doi.org/10.3390/plants14162552





