Effects of Light–Nitrogen Interactions on Leaf Functional Traits of (Picea neoveitchii Mast.)
Abstract
1. Introduction
2. Results
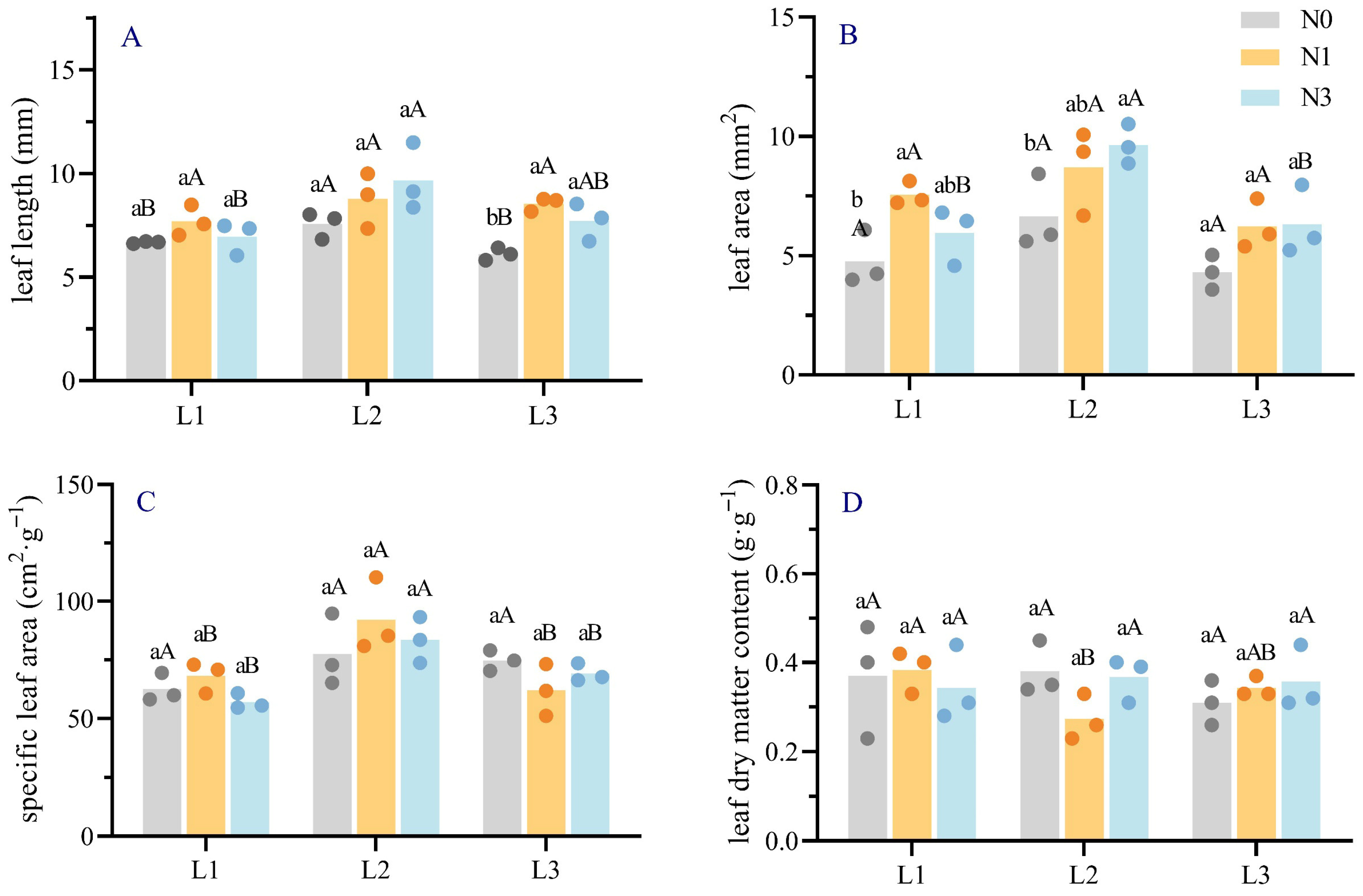
2.1. Effects of Light–Nitrogen Interaction on Needle Morphological and Anatomical Traits of Picea neoveitchii Mast. Seedlings
2.2. Effects of Light–Nitrogen Interaction on N and P Stoichiometric Characteristics of Picea neoveitchii Mast. Seedling Needles
2.3. Effects of Light–Nitrogen Interaction on Photosynthetic Physiological Traits of Picea neoveitchii Mast. Seedling Needles
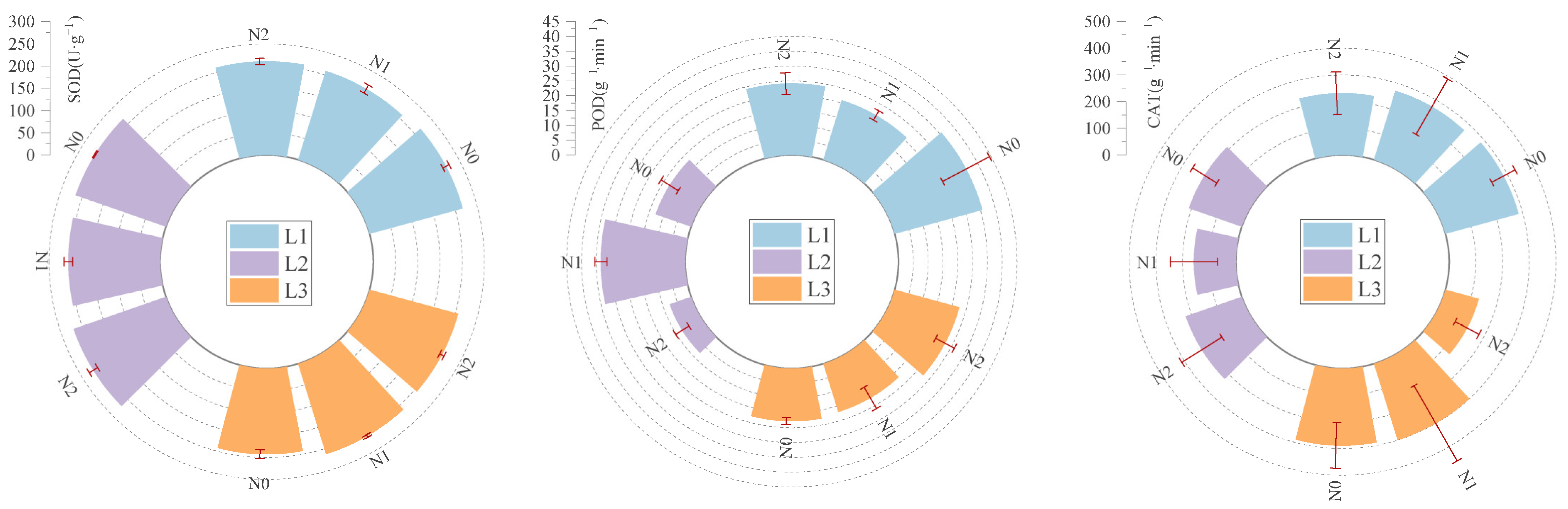
2.4. Effects of Light–Nitrogen Interaction on Stress Resistance Physiological Traits of Picea neoveitchii Mast. Seedling Needles
2.5. Membership Function Analysis of Leaf Functional Traits of Picea neoveitchii Mast. Seedlings
3. Discussion
3.1. Effects of Light–Nitrogen Interaction on Needle Morphological Traits of Picea neoveitchii Mast. Seedlings
3.2. Effects of Light–Nitrogen Interaction on Photosynthetic Physiological Traits of Picea neoveitchii Mast. Seedling Needles
3.3. Effects of Light–Nitrogen Interaction on Stress Resistance Physiological Traits of Picea neoveitchii Mast. Seedling Needles
4. Materials and Methods
4.1. Study Area
4.2. Experimental Materials
4.3. Experimental Design
4.4. Index Measurements
4.4.1. Basic Measurements
4.4.2. Photosynthetic and Chlorophyll Fluorescence Parameters
4.4.3. Photosynthetic Pigments and Relative Conductivity
4.4.4. Protective Enzyme Activity
4.4.5. Osmoregulatory Substances
4.5. Membership Function Calculation
4.6. Data Analysis
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, P.; Zhu, L.; Zhao, M.; Li, C.; Zhang, Y.; Li, L. The First Transcriptome Sequencing and Analysis of the Endangered Plant Species Picea neoveitchii Mast. and Potential EST-SSR Markers Development. Biotechnol. Biotechnol. Equip. 2019, 33, 967–973. [Google Scholar] [CrossRef]
- Xue, N.; Li, K.; Chen, K.; Li, P.; Ji, X.; Ma, Z.; Ji, W. Predicting Climate Change Impacts on Distribution and Conservation of Critically Endangered Picea neoveitchii Using MaxEnt. Front. For. Glob. Change 2024, 7, 1472857. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Bush, E.R.; Whytock, R.C.; Bahaa-El-Din, L.; Bourgeois, S.; Bunnefeld, N.; Cardoso, A.W.; Dikangadissi, J.T.; Dimbonda, P.; Dimoto, E.; Ndong, J.E.; et al. Long-Term Collapse in Fruit Availability Threatens Central African Forest Megafauna. Science 2020, 370, 1219–1221. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Cun, Z.; Wu, H.-M.; Zhang, J.-Y.; Shuang, S.-P.; Hong, J.; An, T.-X.; Chen, J.-W. High Nitrogen Inhibits Biomass and Saponins Accumulation in a Medicinal Plant Panax Notoginseng. PeerJ 2023, 11, e14933. [Google Scholar] [CrossRef]
- Sugiura, D.; Tateno, M. Optimal Leaf-to-Root Ratio and Leaf Nitrogen Content Determined by Light and Nitrogen Availabilities. PLoS ONE 2011, 6, e22236. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, C.; Gou, L.; Yang, H.; Liu, G. Functional Identification and Genetic Transformation of the Ammonium Transporter PtrAMT1;6 in Populus. Int. J. Mol. Sci. 2023, 24, 8511. [Google Scholar] [CrossRef]
- Alanazi, A.K.; Abo-Dief, H.M.; Alothman, Z.A.; Mohamed, A.T.; Pramanik, T.; Alotaibi, S.H. Effect of Plant Nanocellulose Electrolyte, Zinc Oxide Nanoparticles, and Nano-Chlorophyll Sensitiser on the Dye-Sensitised Solar Cell Performance. Crystals 2022, 12, 1771. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of Different Light Sources on the Growth of Non-Heading Chinese Cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Poorter, H.; Yin, X.; Alyami, N.; Gibon, Y.; Pons, T.L. MetaPhenomics: Quantifying the Many Ways Plants Respond to Their Abiotic Environment, Using Light Intensity as an Example. Plant Soil 2022, 476, 421–454. [Google Scholar] [CrossRef]
- Monib, A.W.; Alimyar, O.; Mohammad, M.U.; Akhundzada, M.S.; Niazi, P. Macronutrients for Plants Growth and Humans Health. J. Res. Appl. Sci. Biotechnol. 2023, 2, 268–279. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Zhang, R.; Nie, L.; Huang, M.; Yang, H.; Shi, C.; Wei, Y.; Song, L.; Zhu, J.; Bo, H.; Wang, J.; et al. Effects of Irrigation and Nitrogen Application on Soil Nutrients in Triploid Populus tomentosa Stands. Forests 2022, 13, 1046. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dise, N.B.; Gowing, D.J.G.; Mountford, J.O. Loss of Forb Diversity in Relation to Nitrogen Deposition in the UK: Regional Trends and Potential Controls. Glob. Change Biol. 2006, 12, 1823–1833. [Google Scholar] [CrossRef]
- Joseph Wright, S.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Diaz, S.; et al. Functional Traits and the Growth-Mortality Trade-off in Tropical Trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, U. Glandular Trichomes as a Barrier against Atmospheric Oxidative Stress: Relationships with Ozone Uptake, Leaf Damage, and Emission of LOX Products across a Diverse Set of Species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Teraa, S.; Bencherif, M. From Hygrothermal Adaptation of Endemic Plants to Meteorosensitive Biomimetic Architecture: Case of Mediterranean Biodiversity Hotspot in Northeastern Algeria. Environ Dev Sustain 2022, 24, 10876–10901. [Google Scholar] [CrossRef]
- Lv, Y.; Shao, G.; Qiu, J.; Jiao, G.; Sheng, Z.; Xie, L.; Wu, Y.; Tang, S.; Wei, X.; Hu, P. White Leaf and Panicle 2, Encoding a PEP-Associated Protein, Is Required for Chloroplast Biogenesis under Heat Stress in Rice. J. Exp. Bot. 2017, 68, 5147–5160. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf Nitrogen and Phosphorus Stoichiometry across 753 Terrestrial Plant Species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Hua, J.; Wang, W.; Huo, J.; Wu, L.; Huang, L.; Zhong, H. Effects of Ecosystem Recovery Types on Soil Phosphorus Bioavailability, Roles of Plant and Microbial Diversity: A Meta-analysis. Ecol. Evol. 2025, 15, e71172. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global Patterns of Plant Leaf N and P in Relation to Temperature and Latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Rumyantseva, N.I.; Valieva, A.I.; Kostyukova, Y.A.; Ageeva, M.V. The Effect of Leaf Plasticity on the Isolation of Apoplastic Fluid from Leaves of Tartary Buckwheat Plants Grown In Vivo and In Vitro. Plants 2023, 12, 4048. [Google Scholar] [CrossRef]
- Argus, R.E.; Colmer, T.D.; Grierson, P.F. Early Physiological Flood Tolerance Is Followed by Slow Post-Flooding Root Recovery in the Dryland Riparian Tree Eucalyptus camaldulensis Subsp Refulgens. Plant Cell Environ. 2015, 38, 1189–1199. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, Noninvasive Screening for Perturbations of Metabolism and Plant Growth Using Chlorophyll Fluorescence Imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Sehar, Z.; Iqbal, N.; Alvi, A.F.; Abdi, G.; Proestos, C.; Khan, N.A. Sulfur Supplementation Enhances Nitric Oxide Efficacy in Reversal of Chromium-Inhibited Calvin Cycle Enzymes, Photosynthetic Activity, and Carbohydrate Metabolism in Wheat. Sci. Rep. 2023, 13, 6858. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-analyses of Interspecific Variation and Environmental Control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Q.; Tigabu, M.; Jin, S.; Ma, X.; Liu, B. Plastic Responses in Growth, Morphology, and Biomass Allocation of Five Subtropical Tree Species to Different Degrees of Shading. Forests 2022, 13, 996. [Google Scholar] [CrossRef]
- Modrzynski, J.; Chmura, D.J.; Tjoelker, M.G. Seedling Growth and Biomass Allocation in Relation to Leaf Habit and Shade Tolerance among 10 Temperate Tree Species. Tree Physiol. 2015, 35, 879–893. [Google Scholar] [CrossRef]
- Gong, W.; Qi, P.; Du, J.; Sun, X.; Wu, X.; Song, C.; Liu, W.; Wu, Y.; Yu, X.; Yong, T.; et al. Transcriptome Analysis of Shade-Induced Inhibition on Leaf Size in Relay Intercropped Soybean. PLoS ONE 2014, 9, e98465. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Sembiring, M.; Sabrina, T. Effectiveness of Dyella Japonica and Enterobacter Cloacae as Biofertilizers to Increase Maize (Zea mays) Production on Andisol Soil. Biodiversitas J. Biol. Divers. 2022, 23, 3338–3343. [Google Scholar] [CrossRef]
- Wyka, T.P.; Oleksyn, J.; Zytkowiak, R.; Karolewski, P.; Jagodzinski, A.M.; Reich, P.B. Responses of Leaf Structure and Photosynthetic Properties to Intra-Canopy Light Gradients: A Common Garden Test with Four Broadleaf Deciduous Angiosperm and Seven Evergreen Conifer Tree Species. Oecologia 2012, 170, 11–24. [Google Scholar] [CrossRef]
- Li, A.-M.; Wang, M.; Chen, Z.-L.; Qin, C.-X.; Liao, F.; Wu, Z.; He, W.-Z.; Lakshmanan, P.; Pan, Y.-Q.; Huang, D.-L. Integrated Transcriptome and Metabolome Analysis to Identify Sugarcane Gene Defense against Fall Armyworm (Spodoptera frugiperda) Herbivory. Int. J. Mol. Sci. 2022, 23, 13712. [Google Scholar] [CrossRef]
- Van Leur, H.; Vet, L.E.M.; Van Der Putten, W.H.; Van Dam, N.M. Barbarea vulgaris Glucosinolate Phenotypes Differentially Affect Performance and Preference of Two Different Species of Lepidopteran Herbivores. J. Chem. Ecol. 2008, 34, 121–131. [Google Scholar] [CrossRef]
- Güsewell, S. N:P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Zhao, L.; Cai, B.; Zhang, X.; Zhang, B.; Feng, J.; Zhou, D.; Chen, Y.; Zhang, M.; Qi, D.; Wang, W.; et al. Physiological and Transcriptional Characteristics of Banana Seedlings in Response to Nitrogen Deficiency Stress. Horticulturae 2024, 10, 290. [Google Scholar] [CrossRef]
- Perring, M.P.; Hedin, L.O.; Levin, S.A.; McGroddy, M.; De Mazancourt, C. Increased Plant Growth from Nitrogen Addition Should Conserve Phosphorus in Terrestrial Ecosystems. Proc. Natl. Acad. Sci. USA 2008, 105, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.Y.; van Geloof, I.; Rietkerk, M. High Solar Radiation Hinders Tree Regeneration above the Alpine Treeline in Northern Ecuador. Plant Ecol. 2007, 191, 33–45. [Google Scholar] [CrossRef]
- Naresh, K.S.; Kasturi Bai, K.V. Photo-Oxidative Stress in Coconut Seedlings: Early Events to Leaf Scorching and Seedling Death. Braz. J. Plant Physiol. 2009, 21, 223–232. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Sun, J.; Ye, M.; Peng, S.; Li, Y. Nitrogen Can Improve the Rapid Response of Photosynthesis to Changing Irradiance in Rice (Oryza sativa L.) Plants. Sci. Rep. 2016, 6, 31305. [Google Scholar] [CrossRef]
- Balazadeh, S.; Schildhauer, J.; Araujo, W.L.; Munne-Bosch, S.; Fernie, A.R.; Proost, S.; Humbeck, K.; Mueller-Roeber, B. Reversal of Senescence by N Resupply to N-Starved Arabidopsis Thaliana: Transcriptomic and Metabolomic Consequences. J. Exp. Bot. 2014, 65, 3975–3992. [Google Scholar] [CrossRef]
- Aliarab, A.; Vazifekhah, E.O.; Sadati, S.-E. Effect of Soil Moisture Content and Nitrogen Fertilizer on Survival, Growth and Some Physiological Characteristics of Platycladus orientalis Seedlings. J. For. Sci. 2020, 66, 511–523. [Google Scholar] [CrossRef]
- Wang, G.; Mao, J.; Ji, M.; Wang, W.; Fu, J. A Comprehensive Assessment of Photosynthetic Acclimation to Shade in C4 Grass (Cynodon dactylon (L.) Pers.). BMC Plant Biol. 2024, 24, 591. [Google Scholar] [CrossRef]
- Losacco, D.; Ancona, V.; De Paola, D.; Tumolo, M.; Massarelli, C.; Gatto, A.; Uricchio, V.F. Development of Ecological Strategies for the Recovery of the Main Nitrogen Agricultural Pollutants: A Review on Environmental Sustainability in Agroecosystems. Sustainability 2021, 13, 7163. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Harkess, R.L.; Denny, G.C.; Scagel, C. Nitrogen Fertilization and Irrigation Frequency Affect Hydrangea Growth and Nutrient Uptake in Two Container Types. Hortscience 2019, 54, 167–174. [Google Scholar] [CrossRef]
- Zlobin, I.E.; Kartashov, A.V.; Pashkovskiy, P.P.; Ivanov, Y.V.; Kreslavski, V.D.; Kuznetsov, V.V. Comparative Photosynthetic Responses of Norway Spruce and Scots Pine Seedlings to Prolonged Water Deficiency. J. Photochem. Photobiol. B-Biol. 2019, 201, 111659. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Zhao, D.; Liu, H.; Fu, W.; Li, G.; Hao, L. Paraburkholderia Sp. GD17 Improves Tomato Plant Growth and Resistance to Botrytis Cinerea-Induced Disease. Plant Soil 2023, 486, 487–502. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. IJMS 2021, 22, 9326. [Google Scholar] [CrossRef]
- Abdulfatah, H.F. Non-Enzymatic Antioxidants in Stressed Plants: A Review. J. Univ. Anbar Pure Sci. 2022, 16, 25–37. [Google Scholar] [CrossRef]
- Rinalducci, S.; Murgiano, L.; Zolla, L. Redox Proteomics: Basic Principles and Future Perspectives for the Detection of Protein Oxidation in Plants. J. Exp. Bot. 2008, 59, 3781–3801. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Sikder, R.K.; Wang, X.; Zhang, H.; Gui, H.; Dong, Q.; Jin, D.; Song, M. Nitrogen Enhances Salt Tolerance by Modulating the Antioxidant Defense System and Osmoregulation Substance Content in Gossypium hirsutum. Plants 2020, 9, 450. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Ajmal, M.; Ullah, R.; Muhammad, Z.; Khan, M.N.; Kakar, H.A.; Kaplan, A.; Okla, M.K.; Saleh, I.A.; Kamal, A.; Abdullah, A.; et al. Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung bean). Molecules 2023, 28, 5059. [Google Scholar] [CrossRef]
- Jhanzab, H.M.; Qayyum, A.; Bibi, Y.; Sher, A.; Hayat, M.T.; Iqbal, J.; Qamar, M.; Elesawy, B.H.; Ismail, K.A.; Gharib, A.F.; et al. Chemo-Blended Ag & Fe Nanoparticles Effect on Growth, Physiochemical and Yield Traits of Wheat (Triticum aestivum). Agronomy 2022, 12, 757. [Google Scholar] [CrossRef]
- Addor, F.A.S. Antioxidants in Dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Sewell, R.D.E.; Rafieian-Kopaei, M. Antioxidants and Atherosclerosis: Mechanistic Aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, X.; Song, C.; Wang, Q.; Deng, L.; Zhong, S. Cenozoic Evolution of the Western Qinling Mt. Range Based on Thermochronologic and Sedimentary Records from the Wudu Basin, NE Tibetan Plateau. J. Asian Earth Sci. 2017, 138, 484–494. [Google Scholar] [CrossRef]
- He, S.; Wang, D.; Li, Y.; Zhao, P. Land Use Changes and Their Driving Forces in a Debris Flow Active Area of Gansu Province, China. Sustainability 2018, 10, 2759. [Google Scholar] [CrossRef]
- Yang, P.; Xu, L.; Xu, H.; Tang, Y.; He, G.; Cao, Y.; Feng, Y.; Yuan, S.; Ming, J. Histological and Transcriptomic Analysis during Bulbil Formation in Lilium Lancifolium. Front. Plant Sci. 2017, 8, 1508. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Yin, B.; Downing, A. Sensitivity of the Xerophytic Moss Syntrichia caninervis to Prolonged Simulated Nitrogen Deposition. Ann. Bot. 2016, 117, 1153–1161. [Google Scholar] [CrossRef]
- Bebıtoglu, B.T.; Oguz, E.; Acet, N.G.; Hodzıc, A.; Temel, F.; Ada, S.; Kılıckap, A. The Neuroprotective Effect of Lamotrigine against Glutamate Excitotoxicity in SH-SY5Y Human Neuroblastoma Cells. Marmara Med. J. 2020, 33, 146–152. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C.; Chance, B. The Assay of Catalases and Peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef] [PubMed]


| Anatomical Characteristics | N Treatment | Shading Treatment | ||
|---|---|---|---|---|
| L1 100% Full Light | L2 70% Full Light | L3 40% Full Light | ||
| Needle thickness (mm) | N0 | 0.60 ± 0.14 aA | 0.79 ± 0.06 aA | 0.60 ± 0.03 aA |
| N1 | 0.62 ± 0.14 aA | 0.68 ± 0.02 abA | 0.55 ± 0.05 aA | |
| N2 | 0.66 ± 0.06 aA | 0.54 ± 0.11 bA | 0.62 ± 0.09 aA | |
| Stomatal density (No. mm2) | N0 | 56.19 ± 20.72 aA | 23.32 ± 3.13 bB | 31.19 ± 3.53 aB |
| N1 | 33.19 ± 7.36 bA | 23.72 ± 3.84 bA | 27.95 ± 1.95 aA | |
| N2 | 35.82 ± 4.83 abA | 37.81 ± 5.22 aA | 32.90 ± 3.81 aA | |
| Needle cross-sectional area (10−2 mm2) | N0 | 40.01 ± 1.84 aB | 81.07 ± 11.60 aA | 54 ± 6.67 aB |
| N1 | 49.08 ± 6.34 aA | 55.72 ± 6.15 bA | 47.6 ± 3.30 aA | |
| N2 | 52.33 ± 9.84 aA | 50.75 ± 8.19 bA | 50.2 ± 2.59 aA | |
| Perimeter of needle section (mm) | N0 | 2.88 ± 0.47 aA | 3.58 ± 0.43 aA | 3.01 ± 0.28 aA |
| N1 | 2.78 ± 0.19 aA | 2.94 ± 0.16 bA | 2.85 ± 0.01 aA | |
| N2 | 2.83 ± 0.28 aA | 3.1 ± 0.20 abA | 2.87 ± 0.17 aA | |
| Epidermal tissue area (10−2 mm2) | N0 | 6.03 ± 1.49 aA | 8.85 ± 1.81 aA | 6.59 ± 0.47 aA |
| N1 | 5.93 ± 1.22 aA | 6.49 ± 0.96 aA | 6.85 ± 0.57 aA | |
| N2 | 7.13 ± 1.44 aA | 6.83 ± 0.84 aA | 6.51 ± 0.84 aA | |
| Mesophyll tissue area (10−2 mm2) | N0 | 30.72 ± 1.73 aB | 66.81 ± 9.87 aA | 42.3 ± 5.93 aB |
| N1 | 38.12 ± 7.04 aA | 45.02 ± 5.32 bA | 36.38 ± 3.19 aA | |
| N2 | 40.15 ± 7.28 aA | 40.25 ± 5.83 bA | 39.71 ± 2.25 aA | |
| Resin cavity area (10−2 mm2) | N0 | 0.08 ± 0.02 bB | 0.16 ± 0.09 aB | 0.38 ± 0.13 aA |
| N1 | 0.18 ± 0.04 aA | 0.19 ± 0.04 aA | 0.26 ± 0.06 abA | |
| N2 | 0.18 ± 0.03 aA | 0.08 ± 0.02 aA | 0.16 ± 0.08 bA | |
| Endocortical area (10−2 mm2) | N0 | 1.35 ± 0.15 aB | 1.88 ± 0.2 aA | 1.56 ± 0.15 aAB |
| N1 | 1.27 ± 0.16 aA | 1.49 ± 0.14 abA | 1.38 ± 0.15 abA | |
| N2 | 1.41 ± 0.27 aA | 1.22 ± 0.31 bA | 1.20 ± 0.06 bA | |
| Central column area (10−2 mm2) | N0 | 2.27 ± 0.20 bB | 3.36 ± 0.18 aA | 3.29 ± 0.11 aA |
| N1 | 2.53 ± 0.28 bA | 2.55 ± 0.24 bA | 2.73 ± 0.5 aA | |
| N2 | 3.14 ± 0.26 aA | 1.88 ± 0.27 cB | 2.61 ± 0.35 aA | |
| Mesophyll tissue area ratio (%) | N0 | 76.76 ± 0.91 bB | 82.38 ± 0.47 aA | 78.23 ± 1.51 aB |
| N1 | 79.89 ± 1.02 aA | 80.76 ± 1.20 abA | 76.37 ± 1.35 aB | |
| N2 | 76.79 ± 0.59 bA | 79.43 ± 1.29 bA | 79.11 ± 1.89 aA | |
| Resin cavity area ratio (%) | N0 | 0.19 ± 0.057 bB | 0.19 ± 0.077 bB | 0.70 ± 0.149 aA |
| N1 | 0.36 ± 0.10 aA | 0.35 ± 0.099 aA | 0.55 ± 0.10 abA | |
| N2 | 0.35 ± 0.08 aA | 0.16 ± 0.017 bB | 0.33 ± 0.18 bAB | |
| Area ratio of central column (%) | N0 | 5.61 ± 0.28 aA | 4.21 ± 0.74 aB | 6.13 ± 0.59 aA |
| N1 | 5.19 ± 0.51 aAB | 4.59 ± 0.36 aB | 5.69 ± 0.68 aA | |
| N2 | 6.10 ± 0.88 aA | 3.71 ± 0.20 aB | 5.20 ± 0.57 aA | |
| Photosynthetic Pigment | N Treatment | Shading Treatment | ||
|---|---|---|---|---|
| L1 100% Full Light | L2 70% Full Light | L3 40% Full Light | ||
| Chlorophyll a content (mg·g−1) | N0 | 0.245 ± 0.058 aA | 0.162 ± 0.034 aA | 0.216 ± 0.03 aA |
| N1 | 0.162 ± 0.027 bA | 0.152 ± 0.046 aA | 0.127 ± 0.027 bA | |
| N2 | 0.143 ± 0.018 bB | 0.171 ± 0.036 aB | 0.261 ± 0.025 aA | |
| Chlorophyll b content (mg·g−1) | N0 | 0.151 ± 0.042 aA | 0.086 ± 0.009 aA | 0.124 ± 0.043 bA |
| N1 | 0.093 ± 0.021 bA | 0.089 ± 0.041 aA | 0.046 ± 0.011 cA | |
| N2 | 0.063 ± 0.006 bB | 0.092 ± 0.029 aB | 0.205 ± 0.033 aA | |
| Total chlorophyll content (mg·g−1) | N0 | 0.396 ± 0.100 aA | 0.248 ± 0.043 aB | 0.339 ± 0.054 bAB |
| N1 | 0.255 ± 0.048 bA | 0.240 ± 0.087 aA | 0.173 ± 0.038 cA | |
| N2 | 0.206 ± 0.015 bB | 0.264 ± 0.065 aB | 0.465 ± 0.052 aA | |
| Carotenoid content (mg·g−1) | N0 | 0.061 ± 0.015 aA | 0.056 ± 0.005 aA | 0.052 ± 0.017 aA |
| N1 | 0.053 ± 0.010 aA | 0.044 ± 0.011 aA | 0.050 ± 0.010 aA | |
| N2 | 0.056 ± 0.004 aA | 0.055 ± 0.010 aA | 0.038 ± 0.013 aA | |
| Resistance Physiological traits | N Treatment | Shading Treatment | ||
|---|---|---|---|---|
| L1 100% Full Light | L2 70% Full Light | L3 40% Full Light | ||
| Soluble protein content (mg·g−1) | N0 | 1.71 ± 0.27 aA | 1.47 ± 0.39 abA | 1.44 ± 0.04 aA |
| N1 | 1.68 ± 0.03 aA | 1.19 ± 0.11 bB | 1.18 ± 0.14 aB | |
| N2 | 1.09 ± 0.24 bA | 1.73 ± 0.08 aA | 1.40 ± 0.47 aA | |
| Free proline content (μg·g−1) | N0 | 304.95 ± 28.67 bA | 287.00 ± 22.00 aA | 312.84 ± 15.17 bA |
| N1 | 314.56 ± 10.85 bAB | 352.51 ± 37.09 aA | 281.23 ± 12.48 bB | |
| N2 | 445.05 ± 18.41 aA | 346.15 ± 55.87 aB | 390.19 ± 48.11 aAB | |
| Malondialdehyde content (m·mol g−1) | N0 | 1.3960.815 aA | 0.676 ± 0.217 aAB | 0.472 ± 0.098 aB |
| N1 | 0.453 ± 0.195 bA | 0.550 ± 0.097 aA | 0.686 ± 0.355 aA | |
| N2 | 0.772 ± 0.337 abA | 0.499 ± 0.036 aA | 0.970 ± 0.432 aA | |
| Relative electrical conductivity (%) | N0 | 73.42 ± 3.99 aA | 60.66 ± 7.35 aA | 64.77 ± 9.97 aA |
| N1 | 63.60 ± 4.80 aA | 55.22 ± 3.40 aB | 37.00 ± 0.36 bC | |
| N2 | 64.60 ± 9.95 aA | 38.17 ± 4.17 bB | 52.07 ± 4.92 aA | |
| Soluble sugar content (mg·g−1) | N0 | 58.47 ± 6.04 aB | 70.50 ± 2.43 aA | 48.90 ± 5.99 aB |
| N1 | 65.95 ± 9.43 aA | 51.49 ± 6.53 bB | 47.19 ± 1.35 aB | |
| N2 | 59.17 ± 9.77 aA | 55.61 ± 11.89 abA | 50.38 ± 14.07 aA | |
| Starch content (mg·g−1) | N0 | 9.99 ± 0.53 bB | 11.53 ± 0.97 aAB | 11.82 ± 0.76 aA |
| N1 | 9.96 ± 1.56 bA | 11.66 ± 1.35 aA | 10.01 ± 0.63 bA | |
| N2 | 13.92 ± 2.27 aA | 12.52 ± 2.43 aAB | 9.12 ± 0.98 bB | |
| Unstructured carbohydrates (mg·g−1) | N0 | 68.47 ± 6.49 aB | 82.03 ± 1.76 aA | 60.71 ± 6.54 aB |
| N1 | 75.91 ± 10.88 aA | 63.15 ± 6.14 bAB | 57.19 ± 1.82 aB | |
| N2 | 73.08 ± 11.88 aA | 68.13 ± 13.98 abA | 59.5 ± 15.03 aA | |
| Index | Subordinate Function Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L1N0 | L1N1 | L1N2 | L2N0 | L2N1 | L2N2 | L3N0 | L3N1 | L3N2 | |
| Needle thickness | 0.158 | 0.445 | 0.237 | 0.408 | 0.746 | 1.000 | 0.000 | 0.685 | 0.448 |
| Leaf area | 0.086 | 0.610 | 0.308 | 0.437 | 0.824 | 1.000 | 0.000 | 0.360 | 0.375 |
| Specific leaf area | 0.156 | 0.316 | 0.000 | 0.586 | 1.000 | 0.754 | 0.502 | 0.142 | 0.345 |
| Leaf dry matter content | 0.900 | 1.000 | 0.600 | 1.000 | 0.000 | 0.900 | 0.300 | 0.600 | 0.700 |
| Needle thickness | 0.260 | 0.309 | 0.488 | 1.000 | 0.569 | 0.000 | 0.248 | 0.049 | 0.341 |
| Stomatal density | 1.000 | 0.300 | 0.380 | 0.000 | 0.012 | 0.441 | 0.239 | 0.141 | 0.291 |
| Needle cross-sectional area | 0.000 | 0.221 | 0.300 | 1.000 | 0.383 | 0.262 | 0.341 | 0.185 | 0.248 |
| Perimeter of needle section | 0.125 | 0.000 | 0.063 | 1.000 | 0.200 | 0.400 | 0.288 | 0.088 | 0.113 |
| Epidermal tissue area | 0.034 | 0.000 | 0.411 | 1.000 | 0.192 | 0.308 | 0.226 | 0.315 | 0.199 |
| Mesophyll tissue area | 0.000 | 0.205 | 0.261 | 1.000 | 0.396 | 0.264 | 0.321 | 0.157 | 0.249 |
| Resin cavity area | 0.000 | 0.333 | 0.333 | 0.267 | 0.367 | 0.000 | 1.000 | 0.600 | 0.267 |
| Endocortical area | 0.221 | 0.103 | 0.309 | 1.000 | 0.426 | 0.029 | 0.529 | 0.265 | 0.000 |
| Central column area | 0.264 | 0.439 | 0.851 | 1.000 | 0.453 | 0.000 | 0.953 | 0.574 | 0.493 |
| Mesophyll tissue area ratio | 0.065 | 0.586 | 0.070 | 1.000 | 0.730 | 0.509 | 0.309 | 0.000 | 0.456 |
| Resin cavity area ratio | 0.049 | 0.368 | 0.344 | 0.056 | 0.346 | 0.000 | 1.000 | 0.721 | 0.314 |
| Area ratio of central column | 0.785 | 0.612 | 0.988 | 0.207 | 0.364 | 0.000 | 1.000 | 0.818 | 0.616 |
| N content of needles | 0.275 | 0.237 | 1.000 | 0.000 | 0.397 | 0.971 | 0.369 | 0.437 | 0.724 |
| P content of needles | 1.000 | 0.207 | 0.272 | 0.482 | 0.327 | 0.267 | 0.764 | 0.173 | 0.000 |
| N/P | 1.000 | 0.671 | 0.138 | 0.984 | 0.620 | 0.164 | 0.872 | 0.404 | 0.000 |
| Chlorophyll a content | 0.881 | 0.261 | 0.119 | 0.261 | 0.187 | 0.328 | 0.664 | 0.000 | 1.000 |
| Chlorophyll b content | 0.660 | 0.296 | 0.107 | 0.252 | 0.270 | 0.289 | 0.491 | 0.000 | 1.000 |
| Total chlorophyll content | 0.764 | 0.281 | 0.113 | 0.257 | 0.229 | 0.312 | 0.568 | 0.000 | 1.000 |
| Carotenoid content | 1.000 | 0.652 | 0.783 | 0.783 | 0.261 | 0.739 | 0.609 | 0.522 | 0.000 |
| Net photosynthetic rate | 0.260 | 0.419 | 0.354 | 0.574 | 1.000 | 0.623 | 0.000 | 0.567 | 0.933 |
| Stomatal conductance | 0.056 | 0.022 | 0.377 | 0.242 | 0.593 | 0.288 | 0.000 | 0.375 | 1.000 |
| Intercellular CO2 concentration | 0.303 | 0.000 | 0.722 | 0.443 | 0.628 | 0.530 | 1.000 | 0.487 | 0.642 |
| Transpiration rate | 0.285 | 0.170 | 0.443 | 0.308 | 0.364 | 0.324 | 0.000 | 0.611 | 1.000 |
| Fv/Fm | 0.398 | 0.000 | 0.496 | 1.000 | 0.235 | 0.656 | 0.494 | 0.060 | 0.438 |
| Fv/Fo | 0.237 | 0.000 | 0.301 | 1.000 | 0.123 | 0.488 | 0.313 | 0.029 | 0.285 |
| SOD activity | 0.890 | 0.581 | 0.652 | 0.824 | 0.503 | 1.000 | 0.000 | 0.722 | 0.504 |
| POD activity | 1.000 | 0.581 | 0.719 | 0.219 | 0.909 | 0.000 | 0.443 | 0.456 | 0.650 |
| CAT activity | 0.908 | 0.832 | 0.603 | 0.475 | 0.168 | 0.547 | 0.939 | 1.000 | 0.000 |
| Soluble protein content | 0.969 | 0.922 | 0.000 | 0.594 | 0.156 | 1.000 | 0.547 | 0.141 | 0.484 |
| Free proline content | 0.145 | 0.203 | 1.000 | 0.035 | 0.435 | 0.396 | 0.193 | 0.000 | 0.665 |
| Malondialdehyde content | 0.000 | 1.000 | 0.661 | 0.763 | 0.897 | 0.951 | 0.980 | 0.752 | 0.452 |
| Relative electrical conductivity | 0.000 | 0.075 | 0.040 | 0.316 | 0.366 | 0.611 | 0.034 | 1.000 | 0.476 |
| Soluble sugar content | 0.484 | 0.805 | 0.514 | 1.000 | 0.184 | 0.361 | 0.073 | 0.000 | 0.137 |
| Starch content | 0.181 | 0.175 | 1.000 | 0.502 | 0.529 | 0.708 | 0.563 | 0.185 | 0.000 |
| NSC content | 0.454 | 0.754 | 0.640 | 1.000 | 0.240 | 0.440 | 0.142 | 0.000 | 0.093 |
| Mean | 0.409 | 0.389 | 0.435 | 0.592 | 0.439 | 0.462 | 0.433 | 0.362 | 0.419 |
| Order | 7 | 8 | 4 | 1 | 3 | 2 | 5 | 9 | 6 |
| N Treatment | Shade Levels | ||
|---|---|---|---|
| L1 (100% Full Light) | L2 (70% Full Light) | L3 (40% Full Light) | |
| N0 (0 g m−2·a−1) | L1N0 (CK) | L2N0 | L3N0 |
| N0 (10 g m−2·a−1) | L1N1 | L2N1 | L3N1 |
| N1 (20 g m−2·a−1) | L1N2 | L2N2 | L3N2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yang, S.; Liu, W.; Li, K.; Xue, N.; Ji, W. Effects of Light–Nitrogen Interactions on Leaf Functional Traits of (Picea neoveitchii Mast.). Plants 2025, 14, 2550. https://doi.org/10.3390/plants14162550
Chen S, Yang S, Liu W, Li K, Xue N, Ji W. Effects of Light–Nitrogen Interactions on Leaf Functional Traits of (Picea neoveitchii Mast.). Plants. 2025; 14(16):2550. https://doi.org/10.3390/plants14162550
Chicago/Turabian StyleChen, Sibo, Siyu Yang, Wanting Liu, Kaiyuan Li, Ninghan Xue, and Wenli Ji. 2025. "Effects of Light–Nitrogen Interactions on Leaf Functional Traits of (Picea neoveitchii Mast.)" Plants 14, no. 16: 2550. https://doi.org/10.3390/plants14162550
APA StyleChen, S., Yang, S., Liu, W., Li, K., Xue, N., & Ji, W. (2025). Effects of Light–Nitrogen Interactions on Leaf Functional Traits of (Picea neoveitchii Mast.). Plants, 14(16), 2550. https://doi.org/10.3390/plants14162550





