Intermediate Anthropogenic Disturbances Boost Taxonomic and Phylogenetic Diversities but Reduce Functional Diversity in Subtropical Forests of Eastern China
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
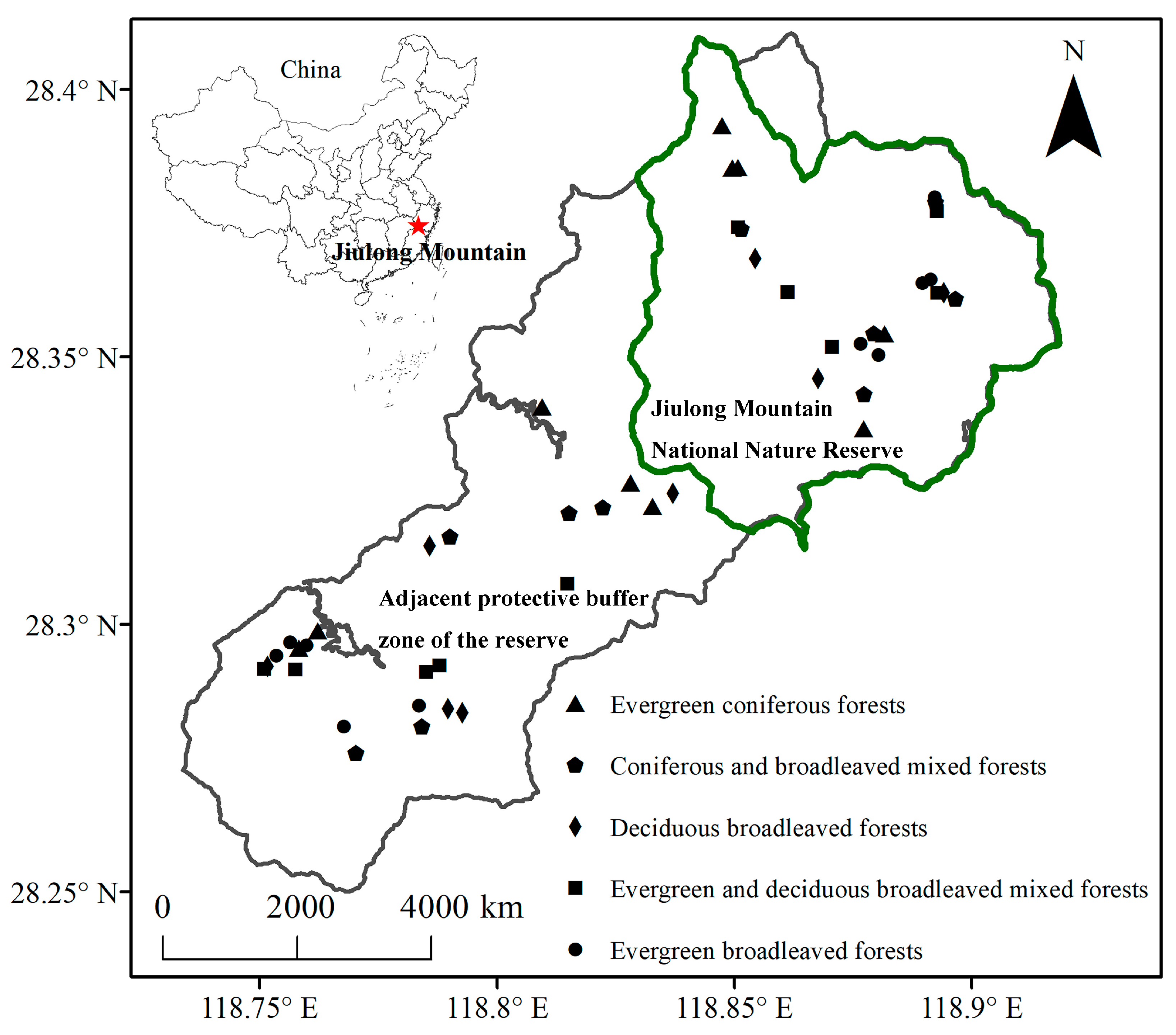
4.1. Study Area
4.2. History of Sampling Sites and Vegetation Survey
4.3. Functional Trait Measurements
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragao, L.E.O.C.; De Camargo, P.B.; Cerri, C.E.; Durigan, M.; De Oliveira, R.C.; Vieira, I.C.G.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Change Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef] [PubMed]
- Bebi, P.; Seidl, R.; Motta, R.; Fuhr, M.; Firm, D.; Krumm, F.; Conedera, M.; Ginzler, C.; Wohlgemuth, T.; Kulakowski, D. Changes of forest cover and disturbance regimes in the mountain forests of the Alps. For. Ecol. Manag. 2017, 388, 43–56. [Google Scholar] [CrossRef]
- Suarez, D.R.; Rozendaal, D.M.A.; De Sy, V.; Decuyper, M.; Málaga, N.; Montesinos, P.D.; Olivos, A.A.; Pai, R.D.; Martius, C.; Herold, M. Forest disturbance and recovery in Peruvian Amazonia. Glob. Change Biol. 2023, 29, 3601–3621. [Google Scholar]
- Smith, M.N.; Stark, S.C.; Taylor, T.C.; Schietti, J.; De Almeida, D.R.A.; Aragón, S.; Torralvo, K.; Lima, A.P.; De Oliveira, G.; De Assis, R.L.; et al. Diverse anthropogenic disturbances shift Amazon forests along a structural spectrum. Front. Ecol. Environ. 2023, 21, 24–32. [Google Scholar] [CrossRef]
- Krüger, K.; Senf, C.; Hagge, J.; Seidl, R. Setting aside areas for conservation does not increase disturbances in temperate forests. J. Appl. Ecol. 2025, 62, 1271–1281. [Google Scholar] [CrossRef]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-term forest degradation surpasses deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Zheng, Z.; Wang, Y.; Chen, J.; Ni, J.; Yu, M.; Zheng, W.; Liu, L. Plant life history strategies vary in subtropical forests with different disturbance histories: An assessment of biodiversity, biomass, and functional traits. Front. Plant Sci. 2024, 14, 1230149. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar]
- Walker, C. Patterns of forest disturbance. Nat. Plants 2024, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Acil, N.; Sadler, J.P.; Senf, C.; Suvanto, S.; Pugh, T.A.M. Landscape patterns in stand-replacing disturbances across the world’s forests. Nat. Sustain. 2025, 8, 86–98. [Google Scholar] [CrossRef]
- Loto, D.; Bravo, S. Species composition, structure, and functional traits in Argentine Chaco forests under two different disturbance histories. Ecol. Indic. 2020, 113, 106232. [Google Scholar] [CrossRef]
- Zemlerová, V.; Kozák, D.; Mikoláš, M.; Svitok, M.; Bače, R.; Smyčková, M.; Buechling, A.; Martin, M.; Larrieu, L.; Paillet, Y.; et al. Natural disturbances are essential determinants of tree-related microhabitat availability in temperate forests. Ecosystems 2023, 26, 1260–1274. [Google Scholar] [CrossRef]
- Maroschek, M.; Seidl, R.; Poschlod, B.; Senf, C. Quantifying patch size distributions of forest disturbances in protected areas across the European Alps. J. Biogeogr. 2024, 51, 368–381. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Xu, W.; Ma, Z.Y.; Jing, X.; He, J.S. Biodiversity and ecosystem multifunctionality: Advances and perspectives. Biodivers. Sci. 2016, 24, 55–71. [Google Scholar] [CrossRef]
- Devictor, V.; Mouillot, D.; Meynard, C.; Jiguet, F.; Thuiller, W.; Mouquet, N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world. Ecol. Lett. 2010, 13, 1030–1040. [Google Scholar] [CrossRef]
- Fu, R.; Dai, L.; Zhang, Z.; Hu, G. Community assembly along a successional chronosequence in the northern tropical karst mountains, South China. Plant Soil. 2023, 491, 317–331. [Google Scholar] [CrossRef]
- Tiwari, R.M.; Liu, J.; Xie, Y.; Yao, S.; Liu, S.; Wu, S.; Liu, J.; Qian, H.; Lei, Z.; Zhang, H.; et al. Decoupling the impact of biodiversity and environmental factors on the biomass and biomass growth of trees in subtropical forests. J. Plant Ecol. 2023, 16, rtac040. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Schweiger, O.; Klotz, S.; Durka, W.; Kühn, I. A comparative test of phylogenetic diversity indices. Oecologia 2008, 157, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hao, M.; He, H.; Zhang, C.; Zhao, X. Relationships between functional diversity and aboveground carbon sink functions and their changes with forest succession in Changbai Mountains, China. Chin. J. Plant Ecol. 2025, 49, 232–243. [Google Scholar]
- Bongers, F.; Poorter, L.; Hawthorne, W.D.; Sheil, D. The intermediate disturbance hypothesis applies to tropical forests, but disturbance contributes little to tree diversity. Ecol. Lett. 2009, 12, 798–805. [Google Scholar] [CrossRef]
- Baraloto, C.; Hérault, B.; Timothy Paine, C.E.; Massot, H.; Blanc, L.; Bonal, D.; Molino, J.F.; Nicolini, E.A.; Sabatier, D. Contrasting taxonomic and functional responses of a tropical tree community to selective logging. J. Appl. Ecol. 2012, 49, 861–870. [Google Scholar] [CrossRef]
- Martin, P.A.; Newton, A.C.; Pfeifer, M.; Khoo, M.; Bullock, J.M. Impacts of tropical selective logging on carbon storage and tree species richness: A meta-analysis. For. Ecol. Manag. 2015, 356, 224–233. [Google Scholar] [CrossRef]
- Yue, Q.; He, H.; Zhang, C.; Zhao, X.; Hao, M. Response of phylogenetic and functional diversity to harvesting disturbance in Korean pine-broadleaved forest. Acta Ecol. Sin. 2024, 44, 8617–8626. [Google Scholar]
- Ernst, R.; Linsenmair, K.E.; Rödel, M.O. Diversity erosion beyond the species level: Dramatic loss of functional diversity after selective logging in two tropical amphibian communities. Biol. Conserv. 2006, 133, 143–155. [Google Scholar] [CrossRef]
- De Avila, A.L.; Ruschel, A.R.; De Carvalho, J.O.P.; Mazzei, L.; Silva, J.N.M.; Do Carmo Lopes, J.; Araujo, M.M.; Dormann, C.F.; Bauhus, J. Medium-term dynamics of tree species composition in response to silvicultural intervention intensities in a tropical rain forest. Biol. Conserv. 2015, 191, 577–586. [Google Scholar] [CrossRef]
- Song, Y.; Chen, X.; Wang, X. Studies on evergreen broad-leaved forests of China: A retrospect and prospect. J. East. China Norm. Univ. 2005, 1, 1–8. [Google Scholar]
- Song, Y. China Evergreen Broad-Leaved Forest: Classification, Ecology, Conservation; Science Press: Beijing, China, 2013. [Google Scholar]
- Liu, L.; Xia, H.; Quan, X.; Wang, Y. Plant trait-based life strategies of overlapping species vary in different succession stages of subtropical forests, Eastern China. Front. Ecol. Evol. 2023, 10, 1103937. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Z.; Jiang, W.; Zheng, Q.; Jiang, X. The effects of human-caused disturbance on species diversity of forest community in northern Fujian Province. Biodivers. Sci. 1997, 5, 263–270. [Google Scholar]
- Yin, C. Disturbance-Dependent Variations in Species and Functional Diversity Relationships Across Spatial Scale and Vegetation Layers in Tiantong National Forest Park, Zhejiang Province. Master’s Thesis, East China Normal University, Shanghai, China, 2022. [Google Scholar]
- Song, K.; Mi, X.; Jia, Q.; Ren, H.; Bebber, D.; Ma, K. Variation in phylogenetic structure of forest communities along a human disturbance gradient in Gutianshan forest, China. Biodivers. Sci. 2011, 19, 190–196. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, L.; Jiao, J.; Lu, C.; Wang, Z.; Li, T.; Yuan, W.; Jiang, B.; Wu, C. Response of functional traits and functional diversity of woody plants to tending in a secondary subtropical evergreen broad-leaved forest. Acta Ecol. Sin. 2024, 44, 4733–4743. [Google Scholar]
- Horn, H.S. Markovian processes of forest succession. In Ecology and Evolution of Communities; Cody, K.L., Diamond, L.M., Eds.; Belknap Press: Cambridge, UK, 1975; pp. 196–213. [Google Scholar]
- Connell, J.H. Diversity in tropical rainforests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Wilson, J.B. Mechanisms of species coexistence: Twelve explanations for Hutchinson’s paradox of the plankton: Evidence from New Zealand plant communities. N. Z. J. Ecol. 1990, 13, 17–42. [Google Scholar]
- Meyer, P.; Schmidt, M.; Feldmann, E.; Willig, J.; Larkin, R. Long-term development of species richness in a central European beech (Fagus sylvatica) forest affected by windthrow—Support for the intermediate disturbance hypothesis? Ecol. Evol. 2021, 11, 12801–12815. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Huang, L.; Yu, Y.; An, X.; Yu, L.; Xue, Y. Leaf functional traits, species diversity and functional diversity of plant community in Tiankeng forests. Acta Ecol. Sin. 2022, 42, 10264–10275. [Google Scholar] [CrossRef]
- Chan, Y.K.S.; Ng, C.S.L.; Tun, K.P.P.; Chou, L.M.; Huang, D.W. Decadal decline of functional diversity despite increasing taxonomic and phylogenetic diversity of coral reefs under chronic urbanisation stress. Ecol. Indic. 2024, 164, 112143. [Google Scholar] [CrossRef]
- Gosper, C.R.; Tates, C.J.; Prober, S.M.; Jiao, F.; Li, Y.H.; Kallenbach, R.L. Floristic diversity in fire-sensitivity eucalypt woodlands shows a ‘U’-shaped relationship with time since fire. J. Appl. Ecol. 2013, 50, 1187–1196. [Google Scholar] [CrossRef]
- Rocha-Santos, L.; Faria, D.; Mariano-Neto, E.; Andrade, E.R.; Bomfim, J.A.; Talora, D.C.; Pessoa, M.S.; Cazetta, E. Taxonomic, phylogenetic and functional response of plant communities in different life-stages to forest cover loss. Perspect. Ecol. Conser. 2023, 21, 136–142. [Google Scholar] [CrossRef]
- Akber, M.A.; Shrestha, R.P. Land use change and its effect on biodiversity in Chiang Rai province of Thailand. J. Land Use Sci. 2013, 1, 807315. [Google Scholar] [CrossRef]
- Schipper, A.M.; Hilbers, J.P.; Meijer, J.P.; Antao, L.H.; Benitez-Lopez, A.; De Jonge, M.M.J.; Leemans, L.H.; Scheper, E.; Alkemade, R.; Doelman, L.H.; et al. Projecting terrestrial biodiversity intactness with GLOBIO 4. Glob. Change Biol. 2020, 26, 760–771. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China (MEEC). China National Biodiversity Conservation Strategy and Action Plan; China Environmental Science Press: Beijing, China, 2011.
- Li, R. The national conservation plants in Jiulongshan Nature Reserve and their principal characteristics. J. Jiangsu For. Sci. Technol. 2008, 35, 27–30, 38. [Google Scholar]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Niu, H.; Cui, X.; Wang, S.; Wang, Y. Three common errors in Chinese ecological experimental design and interpretation. Acta Ecol. Sin. 2009, 29, 3901–3910. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.T.; Morgan, H.D.; Heijden, M.G.A.V.D.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; Kembel, S.W. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 2008, 24, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picanter: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
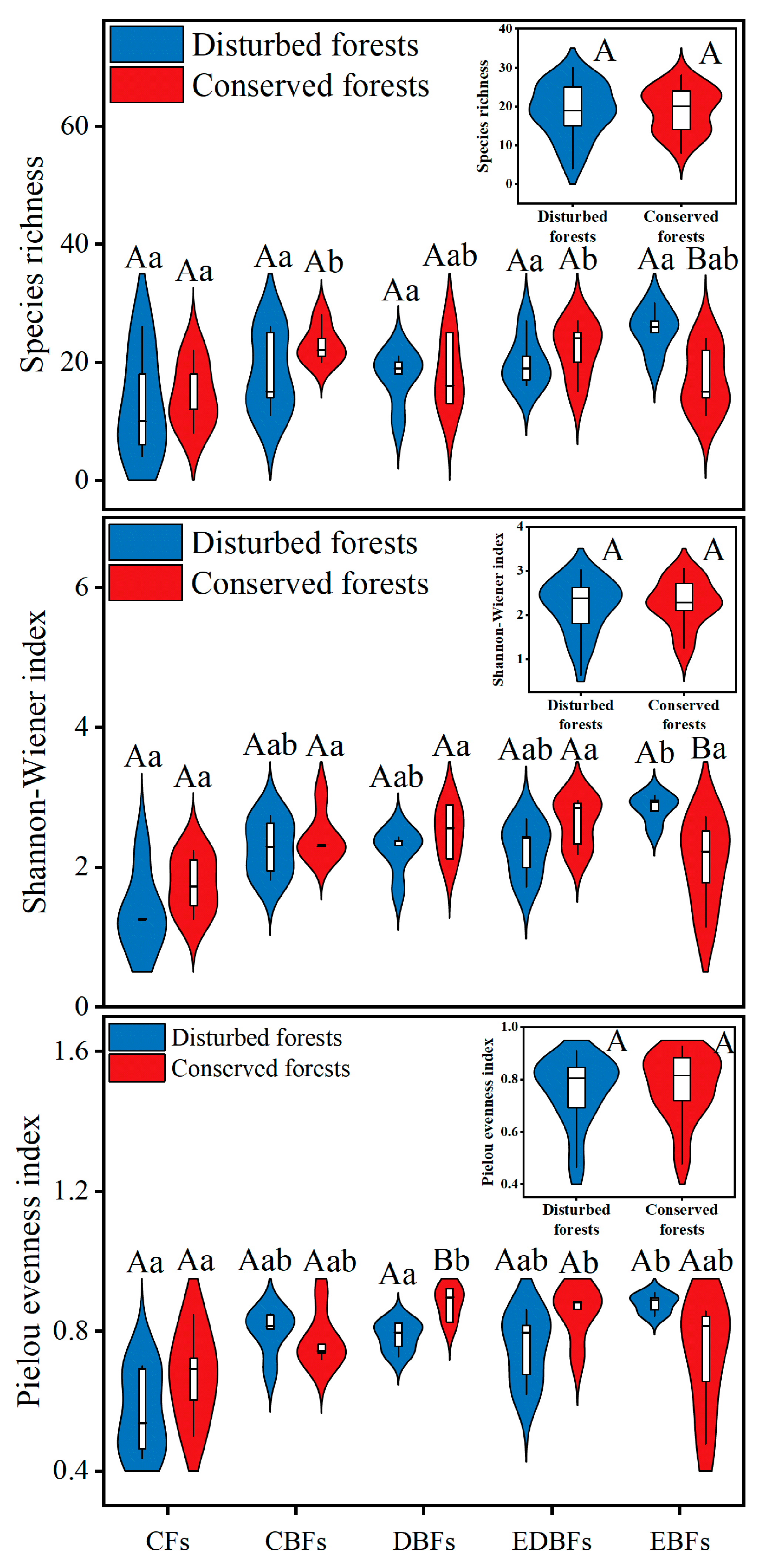
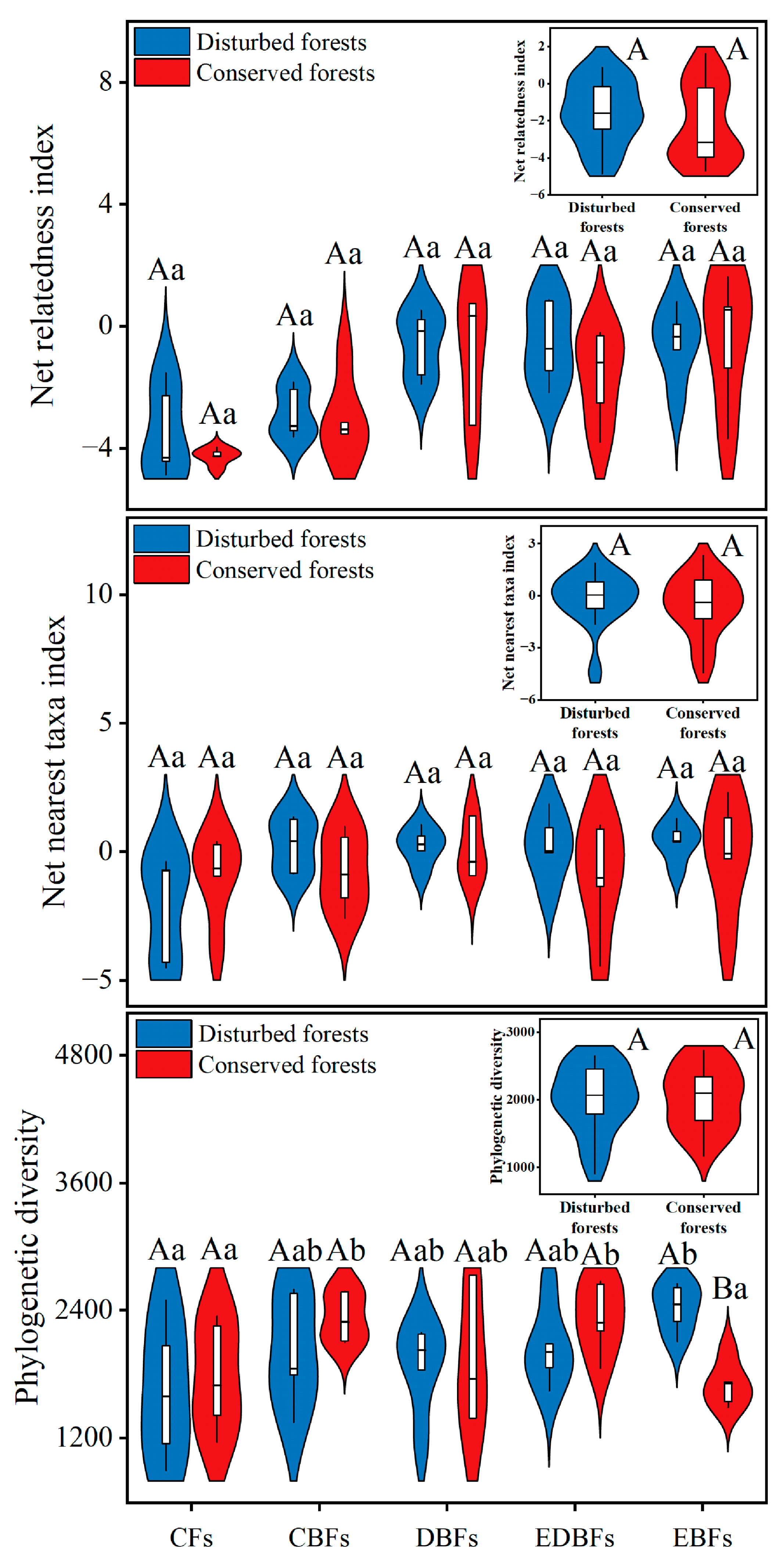
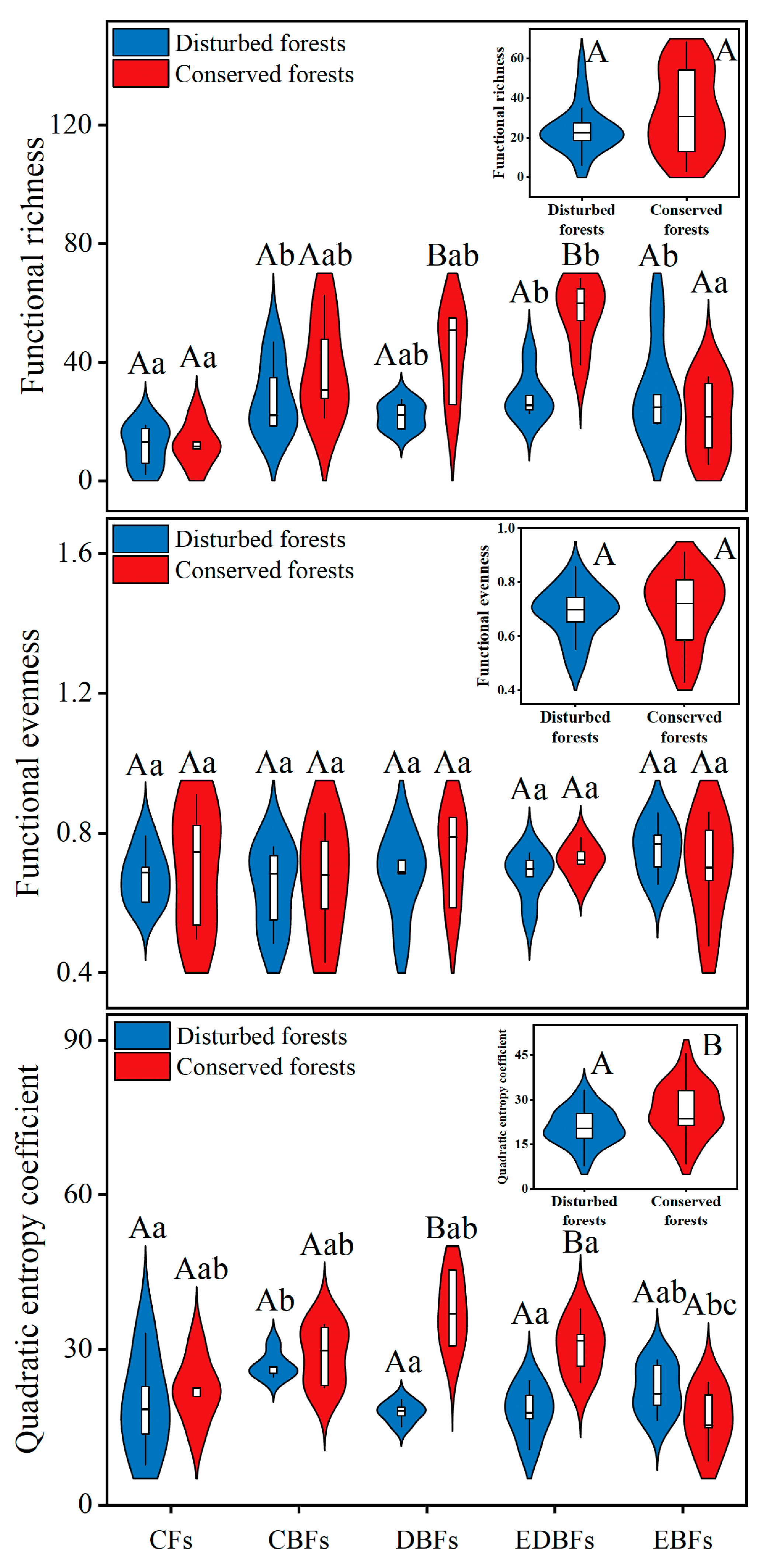
| Index | Calculation | Meaning of Terms |
|---|---|---|
| Species richness (R) | S is the total number of species Pi is the proportion of total abundance of the ith species i is a species within the community | |
| Shannon–Wiener index (H) | ||
| Pielou evenness index (E) | ||
| Net relatedness index (NRI) | MPDs and MNTDs are the observed mean pairwise distance (MPD) values and the mean nearest taxon distance (MNTD) values, respectively MPDmds and MNTDmds are the simulated MPD values and MNTD values, respectively, which are derived from 999 random simulations conducted under the null model SD is the standard deviation C is the sum of shortest path branch lengths of phylogenetic trees composed of all species in the study area c is a branch of C Lc is the branch length of c | |
| Net nearest taxa index (NTI) | ||
| Phylogenetic diversity (PD) | ||
| Functional richness (FRic) | SFic is the niche space occupied by species within the community Rc is the absolute range of trait c S is the total number of species PEWi is the weighted uniformity of specie i dij is the overlap of the probability density function of trait for species i and j Pi and Pj are the proportion of the individuals of species i and species j to the total number of species within the community | |
| Functional evenness (FEve) | ||
| Quadratic entropy coefficient (RaoQ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Guan, X.; Wang, Y.; Chen, J.; Liu, J.; Yu, S.; Zheng, Z.; Yu, M. Intermediate Anthropogenic Disturbances Boost Taxonomic and Phylogenetic Diversities but Reduce Functional Diversity in Subtropical Forests of Eastern China. Plants 2025, 14, 2529. https://doi.org/10.3390/plants14162529
Liu L, Guan X, Wang Y, Chen J, Liu J, Yu S, Zheng Z, Yu M. Intermediate Anthropogenic Disturbances Boost Taxonomic and Phylogenetic Diversities but Reduce Functional Diversity in Subtropical Forests of Eastern China. Plants. 2025; 14(16):2529. https://doi.org/10.3390/plants14162529
Chicago/Turabian StyleLiu, Libin, Xiaoyin Guan, Yunquan Wang, Jianhua Chen, Julian Liu, Shuisheng Yu, Zihong Zheng, and Mingjian Yu. 2025. "Intermediate Anthropogenic Disturbances Boost Taxonomic and Phylogenetic Diversities but Reduce Functional Diversity in Subtropical Forests of Eastern China" Plants 14, no. 16: 2529. https://doi.org/10.3390/plants14162529
APA StyleLiu, L., Guan, X., Wang, Y., Chen, J., Liu, J., Yu, S., Zheng, Z., & Yu, M. (2025). Intermediate Anthropogenic Disturbances Boost Taxonomic and Phylogenetic Diversities but Reduce Functional Diversity in Subtropical Forests of Eastern China. Plants, 14(16), 2529. https://doi.org/10.3390/plants14162529







